Abstract
Background:
Enhanced recovery after surgery (ERAS) protocols are a series of perioperative care to optimize preoperative preparation, prevent postoperative complications, minimize stress, and speed up recovery. This study aimed to assess the impact of ERAS protocols for functional endoscopic sinus surgery (FESS) in patients with chronic rhinosinusitis with nasal polyps (CRSwNP).
Methods:
One hundred and two patients with CRSwNP undergoing FESS were randomly divided into the ERAS group and the control group. The outcomes of the Self-Rating Anxiety Scale (SAS), Visual Analogue Scale (VAS), Medical Outcomes Study Sleep Scale (MOS-SS) and Kolcaba Comfort Scale Questionnaire (GCQ) were determined in both groups. The serum levels of C-reactive protein (CRP) were compared preoperatively and 24 hours postoperatively.
Results:
The ERAS group had a significantly better SAS scores than did the control group (28 [24, 35] vs. 43 [42, 47], Z = 5.968, P < 0.001). The rhinalgia and headache scores at 2, 24 and 48 hours postoperatively were lower in the ERAS group than that in the control group (all P < 0.001). The outcomes of the MOS-SS (43 [42, 39] vs. 28 [22, 35], Z = 7.071, P < 0.001) and GCQ (76 [68, 87] vs. 64 [50, 75], Z = 4.806, P < 0.001) were significantly different between the two groups. No significant difference was found in the preoperative CRP levels between the two groups (1.3 [0.6, 2.8] vs. 0.5 [0.5, 1.2], Z = 3.049, P > 0.05); However, the CRP level in 24 hours postoperatively was significantly lower in the ERAS group than that in the control group (2.5 [1.4, 3.9] vs. 6.6 [3.8, 9.0], Z = 5.027, P < 0.001). The incidence rates of complications, such as nausea/emesis (χ2 = 0.343, P > 0.05), hemorrhage, aspiration and tumble, were not increased in the ERAS group compared with those in the control group. The ERAS group had a significantly shorter length of hospital stay (5 [4, 5] days vs. 8 [8,9] days, Z = 8.939, P < 0.001) and hospitalization expenses ($ 2670 [2375, 2740] vs. $3129 [3116, 3456], Z = 8.514, P < 0.001).
Conclusions:
ERAS protocols might optimize FESS for patients with CRSwNP by reducing psychological and physical stress, shortening the length of hospital stay and lowering hospitalization expenses without increasing postoperative complications.
Trial registration:
Chinese Clinical Trial Registry, No. ChiCTR1800015791; http://www.chictr.org.cn/showproj.aspx?proj=26872
Keywords: Enhanced recovery after surgery, Chronic rhinosinusitis, Perioperative period, Quality of life
Introduction
Enhanced recovery after surgery (ERAS) is a series of optimized protocols adopted in the perioperative phase to reduce psychological and physical stress reactions. The hospitalization experience and quality of life can be potentially improved, and the total length of hospital stay and hospitalization expenses can be reduced.[1] Recently, ERAS protocols have been introduced in orthopedics, cardiothoracic surgery, gynecology and obstetrics, urinary and general surgery. However, ERAS protocols are still being developed in China, and very few reports of ERAS in Otolaryngology have been published.
Chronic rhinosinusitis (CRS) is a chronic local inflammatory disease with a relatively high incidence. The global incidence of CRS ranges between 5% and 12%,[2] and the incidence in China ranges between 2% and 8%.[3] CRS can be divided into 2 types: chronic rhinosinusitis with nasal polyps (CRSwNP) and chronic rhinosinusitis without nasal polyps (CRSsNP). The most commonly recommended treatment for CRSwNP is comprehensive treatment centering on functional endoscopic sinus surgery (FESS). Our study was designed to explore the benefits of ERAS protocols in patients with CRSwNP undergoing FESS.
Methods
Ethical approval
The study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (No. [2018]02-011-01) and registered with the Chinese Clinical Trial Registry (No. ChiCTR1800015791). Informed consent forms were obtained from all patients.
Subjects
One hundred and two patients undergoing FESS were collected from January 2018 to April 2018 in the Department of Otolaryngology at the Third Affiliated Hospital of Sun Yat-sen University. The subjects were divided randomly into the ERAS group and the control group using a random number table. The ERAS group included 38 males and 14 females between 20 and 59 years of age with an average age of (40.0 ± 10.0) years, and the control group included 39 males and 11 females between 18 and 57 years of age with an average age of (38.8 ± 10.0) years.
CRSwNP was diagnosed according to the European Position Paper on Rhinosinusitis and Nasal Polyps 2012 (EPOS2012).[4]
Inclusion criteria: patients confirmed CRSwNP by pathology; patients presented with different degrees of nasal congestion, snot, headache, hyposmia and other clinical symptoms; failure of conservative treatment; patients had no history of FESS before and performed FESS by the same surgeon, and the outcomes of the questionnaires were collected by the same researcher; age between 18 and 60 years.
Exclusion criteria: patients suffered from nasal diseases including ciliary dysfunction, cystic fibrosis or granulomatous diseases; patients had chronic diseases including hypertension, tuberculosis, heart disease, asthma, or psychological disorders who are unsuitable for surgery.
Additionally, patients were excluded if the required data were not completely collected or the treatment failed to progress during the study.
Protocols for ERAS group
ERAS group was scheduled for optimized perioperative treatment [Table 1].
Table 1.
Enhanced recovery after surgery programs include a combination of elements of perioperative care for elective surgery with an aim to optimize preoperative preparation, prevent postoperative complications, minimize stress and speed up recovery
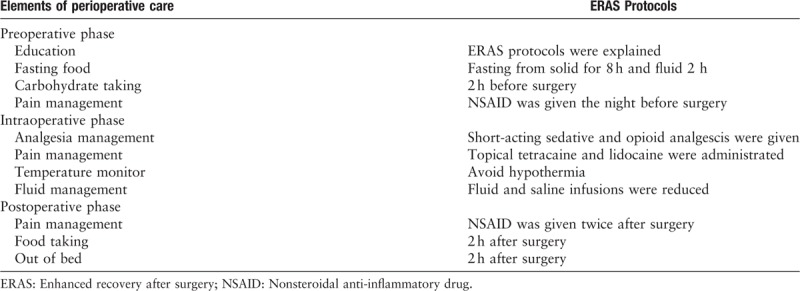
Preoperative treatment
A presurgical verbal explanation of ERAS treatment and preoperative counseling were given to each patient in this group. Antianxiety drugs were administered to improve sleeping quality as needed. If the patient had no contraindications, a nonsteroidal anti-inflammatory drug (NSAID; i.e., 80 mg of loxoprofen) was administered the night before surgery to induce preventive analgesia. Before the patients were taken to the operating room, they fasted for 8 hours from solids and 2 hours from fluids and had a carbohydrate drink (Outfast, 300 mL or 5 mL/kg, Yichang Renfu Pharmaceutical Co. Ltd, China) 2 hours before surgery.
Intraoperative treatment
A preventive antibiotic was given 30 minutes before surgery. Short-acting sedatives and short-acting opioid analgesics were given during surgery. Topical tetracaine anesthesia and local lidocaine infiltration anesthesia were applied to the nasal mucosa before surgery. The body temperature was monitored to avoid intraoperative hypothermia (<36°C). The intraoperative fluid volume was also restricted. The crystalloid solution was reduced when moderate colloid fluid was given. After the operation was finished, degradable hemostatic material (i.e., Nasopore) was used for nasal packing.
Postoperative treatment
Bed rest, electrocardiograph monitoring and oxygen inhalation therapy were given for 2 hours. A NSAID (i.e., 50 mg of flurbiprofen axetil by intravenous injection) for preventive analgesia was given at 2 hours and 12 hours postoperatively. After 2 hours, the patients were encouraged to have warm and soft food; the patients could increase the amount and frequency of food intake according to their gastrointestinal tract tolerance. They were also encouraged to engage in out-of-bed activities that were guided according to their recovery conditions.
Protocols for control group
The control group was scheduled for traditional perioperative treatment.
Preoperative treatment
An ordinary presurgical explanation was given to each patient in this group. Psychological counseling and antianxiety drugs were administered to improve sleep when necessary. No preventive analgesia measures were taken. According to the ordinary preoperative fasting guidelines, the patients fasted from both food and fluids for at least 8 hours before surgery.
Intraoperative treatment
A preventive antibiotic was given 30 minutes before surgery. A long-acting sedative and long-acting opioid analgesic were given during the operation. Traditional treatment was applied to the nasal mucosa before surgery. The intraoperative fluid volume and body temperature were not monitored. The volume was guided by preoperative evaluation of the patients. After the operation was finished, non-degradable hemostatic material (i.e., Merocel) was used for nasal packing.
Postoperative treatment
Bed rest, electrocardiograph monitoring and oxygen inhalation therapy were given for at least 6 hours. No preventive analgesia was given after the operation except as needed. After 6 hours, the patients were allowed to take food and fluids and engage in out-of-bed activities under guidance according to their conditions.
Observation indices
Questionnaires
Questionnaires including the Self-Rating Anxiety Scale (SAS), Kolcaba General Comfort Questionnaire (GCQ) and Medical Outcomes Study Sleep Scale (MOS-SS) were given to each patient, and the same guidance was offered. The data were collected after the questionnaires were completed.
Visual analogue scale
The Visual Analogue Scale was used to assess the patients’ rhinalgia and headache symptoms at 2, 24, and 48 hours postoperatively.
C-reactive protein
C-reactive protein (CRP) was obtained from the serum of patients before the operation and 24 hours postoperatively to evaluate the inflammatory condition.
Postoperative complications
Postoperative complications, such as nausea/emesis, hemorrhage, aspiration and dizziness, were treated and recorded if they were observed. The total length of the hospital stay and the hospitalization expenses were calculated upon discharge.
Discharge criteria
The ERAS group adopted an ERAS criterion. The patients resumed their regular diets completely, and no infusion treatment was needed; the patients finished the first postoperative endoscopic cleaning of the nasal cavities on the second day; the patients had no complications and could mobilize adequately compared to their preoperative ability; the patients agreed to continue rehabilitation treatment and postoperative follow-up visits.
The control group adopted an ordinary criterion. The patients were discharged on the fourth or fifth day with no complications, the day after the first postoperative endoscopic cleaning of the nasal cavities.
Statistical analysis
Values were expressed as mean ± standard deviation, median (quantile), or n. The IBM-SPSS v.20 (SPSS Inc., Chicago, IL, USA) statistical software package was used for data processing and analysis. Normal distributed continuous variables were expressed as mean and standard deviation, and group differences were evaluated through independent sample t-test, otherwise, median and interquartile range and non-parametric test were used respectively. Categorical data (such as the incidence of postoperative complications) were expressed as frequency and analyzed by the Chi-square test. P < 0.05 was considered statistically significant.
Results
Preoperative psychological states
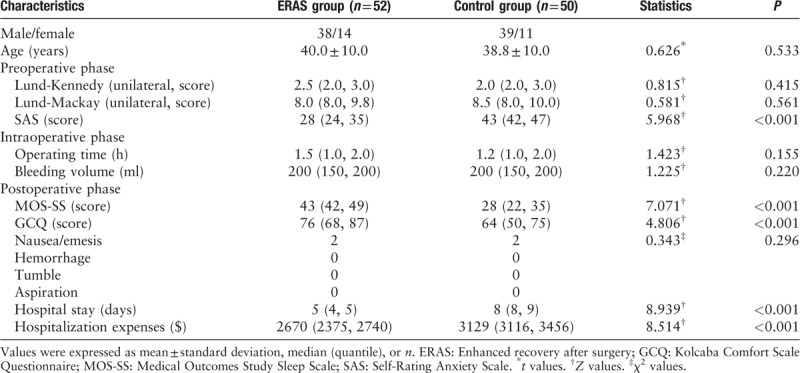
Based on the SAS scores, patients in the ERAS group had a significantly better psychological states than did patients in the control group (28 [24, 35] vs. 43 [42, 47], Z = 5.968, P < 0.001) [Table 2].
Table 2.
Characteristics of patients in the ERAS group and control group and data analysis in the perioperative phase
Postoperative pain
The VAS was used to evaluate the degree of pain. Numbers from 0 to 10 were used to show the degree of pain (ranked as 0–3 mild, 4–7 moderate, and >7 severe). Scores from 0 to 5 indicated that the pain did not reduce the quality of life, whereas scores larger than 5 indicated a reduction in the quality of life.
According to our results, patients in the ERAS group enjoyed a lower pain level. The median and interquartile range of rhinalgia scores of the ERAS group at 2 hours 1 (0, 1), 24 hours 1 (0, 1) and 48 hours 0 (0, 1) postoperatively were lower than those of the control group (all P < 0.001), which were 3 (3, 4), 2 (2, 3) and 2 (1, 2) at 2 hours, 24 hours and 48 hours postoperatively, respectively. The ERAS group also had lower headache scores (1 [0, 1], 1 [0, 1] and 0 [0, 1]) than did the control group (2 [2, 3], 2 [1, 2] and 1 [1, 2]) (all P < 0.001).
Postoperative sleep quality
Patients in the ERAS group enjoyed improved sleep quality due to optimized pain management. The MOS-SS was used to evaluate sleep quality. The scores were significantly higher for the ERAS group than for the control group (43 [42, 39] vs. 28 [22, 35], Z = 7.071, P < 0.001) [Table 2].
Postoperative comfort level
The ERAS group had a higher comfort level due to optimized pain management. The 4 parts of the GCQ were used to evaluate the patients’ comfort levels. The ERAS group had significantly higher scores than did the control group (76 [68, 87] vs. 64 [50, 75], Z = 4.806, P < 0.001) [Table 2].
CRP level
The stress reaction was reduced in the ERAS group due to optimized pain management, improved sleep quality and a higher comfort level. The ERAS group had a higher preoperative CRP level 1.3 (0.6, 2.8) mg/L than that of the control group 0.5 (0.5, 1.2) mg/L (Z = 3.049, P > 0.05). However, the ERAS group had a significantly lower 24 hours postoperative CRP level 2.5 (1.4, 3.9) mg/L than did the control group 6.6 (3.8, 9.0) mg/L (Z = 5.027, P < 0.001) [Figure 1].
Figure 1.
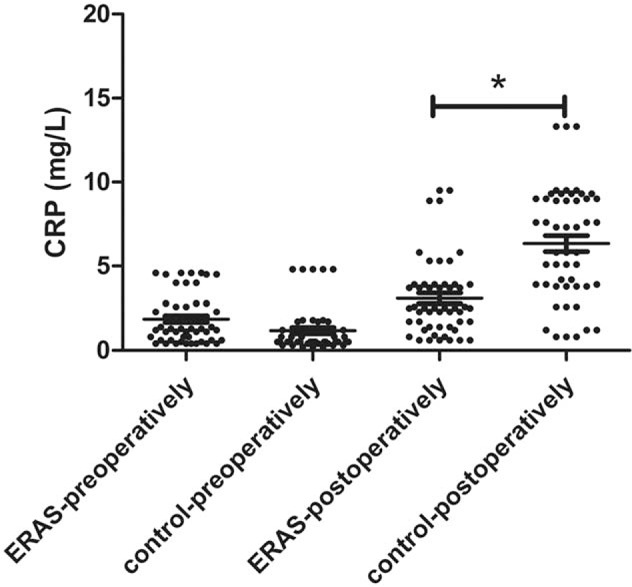
CRP levels preoperatively and postoperatively in the ERAS and control groups showing that the ERAS group had a lower postoperative CRP level than did the control group (∗P < 0.05) (the ERAS group: n = 52; the control group: n = 50). CRP: C-reactive protein; ERAS: Enhanced recovery after surgery.
Postoperative complications
After bed rest with electrocardiograph monitoring and oxygen inhalation therapy for 2 hours, the patients in the ERAS group were encouraged to engage in out-of-bed activities and enjoy soft and warm food according to their recovery status. Conversely, the control group received traditional postoperative treatment. During this period, complications may occur, such as nausea/emesis, hemorrhage, aspiration and dizziness. Two patients from both the ERAS and control groups suffered from nausea (χ2 = 0.343, P > 0.05). No other complications were observed [Table 2].
Total length of hospital stay and hospitalization expenses
According to the results, the ERAS group had a significantly shorter hospital stay than did the control group (5 [4, 5] days vs. 8 [8, 9] days, Z = 8.939, P < 0.001). The hospitalization expenses of the ERAS group were $2670 (2375, 2740), whereas those of the control group were $3129 (3116, 3456) (Z = 8.514, P < 0.001) [Table 2].
Discussion
ERAS protocols are multidisciplinary treatments that aim to improve perioperative patient experiences and outcomes after major elective surgery. The approach was pioneered by Kehlet[5,6] in Denmark and was originally described as an optimal method for the treatment of patients following colorectal surgery. ERAS programs have been applied successfully in various fields of surgery, including orthopedics, cardiothoracic surgery, gynecology and obstetrics, urinary, and general surgery.
The overall strategy of ERAS protocols is three-fold, to optimize the patient's health status before surgery; to provide protocolized evidenced-based care throughout the hospital stay; and to offer the best possible rehabilitation. Substantial level I evidence has shown that implementation of ERAS programs after colorectal resections is associated with faster recovery, reduced complication rates, and shortened primary and overall lengths of hospital stay compared with traditional approaches.[7,8]
Recently, ERAS protocols have been extended and introduced to head and neck oncological patients undergoing surgery.[9–12] However, the application of ERAS in FESS for sinonasal disease has not been reported previously. FESS is the recommended treatment for CRSwNP for relief of symptoms, such as nasal congestion, headache and sneezing, but this approach has disadvantages, including postoperative pain and high hospitalization expenses. Widely used nasal endoscopic techniques, minimally invasive techniques and evidence-based medicine provide probability and feasibility for the application of ERAS protocols in FESS. In our study, ERAS protocols may have optimized FESS for CRSwNP patients by reducing psychological and physical stress, shortening the length of hospital stay and reducing hospitalization expenses without increasing postoperative complications.
Implementation of ERAS protocols in FESS can reduce the preoperative psychological stress reaction. Patients suffer from fear and anxiety preoperatively, which may lead to unpleasant stress reactions and unsatisfactory postoperative recovery. An individualized explanation has been reported to be an independent prognostic factor for the successful implementation of ERAS protocols.[13] In the perioperative phase of our study, an explanation of ERAS protocols and psychological counseling were offered. The details of the minimally invasive treatment, analgesic treatment for anesthesia and perioperative phase were provided to patients to relieve their anxiety and fears and to acquire the trust and cooperation of both the patients and their families. We used SAS to evaluate preoperative psychological anxiety and found that the ERAS group had a better psychological status than did the control group.
Implementation of ERAS protocols in FESS can reduce the systemic stress reaction. The original long-term fasting, postoperative pain and injury associated with FESS can aggravate the stress reaction, which may affect postoperative recovery. For ordinary surgery under general anesthesia, patients need to fast for longer than 8 hours preoperatively and must fast for 6 hours postoperatively. Lying without a pillow for 6 hours postoperatively is also required, resulting in hunger and dysphoria. ERAS protocols can optimize the endoscopic perioperative treatment, improve bowel preparation and gastrointestinal function recovery and relieve hunger and dysphoria. These protocols can even reduce the total length of hospital stay by reducing inflammatory reactions.[14] ERAS protocols recommend a preoperative carbohydrate drink (≤400 ml).[15] In our study, patients in the ERAS group fasted for 8 hours for solids and 2 hours for fluids and were given a carbohydrate drink 2 hours before surgery. Two hours after the operation was finished, the patients were encouraged to eat carbohydrate-rich foods and to take fluids according to their conditions; thus, the ERAS group patients experienced less hunger and dysphoria.
Implementation of ERAS protocols in FESS can optimize postoperative pain management and reduce physiological stress. The pain induced by FESS was the most unpleasant feeling experienced in the hospital. Helping patients endure the perioperative phase without pain is an important objective. During the implementation of ERAS protocols, pain management with effective pain control (VAS≤3) based on preventive and multimodal analgesia [16] enabled the patients to take foods and fluids and engage in out-of-bed activities in the early stages.[17] With the provision of local and general analgesia during the perioperative stage, the VAS scores for rhinalgia and headache in the ERAS group were less than 3 at 2, 24, and 48 hours postoperatively and were significantly lower than the scores of the control group. In addition, with strengthened perioperative pain management, their sleep quality and comfort level were significantly better than those of the control group.
Implementation of ERAS protocols in FESS can reduce postoperative systemic inflammatory reactions. Although FESS provides benefits in terms of a short operation time and less tissue injury, general anesthesia and the psychological stress reaction remain important factors in systemic inflammatory reactions.[18] CRP plays an important role in postoperative systemic inflammatory reactions and is regarded as an important inflammatory marker.[19,20] The CRP levels of the ERAS and the control groups revealed that the ERAS protocols reduced postoperative psychological and systemic stress.
Implementation of ERAS protocols in FESS can reduce hospital stay and hospitalization expenses. Compared with traditional approaches, ERAS has been reported to reduce complication rates and shorten the primary and overall lengths of hospital stay in patients undergoing head and neck surgery.[9,11] In our study, new discharge criteria were adopted. Thus, the ERAS protocols effectively reduced hospital stay and hospitalization expenses without increasing the incidence of postoperative complications.
In this study, the short-term prognosis of ERAS in perioperative period was better than the control group. However, the time of ERAS implementation in FESS is not long, and the effects of ERAS on patients’ long-term prognosis have not been stated. Next we will continue to follow up the patients and collect the data to describe the long-time prognosis.
In conclusion, we showed that ERAS protocols might optimize FESS by reducing psychological and physical stress reactions, the length of the hospital stay and hospitalization expenses without increasing postoperative complications.
Acknowledgement
We would like to thank Prof. Ge-Hua Zhang for the important advice on this study.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81670912), Science and Technology Planning Project of Guangdong Province (No. 2014A020212138), and Industry-Academic Cooperation Foundation of Guangzhou (No. 201704030046).
Conflicts of interest
None.
Footnotes
How to cite this article: Wu XF, Kong WF, Wang WH, Yuan LX, Xu HQ, Qi M, Zhou SL, Yang QT. Enhanced recovery after surgery protocols in functional endoscopic sinus surgery for patients with chronic rhinosinusitis with nasal polyps: A randomized clinical trial. Chin Med J 2019;00. doi: 10.1097/CM9.0000000000000060
References
- 1.Chinese Society of Surgery, Chinese Society of Anesthesiology. The Chinese expert consensus and clinical pathway guidelines on ERAS 2018 (in Chinese). Chin J Pract Surg 2018; 38:1–20. doi: 10.19538/j.cjps.issn1005-2208.2018.01.01. [Google Scholar]
- 2.Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol 2015; 136:1431–1440. doi: 10.1016/j.jaci.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Shi JB, Fu QL, Zhang H, Cheng L, Wang YJ, Zhu DD, et al. Epidemiology of chronic Rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy 2015; 70:533–539. doi: 10.1111/all.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012; 50:1–12. doi: 10.4193/Rhino50E2. [DOI] [PubMed] [Google Scholar]
- 5.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997; 78:606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 6.Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg 1999; 86:227–230. doi: 10.1046/j.1365-2168.1999.01023.x. [DOI] [PubMed] [Google Scholar]
- 7.Eskicioglu C, Forbes SS, Aarts MA, Okrainec A, McLeod RS. Enhanced recovery after surgery (ERAS) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointest Surg 2009; 13:2321–2329. doi: 10.1007/s11605-009-0927-2. [DOI] [PubMed] [Google Scholar]
- 8.Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 2010; 29:434–440. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Coyle MJ, Main B, Hughes C, Craven R, Alexander R, Porter G, et al. Enhanced recovery after surgery (ERAS) for head and neck oncology patients. Clin Otolaryngol 2015; 41:118–126. doi: 10.1111/coa.12482. [DOI] [PubMed] [Google Scholar]
- 10.Gemma M, Toma S, Luce FL, Beretta L, Braga M, Bussi M. Enhanced recovery program (ERP) in major laryngeal surgery: building a protocol and testing its feasibility. Acta Otorhinolaryngol Ital 2017; 37:475–478. doi: 10.14639/0392-100X-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dort JC, Farwell DG, Findlay M, Huber GF, Kerr P, Shea-Budgell MA, et al. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction: a consensus review and recommendations from the enhanced recovery after surgery society. JAMA Otolaryngol Head Neck Surg 2017; 143:292–303. doi: 10.1001/jamaoto.2016.2981. [DOI] [PubMed] [Google Scholar]
- 12.McMahon J, Handley TPB, Bobinskas A, Elsapagh M, Anwar HS, Ricciardo PV, et al. Postoperative complications after head and neck operations that require free tissue transfer - prevalent, morbid, and costly. Br J Oral Maxillofac Surg 2017; 55:809–814. doi: 10.1016/j.bjoms.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Aarts MA, Okrainec A, Glicksman A, Pearsall E, Victor JC, McLeod RS. Adoption of enhanced recovery after surgery (ERAS) strategies for colorectal surgery at academic teaching hospitals and impact on total length of hospital stay. Surg Endosc 2012; 26:442–450. doi: 10.1007/s00464-011-1897-5. [DOI] [PubMed] [Google Scholar]
- 14.Nygren J, Thorell A, Ljungqvist O. Preoperative oral carbohydrate therapy. Curr Opin Anaesthesiol 2015; 28:364–369. doi: 10.1097/ACO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 15.Feldheiser A, Aziz O, Baldini G, Cox BP, Fearon KC, Feldman LS, et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 2016; 60:289–334. doi: 10.1111/aas.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vadivelu N, Mitra S, Schermer E, Kodumudi V, Kaye AD, Urman RD. Preventive analgesia for postoperative pain control: a broader concept. Local Reg Anesth 2014; 7:17–22. doi: 10.2147/LRA.S62160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am 2015; 95:301–318. doi: 10.1016/j.suc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Cabellos OM, Martinez ML, Torralba M, Rodriguez FJR, Atance MJC. C-reactive protein as a marker of the surgical stress reduction within an ERAS protocol (enhanced recovery after surgery) in colorectal surgery: a prospective cohort study. J Surg Oncol 2018; 117:717–724. doi: 10.1002/jso.24909. [DOI] [PubMed] [Google Scholar]
- 19.Mari G, Crippa J, Costanzi A, Mazzola M, Rossi M, Maggioni D. ERAS protocol reduces IL-6 secretion in colorectal Laparoscopic surgery: Results from a randomized clinical trial. Surg Laparosc Endosc Percutan Tech 2016; 26:444–448. doi: 10.1097/SLE.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Tovar J, Munoz JL, Gonzalez J, Garcia A, Ferrigni C, Jimenez M, et al. C-reactive protein, fibrinogen, and procalcitonin levels as early markers of staple line leak after laparoscopic sleeve gastrectomy in morbidly obese patients within an enhanced recovery after surgery (ERAS) program. Surg Endosc 2017; 31:5283–5288. doi: 10.1007/s00464-017-5602-1. [DOI] [PubMed] [Google Scholar]


