Abstract
Background:
Eucommia ulmoides Oliv. is a medicinal plant native to China, with its bark (Eucommiae Cortex) traditionally being used for medicinal purposes. Previous research has shown that Eucommia male flowers can exert anti-inflammatory, analgesic, antibacterial, and other pharmacological effects, including immune regulation. This study explored the anti-inflammatory effects of the 70% ethanol extract of male flowers (EF) of E. ulmoides in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells and LPS-administered mice.
Methods:
Cytotoxicity of EF for RAW 264.7 cells was investigated using Cell Counting Kit-8. The production of proinflammatory mediators, nitric oxide (NO), tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 was determined using enzyme-linked immunosorbent assays. IL-17, IL-23, and IL-10 mRNA levels were determined using quantitative real-time polymerase chain reaction. Activation of the nuclear factor (NF)-κB pathway in RAW 264.7 cells was investigated via Western blotting. In vivo anti-inflammatory effects of EF were studied in an LPS-induced acute inflammation mouse model by analyzing lung tissue histopathology, serum TNF-α and IL-6 levels, and myeloperoxidase (MPO) activity in lung tissue.
Results:
EF showed no significant cytotoxicity at concentrations from 10 to 60 μg/mL (cell viability > 80%) in the CCK-8 cell viability assay. EF inhibited the RAW 264.7 cell proliferation (EF 60 μg/mL, 120 μg/mL, and 250 μg/mL vs. negative control: 87.31 ± 2.39% vs. 100.00 ± 2.50%, P = 0.001; 79.01 ± 2.56 vs. 100.00 ± 2.50%, P < 0.001; and 64.83 ± 2.50 vs. 100.00 ± 2.50%, P < 0.001), suppressed NO (EF 20 μg/mL and 30 μg/mL vs. LPS only, 288.81 ± 38.01 vs. 447.68 ± 19.07 μmol/L, P = 0.004; and 158.80 ± 45.14 vs. 447.68 ± 19.07 μmol/L, P < 0.001), TNF-α (LPS+EF vs. LPS only, 210.20 ± 13.85 vs. 577.70 ± 5.35 pg/mL, P < 0.001), IL-1β (LPS+EF vs. LPS only, 193.30 ± 10.80 vs. 411.03 ± 42.28 pg/mL, P < 0.001), and IL-6 (LPS+EF vs. LPS only, 149.67 ± 11.60 vs. 524.80 ± 6.24 pg/mL, P < 0.001) secretion, and downregulated the mRNA expression of IL-17 (LPS+EF vs. LPS only, 0.23 ± 0.02 vs. 0.43 ± 0.12, P < 0.001), IL-23 (LPS+EF vs. LPS only, 0.29 ± 0.01 vs. 0.42 ± 0.06, P=0.002), and IL-10 (LPS+EF vs. LPS only, 0.30 ± 0.01 vs. 0.47 ± 0.01, P=0.008) in LPS-stimulated RAW 264.7 cells. EF inhibited the LPS-induced NF-κB p65 (LPS+EF 20 μg/mL and 30 μg/mL vs. LPS only: 0.78 ± 0.06 vs. 1.17 ± 0.08, P < 0.001; and 0.90 ± 0.06 vs. 1.17 ± 0.08, P =0.002) and inhibitor of kappa B (IκBα) phosphorylation (LPS+EF 20 μg/mL and 30 μg/mL vs. LPS only: 0.25 ± 0.01 vs. 0.63 ± 0.03, P < 0.001; and 0.31 ± 0.01 vs. 0.63 ± 0.03, P < 0.001), LPS+EF 30 μg/mL inhibited IκB kinase (IKKα/β) phosphorylation (LPS+EF 30 μg/mL vs. LPS only, 1.12 ± 0.14 vs. 1.71 ± 0.25, P = 0.002) in RAW 264.7 cells. Furthermore, EF 10 mg/kg and EF 20 mg/kg inhibited lung tissue inflammation in vivo and suppressed the serum TNF-α (LPS+EF 10 mg/kg and 20 mg/kg vs. LPS only, 199.99 ± 186.49 vs. 527.90 ± 263.93 pg/mL, P=0.001; and 260.56 ± 175.83 vs. 527.90 ± 263.93 pg/mL, P = 0.005), and IL-6 (LPS+EF 10 mg/kg and 20 mg/kg vs. LPS only, 41.26 ± 30.42 vs. 79.45 ± 14.16 pg/ ml, P = 0.011; and 42.01 ± 26.26 vs. 79.45 ± 14.16 pg/mL, P = 0.012) levels and MPO (LPS+EF 10 mg/kg and 20 mg/kg vs. LPS only, 3.19 ± 1.78 vs. 5.39 ± 1.51 U/g, P = 0.004; and 3.32 ± 1.57 vs. 5.39 ± 1.51 U/g, P = 0.006) activity in lung tissue.
Conclusions:
EF could effectively inhibit the expression of inflammatory factors and overactivation of neutrophils. Further investigation is needed to evaluate its potential for anti-inflammation therapy.
Keywords: Eucommia ulmoides Oliv., Male flower, Lipopolysaccharide, Inflammation, Cytokine, Nuclear factor-κB
Introduction
Eucommia ulmoides Oliv. is a traditional medicinal plant that is native to China, and its bark has been reported to be able to lower blood pressure, act as a diuretic, regulate the immune system, exhibit an anti-complement activity, prevent osteoporosis, and provide a range of other benefits, including anti-aging, antitumor, antibacterial, anti-inflammatory, and analgesic effects.[1] It has previously been shown that Eucommiae Cortex exhibited anti-inflammatory effects in a rat model of collagen-induced arthritis.[2] However, the annual production rate of Eucommiae Cortex is very low, limiting its wider usage.
E. ulmoides blooms from April to May.[3]Eucommia male flowers can be harvested every year and have recently been marketed as a health food in China.[4] Unlike Eucommiae Cortex, Eucommia male flowers are available in relatively large yields and are easy to harvest. Previous research has shown that Eucommia male flowers can exert anti-inflammatory, analgesic, antibacterial, and other pharmacological effects, including immune regulation.[5,6]
Inflammation is an important pathological process, common to many organisms. Adequate inflammatory responses are beneficial against injury and help ameliorate infection and promote wound healing. However, long-term or excessive inflammation can cause permanent tissue damage. In recent years, the development of naturally derived anti-inflammatory therapeutics, such as plant extracts, has received broad attention. Research into natural product medicine has become a focus for innovation and a hot topic in medical research.[7]
In early stages of inflammation, macrophages can release interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and other inflammatory cytokines, promoting neutrophil activation and eventually leading to inflammatory injury.[8] Lipopolysaccharide (LPS) is the main component of the outer membrane of gram-negative bacteria. It has various biological activities such as inducing non-specific immunity, promoting the release of proinflammatory cytokines (eg, TNF-α, IL-6, and prostaglandins), and stimulating the body's immune inflammatory response.[9] To explore the possible therapeutic uses of Eucommia male flowers, in this study, we investigated the anti-inflammatory activity of the male flower extract in an LPS-stimulated inflammatory cell model in vitro and in a mouse model of acute inflammation in vivo.
Methods
Plant material
Eucommia male flowers were purchased from Zhangjiajie City, Hunan Province, China, and identified as belonging to the family Eucommiaceae by Prof. Jin-Rong Wu of the Shanghai University of Traditional Chinese Medicine. A voucher specimen (9523) has been deposited at the Department of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine.
Extraction
The flowers (1 kg) were washed, sliced, dried, and then extracted twice with 70% ethanol (1:8 and 1:6, w/v) at 60°C for 3 days each. The 70% ethanol extract (hereinafter referred to as EF) was then evaporated under vacuum so that 1 ml of the extract corresponded to 1 g of dried flowers. The yield of the extract was 20.47%, and the total flavonoid content was 1.72%.
Cell growth inhibition
The mouse macrophage cell line RAW 264.7 was purchased from Shanghai Rochen Pharma Co., Ltd. (Shanghai, China) and cultured in Dulbecco modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and a 1% penicillin-streptomycin solution (Gibco, CA, USA; hereinafter referred to as “standard growth medium”) in a humidified atmosphere of 5% CO2/95% air at 37°C (hereinafter referred to as “standard culture conditions”). RAW 264.7 cells in the exponential phase were seeded into a 96-well plate at a density of 2000 cells/well in 200 μL of medium. After overnight incubation, various concentrations of EF and Tripterygium glycosides (TGs) were added to the RAW 264.7 cell culture in triplicate. The medium was used as a blank. Cell Counting Kit-8 (CCK-8, Dojindo, Japan) was used to assess growth inhibition after 24 h at 37°C. Briefly, 10 μL of the CCK-8 solution was added to each well. After incubation for 4 h, the absorbance was determined at 450 nm.
Determination of nitric oxide production by LPS-stimulated RAW 264.7 cells
RAW 264.7 cells were cultured in the standard growth medium under standard conditions in a 96-well plate at a cell density of 2 × 104 cells/mL (2000 cells/well). After incubation for 24 h, the medium was removed, and EF or TGs, diluted in a serum-free medium, were added to final concentrations of 10, 20, and 30 μg/mL. After incubation for 4 h, the media were removed, and 1 μg/mL LPS in DMEM containing 10% FBS was added to each well, except the negative control wells. After incubation for 48 h, cell supernatants were collected, and the nitric oxide (NO) concentrations in the media were determined by measuring the absorbance at 550 nm.
Secretion of TNF-α, IL-1β, and IL-6 by LPS-stimulated RAW 264.7 cells
RAW 264.7 cells were cultured in the standard growth medium under standard conditions in 96-well plates at a density of 2 × 104 cells/mL (2000 cells/well). After incubation for 24 h, the growth medium was removed, and EF or TGs, diluted in a serum-free medium, were added to a final concentration of 30 μg/mL. After incubation for 4 h, the media were removed, and 1 μg/mL LPS in DMEM containing 10% FBS was added to each well, except the negative control wells. After incubation for 24 h, the supernatants were collected to determine the concentrations of TNF-α, IL-1β, and IL-6 by using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions.
Determination of IL-17, IL-23, and IL-10 mRNA expression levels in LPS-stimulated RAW 264.7 cells
RAW 264.7 cells were cultured in the standard growth medium in a 96-well plate under standard culture conditions at a density of 2 × 104 cells/mL (2000 cells/well). After incubation for 24 h, the growth medium was removed, and EF or TGs, diluted in a serum-free medium, were added to a final concentration of 30 μg/mL. After incubation for 4 h, the media were removed, and 1 μg/mL LPS in DMEM containing 10% FBS was added to each well, except for blank control wells. After incubation for 24 h, RNA was extracted and purified using the PureLink RNA mini kit (Ambion, Austin, TX, USA) and reverse transcribed into cDNA by using the SuperScript III first-strand synthesis supermix (Invitrogen, USA). Quantitative real-time polymerase chain reaction (qPCR) was performed using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a reference and SYBR Green as the fluorescent dye. Amplification was performed using the following conditions: pre-denaturation at 50°C for 2 min and polymerase activation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 60 s. Primer Premier (Premier, Canada) was used to design primers. The primers were as follows (5′–3′): IL-17-F, GTGTCTCTGATGCTGTTG; IL-17-R, AACGGTTGAGGTAGTCTG; IL-23-F, GACTCAGCCAACTCCTCCAGCCAG; IL-23-R, TTGGCACTAAGGGCTCAGTCAGA; IL-10-F, GGTTGCCAAGCCTTATCGGA; IL-10-R, ACCTGCTCCACTGCCTTGCT; and GAPDH-F, GGAAAGCTGTGGCGTGATGG; GAPDH-R, TATCCTTGCTGGGCTGGGTG.
Activation of the nuclear factor-κB pathway in RAW 264.7 cells
The effects of EF on LPS-induced activation of the nuclear factor (NF)-κB pathway in RAW 264.7 cells were investigated using Western blotting. Cells were seeded in 24-well plates and cultured in the standard medium under standard growth conditions at a density of 2 × 105 cells/mL. The cells were pretreated with EF at 10, 20, and 30 μg/mL in a serum-free medium for 4 h. Pretreatment media were removed, and 1 μg/mL LPS in Dulbecco modified Eagle's medium containing 10% FBS was added to each well, except the blank control wells, to which only the medium was added. After the incubation for 20 min, the cells were collected and washed twice with phosphate-buffered saline (PBS). The cell pellets were resuspended in lysis buffer containing 50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS), 1 mmol/L Na3VO4, 1 mmol/L NaF, 1 mmol/L phenylmethane sulphonyl fluoride, and 1 mmol/L protease inhibitor cocktail.
The cell lysates were resolved via 10% SDS-polyacrylamide gel electrophoresis, and the proteins were transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat milk and then incubated at 4°C with the following specific primary antibodies (Cell Signaling Technology, Danvers, MA, USA): phospho-NF-κB p65 (Ser536) rabbit monoclonal antibody (mAb; #3033), phospho-inhibitor of kappa B (IκBα; Ser32) rabbit mAb (#2859), and phospho-IκB kinase (IKKα/β; Ser176/180) rabbit mAb (#2697). After being washed three times with PBS containing 0.05% Tween 20 (PBST), the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology) for 1 h at 25°C, followed by washing 3 times with PBST. The signals were visualized via enhanced chemiluminescence (Pierce, Rockford, IL, USA) according to the manufacturer's instructions. The relative band intensity was determined using ImageJ v. 1.47 (National Institutes of Health, USA).
LPS-induced acute inflammation in mice
In total, 16 female and 16 male ICR mice (SLRC, Shanghai, China) were housed under specific pathogen-free conditions. All animal procedures were performed in accordance with the ethical guidelines of the Institutional Laboratory Animal Welfare and Animal Experimental Ethics Committee (approval number: SZY201704008).
The mice were randomly allocated to the following 4 groups, containing four male and four female mice each: a low-dose EF group (LPS + 10 mg/kg EF), high-dose EF group (LPS + 20 mg/kg EF), control group, and LPS only group. On day 0, the mice in the low- and high-dose EF groups were intragastrically infused once daily with 10 and 20 mg/kg EF, dissolved in PBS, respectively. The doses of EF were based on a previous report.[7] On day 7, the animals, except for the control group, were intraperitoneally injected with 10 mg/kg LPS. The control group was injected with the same volume of PBS.
Sample collection
On day 8, 24 h after LPS injection, all mice were euthanized, and blood was collected from the heart. The blood was centrifuged at 4°C, 3000 × g for 10 min to obtain serum, which was stored at −80°C. The right lungs of all mice were fixed in 10% formaldehyde for subsequent experiments, and the left lungs were frozen in liquid nitrogen.
Hematoxylin and eosin staining
The formaldehyde-fixed right lungs of the mice were dehydrated in a graded ethanol series, embedded in paraffin, serially sectioned into 5-μm slices, and stained with hematoxylin and eosin (H&E). The sections were examined under an optical microscope at a 200 × magnification.
Serum TNF-α and IL-6 levels
Serum TNF-α and IL-6 levels were determined after treatment with EF using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience) according to the manufacturer's instructions.
Myeloperoxidase activity in lung homogenates
Lung tissue was accurately weighed, and homogenization medium was added at a ratio of 1:19 (w/v) to obtain 5% tissue homogenates. Myeloperoxidase (MPO) levels were determined using MPO kits (Nanjing Jiancheng Bioengineering Institute, China).
Statistical analysis
Data are presented as the mean ± standard deviation (SD). All statistical analyses were performed with the SPSS 19.0 software package (SPSS, Inc., Chicago, IL, USA). Statistical differences were calculated using one-way analysis of variance (ANOVA) and Student-Newman-Keuls Q test and factorial analysis. Spearman correlation coefficient was used for nonparametric tests. A P < 0.05 indicated statistically significant differences.
Results
EF inhibited RAW 264.7 cell proliferation
The CCK-8 cell viability assay was performed to evaluate whether RAW 264.7 cell proliferation was inhibited by EF. As shown in Figure 1A and 1B, EF inhibited the cell proliferation at 24 h in a concentration-dependent manner (EF 60 μg/mL vs. negative control, 87.31 ± 2.39% vs. 100.00 ± 2.50%, P = 0.001; EF 120 μg/mL vs. negative control, 79.01 ± 2.56% vs. 100.00 ± 2.50%, P < 0.001; and EF 250 μg/mL vs. negative control, 64.83 ± 2.50% vs. 100.00 ± 2.50%, P < 0.001). EF showed no significant cytotoxicity at concentrations from 10 to 60 μg/mL (cell viability > 80%).
Figure 1.
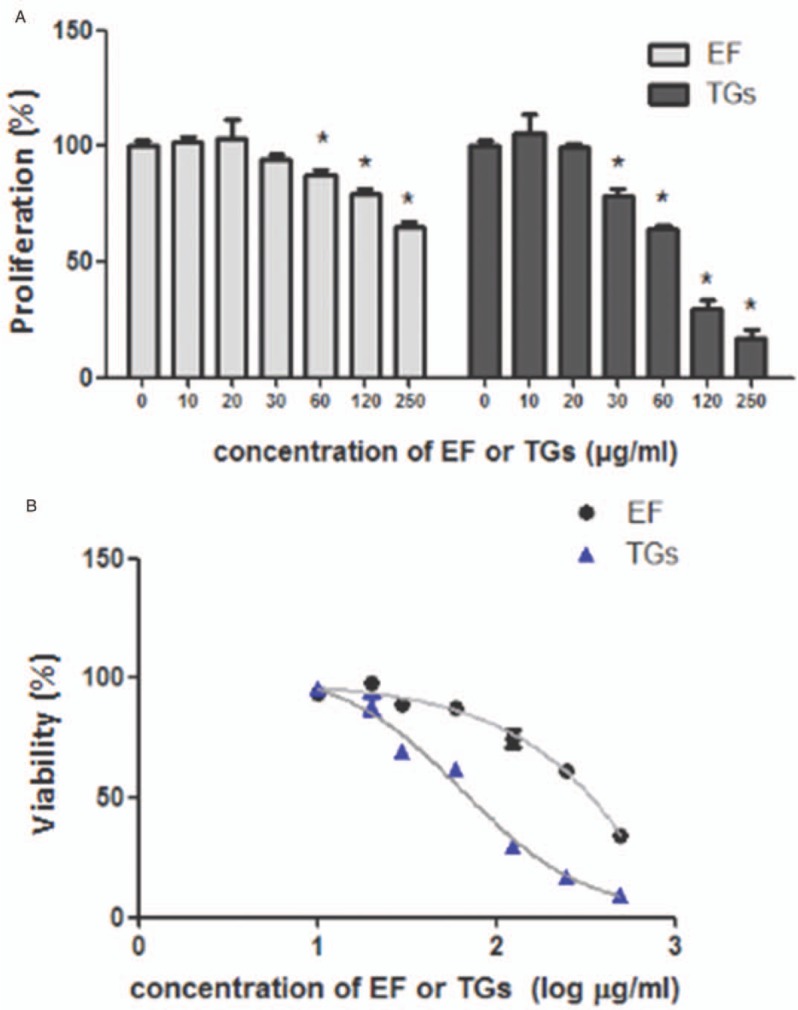
Effects of 70% ethanol extract of male flowers of E. ulmoides on the proliferation of RAW 264.7 cells. (A) Cell proliferation at 24 h. (B) Cell viability at 24 h. ∗P < 0.05 vs. negative control, n = 3. EF: 70% ethanol extract of male flowers of E. ulmoides; TGs: Tripterygium glycosides.
EF suppressed NO release from LPS-stimulated RAW 264.7 cells
As shown in Figure 2A, EF significantly inhibited the release of NO from LPS-stimulated RAW 264.7 cells at concentrations of 20 and 30 μg/mL compared with that in the LPS only control (EF 20 μg/mL vs. LPS only, 288.81 ± 38.01 vs. 447.68 ± 19.07 μmol/L, P = 0.004; EF 30 μg/mL vs. LPS only, 158.80 ± 45.14 vs. 447.68 ± 19.07 μmol/L, P < 0.001). Factorial analysis showed that for NO secreted by RAW264.7 cells after administration of EF or TG, the difference was not obvious. As shown in Figure 2B, Spearman correlation coefficient showed that the inhibitory effect of EF and TGs on NO secretion by RAW264.7 cells was positively correlated with the concentration (r2 = 0.945, P < 0.001).
Figure 2.
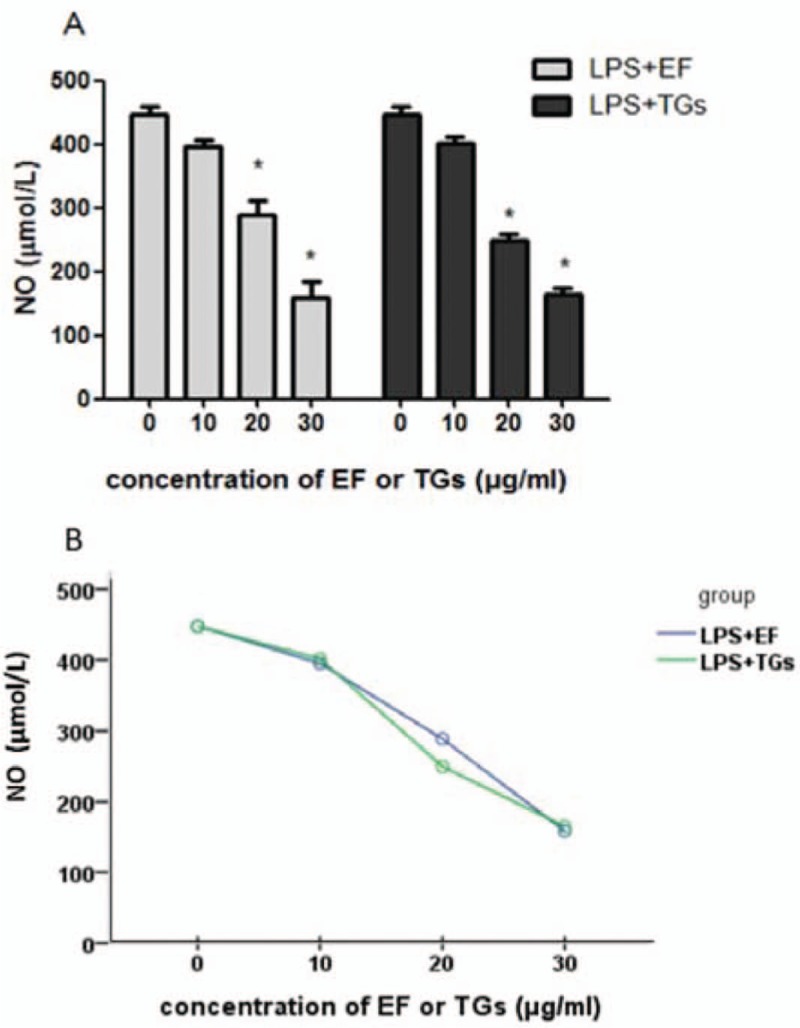
The 70% ethanol extract of male flowers of E. ulmoides suppressed the lipopolysaccharide-induced release of nitric oxide in the RAW 264.7 cells. (A) Secretion of NO in LPS stimulated RAW 264.7 cells. ∗P < 0.05 vs. LPS only treatment (n = 3). (B) Nonparametric tests of NO level, r2 = 0.945. LPS only treatment: Cells were treated with 1 μg/mL LPS in Dulbecco modified Eagle's medium containing 10% fetal bovine serum. EF: 70% ethanol extract of male flowers of E. ulmoides; TGs: Tripterygium glycosides; NO: Nitric oxide; LPS: Lipopolysaccharide.
EF reduced the production of proinflammatory cytokines by LPS-stimulated RAW 264.7 cells
ELISAs were performed to evaluate the effects of EF on LPS-induced secretion of TNF-α, IL-1β, and IL-6 by RAW 264.7 cells. As shown in Figure 3A, LPS-stimulated macrophages released large amounts of inflammatory cytokines. The secretion levels of TNF-α (LPS only vs. negative control, 577.70 ± 5.35 vs. 4.90 ± 1.18 pg/mL, P < 0.001), IL-1β (LPS only vs. negative control, 411.03 ± 42.28 vs. 5.63 ± 1.80 pg/mL, P < 0.001), and IL-6 (LPS only vs. negative control, 524.80 ± 6.24 vs. 4.90 ± 2.29 pg/mL, P < 0.001) in the LPS-stimulated cells were higher than those in the negative control cells, and EF could significantly suppress the secretion of TNF-α (LPS+EF vs. LPS only, 210.20 ± 13.85 vs. 577.70 ± 5.35 pg/mL, P < 0.001), IL-1β (LPS+EF vs. LPS only, 193.30 ± 10.80 vs. 411.03 ± 42.28 pg/mL, P < 0.001), and IL-6 (LPS+EF vs. LPS only, 149.67 ± 11.60 vs. 524.80 ± 6.24 pg/mL, P < 0.001) compared with that in cells treated with LPS alone. The qPCR was performed to evaluate the effects of EF on the expression of IL-17, IL-23, and IL-10 mRNA. As shown in Figure 3B, the mRNA levels of IL-17 (LPS only vs. negative control, 0.43 ± 0.12 vs. 0.08 ± 0.01, P < 0.001), IL-23 (LPS only vs. negative control, 0.42 ± 0.06 vs. 0.26 ± 0.01, P = 0.001), and IL-10 (LPS only vs. negative control, 0.47 ± 0.01 vs. 0.23 ± 0.03, P < 0.001) in LPS-treated RAW 264.7 cells were significantly elevated compared with those in the negative control cells, whereas EF was found to downregulate the IL-17 (LPS+EF vs. LPS only, 0.23 ± 0.02 vs. 0.43 ± 0.12, P < 0.001), IL-23 (LPS+EF vs. LPS only, 0.29 ± 0.01 vs. 0.42 ± 0.06, P = 0.002) and IL-10 (LPS+EF vs. LPS only, 0.30 ± 0.01 vs. 0.47 ± 0.01, P = 0.008) mRNA levels compared with those in the cells treated with LPS only.
Figure 3.
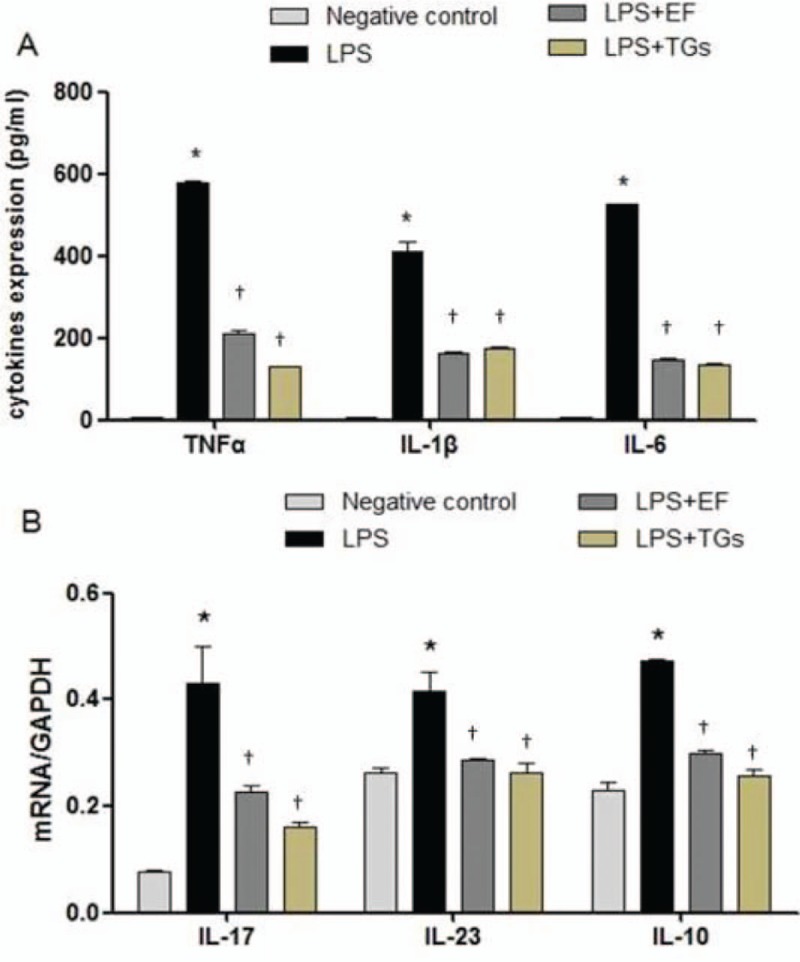
The 70% ethanol extract of male flowers of E. ulmoides reduced proinflammatory cytokine production. (A) Secretion of TNF-α, IL-1β, and IL-6 by LPS-stimulated RAW 264.7 cells. (B) IL-17, IL-23, and IL-10 mRNA expression levels in LPS-stimulated RAW 264.7 cells. ∗P < 0.05 vs. negative control; †P < 0.05, vs. LPS only treatment (n = 3). LPS only treatment: Cells were treated with 1 μg/mL LPS in Dulbecco modified Eagle's medium containing 10% fetal bovine serum. EF: 70% ethanol extract of male flowers of E. ulmoides; TGs: Tripterygium glycosides; TNF-α: Tumor necrosis factor-α; IL-1β: Interleukin-1β; IL-6: Interleukin-6; IL-17: Interleukin-17; IL-23: Interleukin-23; IL-10: Interleukin-10; LPS: Lipopolysaccharide.
EF inhibited the activation of the NF-κB signaling pathway in LPS-stimulated RAW 264.7 cells
To elucidate the mechanism of the anti-inflammatory effects of EF, the activation of IKK and NF-κB, as indicated by their phosphorylation, was determined using Western blotting analysis. As shown in Figure 4, the phosphorylation of NF-κB p65 (LPS only vs. negative control, 1.17 ± 0.08 vs. 0.51 ± 0.01, P < 0.001), IκBα (LPS only vs. negative control, 0.10 ± 0.01 vs. 0.63 ± 0.03, P < 0.001), and IKKα/β (LPS only vs. negative control, 1.29 ± 0.16 vs. 1.71 ± 0.25, P = 0.018) in RAW 264.7 cells increased after LPS stimulation compared with those in the negative control cells. Treatment with 20 μg/mL and 30 μg/mL EF suppressed the phosphorylation of p65 (LPS+EF 30 μg/mL vs. LPS only, 0.90 ± 0.06 vs. 1.17 ± 0.08, P = 0.002; LPS+EF 20 μg/mL vs. LPS only, 0.78 ± 0.06 vs. 1.17 ± 0.08, P < 0.001) and IκBα (LPS+EF 30 μg/mL vs. LPS only, 0.31 ± 0.01 vs. 0.63 ± 0.03, P < 0.001; LPS+EF 20 μg/mL vs. LPS only, 0.25 ± 0.01 vs. 0.63 ± 0.03, P < 0.001) whereas that with 30 μg/mL EF suppressed the phosphorylation of IKKα/β (LPS+EF 30 μg/mL vs. LPS only, 1.12 ± 0.14 vs. 1.71 ± 0.25, P = 0.002).
Figure 4.

The 70% ethanol extract of male flowers of E. ulmoides inhibited the activation of the nuclear factor-κB pathway in lipopolysaccharide-stimulated RAW 264.7 cells. (A) Representative expression for NF-κB p65, pIκBα and pIKKα/β detected by Western blotting. (B) Densitometric quantification of NF-κB p65 with GAPDH as loading control. (C) Densitometric quantification of pIκBα with GAPDH as loading control. (D) Densitometric quantification of pIKKα/β with GAPDH as loading control. 1. Negative control, 2. LPS, 3. LPS+EF 30 μg/mL, 4. LPS+EF 20 μg/mL, 5. LPS+EF 10 μg/mL. ∗P < 0.05 vs. negative control; †P < 0.05 vs. LPS only treatment (n = 3). LPS only treatment: Cells were treated with 1 μg/mL LPS in Dulbecco modified Eagle's medium containing 10% fetal bovine serum. EF:70% ethanol extract of male flowers of E. ulmoides; TGs: Tripterygium glycosides; NF-κB: Nuclear factor-κB; IκB: Inhibitor of kappa B; IKK: Inhibited IκB kinase; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; LPS: Lipopolysaccharide.
EF reduced lung inflammation
H&E staining was used to examine the lung inflammation in mice. As shown in Figure 5A, no significant pathological indicators were observed in the lung tissue sections of the control mice. In the LPS-administered mice [Figure 5B], local consolidation of lung tissue was visible, along with considerable inflammatory cell infiltration and granuloma formation. In the mice pre-dosed with 10 mg/kg EF prior to LPS administration [Figure 5C], some inflammatory cell infiltration was visible, along with a moderate degree of pulmonary microvascular and perivascular inflammation. In the mice pre-dosed with 20 mg/kg EF prior to LPS administration [Figure 5D], only a small amount of inflammatory cell infiltration and pulmonary microvascular and perivascular inflammation was apparent.
Figure 5.
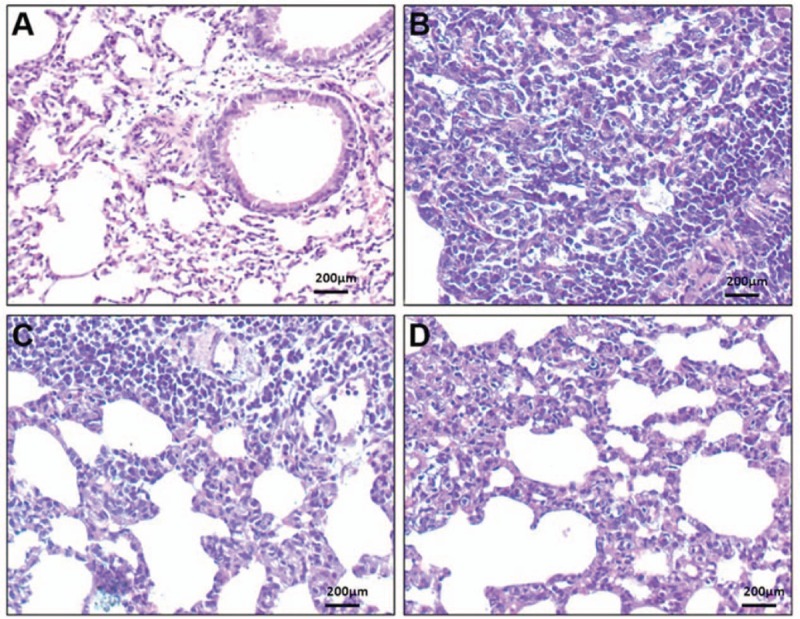
Lung histopathology of lipopolysaccharide-administered mice. Hematoxylin-eosin-stained lung tissue sections from (A) control mice, (B) LPS-administered mice, (C) LPS-administered mice pre-dosed with 10 mg/kg EF, and (D) LPS-administered mice pre-dosed with 20 mg/kg EF. Scale bar = 200 μm. EF: 70% ethanol extract of male flowers of E. ulmoides; TGs: Tripterygium glycosides; LPS: Lipopolysaccharide.
EF reduced serum concentrations of TNF-α and IL-6 in LPS-administered mice
TNF-α and IL-6 concentrations were determined in the mouse serum by ELISA. As shown in Figure 6A, the TNF-α (LPS only vs. negative control, 527.90 ± 263.93 vs. 129.78 ± 63.89 pg/mL, P < 0.001) and IL-6 (LPS only vs. negative control, 79.45 ± 14.16 vs. 27.87 ± 16.75 pg/mL, P = 0.001) levels in the serum of the LPS-administered mice were significantly elevated compared with that in the negative control group, whereas the mice treated with 10 mg/kg or 20 mg/kg EF showed reduced levels of TNF-α (LPS+EF 10 mg/kg vs. LPS only, 199.99 ± 186.49 vs. 527.90 ± 263.93 pg/mL, P = 0.001; LPS+EF 20 mg/kg vs. LPS only, 260.56 ± 175.83 vs. 527.90 ± 263.93 pg/mL, P = 0.005) and IL-6 (LPS+EF 10 mg/kg vs. LPS only, 41.26 ± 30.42 vs. 79.45 ± 14.16 pg/mL, P = 0.011; LPS+EF 20 mg/kg vs. LPS only, 42.01 ± 26.26 vs. 79.45 ± 14.16 pg/mL, P = 0.012) compared with the LPS group.
Figure 6.
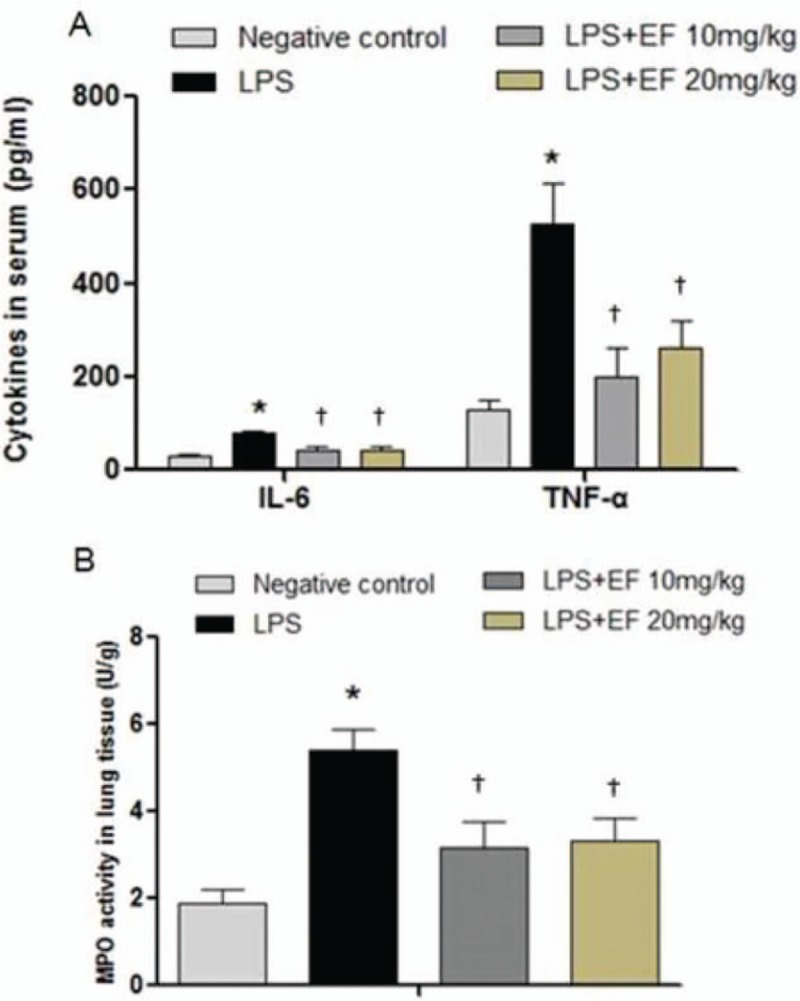
The 70% ethanol extract of male flowers of E. ulmoides reduced proinflammatory cytokines in lipopolysaccharide-administered mice. (A) EF inhibited cytokines in mouse serum (n = 8). ∗P < 0.05 vs. negative control; †P < 0.05 vs. LPS only treatment. LPS only treatment: Mice were intraperitoneally injected with 10 mg/kg LPS. (B) Effects of EF on MPO activity in mouse lung tissue. ∗P < 0.05 vs. negative control; †P < 0.05 vs. LPS only treatment (n = 8). LPS only treatment: Mice were intraperitoneally injected with 10 mg/kg LPS. EF: 70% ethanol extract of male flowers of E. ulmoides; TGs: Tripterygium glycosides; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α. MPO: Myeloperoxidase; LPS: Lipopolysaccharide.
EF inhibited the MPO activity in lung tissue of LPS-administered mice
The MPO activity was assessed as a further indicator of inflammatory responses in the LPS-administered mice. As shown in Figure 6B, the MPO activity in the LPS group was significantly elevated compared with that in the negative control group (LPS only vs. negative control, 5.39 ± 1.51 vs. 1.88 ± 1.03 U/g, P < 0.001) In the mice pre-administered 10 mg/kg or 20 mg/kg EF, the MPO activity was significantly lower than that in the LPS group (LPS+EF 10 mg/kg vs. LPS only, 3.19 ± 1.78 vs. 5.39 ± 1.51 U/g, P = 0.004; LPS+EF 20 mg/kg vs. LPS only, 3.32 ± 1.57 vs. 5.39 ± 1.51 U/g, P = 0.006)
Discussion
Inflammation is a common pathological phenomenon that occurs during disease development. In the present study, the murine peritoneal macrophage cell line RAW 264.7 was used as an in vitro model of LPS-induced inflammation to assess the anti-inflammatory potential of Eucommia male flowers. Excessive NO can promote the occurrence and development of inflammation and upregulate the expression of IL-6 and other inflammatory factors.[10,11] IL-6 and IL-10 are two common inflammatory factors. IL-6 is a proinflammatory factor, which initiates an inflammatory response when inflammation is stimulated in the body. IL-10 is an anti-inflammatory factor, suppressing the inflammatory response, and its levels can rapidly increase to trigger a compensatory anti-inflammatory mechanism to prevent tissue injury.[12] Studies of the IL-23/IL-17 inflammatory axis have shown that IL-23 induces the differentiation of naïve CD4+ T cells into highly pathogenic T-helper 17 cells, which produce IL-17, IL-6, and TNF-α. The IL-23/IL-17 pathway may be a novel therapeutic target for the treatment of chronic inflammatory diseases.[13,14] Our results showed that EF exhibited no significant cytotoxicity at concentrations ranging from 10 to 60 μg/mL. Meanwhile, EF inhibited the release of NO, and the effect was positively correlated with the concentration, which is similar to TGs. EF inhibited IL-6, TNF-α, and IL-1β secretion in LPS-stimulated RAW 264.7 cells, and downregulated the mRNA expression of IL-17, IL-23, and IL-10 in LPS-stimulated RAW 264.7 cells. It was shown that EF inhibited the release of inflammatory cytokines, which is also similar to TGs. It is well known that NF-κB is involved in the regulation of inflammatory cytokines. LPS mediates the activation of monocytes, macrophages, endothelial cells, and other cells through NF-κB activation, which occurs via the binding of LPS to the membrane surface receptor Toll-like receptor 4, followed by activation of IKKα/β and IκB. NF-κB releases and translocates to the nucleus to induce the gene transcription and expression of inflammatory cytokines,[15] including IL-1β, IL-6, and TNF-α.[16–18] The present study showed that high and moderate concentrations of EF could inhibit the LPS-induced phosphorylation of NF-κB p65 and IκB in RAW 264.7 cells. High concentrations of EF were found to downregulate the phosphorylation of IKKα/β, thus inhibiting the release of various downstream inflammatory cytokines.
Intraperitoneal injection and intratracheal instillation of LPS can cause acute lung injury in animals, activate neutrophils, lymphocytes, and endothelial cells to release MPO.[19] MPO is a functional and activation marker of neutrophils. The level and activity of MPO represent the function and activity of neutrophilic polymorphonuclear leukocytes. TNF-α, produced by activated monocytes and macrophages, can kill and inhibit tumor cells and can induce and stimulate the secretion of other cytokines, such as IL-1β and IL-6. TNF-α is known to play an important role in rheumatoid arthritis, cancer, and other diseases.[20] The main function of IL-6 is to activate B cells to proliferate and secrete antibodies, stimulate T cell proliferation, and activate cytotoxic T lymphocytes.[21]
In this study, a mouse model of acute inflammation was established by an intraperitoneal injection of LPS. Analyses of the effects of EF on the LPS-induced expression of the inflammatory cytokines TNF-α and IL-6 in the serum and MPO activity in lung homogenates showed that EF could suppress the TNF-α and IL-6 induction and MPO activity. These findings indicated that EF could effectively inhibit the expression of inflammatory factors and the overactivation of neutrophils, suggesting that its potential should be further investigated for anti-inflammatory therapy.
In conclusion, we demonstrated that the extract of male flowers of E. ulmoides showed anti-inflammatory effects in both in vitro and in vivo experimental models and that its anti-inflammatory effects might be related to the inhibition of proinflammatory cytokine production and the suppression of neutrophil activation. Additionally, the extract could inhibit the phosphorylation of NF-κB p65, IκBα, and IKKα/β. In the future, compounds isolated from male flowers of E. ulmoides will be screened to identify the active anti-inflammatory constituents, and their anti-inflammatory mechanism will be further explored.
Funding
This work was supported by grants from the Natural Science Foundation of China (Nos. 81573814, 81773922), the Shanghai Construction Project of the Establishment of Innovation Center (No. U163020201), and the Shanghai University of Traditional Chinese Medicine (No. 2016YSN10).
Conflicts of interest
None.
Author contributions
Yuan Y and Wang JY conceptualized and designed the research study. Chen XJ supervised and performed the detailed experiments. Zhang L carried out data analysis and interpretation; Pan YY and Gu ZX participated in generating the mouse model and in pathological evaluation. Wang JY and Yuan Y wrote the manuscript and checked for revisions and submission considerations. All the authors read and approved the final manuscript.
Footnotes
How to cite this article: Wang JY, Chen XJ, Zhang L, Pan YY, Gu ZX, Yuan Y. Anti-inflammatory effects of Eucommia ulmoides Oliv. male flower extract on lipopolysaccharide-induced inflammation. Chin Med J 2019;00:000–000. doi: 10.1097/CM9.0000000000000066
References
- 1.Li QX. Advance in chemical component and pharmacological action of Eucommia ulmoides Oliv. (in Chinese). J Anhui Agric Sci 2016; 44:153–156. doi: 10.13989/j.cnki.0517-6611.2016.09.053. [Google Scholar]
- 2.Wang JY, Yuan Y, Chen XJ, Fu SG, Zhang L, Hong YL, et al. Extract from Eucommia ulmoides Oliv. ameliorates arthritis via regulation of inflammation, synoviocyte proliferation and osteoclastogenesis in vitro and in vivo. J Ethnopharmacol 2016; 194:609–616. doi: 10.1016/j.jep.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Hu HZ, Yang H, Li XS. Eucommia ulmoides Oliv. male flower (in Chinese). Biotic Resour 2017; 39:230.doi: 10.3969/j.issn.1006-8376.2017.03.013. [Google Scholar]
- 4.Du QX, Wei YX, Liu PF, Du HY. Diversity of the content of main active components in Eucommia ulmoides male flowers (in Chinese). Sci Silv Sin 2017; 53:35–43. doi: 10.11707/j.1001-7488.20170205. [Google Scholar]
- 5.Chen XJ, Wang FC, Yuan Y, Zhang L, Li XD, Jin SA, et al. Comparative study of bark, leaf and male flower of Eucommia on pharmacodynamics (in Chinese). J Gansu Uni Chin Med 2016; 33:5–8. doi: 10.16841/j.issn1003-8450.2016.05.02. [Google Scholar]
- 6.Lou LJ. The pharmacodynamics research of Eucommia ulmoides male flower tea (in Chinese). Kaifeng:Henan University; 2010. [Google Scholar]
- 7.Wang JY, Chen XJ, Lei Z, Wang K, Hou JW, Fu SG, et al. Effects of alcohol extracts of bark and male flower of Eucommia ulmoides Oliv. on airway allergic inflammation of model mice (in Chinese). Chin J Inf Tradit Chin Med 2018; 25:42–47. doi: 10.3969/j.issn.1005-5304.2018.03.010. [Google Scholar]
- 8.Ishii D, Schenk AD, Baba S, Fairchild R. Role of TNF alpha in early chemokine production and leukocyte infiltration into heart allografts. Am J Transplant 2010; 10:59–68. doi:10.1111/j.1600-6143.2009.02921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou YH, Zhao L, Xu YK, Bao JM, Liu X, Zhang JS, et al. Anti-inflammatory sesquiterpenoids from the traditional Chinese medicine salvia plebeia: Regulates pro-inflammatory mediators through inhibition of NF-κB and Erk1/2 signaling pathways in LPS-induced Raw264.7 cells. J Ethnopharmacol 2018; 210:95–106. doi: 10.1016/j.jep.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Siednienko J, Nowak J, Moynagh PN, Gorczyca WA. Nitric oxide affects IL-6 expression in human peripheral blood mononuclear cells involving cGMP-dependent modulation of NF-κB activity. Cytokine 2011; 54:282–288. doi: 10.1016/j.cyto.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Morita R, Uchiyama T, Hori T. Nitric oxide inhibits IFN-α production of human plasmacytoid dendritic cells partly via a guanosine 3′,5′-cyclic monophosphate-dependent pathway. J Immunol 2005; 175:806–812. doi: 10.4049/jimmunol.175.2.806. [DOI] [PubMed] [Google Scholar]
- 12.Dillow AF, Cardwell LN, Smith TJ, Groppe BD, Peterson BA, Sickman MA, et al. Temporal transcriptional regulation of IL-10-induced anti-inflammatory genes in LPS-triggered macrophages. Open J Immunol 2016; 4:96–116. doi: 10.4236/oji.2014.43013. [Google Scholar]
- 13.Li N, Xu W, Yuan Y, Ayithan N, Imai Y, Wu X, et al. Immune-checkpoint protein VISTA critically regulates the IL-23/IL-17 inflammatory axis. Sci Rep 2017; 7:1485.doi: 10.1038/s41598-017-01411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxwell JR, Zhang Y, Brown WA, Smith CL, Byrne FR, Fiorino M, et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immuno regulation. Immunity 2015; 43:739–750. doi: 10.1016/j.immuni.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Smale ST. Hierarchies of NF-κB target-gene regulation. Nat Immunol 2011; 12:689–694. doi: 10.1038/ni.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ianaro A, Tersigni M, D’Acquisto F. New insight in LPS antagonist. Mini Rev Med Chem 2009; 9:306–317. doi: 10.2174/1389557510909030306. [DOI] [PubMed] [Google Scholar]
- 17.Ionita MG, Arslan F, de Kleijn DP, Pasterkamp G. Endogenous inflammatory molecules engage Toll-like receptors in cardiovascular disease. J Innate Immun 2010; 2:307–315. doi: 10.1159/000314270. [DOI] [PubMed] [Google Scholar]
- 18.Drexler SK, Foxwell BM. The role of Toll-like receptors in chronic inflammation. Int J Biochem Cell Biol 2010; 42:506–518. doi: 10.1016/j.biocel.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Tsushima K, King LS, Aggarwal NR, De Gorordo A, D’Alessio FR, Kubo K. Acute lung injury review. Inter Med 2009; 48:621–630. doi: 10.2169/internalmedicine.48.1741. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Li J, Chen R, Cai G. Targeting NF-κB and TNF-α activation by electroacupuncture to suppress collagen-induced rheumatoid arthritis in model rats. Altern Ther Health Med 2015; 21:26–34. [PubMed] [Google Scholar]
- 21.Park JY, Chung TW, Jeong YJ, Kwak CH, Ha SH, Kwon KM, et al. Ascofuranone inhibits lipopolysaccharide-induced inflammatory response via NF-kappaB and AP-1, p-ERK, TNF-α, IL-6 and IL-1β in RAW 264.7 macrophages. PLoS One 2017; 12:e0171322.doi: 10.1371/journal.pone.0171322. [DOI] [PMC free article] [PubMed] [Google Scholar]


