Abstract
Background:
Medullary thyroid carcinoma (MTC) is a rare disease, but it exhibits more aggressive behaviors. The aim of this study was to improve the diagnostic accuracy of MTC before surgery by analyzing the clinical and ultrasonic data of patients with MTC.
Methods:
The study included 71 patients (96 lesions) with histopathologically proven MTC between April 2011 and September 2016 in the Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College. The clinical characteristics and sonographic findings were retrospectively reviewed and compared between the ultrasonic correct diagnosis group and the ultrasonic misdiagnosis group with the t test or Mann-Whitney U test for quantitative parameters and the χ2 test or Fisher exact test for qualitative parameters.
Results:
Compared with the ultrasonic correct diagnosis group, the proportion of the cystic change in the ultrasonic misdiagnosed group was high (25.0% vs. 4.2%), the uncircumscribed margin and irregular shape proportions were low (20.8%, 58.3% vs. 74.7%, 87.3%), calcification was relatively rare (20.8% vs. 56.3%), and rich vascularity was relatively rare (25.0% vs. 78.9%).
Conclusions:
In the case of atypical MTC, such as cystic change, circumscribed margin, regular shape, no calcification, no rich vascularity, and normal cervical lymph nodes, MTC is easily misdiagnosed as benign by ultrasound. Therefore, ultrasound, cytology and serum calcitonin should be comprehensively evaluated for a preoperative diagnosis of MTC.
Keywords: Thyroid cancer, Medullary, Ultrasonography, Diagnostic errors
Introduction
Medullary thyroid carcinoma (MTC) is a rare disease, representing approximately 2% to 5% of all thyroid malignancies globally.[1–4] However, MTC exhibits more aggressive behavior, such as lymph node metastasis and recrudescence, than other types of thyroid carcinoma. Therefore, a diagnosis of the illness as MTC before surgery is important for patients. The aim of this study was to investigate the clinical characteristics and sonographic findings of an ultrasonic correct diagnosis group and an ultrasonic misdiagnosis group, to improve the diagnostic accuracy of MTC before surgery.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College (No. LC2017A05). Patient consent was waived by the Ethics Committee due to the retrospective nature of this study.
Patients
From a retrospective review of the pathological database at our institute between April 2011 and September 2016, 71 patients were included in this study. All patients underwent surgery. We retrospectively reviewed the clinical characteristics and sonographic features of these patients.
Inclusion criteria were as follows: (1) Initial treatment between April 2011 and September 2016 in the Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College; (2) Complete ultrasound data; and (3) Postoperative pathology confirmed as MTC.
Exclusion criteria included: Cases with poor quality ultrasound images that affected diagnosis.
In this study, all patients were divided into two groups according to the ultrasonic results. Patients whose ultrasound data suggested cancer, malignancy, a possibility of malignancy, or a malignant tendency were allocated to the ultrasonic correct diagnosis group. Patients whose ultrasound data suggested the need for close follow up, a benign tendency, a benign possibility, and a benign condition were classified into the ultrasonic misdiagnosed group. One case was missed by ultrasound, but the data suggested lymph node metastasis derived from the thyroid. The maximum diameter of the primary lesion was 0.2 cm by histopathology. The case was classified into the ultrasonic correct diagnosis group.
Procedure
Radiological screening for thyroid pathologies was performed by ultrasound, using one of four scanners (GE logic 9-General Electric Company, USA; GE logic E9-General Electric Company, USA; Philips IU 22-Royal Dutch Philips Electronics Ltd, the Netherlands; and Siemens Acuson S2000-Siemens AG FWB:SIE, Germany) with a 5 to 12 MHz high frequency linear transducer. The patient was lying in the supine position, the neck was exposed sufficiently, and the thyroid gland was scanned through the anterior cervical multisection. The thyroid lesions in the ultrasound screen were recorded in detail with data for nodule size, margin, shape, echogenicity, echotexture, calcification, and vascularity. Conventional ultrasonography of cervical lymph nodes was also performed. Some patients were examined by serum calcitonin and fine needle aspiration (FNA) before operation.
Imaging interpretation
Hypoechoic was defined as an echo of a lesion that was lower than that of the normal gland. Hyperecho was defined as an echo of a lesion that was higher than that of the normal gland. Calcification in the gland was divided into two categories: microcalcification and coarse calcification. Microcalcification was defined as less than 2 mm of punctate calcification, and coarse calcification was defined as calcification greater than 2 mm. Vascularity was defined as “absent,” “peripheral vascularity,” “internal not rich vascularity (less than five vascular shadows),” or “internal rich vascularity (>5 vascular shadows).” According to Antonelli et al,[5] the sonographic characteristics of lymph node enlargement were a round shape, heterogeneous echoes, a cystic aspect, microcalcifications, and an abnormal lymphatic portal.
Statistical analysis
Clinical and sonographic characteristics were compared between ultrasonic correct diagnosis group and ultrasonic misdiagnosed group with the t test or Mann-Whitney U test for quantitative parameters and the χ2 test or Fisher exact test for qualitative parameters. Statistical analyses were performed with SPSS version 22.0 software (IBM Corporation, Armonk, NY, USA), and P < 0.05 was considered statistically significant.
Results
All 71 MTC patients, including 34 males and 37 females, were confirmed by surgery and histopathology. The age was 13 to 71 years (average age, 47.0±13.4 years). The median follow-up time was 27 months. The 71 patients included 55 cases of sporadic medullary thyroid carcinoma (SMTC), six cases of hereditary medullary thyroid carcinoma (HMTC), and ten cases with a family history of other malignant tumors (three cases of esophageal cancer, three cases of breast cancer, two cases of colorectal cancer, one case of renal cancer, and one case of brain tumor). Among the 71 patients included in the study, 52 (73.2%) patients had single lesions, 18 (25.4%) patients had multiple lesions, and one (1.4%) patient had a missed diagnosis.
The clinical characteristics are summarized in Table 1. There were 96 lesions in the 71 patients; 71 lesions in 52 patients belonged to ultrasonic correct diagnosis group, 24 lesions in 18 patients belonged to ultrasonic misdiagnosed group, and one lesion was a missed diagnosis. The diagnostic accuracies of the thyroid lesions and cervical lymph nodes were 74.0% (71/96) and 71.4% (35/49), respectively. There were no significant differences in the mean age (P = 0.666) or sex of the two groups (P = 0.424). Nineteen (35.8%) patients in ultrasonic correct diagnosis group and two (11.1%) patients in ultrasonic misdiagnosed group had symptom of palpable cervical masses. Although there were statistical differences in the reasons for patients’ visits, this might be related to the insufficient sample size. Further analysis showed that there was no significant difference between physical examination and neck mass. In addition, there were other complications in ultrasonic correct diagnosis group, including diarrhea in four cases, hoarseness in four cases, suffocation in four cases, pain in three cases, dysphagia in three cases and cough in three cases. However, there were no other compression symptoms in ultrasonic misdiagnosed group.
Table 1.
Comparison of the clinical characteristics between ultrasonic correct diagnosis group and ultrasonic misdiagnosed group.
Thirty-seven cases were examined by FNA before operation. The results of cytology showed that there were 25 cases of MTC and 12 cases without MTC (seven cases with indeterminate malignant tumor cells, three cases diagnosed as papillary carcinoma and two cases considered benign), and the accuracy of cytological analysis was 67.6%. One patient was examined by FNA three times before surgery, and the cytology results were all benign. Because of the high level of serum calcitonin, the patient required surgical treatment, and MTC was confirmed by histopathology.
Among the 71 cases included in the study, 68 had measurements of their serum calcitonin levels. The ultrasonic correct diagnosis group had 53 cases, and of these, 52 showed elevations. The range of elevation was approximately 15.0 to 10,427.0 ng/mL, and the median was 932.0 ng/mL. The ultrasonic misdiagnosed group had 16 cases, and all cases were elevated. The range of elevation was approximately 12.8 to 24,367.0 ng/mL, and the median was 86.0 ng/mL. The level of serum calcitonin in ultrasonic correct diagnosis group was higher than that in ultrasonic misdiagnosed group (P = 0.032).
The sonographic characteristics are summarized in Table 2. There was a certain similarity between ultrasonic correct diagnosis group and ultrasonic misdiagnosed group, mostly manifested as hypoechoic lesions which aspect ratios less than one. Only one case presented with hyperecho [Figure 1]. However, there were significant differences in nodule size, margin, shape, echogenicity, echotexture, calcification, and vascularity between the two groups. The accuracy of the ultrasound diagnosis was higher for the lesions that were greater than 1 cm in diameter. The proportion of cystic change in ultrasonic correct diagnosis group was lower (3/71, 4.2%); however, ultrasonic misdiagnosed group had six lesions (6/24, 25.0%), but the majority of the lesions were solid [Figures 2 and 3]. The proportions of an uncircumscribed margin and irregular shape were lower in ultrasonic misdiagnosed group than in ultrasonic correct diagnosis group (20.8%, 58.3% vs. 74.7%, 87.3%). In ultrasonic correct diagnosis group, 40 lesions (56.3%) had calcifications, and the number of microcalcifications was similar to the number of coarse calcifications; however, only five lesions (20.8%) had calcifications in ultrasonic misdiagnosed group [Figures 4 and 5]. Regarding the vascularity of the lesions, in ultrasonic correct diagnosis group, 56 cases (78.9%) had internal rich vascularity, and only four cases (5.6%) did not have vascularity. However, in ultrasonic misdiagnosed group, six cases (25.0%) had internal rich vascularity, and ten cases (41.7%) did not have vascularity. Concomitantly, 29 cases of cavernous nodules were detected by preoperative ultrasonography, of which 16 cases (30.2%) were in ultrasonic correct diagnosis group and 13 cases (72.2%) were in ultrasonic misdiagnosed group (72.2%); the difference was statistically significant (P = 0.002). There were 35 cases of cervical lymph node abnormalities in ultrasonic correct diagnosis group. The cervical lymph nodes in ultrasonic misdiagnosed group were all normal, and the difference between the two groups was statistically significant (P < 0.001).
Table 2.
Comparison of the sonographic characteristics between ultrasonic correct diagnosis group and ultrasonic misdiagnosed group, n (%).
Figure 1.
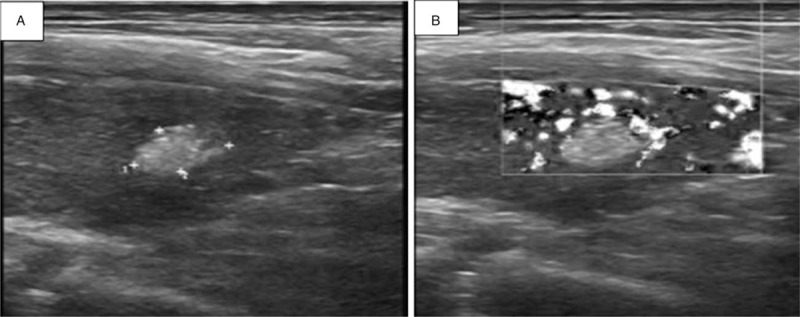
Ultrasonography of MTC in a 58-year-old man found on physical examination for 20 days. (A) Longitudinal image of ultrasonography shows a nodular type of MTC. There is an irregularly shaped hyperechoic nodule in the left lobe of the thyroid. (B) Color Doppler imaging shows peripheral blood flow.
Figure 2.
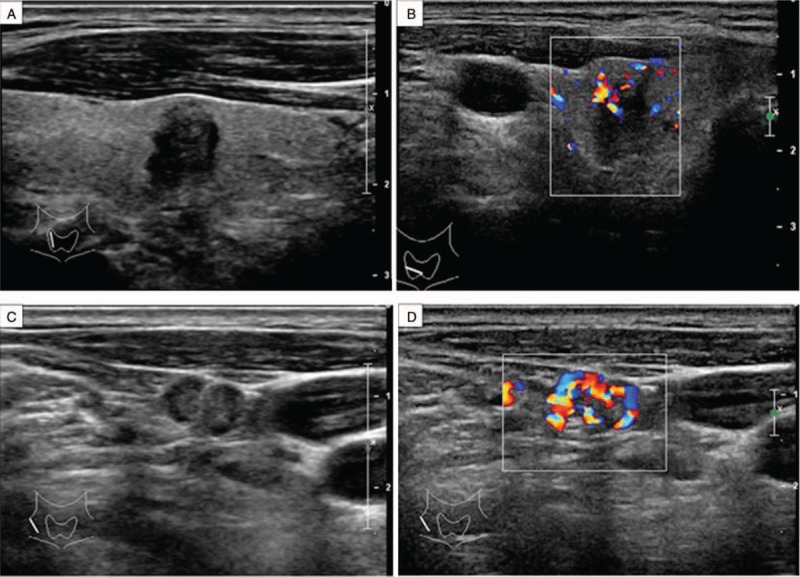
Ultrasonography of MTC in a 64-year-old man found on physical examination for 2 months. (A) Longitudinal image of ultrasonography shows a nodular type of MTC. There is an irregularly shaped hypoechoic mass in the right lobe of the thyroid. (B) Color Doppler imaging shows rich internal blood flow. (C) Longitudinal image of ultrasonography shows pathologically confirmed, involved lymph nodes in the right cervical IV region. (D) Color Doppler imaging shows rich internal blood flow.
Figure 3.
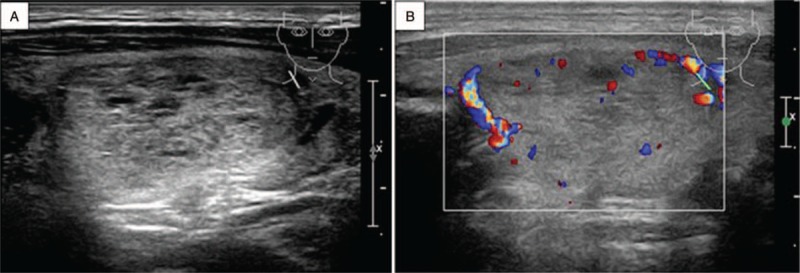
Ultrasonography of MTC in a 48-year-old man found on physical examination for 10 days. (A) Longitudinal image of ultrasonography shows a nodular type of MTC. There is a regularly shaped hypoechoic mass with cystic change in the right lobe of the thyroid. (B) Color Doppler imaging shows peripheral blood flow.
Figure 4.
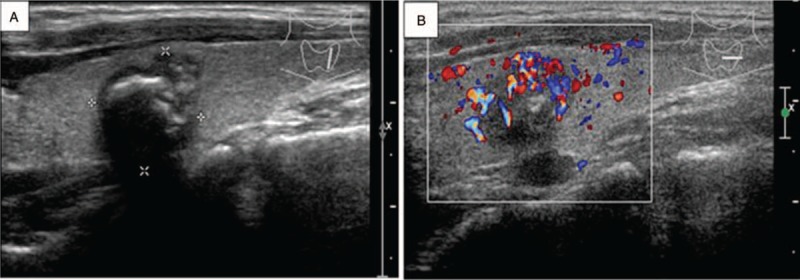
Ultrasonography of MTC in a 31-year-old woman who presented with a neck mass for 8 months. (A) Longitudinal image of ultrasonography shows a nodular type of MTC. There is an irregularly shaped hypoechoic mass with coarse calcifications in the left lobe of the thyroid. (B) Color Doppler imaging shows rich internal blood flow.
Figure 5.
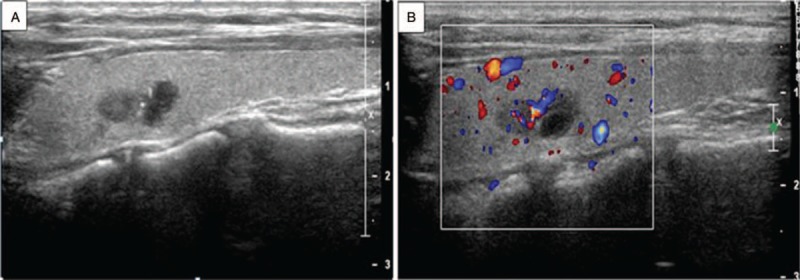
Ultrasonography of MTC in a 25-year-old woman found on physical examination for 1 month. (A) Longitudinal image of ultrasonography shows a nodular type of MTC. There is an irregularly shaped hypoechoic mass with microcalcifications in the left lobe of the thyroid. (B) Color Doppler imaging shows rich internal blood flow.
Discussion
MTC accounts for only 2% to 5% of all thyroid malignant tumors,[1–4] but its behavior is more aggressive, accounting for 13% of all thyroid cancer patient deaths.[6,7] Therefore, it is more positive for the primary lesions and cervical lymph nodes in patients with MTC. A diagnosis of MTC before surgery would be very helpful for clinicians in choosing the surgical procedure.
MTC is derived from parafollicular cells. Its most notable clinical feature is elevated serum calcitonin. The positivity rate of serum calcitonin in the group in the present study was 98.5%. There was a difference in the level of serum calcitonin between the two groups, which may be related to the nodule size, secretory activity of the lesion subtype of the lesion or RET mutation.[8–11] The level of serum calcitonin was normal in one case, and the size of this lesion was 0.3 cm, which may be related to the nodule size or dedifferentiation state of the lesion.[12,13] Ninety-two patients who had other types of thyroid carcinoma during the same period were investigated, including 60 cases of papillary thyroid carcinoma (PTC) and 32 cases of follicular thyroid carcinoma (FTC), and the level of serum calcitonin was all within the normal range. Therefore, patients with suspected MTC must have serum calcitonin examinations. Due to the low incidence of MTC and because many other diseases, such as small cell lung cancer and acute and chronic renal dysfunction, can lead to increases in serum calcitonin,[1] serum calcitonin is not recognized as a routine examination for patients with thyroid nodules.[14] However, this study suggests that the routine examination of serum calcitonin in patients with thyroid nodules may be beneficial for MTC patients.
This study found that there was no significant difference between the two groups in the mean age or sex. While Wolinski et al[15] found that MTC occurs more frequently in older patients (>50) and the incidence in females was higher than in males. In addition, patients in the ultrasonic correct diagnosis group were associated with diarrhea, hoarseness, suffocation, pain, dysphagia, cough, and other symptoms, and the misdiagnosis group has no other concurrent symptoms. Therefore, it is not reliable to diagnose MTC based on patient age and gender. However, the clinical symptoms have some suggestive significance.
Ultrasound is considered to be the most accurate method for evaluating the characteristics of thyroid nodules. The ultrasonographic features of malignant tumors include irregular margins, microcalcification, an aspect ratio >1, hypoechoic outbreaks of calcification, and invasion of the thyroid membrane.[16] However, these manifestations are mainly for PTC. Because of the differences in the pathological basis in PTC and MTC, there are certain differences in the ultrasonic images. There was a certain similarity between ultrasonic correct diagnosis group and ultrasonic misdiagnosed group, mostly manifested as hypoechoic lesions with aspect ratios less than one. First, this study found that the lesions mostly manifested as hypoechoic lesions with aspect ratios less than one, and only six (6.3%) lesions had aspect ratios greater than one. The result was lower than that of the metanalysis by Wolinski et al[15] which was 14.4%, and similar to that by Liu et al,[17] which was 6.8%. The study by Zhou et al[18] found that the diagnostic accuracy of an aspect ratio greater than one decreased with nodule enlargement. In this study, among the six cases with an aspect ratio greater than one, four cases were less than 1 cm and two cases were more than 1 cm, which was mutually confirmed by the results of Zhou et al.[18] Second, regarding echogenicity, both ultrasonic correct diagnosis group and ultrasonic misdiagnosed group had mainly hypoechoic lesions, and only one lesion was hyperechoic in ultrasonic misdiagnosed group. In a study that included 157 patients, there were no hyperechoic lesions[15]. Therefore, the risk of hyperechoic lesions indicated a lower risk for MTC. There were differences in the findings of the echotexture in the MTC lesions, in which the studies by Lee et al[19] found that the MTC lesion was more likely to show a cystic change than the PTC lesion, while the studies by Kim et al[20] suggested that a cystic change in an MTC lesion was rare. In this study, there were nine nodules with cystic change, the average maximum diameter was 2.8 cm, and the difference in the cystic change in the two groups was statistically significant (P = 0.007). Therefore, we should consider the possibility of MTC for larger cystic solid nodules. If necessary, we can measure serum calcitonin and/or perform FNA in larger cystic solid nodules. Third, regarding the margin and shape of the lesions, the studies by Kim et al[20] suggested that the margin of the MTC lesion was circumscribed, while the studies by Liu et al[17] found that the margin was uncircumscribed. This study found that there was a difference between the two groups. The possibility of misdiagnosis was increased for the lesion with a circumscribed margin or regular shape. Fourth, regarding the calcification of the lesions, there were 19 lesions of microcalcification and 26 lesions of coarse calcification. There was no significant difference in the type of calcification in ultrasonic correct diagnosis group. However, there were more patients without calcification in ultrasonic misdiagnosed group (19/24, 79.2%). Because calcification in the MTC lesion is composed of amyloid material wrapped around local calcium salt, its incidence is relatively low.[18] Fifth, there were differences in the distribution of the blood sinus and the degree of fibrosis among the different pathological types, so the value of vascularity is different in malignant nodules.[16] In this study, the proportion of internal rich vascularity in ultrasonic correct diagnosis group was higher (56/71, 78.9%), but the proportion of absent vascularity in ultrasonic misdiagnosed group was higher (10/24, 41.7%); the difference was statistically significant (P < 0.001). However, a previous study found that there was no significant value for color Doppler in the diagnosis of MTC.[21] This difference may be associated with the composition and division of blood vessels.[22]
Meanwhile, cavernous nodules were detected by preoperative ultrasonography in 29 patients. There were 16 cases (30.2%) in ultrasonic correct diagnosis group and 13 cases (72.2%) in ultrasonic misdiagnosed group, and the difference was statistically significant (P = 0.002). This may be related to imprecise observations.
MTC has a tendency toward lymph node metastasis. The studies by Gimm et al[23] show that the incidence of cervical lymph node metastasis in MTC can reach 75% to 80%. In this study, 49 patients (49/71, 69%) were diagnosed with lymph node metastases after surgery, and 35 cases (35/71, 49.3%) were found to be abnormal before surgery, which showed that preoperative ultrasound had some limitations in cervical lymph node metastasis.
FNA cytological examination was a common preoperative diagnostic method. Thirty-seven cases were examined by FNA before surgery, and the accuracy of the cytological diagnosis was 67.6%. This rate is similar to that of previous reports[24,25] but far below that for PTC.[26,27] This indicated that FNA cytology had some limitations in the diagnosis of MTC. But the diagnostic accuracy of FNA analysis has been reported to be markedly increased by immunohistochemical analysis of the FNA specimen and additionally by measuring calcitonin levels in the FNA washout fluid.[28–30] Therefore, the application prospect of FNA is relatively broad.
In summary, in cases of atypical MTC, such as cystic change, circumscribed margin, regular shape, no calcification, no rich vascularity, and normal cervical lymph nodes, lesions are easily misdiagnosed as benign by ultrasound. In addition to ultrasound examination, serum calcitonin examination should be included in the routine which may be beneficial for MTC patients. And FNA is an important means to diagnose MTC, especially immunohistochemical analysis of the FNA specimen and measuring calcitonin levels in the FNA washout fluid. Therefore, ultrasound, cytology and serum calcitonin should be comprehensively evaluated for a preoperative diagnosis of MTC.
Funding
This study was supported by grants from Beijing Hope Run Special Fund of Cancer Foundation of China (No. LC2017A05) and Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No.2017PT32003).
Conflicts of interest
None.
Footnotes
How to cite this article: Guo QQ, Zhang SH, Niu LJ, Zhang YK, Li ZJ, Chang Q. Comprehensive evaluation of medullary thyroid carcinoma before surgery. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000160
Qian-Qian Guo and Shao-Hang Zhang contributed equally to this work.
References
- 1.Wells SJ, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25:567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 2009; 19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 3.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Invest 2010; 33:287–291. doi: 10.1007/BF03346587. [DOI] [PubMed] [Google Scholar]
- 4.Fagin JA, Wells SJ. Biologic and clinical perspectives on thyroid cancer. N Engl J Med 2016; 375:1054–1067. doi: 10.1056/NEJMra1501993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonelli A, Miccoli P, Fallahi P, Grosso M, Nesti C, Spinelli C, et al. Role of neck ultrasonography in the follow-up of children operated on for thyroid papillary cancer. Thyroid 2003; 13:479–484. doi: 10.1089/105072503322021142. [DOI] [PubMed] [Google Scholar]
- 6.Roman S, Mehta P, Sosa JA. Medullary thyroid cancer: early detection and novel treatments. Curr Opin Oncol 2009; 21:5–10. doi: 10.1097/CCO.0b013e32831ba0b3. [DOI] [PubMed] [Google Scholar]
- 7.Das DK, Mallik MK, George SS, Sheikh ZA, Pathan SK, Haji BE, et al. Secretory activity in medullary thyroid carcinoma: a cytomorphological and immunocytochemical study. Diagn Cytopathol 2007; 35:329–337. doi: 10.1002/dc.20637. [DOI] [PubMed] [Google Scholar]
- 8.Matias-Guiu X, De Lellis R. Medullary thyroid carcinoma: a 25-year perspective. Endocr Pathol 2014; 25:21–29. doi: 10.1007/s12022-013-9287-2. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Pyo JS, Cho WJ. Clinicopathological significance and prognosis of medullary thyroid microcarcinoma: a meta-analysis. World J Surg 2017; 41:2551–2558. doi: 10.1007/s00268-017-4031-6. [DOI] [PubMed] [Google Scholar]
- 10.Kudo T, Miyauchi A, Ito Y, Yabuta T, Inoue H, Higashiyama T, et al. Serum calcitonin levels with calcium loading tests before and after total thyroidectomy in patients with thyroid diseases other than medullary thyroid carcinoma. Endocr J 2011; 58:217–221. doi: 10.1507/endocrj.k10e-359. [DOI] [PubMed] [Google Scholar]
- 11.Grubbs EG, Ng PK, Bui J, Busaidy NL, Chen K, Lee JE, et al. RET fusion as a novel driver of medullary thyroid carcinoma. J Clin Endocrinol Metab 2015; 100:788–793. doi: 10.1210/jc.2014-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trimboli P, Giovanella L. Serum calcitonin negative medullary thyroid carcinoma: a systematic review of the literature. Clin Chem Lab Med 2015; 53:1507–1514. doi: 10.1515/cclm-2015-0058. [DOI] [PubMed] [Google Scholar]
- 13.Mendelsohn G, Wells SJ, Baylin SB. Relationship of tissue carcinoembryonic antigen and calcitonin to tumor virulence in medullary thyroid carcinoma. An immunohistochemical study in early, localized, and virulent disseminated stages of disease. Cancer 1984; 54:657–662. doi: 10.1002/1097-0142(1984)54:4<657::aid-cncr2820540412>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Daniels GH. Screening for medullary thyroid carcinoma with serum calcitonin measurements in patients with thyroid nodules in the United States and Canada. Thyroid 2011; 21:1199–1207. doi: 10.1089/thy.2010.0297. [DOI] [PubMed] [Google Scholar]
- 15.Wolinski K, Rewaj-Losyk M, Ruchala M. Sonographic features of medullary thyroid carcinomas–a systematic review and meta-analysis. Endokrynol Pol 2014; 65:314–318. doi: 10.5603/EP.2014.0043. [DOI] [PubMed] [Google Scholar]
- 16.Haugen BR. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer 2017; 123:372–381. doi: 10.1002/cncr.30360. [DOI] [PubMed] [Google Scholar]
- 17.Liu MJ, Liu ZF, Hou YY, Men YM, Zhang YX, Gao LY, et al. Ultrasonographic characteristics of medullary thyroid carcinoma: a comparison with papillary thyroid carcinoma. Oncotarget 2017; 8:27520–27528. doi: 10.18632/oncotarget.15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Chen B, Zhao M, Zhang H, Liang B. Sonographic features of medullary thyroid carcinomas according to tumor size: comparison with papillary thyroid carcinomas. J Ultrasound Med 2015; 34:1003–1009. doi: 10.7863/ultra.34.6.1003. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Shin JH, Han BK, Ko EY. Medullary thyroid carcinoma: comparison with papillary thyroid carcinoma and application of current sonographic criteria. AJR Am J Roentgenol 2010; 194:1090–1094. doi: 10.2214/AJR.09.3276. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Kim BS, Jung SL, Lee JW, Yang PS, Kang BJ, et al. Ultrasonographic findings of medullary thyroid carcinoma: a comparison with papillary thyroid carcinoma. Korean J Radiol 2009; 10:101–105. doi: 10.3348/kjr.2009.10.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saller B, Moeller L, Gorges R, Janssen OE, Mann K. Role of conventional ultrasound and color Doppler sonography in the diagnosis of medullary thyroid carcinoma. Exp Clin Endocrinol Diabetes 2002; 110:403–407. doi: 10.1055/s-2002-36546. [DOI] [PubMed] [Google Scholar]
- 22.Foschini MP, Ragazzi M, Parmeggiani AL, Righi A, Flamminio F, Meringolo D, et al. Comparison between echo-color Doppler sonography features and angioarchitecture of thyroid nodules. Int J Surg Pathol 2007; 15:135–142. doi: 10.1177/1066896906299118. [DOI] [PubMed] [Google Scholar]
- 23.Gimm O. Extent of surgery in clinically evident but operable MTC - when is central and/or lateral lympadenectomy indicated? Thyroid Res 2013; 6 suppl 1:S3.doi: 10.1186/1756-6614-6-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Essig GJ, Porter K, Schneider D, Debora A, Lindsey SC, Busonero G, et al. Fine needle aspiration and medullary thyroid carcinoma: the risk of inadequate preoperative evaluation and initial surgery when relying upon FNAB cytology alone. Endocr Pract 2013; 19:920–927. doi: 10.4158/EP13143.OR. [DOI] [PubMed] [Google Scholar]
- 25.Trimboli P, Treglia G, Guidobaldi L, Romanelli F, Nigri G, Valabrega S, et al. Detection rate of FNA cytology in medullary thyroid carcinoma: a meta-analysis. Clin Endocrinol (Oxf) 2015; 82:280–285. doi: 10.1111/cen.12563. [DOI] [PubMed] [Google Scholar]
- 26.Song H, Wei C, Li D, Hua K, Song J, Maskey N, et al. Comparison of fine needle aspiration and fine needle nonaspiration cytology of thyroid nodules: a meta-analysis. Biomed Res Int 2015; 2015:796120.doi: 10.1155/2015/796120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Fu HJ, Xu HX, Guo LH, Li XL, He YP, et al. Comparison of fine needle aspiration and non-aspiration cytology for diagnosis of thyroid nodules: A prospective, randomized, and controlled trial. Clin Hemorheol Microcirc 2017; 66:67–81. doi: 10.3233/CH-160222. [DOI] [PubMed] [Google Scholar]
- 28.Trimboli P, Cremonini N, Ceriani L, Saggiorato E, Guidobaldi L, Romanelli F, et al. Calcitonin measurement in aspiration needle washout fluids has higher sensitivity than cytology in detecting medullary thyroid cancer: a retrospective multicentre study. Clin Endocrinol (Oxf) 2014; 80:135–140. doi: 10.1111/cen.12234. [DOI] [PubMed] [Google Scholar]
- 29.Giovanella L, Imperiali M, Piccardo A, Taborelli M, Verburg FA, Daurizio F, et al. Procalcitonin measurement to screen medullary thyroid carcinoma: a prospective evaluation in a series of 2705 patients with thyroid nodules. Eur J Clin Invest 2018; 48:e12934.doi: 10.1111/eci.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki A, Hirokawa M, Takada N, Higuchi M, Ito A, Yamao N, et al. Fine-needle aspiration cytology for medullary thyroid carcinoma: a single institutional experience in Japan. Endocr J 2017; 64:1099–1104. doi: 10.1507/endocrj.EJ17-0238. [DOI] [PubMed] [Google Scholar]


