Abstract
Background:
Helicobacter pylori (H. pylori) eradication has been widely used. The recurrence rate of H. pylori after eradication and its related factors are gaining more and more attention. Our study aimed to determine the recurrence rate of H. pylori infection after successful eradication, and analyze its influential factors.
Methods:
We prospectively studied 1050 patients with upper gastrointestinal symptoms who were diagnosed as H. pylori infection by gastroscopy and underwent eradication therapies from April 2013 to January 2014. The 13C-urea breath test (UBT) or Warthin-Starry (WS) staining was done at 8 to 12 weeks after the therapy. Patients with successful eradication were followed by repeated UBT or gastroscopy at one year and 3 years after therapy, as well as, questionnaire surveys. Recurrence was considered if the UBTs or WS staining of biopsy were positive. One-year and 3-year recurrence rates were calculated, and analyzed the differences between recurred patients and others in basic data, sociological characteristics, lifestyle.
Results:
A total of 743 patients finished the 1-year follow-up, and the 1-year recurrence rate was 1.75%. Of the 607 patients who finished the 3-year follow-up, 28 patients recurred, and the 3-year recurrence rate was 4.61%. Analysis of variance showed that low-income, poor hygiene condition of dining out place, and receiving invasive diagnoses or treatments were significant risk factors for H. pylori infection recurrence. Logistic regression analysis demonstrated that the combination of invasive diagnoses or treatments, the level of income, and the hygiene standard of dining out place were significant and independent influential factors of the recurrence of H. pylori.
Conclusions:
The 1-year and 3-year recurrence rates of H. pylori infection after eradication therapy are 1.75% and 4.61%. Low-income, poor hygiene condition of dining out place, and a combination of invasive diagnoses or treatments are independent risk factors of H. pylori recurrence.
Keywords: Affecting factors, Follow-up study, Helicobacter pylori, Recurrence
Introduction
Helicobacter pylori (H. pylori) infection is associated with many upper gastrointestinal tract diseases like chronic gastritis, peptic ulcer, and gastric mucosa associated lymphoid tissue (MALT) lymphoma.[1] Eradication therapy has been proved to be beneficial in alleviating active gastritis, cure peptic ulcer and MALT lymphoma, and even prevent gastric mucosa cancerization. However, the recurrence of H. pylori may erase these benefits of eradication, and lead to the recurrence of ulcer or lymphoma.[2,3] Owing to the wide application of eradication therapy, the recurrence of H. pylori infection and its affecting factors are gaining more and more attention. The recurrence rate varies among different countries, and has negative correlation with the socioeconomic level.[4] Some previous studies found that the recurrence rate was related to factors such as prevalence of H. pylori infection, hygiene conditions, and population susceptibility, while few other studies gave contrary conclusions. Till now, there are no large-scale studies on H. pylori recurrence, or studies focusing on the factors affecting the recurrence in our country. Therefore, we aimed to determine the recurrence rate of H. pylori infection after successful eradication in Chinese population, and analyze its affecting factors.
Methods
Ethical approval
The study was approved by the Ethics Committee of Peking University Third Hospital. All the participants have signed the informed consent form.
Patients and study design
Patients who have received H. pylori eradication therapy successfully for the first time from April 2013 to January 2014 in a previous study[5] from Peking University Third Hospital, Qilu Hospital of Shandong University, and Peking Union Medical College Hospital were enrolled in our study. All patients were informed in detail about the aim, process, benefits, and possible risks of the study before participation.
The inclusion criteria of the previous study were patients aged 18 to 70 years, having upper digestive tract symptoms, and in agreement with endoscope examination.
The exclusion criteria were as follows: Patients receiving eradication therapy in the past; taking proton pump inhibitors, H2-receptor antagonists, bismuth, antibiotics or other medications that interfere with the result of the examination in the recent 4 weeks; history of digestive cancer; operation history of stomach or esophagus; Zollinger-Ellison syndrome; with severe diseases of liver, kidney, cardiovascular system, respiratory system, blood system, nervous and mental system, or endocrine system; allergic to medications used for treatment; women in gestation or lactation period; alcohol abuse or any other clinical situation which may increase the risk of therapeutic side-effects.
The diagnosis of H. pylori infection in the previous study was confirmed when both the rapid urease test and WS staining were positive.
A total of 1050 H. pylori infected patients received who received eradication therapy were randomly divided into three groups with 10-day therapeutic schemes: tailored therapy, quadruple therapy (esomeprazole 20 mg bid, amoxicillin 0.5 g bid, clarithromycin 0.5 g bid, Bismuth potassium citrate capsules 220 mg bid), and concomitant therapy (esomeprazole 20 mg bid, amoxicillin 0.5 g bid, clarithromycin 0.5 g bid, tinidazole 0.5 g bid). The 13C urea breath test (13C-UBT) or gastroscope was applied 8 to 12 weeks after the therapy, and eradication was considered successful if the result of 13C-UBT or gastroscope was negative.
A total of 827 patients succeeded eradication, and were enrolled in the following study. Patients were followed-up at 1 and 3 years after the eradication of H. pylori infection using 13C-UBT or gastroscope [Figure 1].
Figure 1.
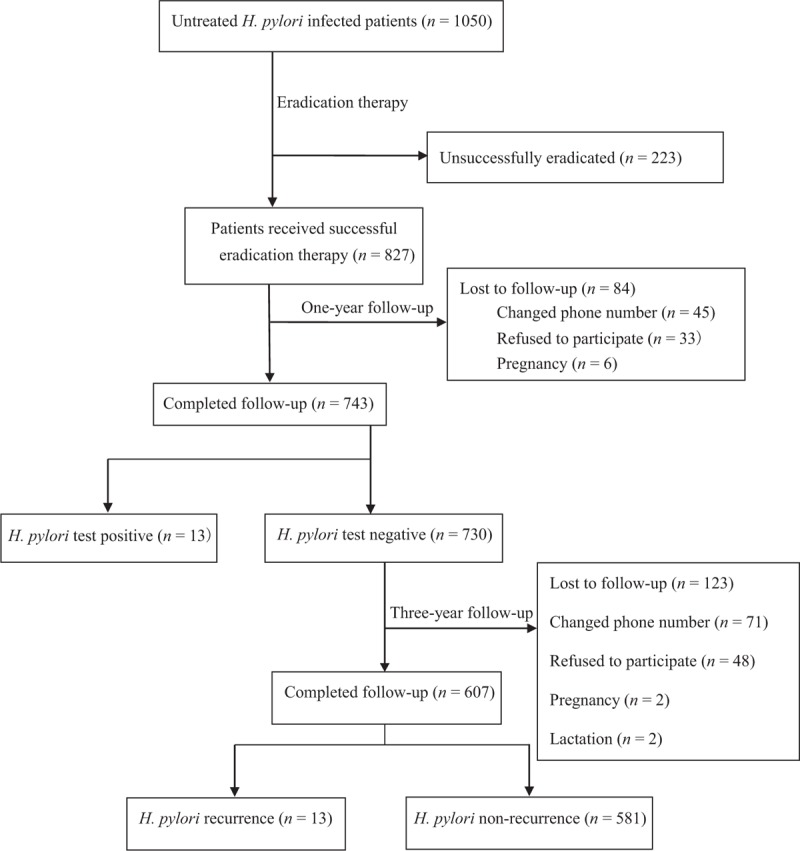
The flow chart of this study.
We calculated 1-year and 3-year eradication rates, and compared the differences between recurred patients and others using their basic data, sociological characteristics, lifestyle, and disease history.
13C-UBT
Patients prior to undergoing 13C-UBT were required to stop taking antibiotics, bismuth, proton pump inhibitors, H2-receptor antagonists, or other medicines that might interfere with the result of examination for at least 4 weeks.
Operation process
(1) The patient should be kept on an empty stomach before the test. (2) The patient's name, age and gender were filled on three labeled gas containers. (3) Breath sample was collected at 0 min: patient's exhalation was collected through a disposable plastic straw connected to the bottom of the first container for 4 to 5 s, then the straw was pulled out, and the cap of the container was closed. (4) The patient was given a capsule containing 75 mg of 13C-labeled urea, and sat still. (5) Breath sample was collected at 30 min as described above. (6) These two samples were analyzed and compared by 13C-exhalation mass spectrometer (type ZHP-2001), and the results were expressed as difference per thousand (δ‰). δ‰ = (isotopic abundance of 13C working sample − isotopic abundance of 13C reference sample)/isotopic abundance of 13C reference sample × 1000. (7) The judgment of H. pylori infection: the test value was expressed as the difference value of breath sample in 30 min and in 0 min, namely, test value = δ‰(30 min)−δ‰(0 min). Infection state of H. pylori was considered positive if the test value exceeded 4.0‰, and otherwise, negative.
Questionnaires
We determined the potential factors that affect the recurrence of H. pylori infection by literature review and formulated questionnaires accordingly. The contents of the questionnaires included basic data (such as occupation, ethnicity, height, mess, the native place of father, the native place of mother, permanent residence, educational level, and income), lifestyle (living space, family members, source of drinking water, cooking habits, and frequency, and way of dining), and disease history (any history of hospitalization in the past 1 year, receiving invasive diagnoses or treatment, contact with other H. pylori infected patients, and combination of underlying diseases). The procedure of survey was conducted by researchers in person, or under the guidance of strict training investigators.
Statistical analysis
EpiData 3.1 was used for data entry and management, and the data entry quality was checked by double data entry verification method. Statistical analyses were performed using SPSS for Windows 22.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were subjected to normality test using one-sample Kolmogorov-Smirnov test. Normally distributed variables were expressed as mean ± standard deviation, while abnormally distributed variables were expressed as medians and quartiles, and categorical variables were described as frequencies and percentages. Normally distributed variables were compared using independent sample t test, while abnormally distributed variables were compared using non-parametric test. Categorical variables were compared using the Chi-squared test. Multivariate analyses were performed using binary non-conditional logistic regression to detect the affecting factors of recurrence of H. pylori infection. Odd's ratio (OR) and 95% confidence interval (95% CI) were applied to measure the degree of its association. All the tests involved were two-tailed, and P < 0.05 was considered statistically significant.
Results
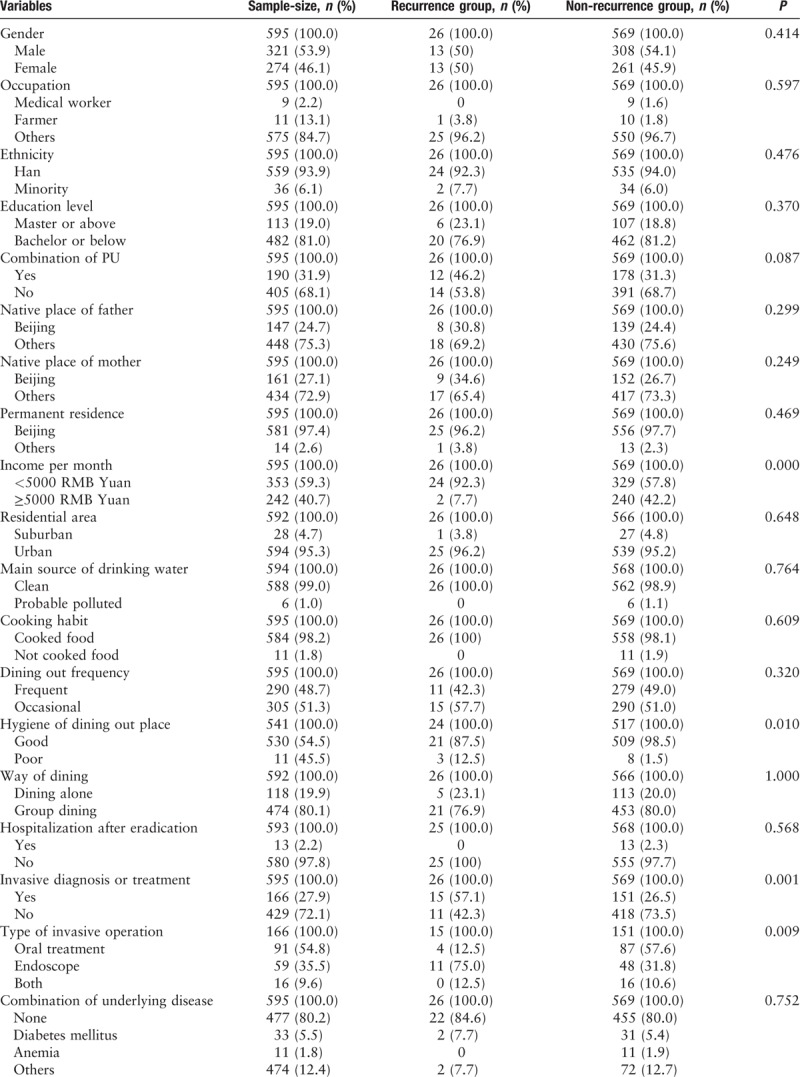
Characteristics of patients
A total of 734 patients completed the follow-up tests 1 year after eradication therapy, and 607 patients completed the tests 3 years after eradication therapy, and included 321 males and 274 females, with a mean age of 48.5 ± 12.9 years. The average body mass index (BMI) was 23.6 ± 3.1 kg/m2, and the average living space was 32.7 ± 21.9 m2. One hundred ninety patients were diagnosed with peptic ulcers, while the remaining 417 patients had non-ulcer dyspepsia [Table 1].
Table 1.
The general factors of the recurrence and non-recurrence patients with H. pylori infection.

Recurrence of H. pylori infection
The recurrence of H. pylori infection 1 year after eradication was positive in 13 patients, and the 1-year recurrence rate was 1.75%. Likewise, a total of 28 patients had recurrence at 3 years after eradication, and the cumulative 3-year recurrence rate of H. pylori infection was 4.61%.
Factors affecting the recurrence of H. pylori infection
Of the 607 patients who completed the follow-up tests, 595 valid questionnaires were returned, with a response rate of 98.02%, including 26 in the recurrence group and 569 in the non-recurrence group.
Single factor analysis of variance showed that low-income, poor hygiene condition of dining out place, and receiving invasive diagnoses or treatments were considered as risk factors of recurrence of H. pylori infection [Table 2].
Table 2.
Univariate analysis of the factors affecting the recurrence of H. pylori infection.
The recurrence rates of patients with a monthly income less than and more than 5000 RMB Yuan were 0.8% and 6.8%, respectively (χ2 = 12.255, P = 0.000).
Five hundred forty-one patients had a habit of dining out. Among them, the recurrence rate was as high as 27.27% with poor hygiene condition of dining out place, while the rate was 3.96% with good hygiene condition (χ2 = 13.812, P = 0.001).
Patients who received invasive diagnoses or treatments, such as oral treatment and gastrointestinal endoscopy examinations, showed a recurrence rate of 9.04%, while the remaining had a recurrence rate of 2.56% (χ2 = 11.997, P = 0.001). The recurrence rate was 18.64% in patients who received gastrointestinal endoscopy examinations, and was higher than the patients who received oral treatment, which was 4.40% (χ2 = 10.600, P = 0.009).
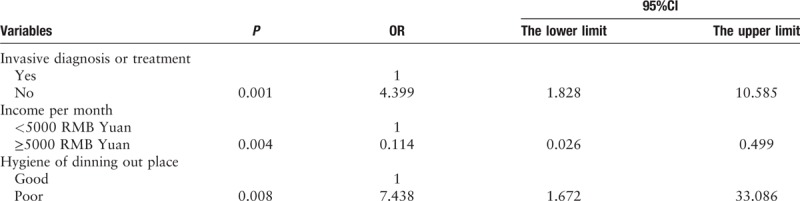
Logistic regression analysis was performed by these three factors, as well as combination of peptic ulcer and hospitalization. Results revealed that the combination of invasive diagnoses or treatments, the level of income, and the hygiene standard of dining out place were independent influential factors of H. pylori recurrence (P = 0.001, 0.004, 0.008, 95%CI 1.828–10.585, 0.026–0.499, 1.672–33.086) [Table 3].
Table 3.
Multivariate analysis of factors affecting the recurrence of H. pylori infection.
Relationship between 13C-UBT value and recurrence of H. pylori
Among all 575 patients who had successful eradication of H. pylori with 13C-UBT, the values of 13C-UBTs in 522 patients were <2, and had a recurrence rate of 4.02%. The values of 13C-UBTs ≥ 2 in 53 patients had a recurrence rate of 7.55%. There were no significant differences between these two groups (F = 1.204, P = 0.601).
Discussion
Eradication therapy of H. pylori play an important role in curing peptic ulcer, prevent the recurrence of ulcer recurrence, and treatment of MALT lymphoma, and even prevents gastric mucosa cancerization. Because of the wide application of eradication therapy in the treatment of H. pylori infection, the recurrence of H. pylori after eradication has been attracting more and more attention.
The recurrence of H. pylori is defined as urea breath test or histological examinations showing negative value of H. pylori infected patient who has finished eradication therapy for at least 4 weeks, and turns positive during the follow-up period.
The recurrence of H. pylori infection was divided into two clinical situations, namely recrudescence and reinfection.[6,7] The recrudescence of H. pylori refers to a situation where the H. pylori stain was suppressed by medicines, thus failing to detect its colonization for the first time with follow-up infection test in 4 weeks or more after eradication, but latter tests become positive with the reproduction of original H. pylori bacterial stain. Reinfection of H. pylori is defined as successful elimination of H. pylori bacterial stain, and when the patient is infected with a new strain or a homogeneous strain of the former one, resulting in positive in latter follow-up infection tests. Many molecular fingerprint techniques could identify the strains of H. pylori. Since the procedures of these techniques are very complicated and the demands on staff and facilities are high, there is still a long way to go for the use of applications. Thus, most former studies classified the recurrence of infection of H. pylori after eradication in less than 1 year as recrudescence, while reinfection if it was more than 1 year. Recurrence in our study patients at 1 year after eradication may be caused by recrudescence or reinfection, and the main cause of recurrence at 3 years after eradication was reinfection.
The recurrence rate varied in different studies, ranging between 0% and 23.4%.[8–14] However, it was deduced that the recurrence rates of developed countries are lower than that of the developing countries. A region with higher prevalence rate of H. pylori has higher recurrence rate correspondingly. Annualized recurrence rates in developed countries like Japan, United Kingdom, and Spain are lower than 1%, while the rates are more than 10% in developing countries such as Latin American countries, Bolivia, and Vietnam.
According to eight research studies conducted 10 years ago in China, with sample sizes were below 300, and subjects with peptic ulcers showed a recurrence rate of H. pylori infection between 1.08% and 17%.[15,16] The factors affecting the recurrence of H. pylori infection was rarely investigated and analyzed. With the wide application of eradication therapy for H. pylori infection, the prevalence of H. pylori was declined in the recent years. Moreover, the recurrence may reveal some new changes and characteristics with the promotion of socioeconomic developing status and improvement of hygiene condition. Hence, we conducted this prospective study to detect the recurrence rate of H. pylori infection in China. In our study, the recurrence rate one year after eradication therapy was 1.75%, and the cumulative recurrence rate in 3 years was 4.61%. Thus, the recurrence rates in the second and third year after eradication therapy are lower than that in the first year, illustrating reinfection, and the reason for the recurrence of H. pylori 1 year after eradication might be due to recrudescence.
A meta-analysis by Yan et al on the recurrence of H. pylori infection covering 77 researches,[14] and 1226 patients (in total 43,525 patient-years) showed a world-wide annual average recurrence rate of H. pylori infection of 2.82 ± 1.16%. In this article, the nation's socioeconomic level was evaluated by human development index (HDI), which is an integrated and objective consideration index based on lifespan, quality of life, education level and so on. The article demonstrated a negative linear correlation between recurrence rate and the nations HDI (r = −0.633), and that the recurrence rate in very-high HDI nations were significantly different compared with nations with high, medium, and low HDI. In the developing countries, the poor populations have a higher recurrence rate of H. pylori, while this rate in the rich populations was similar to the Western countries. Hence, it was considered that the recurrence rate of H. pylori infection was closely related to local economic level. Moreover, hygiene condition, prevalence of H. pylori, and close contact in family members may be associated with recurrence.
We also discovered that the recurrence rate in low-income patients was significantly higher than high-income patients. Besides, hygiene condition of dining out place has been proved to be an influential factor for the recurrence of H. pylori infection as well, with a higher recurrence rate in patients with poor hygiene condition of dining out place. From these views of sociology and public health, it is of great importance to raise the income and health consciousness in people for the prevention of recurrence of H. pylori infection.
Research studies showed that 40% to 50% dental plaques were H. pylori positive. Dental plaque is a bacterial membrane, which can protect microorganisms from antibacterial medicines.[17–19] Thus, the systemic antibiotic therapeutic regimens lose their efficiency due to it. Dental plaques could be regarded as reserve pool, leading to the recurrence of H. pylori infection in the stomach. Meanwhile, inadequate washing and sterilization of endoscope may also spread H. pylori bacteria, thus finally causing the recurrence of H. pylori. In our study, we also observed high recurrence rates in patients receiving oral treatment and gastrointestinal endoscopy examinations. A meta-analysis conducted by Bouziane et al in 2012,[20] which enrolled 298 patients from three studies, demonstrated a significant reduction of recurrence rate in the experimental group (RR = 0.37, 95% CI 0.21–0.64) patients receiving eradication therapy of H. pylori infection compared to control group receiving only eradication therapy. These results suggested that the combination of periodontal scaling can reduce the recurrence of H. pylori infection. On the other side, the procedure of washing and sterilization of endoscopes should be monitored and executed strictly to prevent the spreading of H. pylori infection when performing endoscopy examinations.
The 13C-UBT value after eradication therapy may influence the judgment of recurrence. Patients whose 13C-UBT values are between 2 and 4 have the possibility of false negative, and when 13C-UBT values are found positive in further follow-up tests, these eradication-failed patients may be classified into recurrence group.[21] In our study, we compared the recurrence rate of patients whose values of 13C-UBT were lower than 2 for the first time after eradication therapy and patients whose values of 13C-UBT were between 2 and 4, which eventually showed no statistical difference.
In a word, we have found in our study that the recurrence rate of H. pylori in urban population of China was similar to that of the developed countries. Low-income, poor hygiene condition of dining out place, and combination of invasive diagnoses or treatments are considered as independent risk factors of the recurrence of H. pylori infection. A regular surveillance on infectious state in high-risk population is necessary.
Conflicts of interest
None.
Footnotes
How to cite this article: Xue Y, Zhou LY, Lu HP, Liu JZ. Recurrence of Helicobacter pylori infection: incidence and influential factors. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000146
References
- 1.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut 2017; 66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 2.Lai KC, Hui WM, Wong WM, Wong BC, Hu WH, Ching CK, et al. Treatment of Helicobacter pylori in patients with duodenal ulcer hemorrhage--a long-term randomized, controlled study. Am J Gastroenterol 2000; 95:2225–2232. doi: 10.1111/j.1572-0241.2000.02249.x. [DOI] [PubMed] [Google Scholar]
- 3.Papa A, Cammarota G, Tursi A, Gasbarrini A, Gasbarrini G. Helicobacter pylori eradication and remission of low-grade gastric mucosa-associated lymphoid tissue lymphoma: a long-term follow-up study. J Clin Gastroenterol 2000; 31:169–171. doi: 10.1097/00004836-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Wan JH, Li XY, Zhu Y, Graham DY, Lu NH. Systematic review with meta-analysis: the global recurrence rate of Helicobacter pylori. Aliment Pharmacol Ther 2017; 46:773–779. doi: 10.1111/apt.14319. [DOI] [PubMed] [Google Scholar]
- 5.Liya Zhou, Jianzhong Zhang, Zhiqiang Song, He L, Li Y, Qian J, et al. Tailored versus triple plus bismuth or concomitant therapy as initial helicobacter pylori treatment: a randomized trial. Helicobacter 2016; 21:91–99. doi: 10.1111/hel.12242. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Hyun JJ, Jung SW, Koo JS, Yim HJ, Lee SW. Helicobacter pylori recurrence after first- and second-line eradication therapy in Korea: the problem of recrudescence or reinfection. Helicobacter 2014; 19:202–206. doi: 10.1111/hel.12117. [DOI] [PubMed] [Google Scholar]
- 7.Bell GD, Powell KU, Burridge SM, Harrison G, Rameh B, Weil G, et al. Reinfection or recrudescence after apparently successful eradication of Helicobacter pylori infection: implications for treatment of patients with duodenal ulcer disease. Q J Med 1993; 86:375–382. [PubMed] [Google Scholar]
- 8.Hildebrand P, Bardhan P, Rossi L, Parvin S, Rahman A, Arefin MS, et al. Recrudescence and reinfection with Helicobacter pylori after eradication therapy in Bangladeshi adults. Gastroenterology 2001; 121:792–798. doi: 10.1053/gast.2001.28018. [DOI] [PubMed] [Google Scholar]
- 9.Adachi M, Mizuno M, Yokota K, Miyoshi M, Nagahara Y, Maga T, et al. Reinfection rate following effective therapy against Helicobacter pylori infection in Japan. J Gastroenterol Hepatol 2002; 17:27–31. doi: 10.1046/j.1440-1746.2002.02666.x. [DOI] [PubMed] [Google Scholar]
- 10.Wheeldon TU, Hoang TT, Phung DC, Björkman A, Granström M, Sörberg M. Long-term follow-up of Helicobacter pylori eradication therapy in Vietnam: reinfection and clinical outcome. Aliment Pharmacol Ther 2005; 21:1047–1053. doi: 10.1111/j.1365-2036.2005.02408.x. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez-Ramos A, Gilman RH, Leon-Barua R, Recavarren-Arce S, Watanabe J, Salazar G, et al. Rapid recurrence of Helicobacter pylori infection in Peruvian patients after successful eradication. Gastrointestinal Physiology Working Group of the Universidad Peruana Cayetano Heredia and The Johns Hopkins University. Clin Infect Dis 1997; 25:1027–1031. [DOI] [PubMed] [Google Scholar]
- 12.Seo M, Okada M, Shirotani T, Nishimura H, Maeda K, Aoyagi K, et al. Recurrence of Helicobacter pylori infection and the long-term outcome of peptic ulcer after successful eradication in Japan. J Clin Gastroenterol 2002; 34:129–134. doi: 10.1097/00004836-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Carta M, Dore MP, Idda M, Casu M, Realdi G. Effect of cure rate on reinfection with H. pylori: a three-year follow-up study. Am J Gastroenterol 2000; 95:3324–3325. doi: 10.1111/j.1572-0241.2000.03323.x. [DOI] [PubMed] [Google Scholar]
- 14.Yan TL, Hu QD, Zhang Q, Casu M, Realdi G. National rates of Helicobacter pylori recurrence are significantly and inversely correlated with human development index. Aliment Pharmacol Ther 2013; 37:963–968. doi: 10.1111/j.1572-0241.2000.03323.x. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell HM, Hu P, Chi Y, Chen MH, Li YY, Hazell SL. A low rate of reinfection following effective therapy against Helicobacter pylori in a developing nation (China). Gastroenterology 1998; 114:256–261. [DOI] [PubMed] [Google Scholar]
- 16.Chen TS, Tsay SH, Chang FY, Lee SD. Triple therapy for the eradication of Helicobacter pylori and reduction of duodenal ulcer relapse: comparison of 1 week and 2 week regimens and recrudescence rates over 12 months. J Gastroenterol Hepatol 1995; 10:300–305. [DOI] [PubMed] [Google Scholar]
- 17.Anand PS, Kamath KP, Anil S. Role of dental plaque, saliva and periodontal disease in Helicobacter pylori infection. World J Gastroenterol 2014; 20:5639–5653. doi: 10.3748/wjg.v20.i19.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal S, Jithendra KD. Presence of Helicobacter pylori in subgingival plaque of periodontitis patients with and without dyspepsia, detected by polymerase chain reaction and culture. J Indian Soc Periodontol 2012; 16:398–403. doi: 10.4103/0972-124X.100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Yue H, Li A, Wang J, Jiang B, Zhang Y, et al. An epidemiologic study on the correlation between oral Helicobacter pylori and gastric H. pylori. Curr Microbiol 2009; 58:449–453. doi: 10.1007/s00284-008-9341-3. [DOI] [PubMed] [Google Scholar]
- 20.Bouziane A, Ahid S, Abouqal R, Ennibi O. Effect of periodontal therapy on prevention of gastric Helicobacter pylori recurrence: a systematic review and meta-analysis. J Clin Periodontol 2012; 39:1166–1173. doi: 10.1111/jcpe.12015. [DOI] [PubMed] [Google Scholar]
- 21.Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection -- a critical review. Aliment Pharmacol Ther 2004; 20:1001–1017. doi: 10.1111/j.1365-2036.2004.02203.x. [DOI] [PubMed] [Google Scholar]


