Abstract
Background:
Portosystemic shunts, including surgical portosystemic shunts and transjugular intra-hepatic portosystemic shunt (TIPS), may have benefit over endoscopic therapy (ET) for treatment of variceal bleeding in patients with cirrhotic portal hypertension; however, whether there being a survival benefit among them remains unclear. This study was to compare the effect of three above-mentioned therapies on the short-term and long-term survival in patient with cirrhosis.
Methods:
Using the terms “variceal hemorrhage or variceal bleeding or variceal re-bleeding” OR “esophageal and gastric varices” OR “portal hypertension” and “liver cirrhosis,” the Cochrane Central Register of Controlled Trials, PubMed, Embase, and the references of identified trials were searched for human randomized controlled trials (RCTs) published in any language with full texts or abstracts (last search June 2017). Risk ratio (RR) estimates with 95% confidence interval (CI) were calculated using random effects model by Review Manager. The quality of the included studies was evaluated using the Cochrane Collaboration's tool for the assessment of the risk of bias.
Results:
Twenty-six publications comprising 28 RCTs were included in this analysis. These studies included a total of 2845 patients: 496 (4 RCTs) underwent either surgical portosystemic shunts or TIPS, 1244 (9 RCTs) underwent either surgical portosystemic shunts or ET, and 1105 (15 RCTs) underwent either TIPS or ET. There was no significant difference in overall mortality and 30-day or 6-week survival among three interventions. Compared with TIPS and ET, separately, surgical portosystemic shunts were both associated with a lower bleeding-related mortality (RR = 0.07, 95% CI = 0.01–0.32; P < 0.001; RR = 0.17, 95% CI = 0.06–0.51, P < 0.005) and rate of variceal re-bleeding (RR = 0.23, 95% CI = 0.10–0.51, P < 0.001; RR = 0.10, 95% CI = 0.04–0.24, P < 0.001), without a significant difference in the rate of postoperative hepatic encephalopathy (RR = 0.52, 95% CI = 0.25–1.00, P = 0.14; RR = 1.09, 95% CI = 0.59–2.01, P = 0.78). TIPS showed a trend toward lower variceal re-bleeding (RR = 0.46, 95% CI = 0.36–0.58, P < 0.001), but a higher incidence of hepatic encephalopathy than ET (RR = 1.78, 95% CI = 1.34–2.36, P < 0.001).
Conclusions:
The overall analysis revealed that there seem to be no short-term and long-term survival advantage, but surgical portosystemic shunts are with the lowest bleeding-related mortality among the three therapies. Surgical portosystemic shunts may be the most effective without an increased risk of hepatic encephalopathy and TIPS is superior to ET but at the cost of a higher incidence of hepatic encephalopathy. However, some of findings should be interpreted with caution due to the lower level of evidence and the existence of significant heterogeneity.
Keywords: Portosystemic shunts, Endoscopic therapy, Variceal rebleeding, Cirrhosis, Meta-analysis
Introduction
Esophageal and gastric varices are one of the most serious complications of cirrhotic portal hypertension, which may result in massive gastrointestinal hemorrhage. Studies have shown that gastroesophageal varices develop in approximately 50% of patients with cirrhosis.[1] About one-third of patients with cirrhosis and varices develop hemorrhage,[2] which is a significant cause of early mortality, reaching 30% to 50% for the first variceal bleed.[3,4] Patients who survive the first episode of variceal bleeding are at increased risk of re-bleeding (>60% at 1 year), with a mortality rate of approximately 20%,[5,6] for whom the secondary prophylaxis to prevent recurrence of variceal bleeding should be mandatory.
Surgical portosystemic shunts have played an important role in the treatment of variceal hemorrhage for more than half a century by total, partial, selective, or super-selective decompression of the portal, splenic, mesenteric, and gastroesophageal variceal venous systems, respectively; however, since their peak popularity from the 1960s through the 1980s, surgical portosystemic shunts have been gradually used with less frequency[7] with the introduction of and improvements in the non-surgical therapeutic modalities (such as transjugular intra-hepatic portosystemic shunt (TIPS) and endoscopic therapy [ET]) and the development of liver transplantation over the past few decades. TIPS is a minimally invasive fluoroscopic-guided procedure, which is performed to create a shunt sustained by a metal stent between a hepatic vein and the intra-hepatic portal vein.[8,9]
Because of its lower operative morbidity and mortality, TIPS began to replace surgical shunting as the definitive therapy for cirrhotic portal hypertension complicated by variceal hemorrhage which is refractory or recurs after pharmacologic therapies and ET.[10,11] Furthermore, with the introduction and development of polytetrafluoroethylene (PTFE)-covered stents,[12] it has largely replaced bare stents in many medical institutions owing to the improved patency and a decreased risk of occurrence of postoperative hepatic encephalopathy.[13,14] Some researchers had even concluded that primary unassisted patency rates of PTFE-covered stents are similar to those of surgical shunting.[14] ET (mainly endoscopic injection sclerotherapy [EIS] and endoscopic variceal ligation [EVL]) involves repetitive sessions of intra-variceal injection sclerotherapy, variceal band ligation, or both modalities with the goal of obliterating varices. EIS has been shown to effectively control acute variceal bleeding and reduce the risk of re-bleeding and mortality.[15]
Actually, several randomized controlled trials (RCTs) and meta-analysis have reported the differences in efficacy for treatment of variceal bleeding in patients with cirrhotic portal hypertension between above-mentioned three interventions, separately. And prevention of variceal re-bleeding is clearly the key to improved outcomes[16]; however, most of these studies were not powered to determine whether these therapies resulted in a survival benefit,[17] and few previous reviews have compared surgical portosystemic shunts, TIPS and ET, respectively, to assess the survival advantage. To comprehensively address the question, we performed this meta-analysis of RCTs to compare the outcomes of surgical portosystemic shunts vs. TIPS, surgical portosystemic shunts vs. ET, and TIPS vs. ET in the long-term management of variceal hemorrhage by assessment of overall mortality, 30-day or 6-week survival, bleeding-related mortality, the rate of variceal re-bleeding, as well as the incidence of postoperative hepatic encephalopathy.
Methods
The meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[18,19] and the Cochrane Collaboration's systematic review framework.[20] Because this was a meta-analysis, ethics committee or institutional board approval was not required.
Literature search strategy
We used PubMed, Embase, and the Cochrane Central Register of Controlled Trials in the Cochrane Library to perform a literature search of articles published until June 2017. The following key terms were used: “variceal hemorrhage or variceal bleeding or variceal re-bleeding” OR “esophageal and gastric varices” OR “portal hypertension” and “liver cirrhosis.” The search was limited from the inception up to June 2017 and had no language restrictions. We also searched the reference lists of the retrieved studies (last search performed in June 2017).
Inclusion and exclusion criteria
The study participants were patients with cirrhosis and portal hypertension, with no limitation on nationality or ethnicity. The criteria for inclusion of clinical trials were as follows: (1) RCTs published with full texts or abstract comparing surgical portosystemic shunts with TIPS, surgical portosystemic shunts with ET, or TIPS with ET (ET with or without concomitant long-term drug therapy, such as administration of beta-blockers) were included; (2) study participants aged >16 years with no other liver disorders except cirrhosis(preferably proven by biopsy) and at least one previous episode of gastroesophageal variceal bleeding that had subsequently stabilized, either spontaneously or by the use of non-surgical therapies such as vasoactive drugs and/or balloon tamponade and/or ET; (3) measurement of at least one of the following outcomes as the endpoint: primary study outcomes of overall mortality (death of any cause) and 30-day or 6-week survival, and secondary outcomes of bleeding-related mortality, the rate of variceal re-bleeding as well as the incidence of postoperative hepatic encephalopathy.
The exclusion criteria were as follows: (1) duplicate publication or provision of insufficient data; (2) studies that did not provide details on mortality and studies that involved patients with non-cirrhotic portal hypertension.
Publication selection and data extraction
Two independent reviewers (Zhou and Jiang) selected the publications by screening the titles and abstracts to determine whether they met the inclusion criteria. If necessary, an attempt was made to contact the original investigator for further data. Discrepancies between the two reviewers were resolved by discussion and consensus.
Data were extracted directly from the selected studies and collected to allow intention-to-treat analysis where possible. The relevant information included the first author's last name, publication year, study design, patient characteristics (age, sex, cause of liver disease, and Child-Pugh class), interventions, follow-up, and the following five outcomes: overall mortality, 30-day or 6-week survival, bleeding-related mortality, variceal re-bleeding, and postoperative hepatic encephalopathy.
Quality assessment
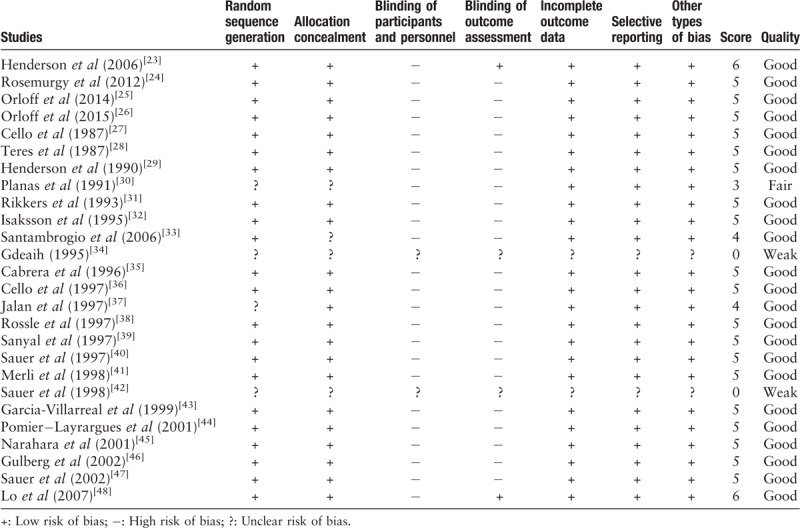
Two investigators (Zhou and Jiang) independently evaluated the quality of the included studies using the Cochrane collaboration's tool for assessing the risk of bias of RCTs.[21] The following aspects were included: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other types of bias specific to the study. Each factor was rated “low risk of bias,” “high risk of bias,” or “unclear risk of bias.” Disagreements between the two investigators were resolved by discussion and consensus. Finally, the overall quality of the included studies was categorized into good, fair, or weak, if ≥4, 3, or <3 domains were rated as low risk of bias, respectively. A summary of the risk of bias assessment is also provided in Supplementary Figure 1.
Statistical analysis
Statistical analysis was performed with Review Manager Software (RevMan 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark). Analysis were performed according to the intention-to-treat method whenever possible, with all randomized patients included in the analysis within the group into which they were randomized. Dichotomous outcomes are expressed as risk ratios (RRs) with 95% confidence intervals (CIs).[22] We assumed that heterogeneity was present even when data were not significant. A random-effects model with the Mantel-Haenszel method was used to ensure that the most conservative estimate was reported. Heterogeneity between studies was assessed with the I2 statistic as calculated by the Chi-squared test; this value indicates the percentage of total variation across studies not attributable to random error.[22] No heterogeneity is present when I2 = 0%. An I2 value of >50% was considered to indicate statistically significant heterogeneity.
When significant heterogeneity was identified (I2 > 50%), we performed subgroup analyses and sensitivity analyses to explore the possible causes of the heterogeneity. Subgroup analyses were used to assess the influence of variables on efficacy of the three interventions in the long-term management of variceal re-bleeding in patients with cirrhosis, as well as to explore the possible causes of heterogeneity. The following important factors were noted, including operation situations (emergent or elective), types of varices (esophageal, gastric, or gastroesophageal varices) and types of surgical shunts (non-selective or selective shunts). Chi-squared test was performed, which was set at a P = 0.05, to identify any subgroup differences. The sensitivity analysis was performed by using the leave-one-out approach, in which the meta-analysis was performed by removing each study in each turn, which was not performed if the number of included trials was small. A P value of <0.05 was considered to indicate statistical significance.
If possible, when the group included more than 10 studies, potential publication bias was qualitatively assessed by the visual symmetry of the funnel plots of the primary outcome. Asymmetry in the funnel plot indicated potential publication bias.
Results
Study selection
We identified 65 publications from the electronic databases by reading the title and abstract. Of these 65 publications, 18 were excluded as duplicates. Eight were excluded based on the selection criteria, and 13 were excluded because of repeated trials. Finally, 26 studies[23–48] involving 28 RCTs (Orloff et al[25,26] included 2 RCTs) and 2845 patients (surgical portosystemic shunts vs. TIPS, n = 496; surgical portosystemic shunts vs. ET, n = 1244; and TIPS vs. ET, n = 1105) were included in our meta-analysis [Figure 1].
Figure 1.
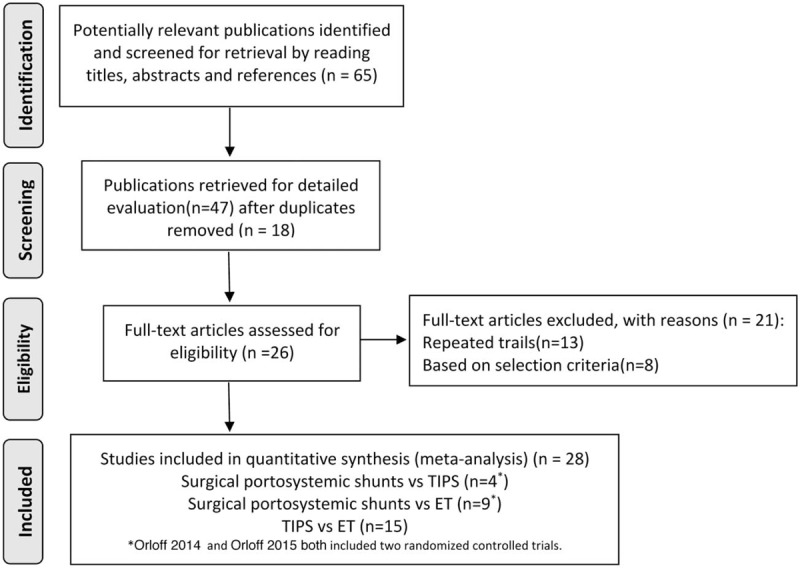
Flow diagram of study selection.
Characteristics of individual studies
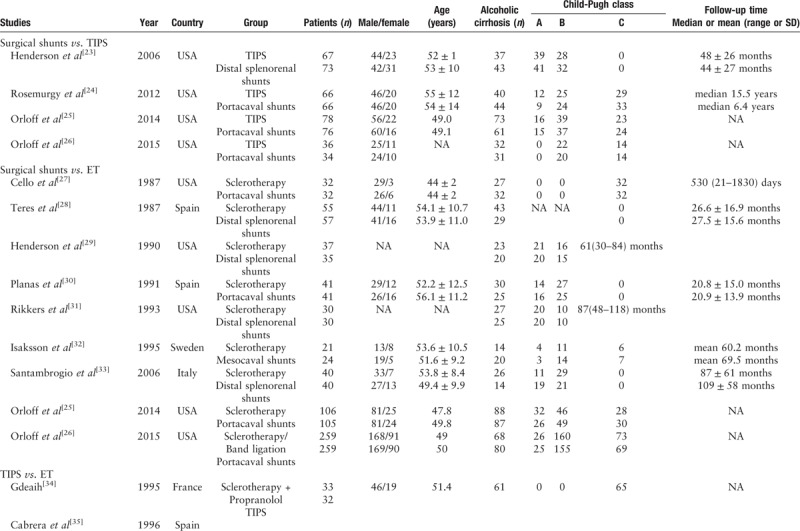
The results from two studies were only reported in abstracts.[34,42] Seven trials employed band ligation in the ET arm.[26,34,37,38,42,44,46,47] In two trials, propranolol was used in addition to sclerotherapy, but only in the ET arm[34,40]; in one trial, propranolol was used in addition to band ligation, but again only in the ET arm.[47] Only two trials performed either sclerotherapy or band ligation),[26,38] and one of these two trials added propranolol in the ET arm.[38] In all other trials, sclerotherapy was used alone in the ET arm. As for the types of surgical shunts, there were two major kinds (portacaval shunts or distal splenorenal shunts). Portacaval shunts were used in five publications (Orloff 2014, Orloff 2015, Rosemurgy 2012, Cello 1987, and Planas 1991)[24–27,30] and distal splenorenal shunts were employed in five trials.[23,28,29,31,33] Furthermore, patients with cirrhosis developed gastric variceal bleeding in three trials (two trials in Orloff et al,[26] one trial in Lo et al[48]). The characteristics of each individual study are presented in Table 1 .
Table 1.
Characteristics of each included studies.
Study quality
The risk of bias in the included studies was strictly evaluated and the results are summarized in Table 2. Among 26 publications included in the systematic review, 23 were categorized as good quality, one was fair quality,[30] and two were weak quality.[34,42] All trials had at least one problem with their methodological approach, which could account for systematic error (bias). Two trials were published as an abstract and the data were not available[34,42]; the risk of bias in these two trials is unclear. No trial used double blinding. Only two trials employed blinded assessment of outcomes.[23,48] Intention-to-treat analysis was used by most researchers, although this was not mentioned in seven trials.[24,27,31,32,34,42,48]
Table 1 (Continued).
Characteristics of each included studies.
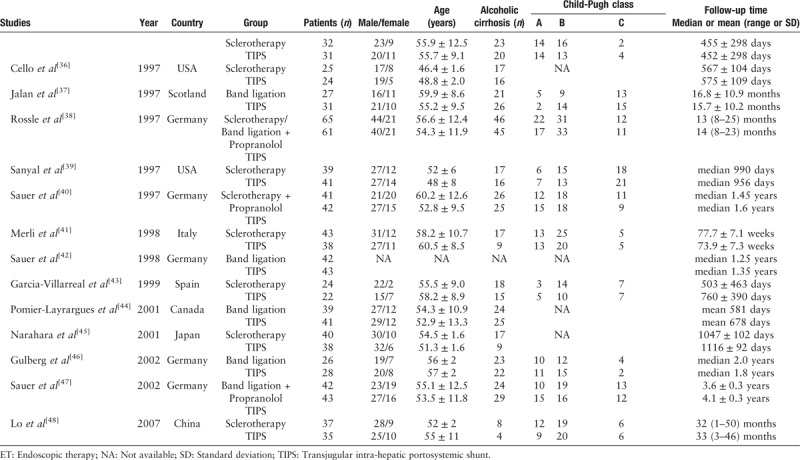
Table 2.
Study quality and risk of bias assessment using the Cochrane Collaboration's tool.
Outcomes measurements
Overall mortality
Firstly, we evaluated the effect of therapy on overall mortality [Figure 2A]. Surgical portosystemic shunts did not significantly differ from TIPS, with significant heterogeneity (52% vs. 75%, RR = 0.64, 95% CI = 0.36–1.13, I2 = 94%, P = 0.12). Comparing surgical portosystemic shunts vs. ET also had no significant difference in overall mortality, with significant heterogeneity (46% vs. 66%; RR = 0.82, 95% CI = 0.64–1.05, I2 = 80%, P = 0.11). Similarly, TIPS vs. ET did not show a statistically significant difference, without heterogeneity (27% vs. 24%, RR = 1.13, 95% CI = 0.93–1.38, I2 = 0%, P = 0.22).
Figure 2.
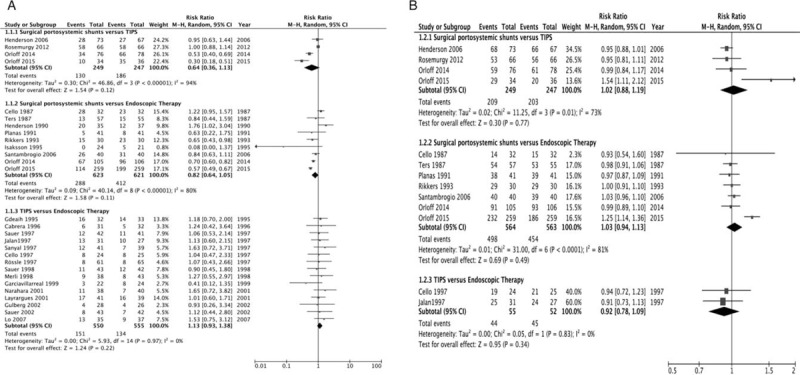
(A) Forest plot showing overall mortality. (B) Forest plot showing 30-day or 6-week survival.
30-day or 6-week survival
Then, we assessed the effect of therapy on 30-day or 6-week survival [Figure 2B]. Surgical portosystemic shunts compared with TIPS were not associated with a significant effect, with significant heterogeneity (84% vs. 82%; RR = 1.02, 95% CI = 0.88–1.19, I2 = 73%, P = 0.77). We also found no significant difference between surgical portosystemic shunts and ET, with significant heterogeneity (88% vs. 81%, RR = 1.03, 95% CI = 0.94–1.13, I2 = 81%, P = 0.49). Similarly, TIPS vs. ET showed no statistically significant difference, without heterogeneity (80% vs. 87%, RR = 0.92, 95% CI = 0.78–1.09, I2 = 0%, P = 0.34).
Bleeding-related mortality
We also assessed the effect of therapy on bleeding-related mortality [Figure 3A]. Surgical portosystemic shunts were associated with significantly lower mortality caused by variceal bleeding than TIPS, without significant heterogeneity (1% vs. 32%, RR = 0.07, 95% CI = 0.01–0.32, I2 = 16%, P = 0.0007). Surgical portosystemic shunts were also associated with significantly lower bleeding-related mortality than ET, without significant heterogeneity (2% vs. 23%, RR = 0.17, 95% CI = 0.06–0.51, I2 = 42%, P = 0.002). However, pooling of all 13 studies comparing TIPS vs. ET resulted in a clear, although not significant, trend toward an advantage of TIPS over ET in terms of bleeding-related death, without significant heterogeneity (4% vs. 9%, RR = 0.53, 95% CI = 0.29–0.99, I2 = 10%, P = 0.05).
Figure 3.
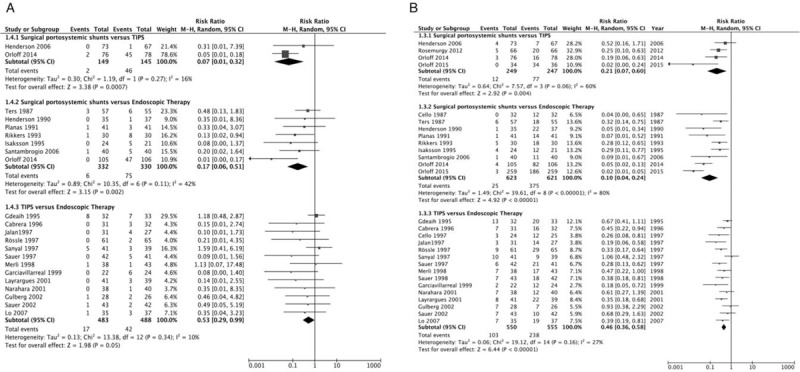
(A) Forest plot showing bleeding-related mortality. (B) Forest plot showing variceal re-bleeding.
Variceal re-bleeding
Next, we assessed the effect of therapy on variceal re-bleeding [Figure 3B]. Surgical portosystemic shunts were associated with a significantly lower rate of re-bleeding caused by gastroesophageal varices than TIPS, without significant heterogeneity (5% vs. 31%, RR = 0.21, 95% CI = 0.07–0.60, I2 = 60%, P = 0.004). Surgical portosystemic shunts were also associated with a significantly lower rate of variceal re-bleeding than ET, with significant heterogeneity (4% vs. 60%, RR = 0.10, 95% CI = 0.04–0.24, I2 = 80%, P < 0.00001). Finally, the rate of variceal re-bleeding was significantly lower with TIPS than ET, but without significant heterogeneity (19% vs. 43%, RR = 0.46, 95% CI = 0.36–0.58, I2 = 27%, P < 0.00001).
Postoperative hepatic encephalopathy
Finally, we evaluated the effect of therapy on postoperative hepatic encephalopathy [Figure 4]. Surgical portosystemic shunts resulted in a clearly, although not significantly, lower rate of hepatic encephalopathy than TIPS with significant heterogeneity (30% vs. 50%; RR = 0.52, 95% CI = 0.22–1.24, I2 = 86%, P = 0.14). Comparing surgical portosystemic shunts vs. ET also had no significant difference in the rate of hepatic encephalopathy, with significant heterogeneity (15% vs. 17%; RR = 1.09, 95% CI = 0.59–2.01, I2 = 75%, P = 0.78). However, TIPS was associated with a significantly higher rate of hepatic encephalopathy than ET, without significant heterogeneity (33% vs. 18%; RR = 1.78, 95% CI = 1.34–2.36, I2 = 33%, P < 0.0001).
Figure 4.
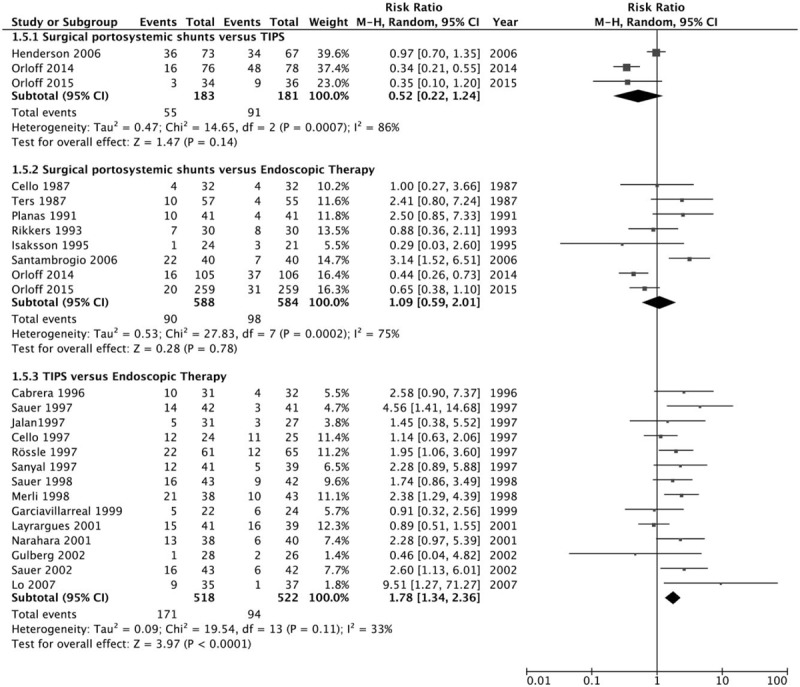
Forest plot showing postoperative hepatic encephalopathy.
Additional analysis and publication bias
Subgroup analysis
Owing to the number of included studies in the comparison between surgical portosystemic shunts vs. TIPS is just four, so it is not fit for subgroup analysis. In the comparison between surgical portosystemic shunts and ET, no significant improvement on heterogeneity was observed in the subgroup of overall mortality. The subgroup analysis of 30-day or 6-week survival and variceal re-bleeding both showed that regarding types of varices, operation situations and types of surgical shunts, most of the 95% CI between the subgroups were overlapped and partially address the heterogeneity, suggesting that there was no significant difference between the most subgroups. Moreover, the subgroup analysis of postoperative hepatic encephalopathy based on operation situations (emergent situation, elective situation, emergent, and elective situation) eliminated the heterogeneity. The other subgroup analysis could not address the heterogeneity of the meta-analysis. The results are presented in Supplementary Table 1.
Sensitivity analysis
For 30-day or 6-week survival and variceal re-bleeding with surgical portosystemic shunts vs. TIPS, the heterogeneity disappeared after removal of Orloff et al[26] (I2 = 0). For postoperative hepatic encephalopathy, the heterogeneity disappeared when we removed Henderson et al[23] (I2 = 0). Comparing surgical portosystemic shunts with ET, for 30-day or 6-week survival, the heterogeneity disappeared after removal of Orloff et al[26] (I2 = 0). The sensitivity analyses did not reveal possible explanations for the other outcomes with significant heterogeneity; that is, the heterogeneity for these outcomes was still significant after removing each study. The results are presented in Supplementary Table 2.
Publication bias
Supplementary Figure 2 shows that in the funnel plots, there was no obviously visual indication of bias favoring TIPS or ET with respect to overall mortality, bleeding-related mortality, variceal re-bleeding, and postoperative hepatic encephalopathy.
Discussion
Esophageal and gastric varices are present in about 30% to 40% of patients with compensated cirrhosis and in 80% of those with decompensated cirrhosis.[8,49] Additionally, variceal bleeding is the cause of about one-third of deaths in patients diagnosed with cirrhosis; even for patients who recover from the first episode of variceal hemorrhage, the re-bleeding risk and mortality rate are high.[5–7,50] Therefore, therapy to prevent re-bleeding should be mandatory for these patients. Four main armamentariums are currently used to prevent re-bleeding. Since surgical portosystemic shunts were introduced into clinical practice in the mid-20th century, which have been a well-established therapy for variceal hemorrhage associated with end-stage liver disease. With the development and widespread utilization of ET and TIPS, variceal bleeding caused by cirrhosis is now almost exclusively treated by gastroenterologists and radiologists who consider ET and TIPS minimally invasive. In addition, with the advent of liver transplantation as the definitive treatment for end-stage liver disease, increasingly more non-surgeons have come to firmly believe that the role of shunts is currently limited to that of a “bridge to transplantation” and that TIPS is able to fulfill this role.[51,52] It seems that surgical shunts are becoming less important in clinical practice. Rosemurgy et al[53] even considered that portal hypertension has disappeared from the purview of surgery and has migrated toward the world of non-surgical therapies, probably never to return. In contrast, Gur et al[54] believed that removing surgical shunts from the surgical armamentarium is premature, and surgical shunts may offer satisfactory control of symptoms and positive long-term prognosis for patients with compensated liver cirrhosis in whom liver transplantation is either premature or not indicated. Thus, the role of surgical shunts remains controversial.
Our study reveals that there was no marked difference in the overall deaths or 30-day or 6-week survival rate among the three therapies. Surgical portosystemic shunts were the most effective at preventing recurrent variceal hemorrhage, and TIPS were superior to ET. With respect to bleeding-related mortality, surgical portosystemic shunts were associated with a lower rate than TIPS which in turn was lower than ET without significant difference. These outcomes clearly indicate that surgical portosystemic shunts are actually the most effective at preventing variceal re-bleeding. In addition, we found that the difference of postoperative hepatic encephalopathy between surgical portosystemic shunts and ET was not notable. However, TIPS were associated with an increased incidence of hepatic encephalopathy, a major disadvantage of shunting that was more obvious following TIPS. Similar findings were also reported by Zheng et al,[55]who concluded that TIPS is related to a lower variceal re-bleeding rate, fewer re-bleeding-related death but at the price of a higher rate of hepatic encephalopathy with no improvement in overall survival. These outcomes are also consistent with the American Association for the Study of Liver Diseases (AASLD) Practice Guidelines which concluded that TIPS will effectively prevent variceal re-bleeding but will increase the incidence of portosystemic encephalopathy and will not improve survival of any of these patients.[56] We found that overall mortality and 30-day or 6-week survival rates were similar between the two forms of portosystemic shunts (surgical shunts and TIPS), and surgical portosystemic shunts are even with lower mortality in patients with variceal bleeding, indicating that surgical portosystemic shunts are at least as safe as TIPS, although the number of included studies were small and the heterogeneity were significant. The outcomes of our study call into the question the widespread practice of using surgical portosystemic shunts only as a salvage treatment for failure of TIPS and other therapies. From an objective point of view, compared with surgical shunts, TIPS is a minimally invasive and relatively uncomplicated procedure, which can be performed in an emergency in awake, mildly sedated patients with compromised liver function under local anesthesia, who are generally considered unsuitable for surgical portosystemic shunts.[57] Hepatic encephalopathy and TIPS dysfunction (occlusion or stenosis) may be the two major complications that have most significantly limited the effectiveness of TIPS. In the present study, we did not evaluate shunt dysfunction, which may necessitate more frequent re-interventions in patients who have undergone TIPS. Surgical portosystemic shunts showed a clear but not significant trend toward an advantage over TIPS with respect to hepatic encephalopathy. Moreover, the development of covered TIPS stents has not only reduced the frequency of shunt dysfunction but has also improved overall survival without increasing the risk of hepatic encephalopathy.[58] Gur et al[54] acknowledged the fact that despite certain shortcomings, TIPS will continue to be considered as a first-line therapy in patients with advanced cirrhosis and variceal bleeding after failed conventional medical therapies and ET. Nevertheless, surgical portosystemic shunts remain an important option in certain circumstances. The AASLD Practice Guidelines state that TIPS is preferred to surgical portosystemic shunts when patients with poor liver function are unresponsive to conventional medical therapies and ET; in patients with good liver function and recurrent variceal bleeding after failed initial medical therapy and ET, both surgical portosystemic shunts and TIPS appear to be equivalent.[56] In addition, surgical portosystemic shunts may offer unmatched long-term patency, the prevention of re-bleeding, and possibly improved survival in these patients, as well as low operative morbidity and mortality.[54] A recent retrospective study also concluded that surgical portosystemic shunts achieved better results than TIPS with respect to shunt failure-free survival and overall survival in patients with complicated portal hypertension and well preserved hepatic function.[59] Although in our study we are uncertain whether there is a difference in short- or long-term survival or the rate of variceal re-bleeding between surgical portosystemic shunts compared with TIPS owing to few included trials and small sample sizes of the individual included trials, our study indicate that surgical shunts may be at least as safe and efficient as TIPS.
ET plays a pivotal role in management of preventing first variceal bleeding, treatment of acute variceal bleeding, as well as prevention of variceal re-bleeding[60]; however, ET is only effective for a short time because the portal pressure and blood flow remain unchanged and the varices frequently recur (about 50% at 2 years).[5] Therefore, strict endoscopic follow-up and repeated courses of therapy are required. Considering the high success rate of TIPS in preventing uncontrolled variceal bleeding and the fact that high-risk patients with Child-Pugh class B or C disease may be better served by TIPS than repeated ET,[16,61] TIPS is generally recommended as salvage therapy in patients who have failed endoscopic treatment among patients with acute variceal hemorrhage or initial combination of EVL plus non-selective beta-blockers for prevention of variceal re-bleeding.[60,62] The precise timing of the procedure is not standardized, but it is usually considered after two occasions of failed endoscopy.[63] Our meta-analysis showed that surgical portosystemic shunts were similar to ET in the outcomes of overall mortality, 30-day or 6-week survival, and the incidence of hepatic encephalopathy, but with a significantly lower rate of variceal re-bleeding. Although significantly heterogeneity was noticed in these results, based on operation situations, subgroup analysis of 30-day or 6-week survival, rate of variceal re-bleeding and hepatic encephalopathy showed the same effect in either emergent or elective situation without significant heterogeneity, indicating we could have certain certainty of the evidence for these results. But we still should be cautious about the conclusion of overall mortality.
Liver transplantation has been known as the ultimate therapy for patients with decompensated cirrhosis and may become the treatment of choice and provide patients with a normal life expectancy,[7] which has also changed the landscape in managing cirrhotic portal hypertension.[64] Hence, it would be highly beneficial to increase the survival of patients with portal hypertension and variceal bleeding while on the waiting list for liver transplantation.[65] Specifically, the prevention of recurrent variceal hemorrhage might be expected to result in improved survival.[66] Consequently, we should take appropriate and personalized interventions for these patients to prevent variceal re-bleeding before liver transplantation is either premature or not indicated.
There is no significant heterogeneity for all five outcomes in the comparison between TIPS vs. ET. However, significant heterogeneity for the overall mortality still existed in both comparison of surgical portosystemic shunts vs. TIPS and ET despite the fact that it was performed with a random-effects model. A significant improvement was not observed in the subgroup analysis according to the operation situations (emergent or elective), types of varices (esophageal varices, gastric varices or gastroesophageal varices) and types of surgical shunts (non-selective or selective shunts). And the resource of heterogeneity was not observed after a sensitivity analysis when excluding any one of the included studies; however, the changes of statistical significance was noted when excluding any one of the studies by Rosemurgy et al,[24] Cello et al,[27] or Henderson et al,[29] which indicated the instability of this outcome in our meta-analysis. Considering the low certainty of this evidence, a conclusion about overall mortality from the present study should be carefully drawn.
Our comparison of surgical portosystemic shunts vs. TIPS for postoperative hepatic encephalopathy showed significant heterogeneity and the sensitivity analysis indicated that the cause of the heterogeneity appeared to be divergent results from the study by Henderson et al.[23] In that study, patients with Child–Pugh class A or B liver disease underwent operations under elective situations at five different clinical centers, which was different from the other two trials. Heterogeneity in 30-day or 6-week survival appeared to result from the study by Orloff et al,[34] in which the patients’ source of hemorrhage was gastric varices, which differed from other trials. Sarin et al[67] concluded that the risk of bleeding from gastric varices is approximately half that of esophageal varices but gastric variceal bleeding would result in a higher mortality rate. Additionally, these patients underwent operations in emergent situations, which may have increased the perioperative mortality rate.
Our meta-analysis has several limitations. Relatively few studies of surgical portosystemic shunts vs. TIPS were available for analysis; that is, the amount of pooled data was small. The results would have been more reliable with an increased amount of data. In addition, subgroup analysis was not performed for some outcomes because of the small number of trials. Moreover, due to the specificity of the interventions, double-blind assessment of methodological quality could not be performed in all included trials, which increases the risk of bias. Notably, the time of follow-up varied among the included studies, which may have thus confounded the conclusions. In addition, some clinical and methodological heterogeneity could not be well addressed by subgroup or sensitivity analysis. Therefore, all results presented in this review were the average effects of three interventions estimated by a random-effects model. Despite this, owing to the uneven methodological quality of the included studies, the results have some inevitable biases. Finally, we did not evaluate procedure-related complications, mortality caused by these complications, the length of hospital stay, or medical expenses; and these factors may influence the patients’ and clinicians’ choice of treatments to various degrees.
In summary, the overall analysis revealed no survival advantage seem to exist among the three therapies, and surgical portosystemic shunts were associated with the lowest bleeding-related mortality. Surgical portosystemic shunts may be the most effective and TIPS is superior to ET but at the cost of a higher incidence of postoperative hepatic encephalopathy. However, some of results in this meta-analysis should be interpreted cautiously.
Acknowledgements
The authors are deeply indebted to Yu Shi (Clinical Epidemiology and EBM Unit, Beijing Friendship Hospital, Capital Medical University, Beijing 100005, China) for her patience, help, and support in the statistics and methodology.
Funding
This study was supported by grants from the Beijing Municipal Administration of Hospitals Ascent Plan (No. DFL20150101), and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. XMLX201815).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Zhou GP, Sun LY, Wei L, Qu W, Zeng ZG, Liu Y, Jiang YZ, Zhu ZJ. Comparision between portosystemic shunts and endoscopic therapy for prevention of variceal re-bleeding: a systematic review and meta-analysis. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000212
References
- 1.Kovalak M, Lake J, Mattek N, Eisen G, Lieberman D, Zaman A. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc 2007; 65:82–88. doi: 10.1016/j.gie.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Burroughs AK, D’Heygere F, McIntyre N. Pitfalls in studies of prophylactic therapy for variceal bleeding in cirrhotics. Hepatology 1986; 6:1407–1413. doi: 10.1002/hep.1840060631. [DOI] [PubMed] [Google Scholar]
- 3.Bornman PC, Krige JE, Terblanche J. Management of oesophageal varices. Lancet 1994; 343:1079–1084. doi: 10.1016/S0140-6736(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 4.Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology 1981; 80:800–809. [PubMed] [Google Scholar]
- 5.Bosch J, Garcia-Pagan JC. Prevention of variceal rebleeding. Lancet 2003; 361:952–954. doi: 10.1016/S0140-6736(03)12778-X. [DOI] [PubMed] [Google Scholar]
- 6.de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis 2001; 5:645–663. doi: 10.1016/S1089-3261(05)70186-0. [DOI] [PubMed] [Google Scholar]
- 7.Costa G, Cruz RJ, Jr, Abu-Elmagd KM. Surgical shunt versus TIPS for treatment of variceal hemorrhage in the current era of liver and multivisceral transplantation. Surg Clin North Am 2010; 90:891–905. doi: 10.1016/j.suc.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Colapinto RF, Stronell RD, Gildiner M, Ritchie AC, Langer B, Taylor BR, et al. Formation of intrahepatic portosystemic shunts using a balloon dilatation catheter: preliminary clinical experience. AJR Am J Roentgenol 1983; 140:709–714. doi: 10.2214/ajr.140.4.709. [DOI] [PubMed] [Google Scholar]
- 9.Rossle M, Haag K, Ochs A, Sellinger M, Noldge G, Perarnau JM, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med 1994; 330:165–171. doi: 10.1056/NEJM199401203300303. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Pagan JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 11.Boyer TD, Henderson JM, Heerey AM, Arrigain S, Konig V, Connor J, et al. Cost of preventing variceal rebleeding with transjugular intrahepatic portal systemic shunt and distal splenorenal shunt. J Hepatol 2008; 48:407–414. doi: 10.1016/j.jhep.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cejna M, Peck-Radosavljevic M, Thurnher SA, Hittmair K, Schoder M, Lammer J. Creation of transjugular intrahepatic portosystemic shunts with stent-grafts: initial experiences with a polytetrafluoroethylene-covered nitinol endoprosthesis. Radiology 2001; 221:437–446. doi: 10.1148/radiol.2212010195. [DOI] [PubMed] [Google Scholar]
- 13.Tripathi D, Redhead D. Transjugular intrahepatic portosystemic stent-shunt: technical factors and new developments. Eur J Gastroenterol Hepatol 2006; 18:1127–1133. doi: 10.1097/01.meg.0000236871.78280.a7. [DOI] [PubMed] [Google Scholar]
- 14.Bureau C, Garcia Pagan JC, Layrargues GP, Metivier S, Bellot P, Perreault P, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int 2007; 27:742–747. doi: 10.1111/j.1478-3231.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 15.Copenhagen Esophageal Varices Sclerotherapy P. Sclerotherapy after first variceal hemorrhage in cirrhosis. A randomized multicenter trial. N Engl J Med 1984; 311:1594–1600. doi: 10.1056/NEJM198412203112502. [DOI] [PubMed] [Google Scholar]
- 16.Corbett C, Mangat K, Olliff S, Tripathi D. The role of transjugular intrahepatic portosystemic stent-shunt (TIPSS) in the management of variceal hemorrhage. Liver Int 2012; 32:1493–1504. doi: 10.1111/j.1478-3231.2012.02861.x. [DOI] [PubMed] [Google Scholar]
- 17.Afdhal NH, Curry MP. Early TIPS to improve survival in acute variceal bleeding. N Engl J Med 2010; 362:2421–2422. doi: 10.1056/NEJMe1003400. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535.doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, Version 5.1.0. The Cochrane Library; 2011. Available at: http://handbook-5-1.cochrane.org/ Accessed on March 2011. [Google Scholar]
- 21.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928.doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.Henderson JM, Boyer TD, Kutner MH, Galloway JR, Rikkers LF, Jeffers LJ, et al. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651. doi: 10.1053/j.gastro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Rosemurgy AS, Frohman HA, Teta AF, Luberice K, Ross SB. Prosthetic H-graft portacaval shunts vs transjugular intrahepatic portasystemic stent shunts: 18-year follow-up of a randomized trial. J Am Coll Surg 2012; 214:445–453. discussion 453-445. doi: 10.1016/j.jamcollsurg.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Orloff MJ. Fifty-three years’ experience with randomized clinical trials of emergency portacaval shunt for bleeding esophageal varices in Cirrhosis: 1958-2011. JAMA Surg 2014; 149:155–169. doi: 10.1001/jamasurg.2013.4045. [DOI] [PubMed] [Google Scholar]
- 26.Orloff MJ, Hye RJ, Wheeler HO, Isenberg JI, Haynes KS, Vaida F, et al. Randomized trials of endoscopic therapy and transjugular intrahepatic portosystemic shunt versus portacaval shunt for emergency and elective treatment of bleeding gastric varices in cirrhosis. Surgery 2015; 157:1028–1045. doi: 10.1016/j.surg.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cello JP, Grendell JH, Crass RA, Weber TE, Trunkey DD. Endoscopic sclerotherapy versus portacaval shunt in patients with severe cirrhosis and acute variceal hemorrhage. Long-term follow-up. N Engl J Med 1987; 316:11–15. doi: 10.1056/NEJM198701013160103. [DOI] [PubMed] [Google Scholar]
- 28.Teres J, Bordas JM, Bravo D, Visa J, Grande L, Garcia-Valdecasas JC, et al. Sclerotherapy vs. distal splenorenal shunt in the elective treatment of variceal hemorrhage: a randomized controlled trial. Hepatology 1987; 7:430–436. doi: 10.1002/hep.1840070303. [DOI] [PubMed] [Google Scholar]
- 29.Henderson JM, Kutner MH, Millikan WJ, Jr, Galambos JT, Riepe SP, Brooks WS, et al. Endoscopic variceal sclerosis compared with distal splenorenal shunt to prevent recurrent variceal bleeding in cirrhosis. A prospective, randomized trial. Ann Intern Med 1990; 112:262–269. doi: 10.7326/0003-4819-112-4-262. [DOI] [PubMed] [Google Scholar]
- 30.Planas R, Boix J, Broggi M, Cabre E, Gomes-Vieira MC, Morillas R, et al. Portacaval shunt versus endoscopic sclerotherapy in the elective treatment of variceal hemorrhage. Gastroenterology 1991; 100:1078–1086. doi: 10.1016/0016-5085(91)90285-S. [DOI] [PubMed] [Google Scholar]
- 31.Rikkers LF, Jin G, Burnett DA, Buchi KN, Cormier RA. Shunt surgery versus endoscopic sclerotherapy for variceal hemorrhage: late results of a randomized trial. Am J Surg 1993; 165:27–32. discussion 32-23. Doi: 10.1016/S0002-9610(05)80400-3. [DOI] [PubMed] [Google Scholar]
- 32.Isaksson B, Jeppsson B, Bengtsson F, Hannesson P, Herlin P, Bengmark S. Mesocaval shunt or repeated sclerotherapy: effects on rebleeding and encephalopathy--a randomized trial. Surgery 1995; 117:498–504. doi: 10.1016/S0039-6060(05)80248-X. [DOI] [PubMed] [Google Scholar]
- 33.Santambrogio R, Opocher E, Costa M, Bruno S, Ceretti AP, Spina GP. Natural history of a randomized trial comparing distal spleno-renal shunt with endoscopic sclerotherapy in the prevention of variceal rebleeding: a lesson from the past. World J Gastroenterol 2006; 12:6331–6338. doi: 10.3748/wjg.v12.i39.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Intra-Hépatiques GdEdA. TIPS vs Sclerotherapy + Propranolol in the prevention of variceal rebleeding: preliminary results of a multicenter randomized trial. Hepatology 1995; 22:A297.doi: 10.1016/0270-9139(95)94909-7. [Google Scholar]
- 35.Cabrera J, Maynar M, Granados R, Gorriz E, Reyes R, Pulido-Duque JM, et al. Transjugular intrahepatic portosystemic shunt versus sclerotherapy in the elective treatment of variceal hemorrhage. Gastroenterology 1996; 110:832–839. doi: 10.1053/gast.1996.v110.pm8608893. [DOI] [PubMed] [Google Scholar]
- 36.Cello JP, Ring EJ, Olcott EW, Koch J, Gordon R, Sandhu J, et al. Endoscopic sclerotherapy compared with percutaneous transjugular intrahepatic portosystemic shunt after initial sclerotherapy in patients with acute variceal hemorrhage. A randomized, controlled trial. Ann Intern Med 1997; 126:858–865. doi: 10.7326/0003-4819-126-11-199706010-00002. [DOI] [PubMed] [Google Scholar]
- 37.Jalan R, Forrest EH, Stanley AJ, Redhead DN, Forbes J, Dillon JF, et al. A randomized trial comparing transjugular intrahepatic portosystemic stent-shunt with variceal band ligation in the prevention of rebleeding from esophageal varices. Hepatology 1997; 26:1115–1122. doi: 10.1002/hep.510260505. [DOI] [PubMed] [Google Scholar]
- 38.Rossle M, Deibert P, Haag K, Ochs A, Olschewski M, Siegerstetter V, et al. Randomised trial of transjugular-intrahepatic-portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet 1997; 349:1043–1049. doi: 10.1016/S0140-6736(96)08189-5. [DOI] [PubMed] [Google Scholar]
- 39.Sanyal AJ, Freedman AM, Luketic VA, Purdum PP3rd, Shiffman ML, Cole PE, et al. Transjugular intrahepatic portosystemic shunts compared with endoscopic sclerotherapy for the prevention of recurrent variceal hemorrhage. A randomized, controlled trial. Ann Intern Med 1997; 126:849–857. doi: 10.7326/0003-4819-126-11-199706010-00001. [DOI] [PubMed] [Google Scholar]
- 40.Sauer P, Theilmann L, Stremmel W, Benz C, Richter GM, Stiehl A. Transjugular intrahepatic portosystemic stent shunt versus sclerotherapy plus propranolol for variceal rebleeding. Gastroenterology 1997; 113:1623–1631. doi: 10.1053/gast.1997.v113.pm9352865. [DOI] [PubMed] [Google Scholar]
- 41.Merli M, Salerno F, Riggio O, de Franchis R, Fiaccadori F, Meddi P, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal bleeding in cirrhosis: a randomized multicenter trial. Gruppo Italiano Studio TIPS (G.I.S.T.). Hepatology 1998; 27:48–53. doi: 10.1002/hep.510270109. [DOI] [PubMed] [Google Scholar]
- 42.Sauer P, Benz C, Theilmann L, Richter G, Stremmel W, Stiehl A. Transjugular intrahepatic portosystemic stent shunt (TIPS) vs. endoscopic banding in the prevention of variceal rebleeding: Final results of a randomized study. Gastroenterology 1998; 114:A1334.doi: 10.1016/S0016-5085(98)85416-4. [Google Scholar]
- 43.Garcia-Villarreal L, Martinez-Lagares F, Sierra A, Guevara C, Marrero JM, Jimenez E, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal rebleeding after recent variceal hemorrhage. Hepatology 1999; 29:27–32. doi: 10.1002/hep.510290125. [DOI] [PubMed] [Google Scholar]
- 44.Pomier-Layrargues G, Villeneuve JP, Deschenes M, Bui B, Perreault P, Fenyves D, et al. Transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic variceal ligation in the prevention of variceal rebleeding in patients with cirrhosis: a randomised trial. Gut 2001; 48:390–396. doi: 10.1136/gut.48.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narahara Y, Kanazawa H, Kawamata H, Tada N, Saitoh H, Matsuzaka S, et al. A randomized clinical trial comparing transjugular intrahepatic portosystemic shunt with endoscopic sclerotherapy in the long-term management of patients with cirrhosis after recent variceal hemorrhage. Hepatol Res 2001; 21:189–198. doi: 10.1016/S1386-6346(01)00104-8. [DOI] [PubMed] [Google Scholar]
- 46.Gulberg V, Schepke M, Geigenberger G, Holl J, Brensing KA, Waggershauser T, et al. Transjugular intrahepatic portosystemic shunting is not superior to endoscopic variceal band ligation for prevention of variceal rebleeding in cirrhotic patients: a randomized, controlled trial. Scand J Gastroenterol 2002; 37:338–343. doi: 10.1080/003655202317284255. [DOI] [PubMed] [Google Scholar]
- 47.Sauer P, Hansmann J, Richter GM, Stremmel W, Stiehl A. Endoscopic variceal ligation plus propranolol vs. transjugular intrahepatic portosystemic stent shunt: a long-term randomized trial. Endoscopy 2002; 34:690–697. doi: 10.1055/s-2002-33565. [DOI] [PubMed] [Google Scholar]
- 48.Lo GH, Liang HL, Chen WC, Chen MH, Lai KH, Hsu PI, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy 2007; 39:679–685. doi: 10.1055/s-2007-966591. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010; 362:823–832. doi: 10.1056/NEJMra0901512. [DOI] [PubMed] [Google Scholar]
- 50.D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995; 22:332–354. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 51.Gimson AE. Transjugular intrahepatic portosystemic stent-shunt and liver transplantation--how safe is the bridge? Eur J Gastroenterol Hepatol 2002; 14:821–822. doi: 10.1097/00042737-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Schneider JA, White EA, Welch DC, Stokes LS, Raiford DS. Transjugular intrahepatic portosystemic shunt for treatment of intractable colonic ischemia associated with portal hypertension: a bridge to liver transplantation. Liver Transpl 2006; 12:1540–1543. doi: 10.1002/lt.20860. [DOI] [PubMed] [Google Scholar]
- 53.Rosemurgy A, Raitano O, Srikumar T, Sawangkum P, Luberice K, Ryan C, et al. Portal hypertension over the last 25 years: where did it go? J Am Coll Surg 2016; 222:1164–1170. doi: 10.1016/j.jamcollsurg.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Gur I, Diggs BS, Orloff SL. Surgical portosystemic shunts in the era of TIPS and liver transplantation are still relevant. HPB (Oxford) 2014; 16:481–493. doi: 10.1111/hpb.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng M, Chen Y, Bai J, Zeng Q, You J, Jin R, et al. Transjugular intrahepatic portosystemic shunt versus endoscopic therapy in the secondary prophylaxis of variceal rebleeding in cirrhotic patients: meta-analysis update. J Clin Gastroenterol 2008; 42:507–516. doi: 10.1097/MCG.0b013e31815576e6. [DOI] [PubMed] [Google Scholar]
- 56.Boyer TD, Haskal ZJ. American Association for the Study of Liver D. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010; 51:306.doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 57.Rosch J, Keller FS. Transjugular intrahepatic portosystemic shunt: present status, comparison with endoscopic therapy and shunt surgery, and future prospectives. World J Surg 2001; 25:337–345. discussion 345-336. doi: 10.1007/s002680020380. [DOI] [PubMed] [Google Scholar]
- 58.Qi X, Tian Y, Zhang W, Yang Z, Guo X. Covered versus bare stents for transjugular intrahepatic portosystemic shunt: an updated meta-analysis of randomized controlled trials. Therap Adv Gastroenterol 2017; 10:32–41. doi: 10.1177/1756283X16671286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hosokawa I, Adam R, Allard MA, Pittau G, Vibert E, Cherqui D, et al. Outcomes of surgical shunts and transjugular intrahepatic portasystemic stent shunts for complicated portal hypertension. Br J Surg 2017; 104:443–451. doi: 10.1002/bjs.10431. [DOI] [PubMed] [Google Scholar]
- 60.Lo GH. Endoscopic treatments for portal hypertension. Hepatol Int 2018; 12 suppl 1:91–101. doi: 10.1007/s12072-017-9828-8. [DOI] [PubMed] [Google Scholar]
- 61.Jalan R, Bzeizi KI, Tripathi D, Lui HF, Redhead DN, Hayes PC. Impact of transjugular intrahepatic portosystemic stent-shunt for secondary prophylaxis of oesophageal variceal haemorrhage: a single-centre study over an 11-year period. Eur J Gastroenterol Hepatol 2002; 14:615–626. doi: 10.1097/00042737-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017; 65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 63.Azoulay D, Castaing D, Majno P, Saliba F, Ichai P, Smail A, et al. Salvage transjugular intrahepatic portosystemic shunt for uncontrolled variceal bleeding in patients with decompensated cirrhosis. J Hepatol 2001; 35:590–597. doi: 10.1016/S0168-8278(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 64.Abouljoud M, Malinzak L, Bruno D. Surgical options for the management of portal hypertension. Curr Hepatology Rep 2015; 14:225–233. doi: 10.1007/s11901-015-0276-4. [Google Scholar]
- 65.Toomey PG, Ross SB, Golkar FC, Hernandez JM, Clark WC, Luberice K, et al. Outcomes after transjugular intrahepatic portosystemic stent shunt: a “bridge” to nowhere. Am J Surg 2013; 205:441–446. doi: 10.1016/j.amjsurg.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Fukui H, Saito H, Ueno Y, Uto H, Obara K, Sakaida I, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol 2016; 51:629–650. doi: 10.1007/s00535-016-1216-y. [DOI] [PubMed] [Google Scholar]
- 67.Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology 1992; 16:1343–1349. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


