Abstract
Background:
Visual-spatial neglect (VSN) is a neuropsychological syndrome, and right-hemisphere stroke is the most common cause. The pathogenetic mechanism of VSN remains unclear. This study aimed to investigate the behavioral and event-related potential (ERP) changes in patients with or without VSN after right-hemisphere stroke.
Methods:
Eleven patients with VSN with right-hemisphere stroke (VSN group) and 11 patients with non-VSN with right-hemisphere stroke (non-VSN group) were recruited along with one control group of 11 age- and gender-matched healthy participants. The visual-spatial function was evaluated using behavioral tests, and ERP examinations were performed.
Results:
The response times in the VSN and non-VSN groups were both prolonged compared with those of normal controls (P < 0.001). In response to either valid or invalid cues in the left side, the accuracy in the VSN group was lower than that in the non-VSN group (P < 0.001), and the accuracy in the non-VSN group was lower than that in controls (P < 0.05). The P1 latency in the VSN group was significantly longer than that in the control group (F[2, 30] = 5.494, P = 0.009), and the N1 amplitude in the VSN group was significantly lower than that in the control group (F[2, 30] = 4.343, P = 0.022). When responding to right targets, the left-hemisphere P300 amplitude in the VSN group was significantly lower than that in the control group (F[2, 30] = 4.255, P = 0.025). With either left or right stimuli, the bilateral-hemisphere P300 latencies in the VSN and non-VSN groups were both significantly prolonged (all P < 0.05), while the P300 latency did not differ significantly between the VSN and non-VSN groups (all P > 0.05).
Conclusions:
Visual-spatial attention function is impaired after right-hemisphere stroke, and clinicians should be aware of the subclinical VSN. Our findings provide neuroelectrophysiological evidence for the lateralization of VSN.
Keywords: Visual-spatial neglect, Right-hemisphere stroke, Behavior, Electrophysiology, Event-related potentials, Response time
Introduction
Visual-spatial neglect (VSN), also known as hemispatial neglect, hemineglect, or hemi-inattention, refers to a neuropsychological syndrome occurring after brain injury, which is pathologically characterized by asymmetric spatial behavior.[1] In the majority of cases, VSN is contralateral to the damaged brain hemisphere, while ipsilesional VSN has also been rarely reported.[2] Visual neglect commonly involves the left side of space; as reported, spatial neglect caused by right hemisphere damage accounts for approximately 13% to 82% of all spatial neglect cases.[3] The pathogenetic mechanism of VSN remains unclear. Previous neuroimaging studies indicate that deficits in visual processing may be the major contributor to unilateral spatial neglect.[4–6]
Currently, the most widely used tool for behavioral assessment of VSN is the paper-and-pencil task. However, its diagnostic sensitivity is quite limited, as some patients with subclinical VSN may show normal performance on paper-and-pencil tasks. Event-related potentials (ERPs) provide a non-invasive and objective method for recording the brain response to a specific sensory, cognitive, and motor event. Recently, ERPs have been used for evaluating the attention function in patients with VSN, and the most commonly used components included P1, N1, and P300. P1 represents the early processing stage of spatial attention, which is essential for maintaining attention and regulated by endogenous attention. Previous studies found that VSN is associated with increased latencies of visual evoked potentials; the latency of P1 evoked by contralesional stimuli is longer than that evoked by ipsilesional stimuli.[7–9] The N1 component is attenuated in neglect patients, indicating an impairment in processing left-side visual input.[10,11] The P300 component represents the late processing stage of spatial attention, which has been found to be associated with the number of missed contralesional targets.[12]
The present study investigated the behavioral and ERP changes in patients with or without VSN following right-hemisphere stroke. The visual processing function in these patients was compared to that in normal controls via analysis of behavioral and electrophysiological parameters, which may provide new insight into the pathogenesis of VSN.
Methods
Ethical approval
This study was approved by the Ethics Committee of Xuanwu Hospital of Capital Medical University. Written informed consent was obtained from each participant.
Participants
Based on the literature and our previous data, we concluded that the minimum sample size was 6. A total of 22 patients who were diagnosed with right-hemisphere stroke were enrolled from our institute. Eleven of these patients who developed VSN were allocated to the VSN group, and the other 11 patients were allocated to the non-VSN group. Additionally, 11 age- and gender-matched healthy participants were recruited as controls from outpatient clinics.
The inclusion criteria were as follows: (1) right handedness evaluated according to the Edinburgh Handedness Inventory Test[13]; (2) age within the range of 18 to 80 years; (3) normal or corrected visual acuity; and (4) first-onset ischemic or hemorrhagic stroke restricted within the right hemisphere with a clinical course of at least 2 weeks.
The exclusion criteria were as follows: (1) achromatopsia or hemianopsia; (2) previous history of stroke; (3) severe psychiatric disorders, such as schizophrenia; (4) neuropsychological diseases, such as dementia; (5) brain tumor; (6) severe cardiac, pulmonary, or kidney diseases; and (7) disturbance of consciousness.
Behavioral assessment
The individual visual-spatial function was evaluated using the following behavioral tests as previously described[14]:
Line bisection task: five parallel line segments were equidistantly distributed on the test paper, and subjects were instructed to mark the midpoint of each line segment. The distance from the marked point to the actual midpoint was measured as α, and the length of the line segment was represented as β. The neglect degree was calculated as [α/(β/2)] × 100%, and >12% indicated VSN.
Line cancellation task: equivalent line segments were randomly scattered in the left and right quadrants of the test paper, and participants were instructed to mark all the visible line segments. The percentage of missed line segments was documented, and visual-spatial lateralization was analyzed.
Star cancellation task: multiple stars, words, and letters were abundantly and symmetrically distributed on the test paper, and participants were instructed to mark all the stars. The percentage of missed star targets was documented.
Clock drawing task: a clock with a random time was presented on a paper, and participants were instructed to copy a duplicate clock on the test paper.
Gap detection task: equivalent circles (two-thirds of the circles have a gap and they are evenly distributed on both sides) were distributed in the left and right quadrants of the test paper, and participants were instructed to mark the circles with a gap. The percentage of missed circle targets was documented.
Text reading task: participants were instructed to read aloud an article in three columns (left, center, right). The number of omitted words was documented.
Electrophysiological evaluation
Participants were tested in a quiet, electromagnetic interference-free testing room. Before the examination, the scalp was washed to reduce electrical impedance. Participants sat 57.5 cm away from the display screen, facing the center of the display screen. During the examination, they were instructed to press the left mouse button when left-side target stimulation was observed, and to press the right mouse button when right-side target stimulation was observed. For this test, the participants were told that accuracy and reaction time were equally important.
Electrophysiological evaluation was performed using a Neuroscan 64-lead ERP workstation (Compumedics USA Inc., Charlotte, NC, USA). The grounding electrode was placed in the forehead, and the reference electrode was placed in the left mastoid. EEG and electrooculography signals were recorded synchronously. The parameters were set as follows: bandpass filter 0.05 to 80 Hz, sampling frequency 1000 Hz, and electrical resistance <5 kΩ.
The ERP task consisted of 16 blocks, each with 40 trials. Participants were instructed to fixate on the center of the screen for the trial duration up until target onset. During each trial, background was presented for 800 to 1000 ms, then the cue was presented for 1400 to 1800 ms, and a target was presented for 100 ms thereafter. The interval between two consecutive trials was 1400 ms. The electrical signals were collected using the International EEG system and processed using E-prime software (Psychology Software Tools, USA).
ERP signals were analyzed between 200 ms before stimulation and 800 ms after stimulation. The artifacts beyond 100 μV were removed. The waveforms of standard stimuli and deviant stimuli were averaged and digitally filtered, respectively. P300 referred to the positive wave occurring 300 ms after stimulation onset, N1 was the negative wave occurring 130 to 230 ms after stimulation onset, and P1 was the positive wave presenting 90 to 160 ms after target onset. For P300 components, the signals from the reference electrode were converted to the mean of signals from bilateral mastoid processes. The analyzed parameters for the P300, N1, and P1 components included presence of waves, latency, amplitude, and electroencephalotopogram. The amplitude of the maximum crest in the time window was defined as the amplitude of specific ERP components, and the interval between the maximum crest and the baseline was defined as the latency. The amplitude and latency of P300, N1, and P1 components were measured using Neuroscan 4.5 software.
Statistical analysis
SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Repeated measures analysis of variance was used to compare the amplitudes and latencies in different conditions, variables including one intra-group factor (VSN group, non-VSN group, and normal group) and two inter-group factors (left target, and right target; left hemisphere, and right hemisphere). Greenhouse-Geisser analysis was used for corrections. Probability (P) values ≤0.05 were considered significant.
Results
Clinical characteristics
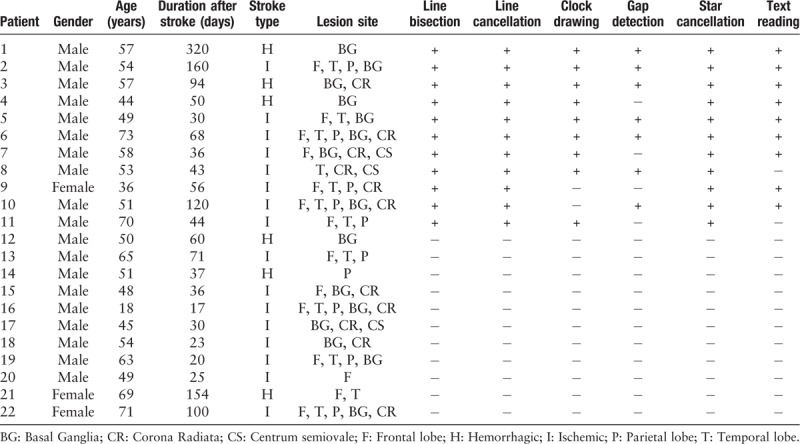
Among the 22 patients with right-hemisphere stroke, 11 patients (10 men and one woman) had VSN with an average age of 54.7 ± 10.5 years (range, 36.0–73.0 years); 11 patients (nine men and two women) had no VSN with an average age of 53.0 ± 14.7 years (range, 18.0–71.0 years). The average age of normal control participants was 46.9 ± 12.8 years (range, 33.0–73.0 years). There was no difference in age or gender distribution among the VSN group, the non-VSN group and the normal control group (F[2, 30] = 1.174, P = 0.323).
Behavioral assessment
In this study, VSN was defined by a >12% rightward deviation in the line bisection task, omission of >3 targets in the line cancellation task, and/or omission of >5 targets in the star cancellation task. All patients in the VSN group showed VSN in the line bisection, line cancellation and star cancellation tests, 9 of 11 patients (81.8%) showed VSN in the clock drawing, and text reading tests, and 7 of 11 patients (63.6%) showed VSN in the gap detection test. In the non-VSN and normal control groups, no patient exhibited VSN in any of these tests. The detailed data are presented in Table 1.
Table 1.
Clinical characteristics of patients and results of behavioral evaluations.
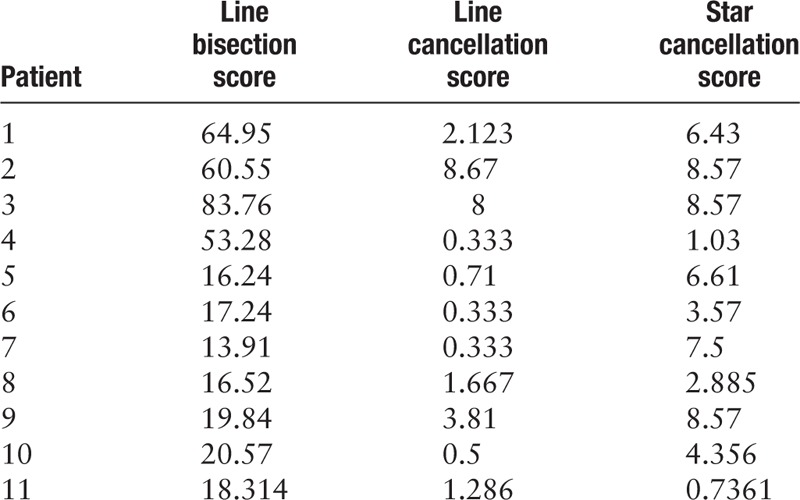
The line bisection mean score of patients in the VSN group was 35.02 ± 25.36, and those of patients in the non-VSN group and the normal control group were 4.00 ± 3.90 and 1.08 ± 2.11, respectively. The relevant data are summarized in Table 2.
Table 2.
Behavioral scores on the paper-and-pencil tasks.
During the electrophysiological visual-spatial task, with valid or invalid cues, the mean response time and accuracy were analyzed [Table 3 and Figure 1]. To either valid or invalid cues occurring in the left or right side, the response times in the VSN and non-VSN groups were significantly prolonged compared with that in normal controls (valid cues in the left side, F [2, 30] = 29.819, P < 0.001; valid cues in the right side, F [2, 30] = 23.707, P < 0.001; invalid cues in the left side, F [2, 30] = 12.143, P < 0.001; invalid cues in the right side, F [2, 30] = 12.011, P < 0.001), while the response time showed no significant difference between the VSN and non-VSN groups (P > 0.05). To left valid and invalid cues, the accuracy in the VSN group was lower than that in non-VSN group (valid cues, F [2, 30] = 18.652, P < 0.001; invalid cues, F [2, 30] = 29.542, P < 0.001), and the accuracy in the non-VSN group was lower than that in controls (valid cues, F [2, 30] = 18.652, P = 0.010; invalid cues, F [2, 30] = 29.542, P = 0.006). To right valid cues, the accuracy in the VSN group and the non-VSN group was lower than that in normal controls (F[2, 30] = 6.142; VSN vs. Control, P = 0.048; non-VSN vs. Control, P = 0.006).
Table 3.
Response time and accuracy on the ERP task.
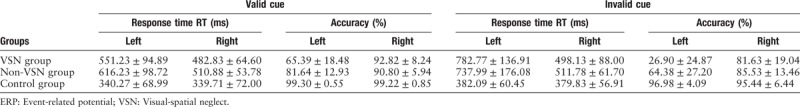
Figure 1.
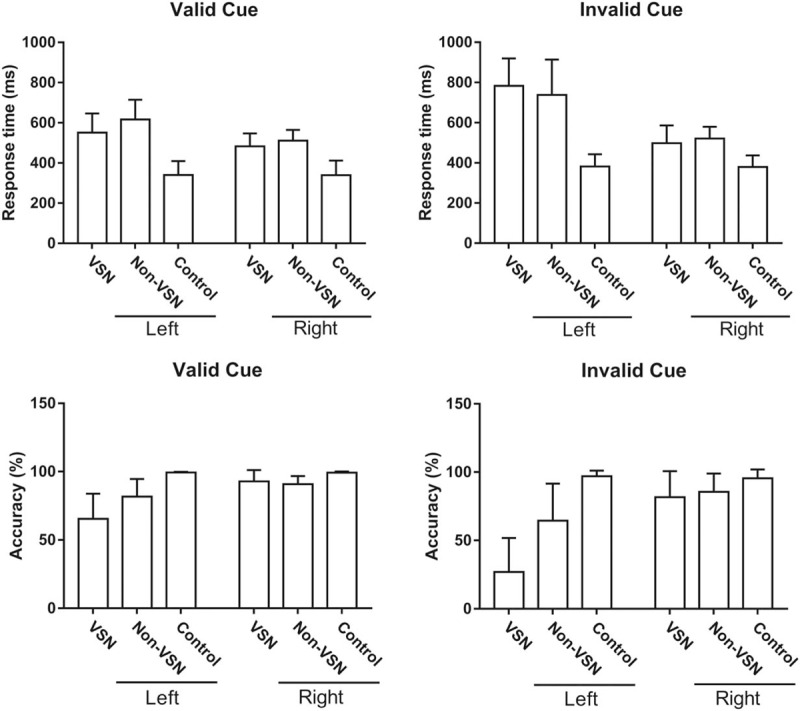
Mean response times and accuracies during the electrophysiological visual-spatial task.
Electrophysiological evaluation
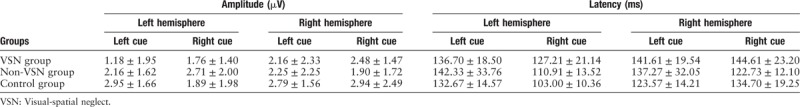
The mean amplitude and latency of P1 are summarized in Table 4. There was no significant difference in the P1 amplitude among the three groups (F[2, 30] = 2.238, P = 0.125). When the target cues occurred on the right side, the left-hemisphere P1 latency in the VSN group was significantly longer than that in the control group (F[2, 30] = 5.494, P = 0.009), and the right-hemisphere P1 latency in the VSN group was significantly longer than that in the non-VSN group (F[2, 30] = 4.586, P = 0.018).
Table 4.
Amplitude and latency of the P1 component.
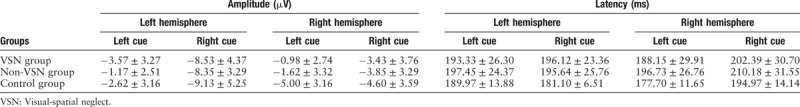
The mean amplitude and latency of N1 are summarized in Table 5. When the target cue occurred on the left side, the right-hemisphere N1 amplitude in the VSN group was significantly lower than that in the control group (F[2, 30] = 4.343, P = 0.022). There was no significant difference in the N1 latency among the three groups (F[2, 30] = 1.267, P = 0.297).
Table 5.
Amplitude and latency of the N1 component
The mean amplitude and latency of P300 are summarized in Table 6. When the target cue occurred on the right side, the left-hemisphere P300 amplitude in the VSN group was significantly lower than that in the control group (F[2, 30] = 4.255, P = 0.025), and the right-hemisphere P300 amplitude in the non-VSN group was significantly lower than that in the control group (F[2, 30] = 4.291, P = 0.024). With either left or right stimuli, the bilateral-hemisphere P300 latencies in the VSN and non-VSN groups were both significantly longer than those in the control group (all P < 0.05), while the P300 latency did not differ significantly between the VSN and non-VSN groups (all P > 0.05). The ERP results are shown in Figure 2.
Table 6.
Amplitude and latency of the P300 component.
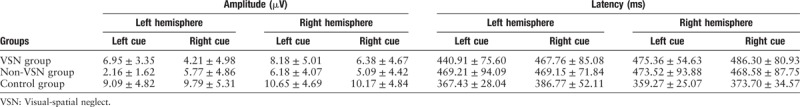
Figure 2.
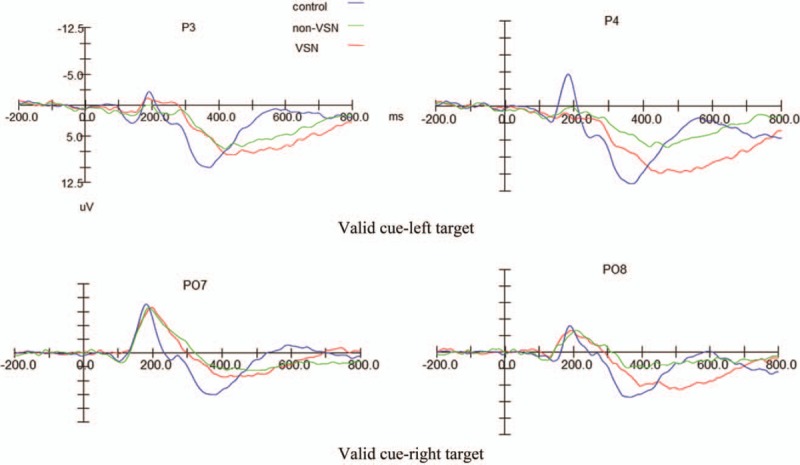
Results of event-related potential examinations.
Discussion
Unilateral VSN is a frequent post-stroke complication, occurring in approximately 25% to 30% of all stroke patients, and the number of patients affected in the United States annually has been estimated to be 250,000.[15–17] Although VSN can be secondary to lesions in either hemisphere, it is more common and severe following right-hemisphere injury.[18] In addition to the attention disturbance, unilateral spatial neglect is also associated with poor motor recovery and poor response to rehabilitation, leading to severe disability.[16] Clinically, unilateral VSN is characterized by difficulties in attention and response to contralesional stimuli.[19] Regarding the pathogenetic mechanisms of VSN, there are several existing theoretical hypotheses. Some scholars have proposed that unilateral VSN is mainly attributed to lateralized perceptual impairment, which may be associated with injured spatial attention function and/or inaccurate perception of the position of a sensory stimulus.[20,21] While some scholars consider unilateral VSN to be a distinct subtype of directional hypokinesia; in this theory, neglect is caused by lateralized deficit of action, causing affected individuals to exhibit delay in initiating movements to the contralesional space.[22,23] Additionally, there is another hypothesis that unilateral VSN arises from the impaired dominant role played by the right hemisphere in regulating visual-spatial attention, which can account for the high prevalence of neglect following right hemisphere damage. Numerous neuroimaging and clinical studies have suggested that VSN is associated with an interaction between the frontoparietal dorsal attention network and the frontoparietal dorsal network.[24] The ventral network, as well as its neural connections to the dorsal network, is anatomically lateralized to the right hemisphere, and this neuroanatomical and functional asymmetry may explain the lateralization of VSN.[25] Due to the paucity of compelling evidence for the above theories, the definitive pathogenesis still requires further validation.
In the current study, we used ERPs to study VSN following right-hemisphere stroke, since this modality offers a high temporal resolution. We divided the subjects into three groups: the VSN group, which had apparent neglect symptoms, the non-VSN group, which was speculated to have subclinical spatial neglect, and normal controls. We monitored multiple ERP components, including early-stage P1 and N1 and late-stage P300.
In the behavioral assessments, the line bisection mean score of patients in the non-VSN group was significantly higher than that of the control participants. This result suggests the left-side spatial attention function may be impaired even though there was no apparent VSN. In the neuroelectrophysiological task, we also noted that the left-target accuracy in the non-VSN group was significantly lower than that in the normal controls, and ERPs showed abnormalities as well, which supports the above behavioral manifestations. Furthermore, we found the accuracy for both left and right targets in the VSN group was significantly lower than that in the other two groups and the response times for both left and right targets were prolonged, indicating that the right-side spatial attention function may be impaired.
In the ERP monitoring, we found significant heterogeneity in the early-stage ERP components (P1 and N1) between bilateral hemispheres; specifically, the response of the left hemisphere to valid cues was more severely impaired than that of the right hemisphere. This result provides neuroelectrophysiological evidence for the lateralization of VSN. There was no significant difference in the P1 amplitude among the three groups; nevertheless, the P1 latency responding to the right stimuli in the VSN group was significantly longer than those in the non-VSN and control groups, while no significant difference was observed in the P1 latency responding to the left stimuli among the three groups. Generally, the latency represents the course of information processing, and amplitude reflects the number of activated neurons.[26] Thus, we speculate that the contralesional VSN may be mainly attributed to the prolonged information processing time rather than a reduction in neurons in the early stage of spatial attention function. For the N1 component, the right-hemisphere amplitude for responding to the left stimuli in the VSN group was significantly lower than that in the control group. This finding was consistent with previous reports. Di et al observed that unilateral stimuli elicited an enhanced amplitude of the contralateral N1 component.[27,28] Lange et al also noted that unilateral stimuli enhanced both the contralateral P1 and N1.[29] There was no significant difference in the N1 latency among the three groups, which is somewhat contradictory to the above speculation; a definitive conclusion requires further studies involving a much larger cohort.
P300 is a late component of ERPs that reflects the late stage of cognitive processing. In the current study, we found that bilateral P300 in response to the right stimuli had a lower amplitude and longer latency in patients with VSN compared with normal controls. These results indicate that the responses of bilateral hemispheres to right-sided targets were both impaired. In the literature, some scholars also noted attenuated P300 when subjects with unilateral VSN fixate on left targets.[11,12,30] Interestingly, with either left or right stimuli, the bilateral-hemisphere P300 latencies in the non-VSN group were both significantly longer than those in the control group. From the above results, in the non-VSN group, even though the patients performed well on the paper-and-pencil task, the ERPs were abnormal, including inter-hemispheric heterogeneity of P1 and N1 components as well as reduced amplitude and prolonged latency of P300, which indicates potential visual-spatial impairment in the non-VSN group. ERP is a non-invasive and objective method with high temporal resolution, which has been widely for evaluating the attention function in patients with VSN. Especially, the P300 component can sensitively reflect the cognitive function.[31,32] Therefore, ERP parameters may be superior to the conventional paper-and-pencil task for the clinical evaluation of VSN.
The present study has some limitations. First, the sample size was relatively small, which may lead to inherent bias. Moreover, we only performed behavioral and neuroelectrophysiological assessments, which could not provide synchronous evidence for the regional activation of cerebral cortices. In future studies, we will recruit a large-scale cohort and use a combination of ERP and functional neuroimaging modalities (such as functional magnetic resonance imaging and magnetoencephalography).
In conclusion, visual-spatial attention function is impaired after right-hemisphere stroke, independent of the presentation of apparent clinical neglect symptoms. Clinicians should be aware of subclinical VSN. This study provides neuroelectrophysiological evidence for the lateralization of VSN, and both the early (P1 and N1) and late (P300) ERP components were altered in patients with VSN after right-hemisphere stroke.
Conflicts of interest
None.
Footnotes
How to cite this article: Ye LL, Cao L, Xie HX, Shan GX, Zhang YM, Song WQ. Visual-spatial neglect after right-hemisphere stroke: behavioral and electrophysiological evidence. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000218
References
- 1.Salazar APS, Vaz PG, Marchese RR, Stein C, Pinto C, Pagnussat AS. Noninvasive brain stimulation improves hemispatial neglect after stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil 2018; 99:355–366. doi: 10.1016/j.apmr.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Beume LA, Martin M, Kaller CP, Kloppel S, Schmidt CS, Urbach H, et al. Visual neglect after left-hemispheric lesions: a voxel-based lesion-symptom mapping study in 121 acute stroke patients. Exp Brain Res 2017; 235:83–95. doi: 10.1007/s00221-016-4771-9. [DOI] [PubMed] [Google Scholar]
- 3.Bowen A, McKenna K, Tallis RC. Reasons for variability in the reported rate of occurrence of unilateral spatial neglect after stroke. Stroke 1999; 30:1196–1202. doi: 10.1161/01.STR.30.6.1196. [DOI] [PubMed] [Google Scholar]
- 4.Baldassarre A, Ramsey L, Hacker CL, Callejas A, Astafiev SV, Metcalf NV, et al. Large-scale changes in network interactions as a physiological signature of spatial neglect. Brain 2014; 137:3267–3283. doi: 10.1093/brain/awu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalaf A, Kersey J, Eldeeb S, Alankus G, Grattan E, Waterstram L, et al. EEG-based neglect assessment: a feasibility study. J Neurosci Methods 2018; 303:169–177. doi: 10.1016/j.jneumeth.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizuno K, Tsuji T, Rossetti Y, Pisella L, Ohde H, Liu M. Early visual processing is affected by clinical subtype in patients with unilateral spatial neglect: a magnetoencephalography study. Front Hum Neurosci 2013; 7:432.doi: 10.3389/fnhum.2013.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitzalis S, Spinelli D, Zoccolotti P. Vertical neglect: behavioral and electrophysiological data. Cortex 1997; 33:679–688. doi: 10.1016/S0010-9452(08)70725-1. [DOI] [PubMed] [Google Scholar]
- 8.Spinelli D, Burr DC, Morrone MC. Spatial neglect is associated with increased latencies of visual evoked potentials. Vis Neurosci 1994; 11:909–918. doi: 10.1017/S0952523800003862. [DOI] [PubMed] [Google Scholar]
- 9.Spinelli D, Di Russo F. Visual evoked potentials are affected by trunk rotation in neglect patients. Neuroreport 1996; 7:553–556. doi: 10.1097/00001756-199601310-00042. [DOI] [PubMed] [Google Scholar]
- 10.Di Russo F, Aprile T, Spitoni G, Spinelli D. Impaired visual processing of contralesional stimuli in neglect patients: a visual-evoked potential study. Brain 2008; 131:842–854. doi: 10.1093/brain/awm281. [DOI] [PubMed] [Google Scholar]
- 11.Verleger R, Heide W, Butt C, Wascher E, Kompf D. On-line brain potential correlates of right parietal patients’ attentional deficit. Electroencephalogr Clin Neurophysiol 1996; 99:444–457. [DOI] [PubMed] [Google Scholar]
- 12.Saevarsson S, Kristjansson A, Bach M, Heinrich SP. P300 in neglect. Clin Neurophysiol 2012; 123:496–506. doi: 10.1016/j.clinph.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9:97–113. [DOI] [PubMed] [Google Scholar]
- 14.Lee BH, Kang SJ, Park JM, Son Y, Lee KH, Adair JC, et al. The character-line bisection task: a new test for hemispatial neglect. Neuropsychologia 2004; 42:1715–1724. doi: 10.1016/j.neuropsychologia.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Appelros P, Karlsson GM, Seiger A, Nydevik I. Neglect and anosognosia after first-ever stroke: incidence and relationship to disability. J Rehabil Med 2002; 34:215–220. doi: 10.1080/165019702760279206. [DOI] [PubMed] [Google Scholar]
- 16.Buxbaum LJ, Ferraro MK, Veramonti T, Farne A, Whyte J, Ladavas E, et al. Hemispatial neglect: subtypes, neuroanatomy, and disability. Neurology 2004; 62:749–756. doi: 10.1212/01.WNL.0000113730.73031.F4. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen PM, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Hemineglect in acute stroke – incidence and prognostic implications. The Copenhagen Stroke Study. Am J Phys Med Rehabil 1997; 76:122–127. [DOI] [PubMed] [Google Scholar]
- 18.Stone SP, Halligan PW, Greenwood RJ. The incidence of neglect phenomena and related disorders in patients with an acute right or left hemisphere stroke. Age Ageing 1993; 22:46–52. doi: 10.1093/ageing/22.1.46. [DOI] [PubMed] [Google Scholar]
- 19.Halligan PW, Fink GR, Marshall JC, Vallar G. Spatial cognition: evidence from visual neglect. Trends Cogn Sci 2003; 7:125–133. doi: 10.1016/S1364-6613(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 20.Behrmann M, Tipper SP. Attention accesses multiple reference frames: evidence from visual neglect. J Exp Psychol Hum Percept Perform 1999; 25:83–101. doi: 10.1037/0096-1523.25.1.83. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich FJ, Egly R, Rafal RD, Beck D. Spatial attention deficits in humans: a comparison of superior parietal and temporal-parietal junction lesions. Neuropsychology 1998; 12:193–207. doi: 10.1037/0894-4105.12.2.193. [DOI] [PubMed] [Google Scholar]
- 22.Husain M, Mattingley JB, Rorden C, Kennard C, Driver J. Distinguishing sensory and motor biases in parietal and frontal neglect. Brain 2000; 123:1643–1659. doi: 10.1093/brain/123.8.1643. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey LE, Siegel JS, Baldassarre A, Metcalf NV, Zinn K, Shulman GL, et al. Normalization of network connectivity in hemispatial neglect recovery. Ann Neurol 2016; 80:127–141. doi: 10.1002/ana.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci 2011; 34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, et al. A lateralized brain network for visuospatial attention. Nat Neurosci 2011; 14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- 26.Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Naatanen R, et al. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol 2009; 120:1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 27.Di Russo F, Martinez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cereb Cortex 2003; 13:486–499. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- 28.Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp 2002; 15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lange JJ, Wijers AA, Mulder LJ, Mulder G. ERP effects of spatial attention and display search with unilateral and bilateral stimulus displays. Biol Psychol 1999; 50:203–233. doi: 10.1016/S0301-0511(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 30.Lhermitte F, Turell E, LeBrigand D, Chain F. Unilateral visual neglect and wave P 300. A study of nine cases with unilateral lesions of the parietal lobes. Arch Neurol 1985; 42:567–573. [DOI] [PubMed] [Google Scholar]
- 31.Chen XS, Lu YZ, Wang JJ, Wang HX, Zhang MD, Lou FY, et al. Relationship between event-related potential P300 and first episode schizophrenia. Chin Med J 2007; 120:339–341. doi: 10.1097/00029330-200702020-00016. [PubMed] [Google Scholar]
- 32.Tong XZ, Xu YL, Fu Z. Long-term P300 in hemispherectomized patients. Chin Med J 2009; 122:1769–1774. doi: 10.3760/cma.j.issn.0366-6999.2009.15.010. [PubMed] [Google Scholar]


