Abstract
Background:
Cardiac rupture (CR) is a major lethal complication of acute myocardial infarction (AMI). However, no valid risk score model was found to predict CR after AMI in previous researches. This study aimed to establish a simple model to assess risk of CR after AMI, which could be easily used in a clinical environment.
Methods:
This was a retrospective case-control study that included 53 consecutive patients with CR after AMI during a period from January 1, 2010 to December 31, 2017. The controls included 524 patients who were selected randomly from 7932 AMI patients without CR at a 1:10 ratio. Risk factors for CR were identified using univariate analysis and multivariate logistic regression. Risk score model was developed based on multiple regression coefficients. Performance of risk model was evaluated using receiver-operating characteristic (ROC) curves and internal validity was explored using bootstrap analysis.
Results:
Among all 7985 AMI patients, 53 (0.67%) had CR (free wall rupture, n = 39; ventricular septal rupture, n = 14). Hospital mortalities were 92.5% and 4.01% in patients with and without CR (P < 0.001). Independent variables associated with CR included: older age, female gender, higher heart rate at admission, body mass index (BMI) <25 kg/m2, lower left ventricular ejection fraction (LVEF) and no primary percutaneous coronary intervention (pPCI) treatment. In ROC analysis, our CR risk assess model demonstrated a very good discriminate power (area under the curve [AUC] = 0.895, 95% confidence interval: 0.845–0.944, optimism-corrected AUC = 0.821, P < 0.001).
Conclusion:
This study developed a novel risk score model to help predict CR after AMI, which had high accuracy and was very simple to use.
Keywords: Acute myocardial infarction, Mechanical complications, Cardiac rupture, Risk score model, Primary percutaneous coronary intervention
Introduction
Coronary artery disease (CAD) is a major cause of mortality and morbidity worldwide. Free wall rupture (FWR) is one of the most serious mechanical complications (MCs) of acute myocardial infarction (AMI).[1,2] The incidence of FWR was 2% to 6.2% in the pre-perfusion era, accounting for up to 30% of mortality after AMI.[3–5] The incidence of ventricular septal rupture (VSR) after AMI was approximately 1% to 3% before the reperfusion era, with in-hospital mortality rates of about 45% for surgical treatment and 90% for those treated medically.[1,6,7] The incidence of MCs after AMI has gone down to less than 1% since the advent of percutaneous coronary intervention (PCI) treatment, but MCs are still associated with extremely poorer outcomes.[4,8,9] However, no valid risk score model was developed to help to predict cardiac rupture (CR) after AMI in previous researches.
CR was specified as FWR and VSR. The aim of our study was to establish a simple risk score to predict CR after AMI, which can help clinicians in making an early diagnosis and choosing appropriate therapy for better outcomes.
Methods
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Beijing Chaoyang Hospital. Since this was a retrospective analysis, informed consent was waived. All data were anonymous and from electronic medical record system.
Patient population and study design
The 53 consecutive patients with CR after AMI referred to Beijing Chaoyang Hospital from January 1, 2010 to December 31, 2017 were retrospectively analyzed. The controls were randomly selected from 7932 AMI patients without CR during the same time period at a ratio of 1:10 (n = 524 after excluding six cases with incomplete record). Selection of the cases is shown in Figure 1. Rupture of papillary muscles was not considered for this study.
Figure 1.

Flow diagram of the case selection in this study. AMI: Acute myocardial infarction; CR: Cardiac rupture.
AMI included ST-segment elevation myocardial infarction (STEMI) and non-STEMI. Diagnostic criteria of STEMI were as follows: (1) typical, prolonged chest pain (>30 min); (2) ST-segment elevation ≥0.2 mV at the J point in two or more contiguous, precordial leads, or ≥0.2 mV in two or more adjacent limb leads on the standard 12-lead electrocardiogram (ECG); and (3) increased serial serum markers of myocardial damage (>2-fold increase over the upper normal range required for troponin-I [TnI]).[10] Non-STEMI was defined by ECG ST-segment depression or prominent T-wave inversion and positive biomarkers of necrosis (eg, TNI) in the absence of ST-segment elevation and in an appropriate clinical setting (chest discomfort or anginal equivalent).[11]
The diagnosis of FWR was made by echocardiography or in cases with either of sudden cardiogenic shock or low blood pressure associated with large pericardial effusion confirmed by pericardiocentesis. VSR was first suggested by physical examination findings such as cardiac systolic murmur, and it was subsequently confirmed using echocardiography.
General data collection and anthropometric measurements
Patient demographics, height, weight, medical and family history, use of medications and smoking status were collected upon patient admission. BMI was calculated as body weight divided by height squared (kg/m2).
The Global Registry of Acute Coronary Events (GRACE) risk score is a validated and established score for risk stratification of patients with ACS, which is calculated from several variables (age, history of heart failure, history of acute myocardial infarction, heart rate and systolic blood pressure at admission, ST-segment depression, serum creatinine at admission, elevated myocardial necrosis markers or enzymes, and lack of percutaneous coronary revascularization during admission).[12–14] Estimated glomerular filtration rate (eGFR) was calculated according to the Cockcroft-Gault formula.[15]
Laboratory parameters
Peripheral blood samples were collected on the first 15 min after admission and analyzed with a Dimension RxL MaxTM automated analyzer (Dade Behring Inc., Chicago, Illinois, USA). All biochemical variables were measured using an automatic analyzer (Hitachi 7600, Hitachi Ltd., Tokyo, Japan).
Statistical analysis
Normality of variables was tested by Kolmogorov-Smirnov test. Normally-distributed continuous variables are presented as mean ± standard deviation (SD), and analyzed using Student's t-test. Abnormally-distributed data are presented as median (interquartile range), and analyzed using Mann-Whitney U test. Dichotomous variables were analyzed with Pearson Chi-squared test, and expressed as percentages. Risk factors for CR were identified using univariate analysis and multivariate logistic regression. Baseline characteristics associated with CR in univariable analyses with P ≤ 0.10 were retained for possible inclusion to the final model and entered in a stepwise-backward manner. Risk score model was developed based on multiple regression coefficients. Each coefficient was divided by the smallest coefficient and rounded to the nearest integer.[16,17] Summation of points assigned for each predictor led to the prediction of CR risk. Discriminatory power was evaluated using the C-index, area under (AUC) the receiver-operating characteristic (ROC) curve with its 95% confidence interval (CI).[18] A C-index of 0.5 indicates the absence of predictive ability, while a C-index of 1.0 represents perfect discriminatory ability.[19,20] All these statistical analyses were performed in SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA). Internal validity was assessed in 1000 bootstrap samples to estimate the optimism-corrected AUC using the ‘validate’ function from package ‘rms’ in R (R statistical software version 3.4.0).[19] The AUCs were compared using the Z test using MedCalc statistical software version 13.0.2.0 (MedCalc Software, Ostend, Belgium). A P < 0.05 (two-sided) was considered statistically significant.
Results
General characteristics
Among the 7985 consecutive AMI patients, 53 cases developed CR (0.67%): FWR occurred in 39 patients (0.49%) and VSR in 14 patients (0.18%). The average observational period from AMI onset to HR was 3.1 days (FWR = 2.7 days, VSR = 3.6 days). The 19 patients (48.7%) developed with FWR and six patients (42.9%) developed with VSR within 24 h after symptoms onset; five FWR (12.8%) and three VSR (21.4%) had already happened at the time of admission. A total of 577 patients (29.8% women) were included in this case-control study. Baseline characteristics of relevant patients are shown in Table 1. The 373 AMI patients (64.6%) received primary percutaneous coronary intervention (pPCI) treatment. No patients received thrombolytic therapy. Vessel disease types of all patients are shown in Table 2. Compared with non-CR patients, CR patients presented more frequently with older in age, female, longer symptom onset time, higher heart rate at admission, KILLIP class, ESR, Nt-proBNP, WBC, CK-MB, CTNI, GRACE risk score, in hospital infection, hemorrhage and mortality (P < 0.05 vs. non-CR patients for all measures). CR patients had significantly lower BMI, ACEI/ARB use in 24 h after admission, RBC, Hb, LVEF, eGFR, and pPCI treatment.
Table 1.
Baseline characteristics of the study population (N = 577).
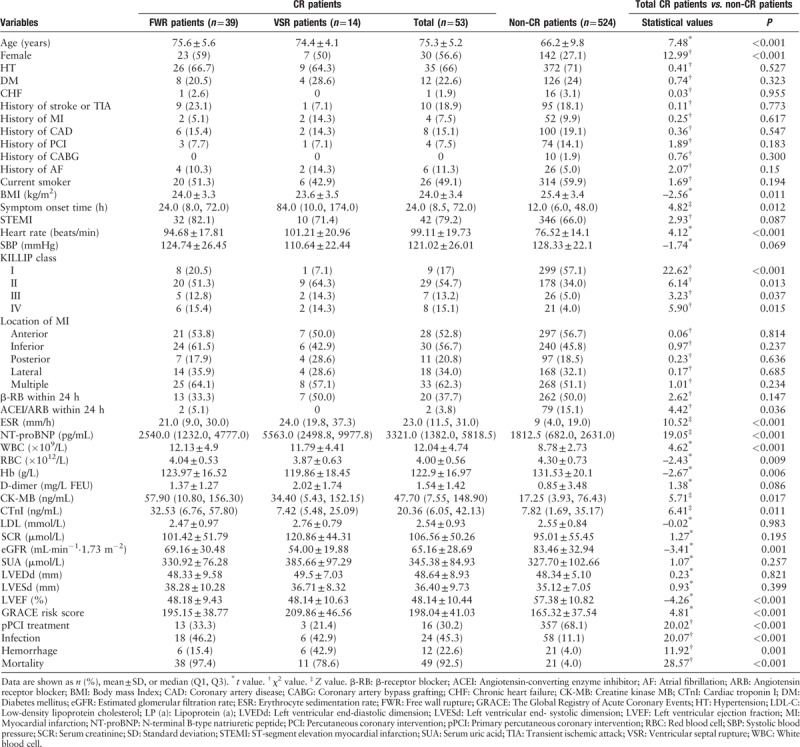
Table 2.
Vessel disease type of CR and non-CR patients receiving CAG during hospitalization (N = 383).

GRACE risk score and ROC curve analysis
The GRACE risk score of FWR and VSR patients was 195.15 ± 38.77 and 209.86 ± 46.56, respectively. CR patients had a significantly higher GRACE risk score than patients without CR (198.04 ± 41.03 vs. 165.32 ± 37.54, P < 0.001). A receiver operating characteristic (ROC) curve analysis was performed and area under the ROC curve (AUC) of the GRACE risk score model to predict CR after AMI was 0.716 (95% CI: 0.634–0.798, P < 0.001) [Figure 2].
Figure 2.
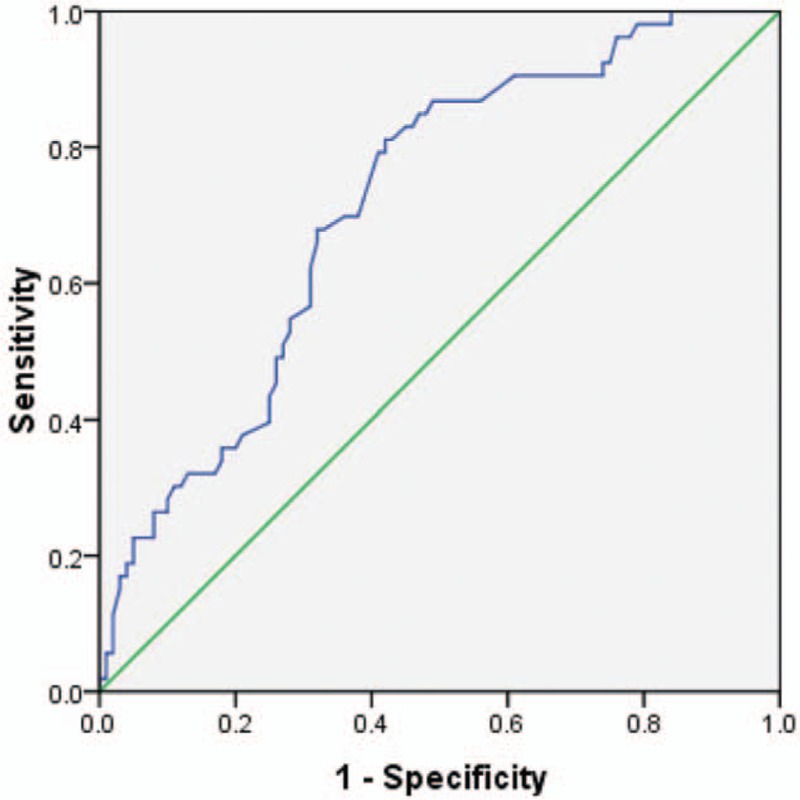
Receiver operating characteristic curve analysis for the GRACE risk score in predicting CR after AMI. The area under the curve was 0.716 (95% CI: 0.634–0.798, P < 0.001). CR: cardiac rupture; AMI: Acute myocardial infarction; GRACE: Global Registry of Acute Coronary Events.
Multivariable analysis and ROC curve analysis
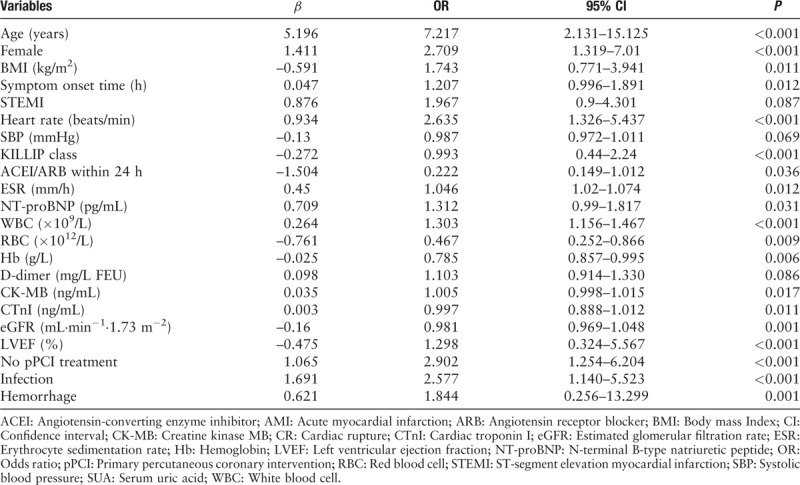
We used the stepwise backward method to fit the multivariate logistic regression model. The inclusion criteria was P ≤ 0.10, and the exclusion criteria was P ≥ 0.05. In multivariate logistic regression, six factors were associated with CR independently: older age, female, higher heart rate at admission, no pPCI treatment, lower LVEF, and lower BMI [Table 3].
Table 3.
Univariable analysis of factors associated with CR in patients with AMI.
A ROC analysis curves served to determine the optimal cut-off point of age, heart rate at admission and LVEF for identifying patients with CR. The criterion for optimal cut-off point selection is the maximum of the Youden index.[21] Age of 68 years, heart rate of 94 beats/min and LVEF of 40% were the optimal cut-off points, respectively. The effect of BMI was assessed as a continuous variable and according to two categories using the cut-off of 25.0 kg/m2, attending to what is considered overweight and obesity.[22,23]
Another multivariable analysis was performed and according to multivariate regression coefficients, different weighted scores were assigned to each risk factor [Table 4]. A risk score model (0–12) was established. The mean score of all participants was 5.59 ± 3.27. CR patients had a significantly higher score than non-CR patients (8.57 ± 1.83 vs. 4.02 ± 2.73, P < 0.001). The mean score of FWR and VSR patients was 8.28 ± 1.9 and 9.35 ± 1.39. The incidence of CR altered from scores and was increased along with the risk score in a nearly linear manner: score from 0 to 12, the risk of CR was 0% (score ≤ 3), 10.0% (score = 4), 26.7% (score = 5), 23.5% (score = 6), 27.8% (score = 7), 66.7% (score = 8), 77.8% (score = 9), 90.9% (score = 10) and 100% (score ≥ 11). We categorized patients into three groups: low risk (score ≤ 3), moderate risk (score 4–7) and high risk (score ≥ 8) groups. We found that the risk of CR in these three groups were 0%, 23.3% and 81.3%, respectively. This CR risk score model demonstrated a very good discriminate power in ROC curve analysis (AUC = 0.843, 95% CI: 0.781–0.905, optimism-corrected AUC = 0.821, P < 0.001) [Figure 3]. The 48 in-hospital CR events could be predicted using our risk score model and the P value of Hosmer-Lemeshow goodness-of-fit was 0.67.
Table 4.
Multiple analyses of factors associated with CR in AMI patients and predicting risk score model.

Figure 3.

Receiver operating characteristic curve analysis for our novel risk score in predicting CR after AMI. The AUC was 0.843 (95% CI: 0.781–0.905; optimism-corrected AUC = 0.821, P < 0.001). AMI: Acute myocardial infarction; AUC: Area under the curve; CR: Cardiac rupture.
Discussion
In the present study, we found several risk factors associated with CR and established a risk score model to predict CR after AMI. To the best of our knowledge, this study early reported such a simple model with very high discriminate power in predicting CR after AMI.
CR was one of the most serious complications after AMI, though its incidence decreased dramatically with the widespread use of thrombolytics, PCI and modern therapies.[4,8,9] In some reports during the pre-reperfusion era, CR occurred in as many as 6% of all cases admitted for AMI.[8] Most of the contemporary studies, including large registries and clinical trials like the GRACE registry, report an incidence of CR after AMI around 1%, similar to the present study (0.67%).[9,24,25] CR, in particular FWR, is considered to be a hopeless complication after AMI. Despite advances in diagnostic procedures and surgical techniques, hospital mortality remains high in patients with CR. The hospital mortality of CR patients was 92.5% in the present study, with 97.4% in FWR and 78.6% in VSR patients, respectively. Similar or a little lower mortality rates have been observed in other modern studies.[1,5,26]
CR occurred more frequently in women and older patients. Longer symptom onset time, higher heart rate at admission, KILLIP class, ESR, Nt-proBNP, WBC, CKMB, CTNI, GRACE risk score, in hospital infection, hemorrhage and mortality was also seen in CR patients. Moreover, CR group had significantly lower BMI, ACEI/ARB use in 24 h after admission, RBC, Hb, eGFR, and pPCI treatment. Most of these CR related factors have also been reported previously.[27–30] In the multivariable analysis, older age, female gender, higher heart rate at admission, lower LVEF, lower BMI and no pPCI treatment related independently to CR.
Of all factors related to CR, age is probably the most relevant. Older age was invariably reported in many previous studies as the leading risk factor for CR.[29,31,32] Same results were shown in our study as well. Many studies have reported the association between BMI and outcomes of patients with acute coronary syndromes (ACS). For example, in a retrospective study of 413,673 patients with AMI, higher BMI patients had the lowest odds of in-hospital mortality.[33] Later, several studies also concluded that in patients with AMI, short-term, medium-term, and long-term mortality rates were all lower in the overweight, obese and morbidly obese groups compared with the normal weight group.[34,35] In the present study, we found BMI level was significantly lower in CR group and BMI < 25 kg/m2 was an independent risk factor for CR in AMI patients.
The frequency of CR has two peaks: an early peak within 24 h and a late one from 4 to 6 days.[36–38] Similar results also found in our study. Early rupture (within 24 h) is related to the initial evolution of infarction before significant collagen deposition, and late rupture (≥24 h) is related to expansion of the infarct-related ventricular wall.[37] Primary PCI treatment seems to provide protection against CR, independent of other factors.[7,8] There are two main factors to explain why CR occurs less frequently with PCI treatment than with traditional thrombolysis therapy: first, PCI treatment achieves the restoration of coronary patency more frequently and second, the risk of bleeding is much lower.[39] In our present study, non-PCI treatment was found to be an independent risk factor for CR, consistent with previous research conclusions. Previous studies have reported coronary single-vessel disease as an independent risk factor for CR.[5,7,27] This study showed that CR patients had more coronary single-vessel disease than non-CR patients (58.3% vs. 53.2%), but without statistical significance (P = 0.431). No patients received thrombolytic therapy in our study due to contraindication or disagreement.
Risk stratification is essential for the comprehensive management of patients with ACS. Prompt diagnosis with appropriate medical therapy and timely surgical intervention are necessary for favorable outcomes of CR patients. To the best of our knowledge, there was no valid risk model to predict CR after AMI reported before. The GRACE risk score has been recognized as a validated predictor of adverse outcomes in ACS patients and current guidelines recommend using the GRACE risk score for risk stratification in ACS patients.[40,41] However, the value of the GRACE risk score in predicting CR after AMI was rarely reported, and should probably not be used to predict CR.[29] Similar results were presented in our study: although CR patients had a significantly higher GRACE risk score than non-CR patients (198.04 ± 41.03 vs. 165.32 ± 37.54, P < 0.001), the discriminate power of GRACE risk score in predicting CR seemed unsatisfactory after ROC curve analysis (AUC = 0.716, 95%CI 0.634–0.798, P < 0.001).
Six factors: female, no pPCI treatment, LVEF < 40%, heart rate ≥ 94 beats/min, BMI < 25 kg/m2 and age ≥ 68 years were found to be associated with CR independently in the present study. After assigned to different weighted scores according to regression coefficients, a simple risk score system ranged from 0 to 12 was established to predict CR after AMI. After ROC curve analysis, this new risk score demonstrated a very good discriminate power (AUC = 0.843, 95% CI: 0.781–0.905, optimism-corrected AUC = 0.821, P < 0.001). Using our risk score model, 48 in-hospital CR events can be predicted (very close to the actual 53 CR events) and the P value of Hosmer-Lemeshow goodness-of-fit was 0.67. The AUC for our risk score model was statistically higher than the AUC for the GRACE risk score (0.843 vs. 0.716, P < 0.001), which demonstrated that the predictive value of our risk model was significantly better than the GRACE risk score.
There were several limitations to the current study. First, as a retrospectively case-control study with relatively small sample size, the potential cause-effect relationship could not be determined. Second, no validation cohort was set up to verify the predictive value of our risk score. Third, we did not obtain all patients’ information about collateral vessels formation.
In conclusion, the risk score model this study established was simple to use, and with high predictive accuracy in predicting CR after AMI.
Conflicts of interest
None.
Footnotes
How to cite this article: Fu Y, Li KB, Yang XC. A risk score model for predicting cardiac rupture after acute myocardial infarction. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000175
References
- 1.Pohjola-Sintonen S, Muller JE, Stone PH, Willich SN, Antman EM, Davis VG, et al. Ventricular septal and free wall rupture complicating acute myocardial infarction: experience in the multicenter investigation of limitation of infarct size. Am Heart J 1989; 117:809–818. doi: 10.1016/0002-8703(89)90617-0. [DOI] [PubMed] [Google Scholar]
- 2.Opaz O, Taylor AL. Interventricular septal rupture complicating acute myocardial infarction: from pathophysiologic features to the role of invasive and noninvasive diagnostic modalities in current management. Am J Med 1992; 93:683–688. doi: 10.1016/0002-9343(92)90203-N. [DOI] [PubMed] [Google Scholar]
- 3.López-Sendón J, González A, de Sá EL, Coma-Canella I, Roldán I, Domínguez F, et al. Diagnosis of subacute ventricular wall rupture after acute myocardial infarction: Sensitivity and specificity of clinical, hemodynamic and echocardiographic criteria. JACC 1992; 19:1145–1153. doi: 10.1016/0735-1097(92)90315-e. [DOI] [PubMed] [Google Scholar]
- 4.Becker RC, Gore JM, Lambrew C, Douglas Weaver W, Michael Rubison R, French WJ, et al. A composite view of cardiac rupture in the United States national registry of myocardial infarction. JACC 1996; 27:1321–1326. doi: 10.1016/0735-1097(96)00008-3. [DOI] [PubMed] [Google Scholar]
- 5.Slater J, Brown RJ, Antonelli TA, Menon V, Boland J, Col J, et al. Cardiogenic shock due to cardiac free-wall rupture or tamponade after acute myocardial infarction: a report from the SHOCK Trial Registry. JACC 2000; 36:1117–1122. doi: 10.1016/s0735-1097(00)00845-7. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj A, Sethi A, Rathor P, Suppogu N, Sethi A. Acute complications of myocardial infarction in the current era: diagnosis and management. J Investig Med 2015; 63:844–855. doi: 10.1097/JIM.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 7.Moreno R, López-Sendón J, García E, de Isla LP, de Sá EL, Ortega A, et al. Primary angioplasty reduces the risk of left ventricular free wall rupture compared with thrombolysis in patients with acute myocardial infarction. JACC 2002; 39:598–603. doi: 10.1016/s0735-1097(01)01796-x. [DOI] [PubMed] [Google Scholar]
- 8.Figueras J, Alcalde O, Barrabes JA, Serra V, Alguersuari J, Cortadellas J, et al. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation 2008; 118:2783–2789. doi: 10.1161/CIRCULATIONAHA.108.776690. [DOI] [PubMed] [Google Scholar]
- 9.Becker RC, Charlesworth A, Wilcox RG, Hampton J, Skene A, Gore JM, et al. Cardiac rupture associated with thrombolytic therapy: impact of time to treatment in the late assessment of thrombolytic efficacy (LATE) study. JACC 1995; 25:1063–1068. doi: 10.1016/0735-1097(94)00524-t. [DOI] [PubMed] [Google Scholar]
- 10.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–executive summary. JACC 2004; 44:671–719. doi: 10.1016/j.jacc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC guideline for the management of patients with non-st-elevation acute coronary syndromes: executive summary. JACC 2014; 64:2645–2687. doi: 10.1016/j.jacc.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 2006; 333:1091.doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox KA, Anderson FA, Jr, Dabbous OH, Steg PG, Lopez-Sendon J, Van de Werf F, et al. Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their risk characteristics? The Global Registry of Acute Coronary Events (GRACE). Heart 2007; 93:177–182. doi: 10.1136/hrt.2005.084830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang EW, Wong C-K, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J 2007; 153:29–35. doi: 10.1016/j.ahj.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Scrutinio D, Ammirati E, Guida P, Passantino A, Raimondo R, Guida V, et al. Clinical utility of N-terminal pro-B-type natriuretic peptide for risk stratification of patients with acute decompensated heart failure. Derivation and validation of the ADHF/NT-proBNP risk score. Int J Cardiol 2013; 168:2120–2126. doi: 10.1016/j.ijcard.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Rassi A, Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, et al. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med 2006; 355:799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004; 23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 19.Meulendijks D, van Hasselt JGC, Huitema ADR, van Tinteren H, Deenen MJ, Beijnen JH, et al. Renal function, body surface area, and age are associated with risk of early-onset fluoropyrimidine-associated toxicity in patients treated with capecitabine-based anticancer regimens in daily clinical care. Eur J Cancer 2016; 54:120–130. doi: 10.1016/j.ejca.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Balan P, Zhao Y, Johnson S, Arain S, Dhoble A, Estrera A, et al. The society of thoracic surgery risk score as a predictor of 30-day mortality in transcatheter vs. surgical aortic valve replacement: a single-center experience and its implications for the development of a TAVR risk-prediction model. J Invasive Cardiol 2017; 29:109–114. [PubMed] [Google Scholar]
- 21.Bantis LE, Nakas CT, Reiser B. Construction of confidence regions in the ROC space after the estimation of the optimal Youden index-based cut-off point. Biometrics 2014; 70:212–223. doi: 10.1111/biom.12107. [DOI] [PubMed] [Google Scholar]
- 22.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med 2015; 163:827–835. doi: 10.7326/M14-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokkinos P, Faselis C, Myers J, Pittaras A, Sui X, Zhang J, et al. Cardiorespiratory fitness and the paradoxical BMI-mortality risk association in male veterans. Mayo Clin Proc 2014; 89:754–762. doi: 10.1016/j.mayocp.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Pujol E, Morales M, Roelandt JR, Perez MJ, Masia R, Sala J, et al. Partial ventricular septal defect (Pacman Heart). Eur J Echocardiogr 2008; 9:316–317. doi: 10.1093/ejechocard/jem068. [DOI] [PubMed] [Google Scholar]
- 25.Fonarow GC, Wright RS, Spencer FA, Fredrick PD, Dong W, Every N, et al. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol 2005; 96:611–616. doi: 10.1016/j.amjcard.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi G, Komiya T, Tamura N, Kobayashi T. Surgical treatment for postinfarction left ventricular free wall rupture. Ann Thorac Surg 2008; 85:1344–1346. doi: 10.1016/j.athoracsur.2007.12.073. [DOI] [PubMed] [Google Scholar]
- 27.Fazlinezhad A, Rezaeian MK, Yousefzadeh H, Ghaffarzadegan K, Khajedaluee M. Plasma Brain Natriuretic Peptide (BNP) as an indicator of left ventricular function, early outcome and mechanical complications after acute myocardial infarction. Clin Med Insights Cardiol 2011; 5:77–83. doi: 10.4137/CMC.S7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gueret P, Khalife K, Jobic Y, Fillipi E, Isaaz K, Tassan-Mangina S, et al. Echocardiographic assessment of the incidence of mechanical complications during the early phase of myocardial infarction in the reperfusion era: a French multicentre prospective registry. Arch Cardiovasc Dis 2008; 101:41–47. doi: 10.1016/s1875-2136(08)70254-7. [DOI] [PubMed] [Google Scholar]
- 29.López-Sendón J, Gurfinkel E, Lopez de Sa E, Agnelli G, Gore J, Steg P, et al. Factors related to heart rupture in acute coronary syndromes in the global registry of acute coronary events. Eur Heart J 2010; 31 12:1449–1456. doi: 10.1093/eurheartj/ehq06110.1093/eurheartj/ehq089. [DOI] [PubMed] [Google Scholar]
- 30.Nozoe M, Sakamoto T, Taguchi E, Miyamoto S, Fukunaga T, Nakao K. Clinical manifestation of early phase left ventricular rupture complicating acute myocardial infarction in the primary PCI era. J Cardiol 2014; 63:14–18. doi: 10.1016/j.jjcc.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Bueno H, Martinez-Selles M, Perez-David E, Lopez-Palop R. Effect of thrombolytic therapy on the risk of cardiac rupture and mortality in older patients with first acute myocardial infarction. Eur Heart J 2005; 26:1705–1711. doi: 10.1093/eurheartj/ehi284. [DOI] [PubMed] [Google Scholar]
- 32.Maggioni AP, Maseri A, Fresco C, Franzosi MG, Mauri F, Santoro E, et al. Age-related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. The investigators of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-2). N Engl J Med 1993; 329:1442–1448. doi: 10.1056/NEJM199311113292002. [DOI] [PubMed] [Google Scholar]
- 33.Dhoot J, Tariq S, Erande A, Amin A, Patel P, Malik S. Effect of morbid obesity on in-hospital mortality and coronary revascularization outcomes after acute myocardial infarction in the United States. Am J Cardiol 2013; 111:1104–1110. doi: 10.1016/j.amjcard.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niedziela J, Hudzik B, Niedziela N, Gąsior M, Gierlotka M, Wasilewski J, et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol 2014; 29:801–812. doi: 10.1007/s10654-014-9961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bundhun PK, Li N, Chen MH. Does an obesity paradox really exist after cardiovascular intervention? A systematic review and meta-analysis of randomized controlled trials and observational studies. Medicine 2015; 94:e1910.doi: 10.1097/MD.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birnbaum Y, Fishbein MC, Blanche C, Siegel RJ. Ventricular septal rupture after acute myocardial infarction. N Engl J Med 2002; 347:1426–1432. doi: 10.1056/NEJMra020228. [DOI] [PubMed] [Google Scholar]
- 37.Crenshaw BS, Granger CB, Birnbaum Y, Pieper KS, Morris DC, Kleiman NS, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation 2000; 101:27–32. doi: 10.1161/01.CIR.101.1.27. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura F, Minamino T, Higashino Y, Ito H, Fujii K, Fujita T, et al. Cardiac free wall rupture in acute myocardial infarction: ameliorative effect of coronary reperfusion. Clin Cardiol 1992; 15:244–250. doi: 10.1002/clc.4960150405. [DOI] [PubMed] [Google Scholar]
- 39.Nakatani D, Sato H, Kinjo K, Mizuno H, Hishida E, Hirayama A, et al. Effect of successful late reperfusion by primary coronary angioplasty on mechanical complications of acute myocardial infarction. Am J Cardiol 2003; 92:785–788. doi: 10.1016/s0002-9149(03)00883-x. [DOI] [PubMed] [Google Scholar]
- 40.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. JACC 2013; 61:e179–e347. doi: 10.1016/j.jacc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011; 32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]


