Abstract
Background:
High on-treatment platelet reactivity (HTPR) has been suggested as a risk factor for patients with ischemic vascular disease. We explored a predictive model of platelet reactivity to clopidogrel and the relationship with clinical outcomes.
Methods:
A total of 441 patients were included. Platelet reactivity was measured by light transmittance aggregometry after receiving dual antiplatelet therapy. HTPR was defined by the consensus cutoff of maximal platelet aggregation >46% by light transmittance aggregometry. CYP2C19 loss-of-function polymorphisms were identified by DNA microarray analysis. The data were compared by binary logistic regression to find the risk factors. The primary endpoint was major adverse clinical events (MACEs), and patients were followed for a median time of 29 months. Survival curves were constructed with Kaplan-Meier estimates and compared by log-rank tests between the patients with HTPR and non-HTPR.
Results:
The rate of HTPR was 17.2%. Logistic regression identified the following predictors of HTPR: age, therapy regimen, body mass index, diabetes history, CYP2C19∗2, or CYP2C19∗3 variant. The area under the curve of receiver operating characteristic for the HTPR predictive model was 0.793 (95% confidence interval: 0.738–0.848). Kaplan-Meier analysis showed that patients with HTPR had a higher incidence of MACE than those with non-HTPR (21.1% vs. 9.9%; χ2 = 7.572, P = 0.010).
Conclusions:
Our results suggest that advanced age, higher body mass index, treatment with regular dual antiplatelet therapy, diabetes, and CYP2C19∗2 or CYP2C19∗3 carriers are significantly associated with HTPR to clopidogrel. The predictive model of HTPR has useful discrimination and good calibration and may predict long-term MACE.
Keywords: High on-treatment platelet reactivity, Clopidogrel, CYP2C19, Light transmittance aggregometry, Ischemic vascular events
Introduction
Ischemic cardiovascular and cerebrovascular diseases pose a serious threat to human health and seriously affect the quality of human life.[1–3] Antiplatelet therapy using acetylsalicylic acid (ASA) or clopidogrel is a standard therapy regimen for secondary prevention of cardiovascular events.[4] However, even if dual antiplatelet therapy (DAPT) was administered, some patients suffered from recurrence.[5–7] Clinical studies have found that the recurrence may be related to high on-treatment platelet reactivity (HTPR).[7,8] Our previous study suggested that HTPR was closely linked to recurrence of stroke.[9,10]
Generally, on-treatment platelet reactivity to adenosine diphosphate (ADP) is influenced by three factors: genetics and cellular and clinical factors. Platelet activation plays a critical role in atherothrombotic thrombosis, and its morphological and functional changes directly affect platelet aggregation. Therefore, changes in platelet morphology and function are significant risk factors for antiplatelet therapy responsiveness.[11,12] Clopidogrel, as a subtype of the ADP receptor and one of the important antiplatelet drugs, can be specifically and irreversibly bound to P2Y12 on the surface of platelets, whereas conversion of clopidogrel to its active compound is more complex, requiring a two-step conversion process to convert to its active compound.[13] It is well known that the P450 system, especially CYP2C19, is essential for the activation of clopidogrel and plays an important role in the metabolism of clopidogrel,[13–15] and CYP2C19 gene polymorphism is considered an important factor in HTPR.[16–21] In addition, diabetes not only destroys blood vessels and promotes thrombosis but also causes changes in platelet morphology and function,[22] so patients with diabetes exhibit increased platelet reactivity.[22,23]
In fact, according to previous studies, although many genetic and non-genetic factors are known, a great portion of clopidogrel variable platelet reactivity remains unexplained, which challenges the personalization of clopidogrel therapy.[24] Therefore, it is necessary to explore more risk factors affecting HTPR. Furthermore, studies on HTPR risk factors are inconsistent. For example, Kim et al[11] thought that patients with large platelets (mean platelet volume [MPV] ≥10.6 fL) had significantly high residual platelet reactivity after clopidogrel (multiple electrode platelet aggregometry [MEA] ADP 21.0 [15.0–30.0] units vs. 24.0 [18.5–40.0] units, P = 0.003) treatment. On the contrary, Verdoia et al[25] held that MPV elevation did not influence the risk of HTPR with clopidogrel. The same argument was made in the correlation between body mass index (BMI) and HTPR,[26,27] so it is necessary to explore a predictive model of HTPR.
In addition, the relationship between HTPR and clinical prognosis still was controversial. The RECLOSE 2-ACS study supported the view that HTPR could predict long-term clinical outcomes,[28] but some studies had opposing opinions.[29] Therefore, it is important to explore the relationship between HTPR and clinical outcomes.
Methods
Ethical approval
The present study was approved by the Human Ethics Committee of Guangdong Provincial People's Hospital and Guangdong Academy of Medical Sciences (No. GDREC2017280H) and adhered to the tenets of the Declaration of Helsinki. Verbal and written informed consent was obtained from all participants included in the study.
Study population
Patients with non-cardioembolic ischemic stroke (NCIS), coronary atherosclerosis heart disease (CAHD), or ischemic perivascular events (IPVEs) who were admitted in Guangdong Provincial People's Hospital between June 2015 and September 2016 were included after admission. All the included patients must have received DAPT (aspirin and clopidogrel) during hospitalization, according to their clinical need. Clinical diagnosis of patients was based on related guidelines of American Heart Association and referred to previously published papers.[9,11,20]
Patients with the following medical conditions were excluded from the study: those being treated with anticoagulants; patients receiving thrombolytic therapy within 1 week of admission; those with thrombocythemia, thrombocytopenia, platelet dysfunction, type 1 diabetes, abnormal coagulation disorders, chronic kidney disease, severe liver disease, hematopoietic dysfunction, malignant tumors; or those who were pregnant.
Therapy regimen
All included patients were divided into two groups according to their therapy methods. If the patients had taken clopidogrel 75 mg and aspirin 100 mg daily for at least 7 days before admission, they were included in the regular DAPT group. Patient who had never taken clopidogrel were assigned to the intensive DAPT group; these patients were given 300 mg of clopidogrel immediately after admission, besides aspirin, and then were switched to 75 mg daily from the following day. After discharge, all patients continued to take 75 mg clopidogrel daily during the follow-up period.
Sample collection and measurements
HbA1c, platelet counts, platelet distribution width (PDW), MPV, plateletcrit (PCT), cholesterol (CHOL), triglyceride (TRIG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and CYP2C19 were measured immediately after admission. Demographic data, clinical data, and lifestyle were collected. All core data had to be verified by two staff members.
HbA1c determination
The ion-exchange high-performance liquid chromatography (HPLC) method was used to determine the HbA1c level. Venous blood was collected.
CYP2C19 genotyping
Briefly, 1 mL of venous blood was collected in BD Vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. Genomic DNA was extracted from whole-blood samples according to the manufacturer's instructions. A genotyping kit (DNA Microarray; BaiO Inc., Shanghai, China) was used to identify the genotypes of CYP2C19. The CYP2C19∗2 and CYP2C19∗3 variant alleles were determined with the BaiO BE-2.0 Biochip diagnostic Analyzer (BaiO Technology Corp, Shanghai, China). The procedures of DNA extraction, polymerase chain reaction amplification, hybridization, gene array detection, and analysis were performed strictly according to the manuals of the BaiO genotype detecting gene array kit and equipment (BaiO Inc.). These two polymorphisms were in Hardy-Weinberg equilibrium (P > 0.05).
On the basis of these procedures, patients were classified as wild-type homozygote (∗1/∗1 allele), heterozygote (∗1/∗2, ∗1/∗3), or variant homozygote (∗2/∗2, ∗2/∗3, ∗3/∗3), corresponding to extensive metabolizers (EMs), intermediate metabolizers (IMs), and poor metabolizers (PMs), respectively.[30]
Platelet reactivity assay
Blood samples that were collected from patients at least 6 h after intensive treatment or 7 days of regular antiplatelet therapy were sent for analysis in a Vacutainer tube containing 3.2% trisodium citrate (ratio, 9:1). The Vacutainer tube was filled to capacity and inverted three to five times to ensure complete mixing of the anticoagulant.
Measurement of ADP-induced platelet aggregation in platelet-rich plasma by light transmittance aggregometry (LTA) assay has long been a classical method for assessing platelet function in relation to clinical outcome. Assessment of platelet function has been reported previously.[31] In brief, platelet-rich plasma, obtained by centrifuging whole blood for 10 min at 200 g, was stimulated with 5 μmol/L ADP. Platelet aggregation was determined by LTA, using an AggRAM Platelet Aggregation Analyzer (Helena Biosciences, Gateshead, UK). It was expressed as maximal platelet aggregation (MPA), which represents the maximal percentage change in light transmittance, using platelet-poor plasma as a reference. MPA >46% by LTA was defined as HTPR.[32]
LTA quality control
All the blood samples were kept in a 15°C to 30°C room and measured within 3 h of collection by trained professional staff. The entire operation was conducted in strict accordance with the approved standard process.
Outcomes and follow-up
The primary endpoint was major adverse clinical events (MACEs), including a composite of vascular death, stroke/transient ischemic attack, gastrointestinal bleeding, stable and unstable angina pectoris, heart failure, and myocardial infarction. Follow-up visits were carried out within 18 to 33 months (median time: 29 months) by hospital visit or telephone after discharge. The following information was recorded at follow-up: Did you take clopidogrel according to doctor's advice? Did MACE occur, and when?
Statistical analysis
All variables were tested for normal distribution, using the Kolmogorov-Smirnov test (KS test). Measurement data were analyzed using the t test, and enumeration data were analyzed using the Chi-squared (χ2) test. One-way analysis of variance (ANOVA) was applied to intergroup comparisons. To analyze the correlation factors affecting clopidogrel HTPR defined by LTA MPA, we performed univariate and then multivariate logistic regression analysis. The predictive model of HTPR based on those risk factors was built, and discrimination and calibration of the model were assessed by the receiver operating characteristic (ROC) curve and Hosmer-Lemeshow goodness-of-fit test. The Kaplan-Meier method was used to assess the cumulative survival of patients with HTPR or non-HTPR, and the log-rank test was used to assess the statistical differences between these two survival curves. A P value <0.05 denoted a statistically significant difference. SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Baseline characteristics
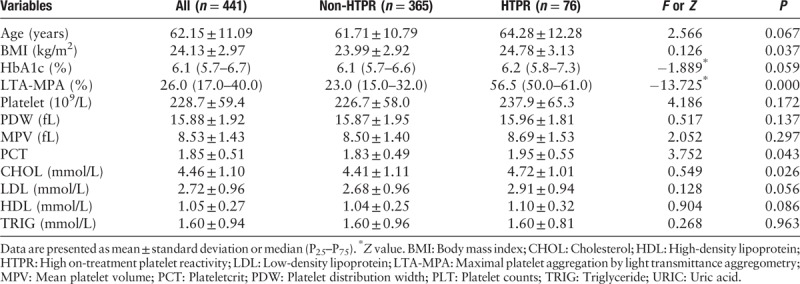
A total of 441 patients were included in our study; 76 (17.2%) patients had HTPR. We collected 92 patients with NCIS, 21 with IPVE, and 328 with CAHD (72 of whom with percutaneous coronary intervention). The study group characteristics are described in Tables 1 and 2. Significant differences in baseline characteristics were observed, including gender, therapy methods, hypertension history, diabetes, BMI, PCT, and CHOL between the HTPR group and the non-HTPR group (LTA-MPA: 57.47 ± 9.10% vs. 23.33 ± 11.37%, P = 0.000). Obviously, there were no significant differences among the other variables between the two groups, for example, current smoking and alcohol consumption, age, HbA1c, platelet counts, PDW, MPV, LDL, HDL, and TRIG.
Table 1.
Demographic and clinical baseline characteristics (categorical data).
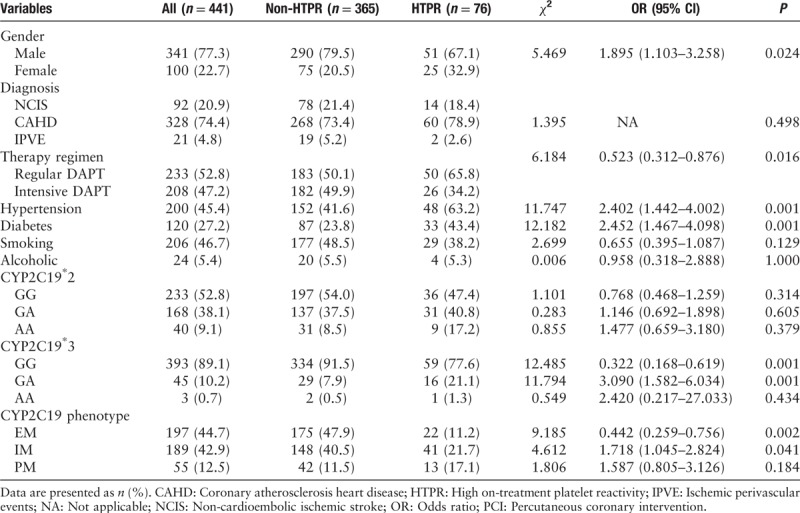
Table 2.
Demographic and clinical baseline characteristics (continuous variables).
CYP2C19 genotyping
Genotyping results are illustrated in Table 1. A total of 47.2% of the participants were carriers of at least one copy of the CYP2C19∗2 LOF allele (681 GA: 38.1%, 681 AA: 9.1%), whereas for CYP2C19∗3, only 10.9% (636 GA: 10.2%, 636 AA: 0.7%) were carriers; 44.7% of the CYP2C19 phenotype was EM (non-HTPR: 47.9% vs. HTPR 11.2%, P = 0.002), 42.9% was IM (40.5% vs. 21.7%, p = 0.041), and 12.5% was PM (11.5% vs. 17.1%, P = 0.184).
Regression analysis
Risk factors of HTPR
All patients’ platelet function was checked by LTA. As summarized in Table 2, the median of LTA MPA was 26%. According to the multivariate logistic regression analysis described in Table 3, the independent factors determining HTPR were age [odds ratio (OR): 1.030; 95% confidence interval (CI): 1.002–1.058; P = 0.036], therapy methods (OR: 2.812; 95% CI: 1.512–5.232; P = 0.001), BMI (OR: 1.313; 95% CI: 1.028–1.245; P = 0.012), diabetes history (OR: 0.322; 95% CI: 0.148–0.699; P = 0.004), CYP2C19∗2 (OR: 0.512; 95% CI: 0.287–0.915; P = 0.024), or CYP2C19∗3 (OR: 0.158; 95% CI: 0.071–0.354; P = 0.000). Figure 1 shows the distribution of non-HTPR and HTPR patients in different risk factor groups.
Table 3.
Multivariate regression analysis to predict HTPR after clopidogrel treatment.
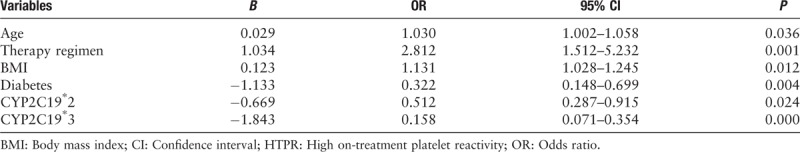
Figure 1.
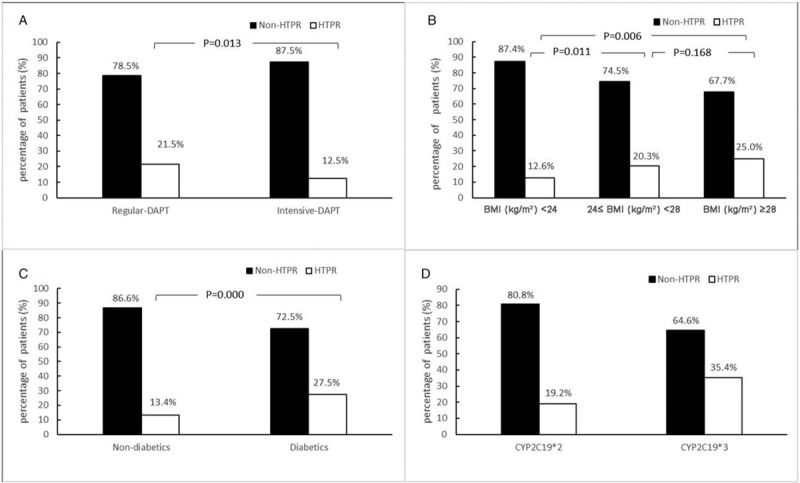
Distribution of non-HTPR and HTPR patients in different risk factor groups. (A) HTPR between therapy groups. (B) HTPR between BMI groups. (C) HTPR between non-diabetics and diabetics groups. (D) HTPR between CYP2C19 variation groups.BMI: Body mass index; DAPT: Dual antiplatelet therapy; HTPR: High on-treatment platelet reactivity.
The predictive model of HTPR
The predictive model of HTPR based on these risk factors was built as follows:
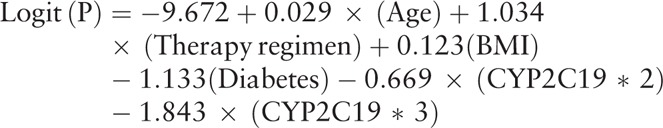 |
Model discrimination and calibration
The area under the ROC curve for the prediction of HTPR was calculated using predicted probabilities and outcomes of HTPR according to the cut-off by LTA MPA of each patient. The area under the ROC curve was only 0.793 (95% CI: 0.738–0.848), sensitivity 76.3%, specificity 74.2%, and Youden index 0.506. The positive likelihood ratio was 2.96, and the negative likelihood ratio was 0.32 [Figure 2].
Figure 2.
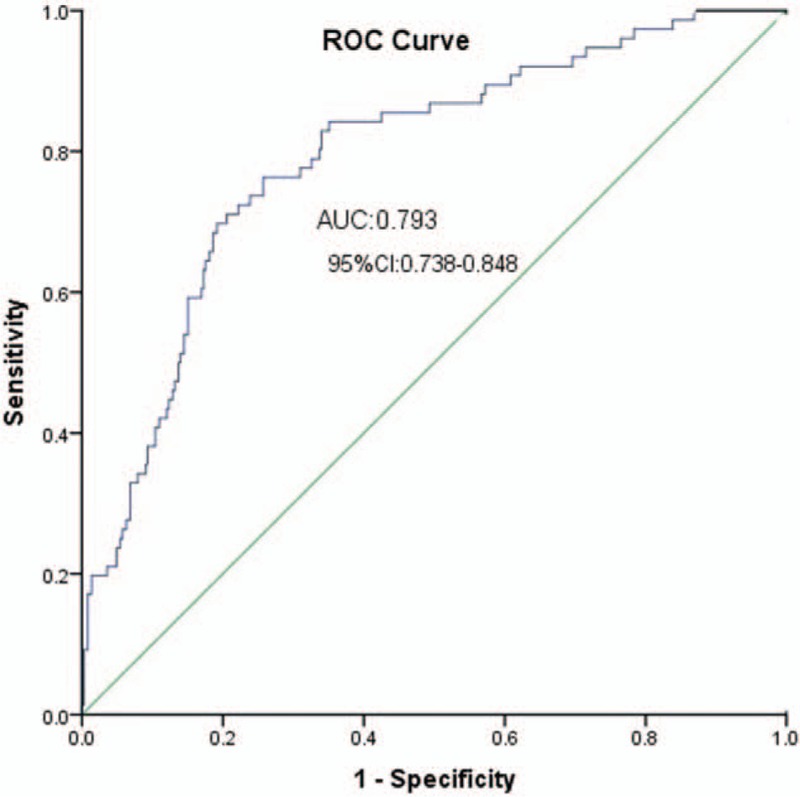
ROC curve analysis to the HTPR predictive model. AUC: Area under curve; CI: Confidence interval; HTPR: High on-treatment platelet reactivity; ROC: Receiver operating characteristic.
The performance of the HTPR predictive model assessed in terms of calibration, using the Hosmer-Lemeshow test, was not significant (P = 0.231). It was also apparent in the calibration plot [Figure 3]. This suggests that there was no statistically significant difference between the predicted and observed outcomes.
Figure 3.
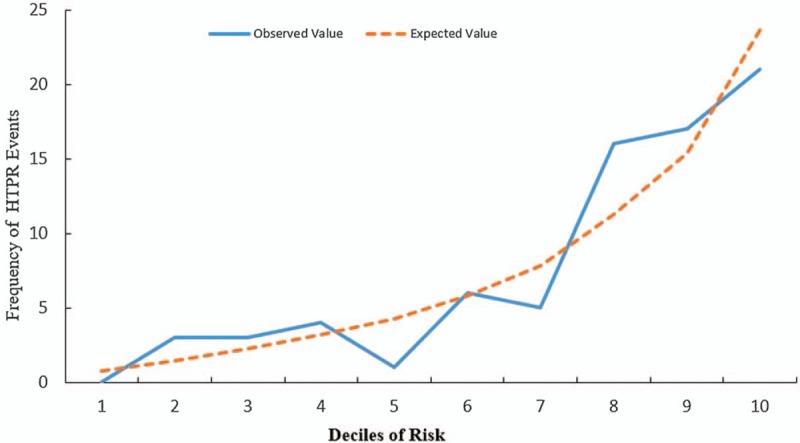
Calibration of the model to predicting HTPR assessed by Hosmer-Lemeshow goodness-of-fit test. HTPR: High on-treatment platelet reactivity.
Clinical outcomes
Clinical follow-up was available in 96.3% (441 patients were available and 17 were lost). A total of 52 patients suffered from MACE by 18 to 33 months of follow-up after discharge. Among them, 30 had stable or unstable angina pectoris, six had myocardial infarction, three had heart failure, 10 had an ischemic stroke or transient ischemic attack, two had gastrointestinal bleeding, and one died. Compared with the non-HTPR group, the HTPR group had a higher risk of MACE (21.1% vs. 9.9%; χ2 = 7.572, P = 0.010) and shorter mean survival time (29.6 vs. 31.2 months; χ2 = 6.880, P = 0.009). The Kaplan-Meier curve was constructed [Figure 4]. A log-rank test was run to determine whether there were differences in treatment failure distribution for the HTPR group compared with the non-HTPR group. The survival distribution for the two groups was statistically different (χ2 = 6.880, P = 0.009). However, CYP2C19∗2 (χ2 = 0.220, P = 0.639) and CYP2C19∗3 (χ2 = 0.070, P = 0.792) were not statistically different in the rate of MACE.
Figure 4.
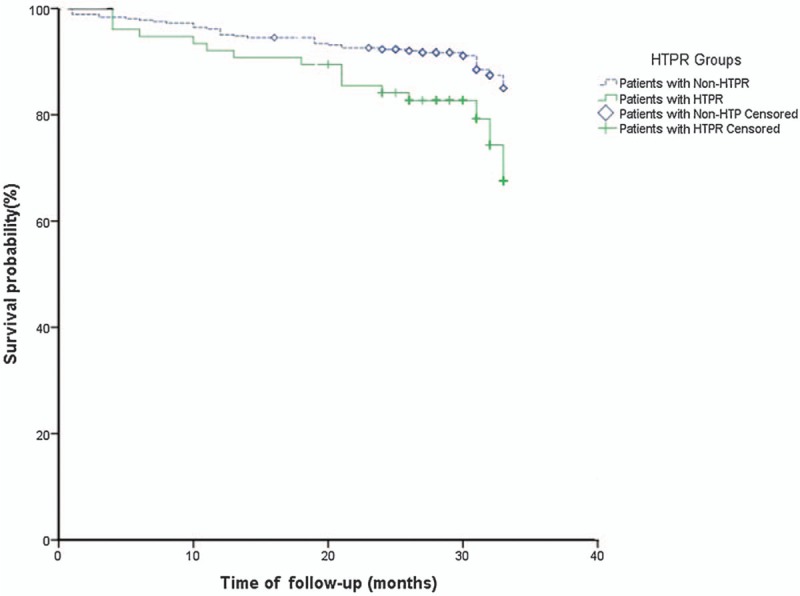
Kaplan-Meier analysis of incidence of MACE, according to platelet function analysis. HTPR: High on-treatment platelet reactivity; MACE: Major adverse clinical events.
Discussion
We confirm that age, therapy method, BMI, diabetes history, and CYP2C19∗2 or CYP2C19∗3 correlated with HTPR on clopidogrel defined by LTA (MPA >46%) in patients with acute ischemic vascular events who received DAPT, whereas gender, current smoking, HbA1c, platelet counts, PDW, and MPV, which were thought to be risk factors for HTPR in previous studies,[11,16] were not significantly correlated with HTPR incidence.
We built the predictive model of HTPR on the basis of these risk factors; the purpose of the predictive model was to assess potential risks, manage treatment, and avoid recurrence of ischemic vascular events. The performance of the predictive model in this study was assessed by the discrimination and calibration of the model. The area under the ROC curve for a prognostic model is classically between 0.6 and 0.85.[33] In our study, the ROC curve was 0.793, so the predictive model of HTPR might have useful discrimination.
The accuracy of the model was assessed by examining the calibration; a better way to assess the fit of a logistic regression model was to compare the predicted and observed values. We found that the predicted values were very close to the observed values in the calibration plot. Therefore, there was excellent agreement between the predicted and observed values in predicting HTPR in this study. The predictive model of HTPR had a good calibration.
In addition, a follow-up of median 29 months revealed that platelet function testing was helpful in judging clinical outcomes, and patients with HTPR seemed to have a higher incidence of MACE.
Genetic factors contributing to clopidogrel HTPR
Genetic factors, especially CYP2C19 polymorphisms, contributed to high HTPR and increased thrombotic events in patients with CAHD and NCIS on clopidogrel, and even induced major complications.[21,34,35] The CYP2C19 genotype is highly polymorphic; more than 25 alleles have variable enzymatic activity levels, but the most extensively studied variants are CYP2C19∗2 and CYP2C19∗3, both of which involve replacement of guanine (G) with adenine (A).[17,36] The presence of at least one allele of the CYP2C19∗2 is observed in 30.26% of East Asians, whereas the CYP2C19∗3 allele is present in less than 7%.[37] In our study, the frequency of CYP2C19∗2 was 47.2% (681 GA: 38.1%; 681 AA 9.1%) in total; of CYP2C19∗3, it was 10.9% (636 GA: 10.2%; 636 AA: 0.7%), which is higher than the East Asian population overall level.
Bouman et al[16] proved that clopidogrel response was significantly influenced by the presence of CYP2C19 polymorphisms. Similar results were reported by many scholars in this field,[17–20] and we confirmed again that the CYP2C19∗2 and CYP2C19∗3 variations were correlated with non-responsiveness to clopidogrel treatment. As is well known, clopidogrel, as a prodrug, must be metabolized to release clopidogrel's active metabolites (clop-AM) by the P450 system in the hepatocytes.[14] During the two-step conversion process from clopidogrel to its active compound in hepatic biotransformation of clopidogrel, the CYP2C19 enzyme is responsible for 45% of the first step and 20% of the second step,[13] and it is estimated that CYP2C19 (a member of the P450 system) contributes to about 50% of the overall formation of clop-AM from clopidogrel and thus plays a substantial role in the bioactivation of clopidogrel.[13] In a linear mixed-effects model based on active metabolite measurements, carriage of CYP2C19∗2 or ∗3 was associated with the most significant reduction (32%, P < 0.001) in area under the curve (AUC) 0–24, compared with the genetic variation in the other cytochrome P450 enzymes involved in clopidogrel metabolism.[15] Therefore, it is not hard to explain that CYP2C19∗2 or ∗3 carriers are prone to HTPR and ischemic events. Furthermore, the U.S. Food and Drug Administration declared in a boxed warning dated March 12, 2010, that CYP2C19∗2 and ∗3 alleles were devoid of the functional metabolism of clopidogrel and likewise made platelets likely to form blood clots.[38]
Most studies confirmed that CYP2C19∗2 was independently associated with HTPR.[16–21] However, concerning CYP2C19∗3 polymorphism, some authors believed that it had no relationship with HTPR,[18–20] and a few studies drew the conclusion that it had nothing to do with CYP2C19∗2 or CYP2C19∗3.[39,40] Maybe the following reasons can explain this phenomenon: first, the study population was relatively small. Golukhova et al[39] collected only 94 patients. Second, the overall contribution of CYP2C19 polymorphism to HTPR is little and can only explain a partial effect.[24] However, in the population we studied, including the HTPR group, the detection rate of CYP2C19∗2 or CYP2C19∗3 was relatively high. Third, the platelet function detection methods were different in the previous studies, and the definition of HTPR was also different, which resulted in statistical errors. Finally, statistical errors may be related to insufficient samples originating from a low frequency of CYP2C19∗3.[37]
Non-genetic factors contributing to clopidogrel HTPR
The failure in clopidogrel response may also be due to non-genetic factors,[13] for example, multiple chronic conditions and therapy options; chronic mechanisms, including age, diabetes mellitus, and elevated BMI, as the results of regression analysis in this study showed.
Ischemic event rates are routinely higher in diabetic patients, and this could be due to an increased rate of clopidogrel non-responsiveness in these patients.[22,24] As found in our research, the frequency of HTPR was 13.4% in the non-diabetics group and 27.5% in the diabetics group (P = 0.000). Compared with non-diabetics, patients with diabetes exhibited increased platelet reactivity; even a loading dose of clopidogrel was not sufficient to overcome increased platelet reactivity adequately in patients with type-2 diabetes.[23] Currently, almost all studies have concluded that diabetes is an independent risk factor for predicting HTPR,[22,23,35] which we confirm again in our study (OR: 0.322, 95% CI: 0.148–0.699; P = 0.004). This possibly stems from the following reasons. At first, diabetes mellitus (DM) subjects tend to have higher MPV on platelet morphology changes[25,41] and are associated with high residual platelet reactivity after DAPT.[11] Second, a mutation of insulin receptor substrate-1 was associated with a significantly higher prevalence of HTPR.[42,43] Third, poor clopidogrel response in patients with DM was attributed to the lower systemic exposure to clop-AM rather than changes in platelet response.[43] Finally, some hypoglycemic agents may affect HTPR on clopidogrel.[44] In addition, quite a few patients with DM have higher BMI. In short, the cause of diabetes leading to clopidogrel response variability is very complicated.
Higher BMI (r = 0.14, P = 0.023) in patients treated with DAPT for coronary artery disease is related to increased platelet reactivity and a higher prevalence of HTPR in clopidogrel-treated patients.[16,45] We believe that BMI (OR: 1.313; 95% CI: 1.028–1.245; P = 0.012) affects platelet reactivity, and it seems that there is a tendency for higher frequency of HTPR as BMI increases (HTPR frequency, BMI ≤23.9 vs. 24 ≤BMI ≤27.9 vs. BMI ≥28; 12.6%, 20.3%, and 25.0%, respectively). Jakubowski et al[46] thought that it was an inverse relationship between body size, exposure to Clop-AM, and platelet reactivity. Similar results were reported by Jiang et al.[47] Moreover, compared with patients with lower body weight (56.4 ± 3.7 kg), patients with higher body weight (84.7 ± 14.9 kg) had about 30% lower clop-AM plasma AUC, which ultimately led to higher on-treatment platelet reactivity in these obese patients.[48]
In patients receiving DAPT, advanced age (≥70 years) is independently associated with reduced effectiveness of ADP antagonists,[49] and similar conclusions were reported by other authors.[47,50] We believe a significant association exists between older age and higher prevalence of HTPR following clopidogrel treatment (OR: 1.030; 95% CI: 1.002–1.058, P = 0.036). A decreased conversion to its active metabolites due to a decline in the activity of the liver CYP450 enzymes in advanced age may be a good explanation.[51]
Clopidogrel dose-related effect could be responsible for the reduced atherothrombotic complications, including less stent thrombosis,[52] and compared with the standard clopidogrel dosage, the high loading regimen resulted in significantly lower mean platelet reactivity to ADP, with a lower proportion of patients exhibiting clopidogrel non-responsiveness (28% vs. 11%, P = 0.004).[53] Our findings were similar to this; patients in the intensive DAPT group had a lower frequency of HTPR than did the regular DAPT group (Figure 1A: 21.5% vs. 12.5%, P = 0.013), and the dose of clopidogrel was correlated with platelet reactivity (OR: 2.812; 95% CI: 1.512–5.32; P = 0.001) [Table 3]. It was not difficult to understand that clopidogrel's pharmacokinetics and pharmacodynamics contributed to the lower frequency of HTPR.[13]
In addition, in this study, we did not find a correlation between current smoking and HTPR, and similar results were reported by Kim et al[54]: cigarette smoking does not enhance clopidogrel responsiveness. As Kim et al[54] explained, it is possible that hemoglobin concentration might have an influence on P2Y12 reaction units (PRUs) and LTA MPA value that is unrelated to intrinsic platelet reactivity.
Platelet function and clinical outcomes
By applying platelet function testing, our study showed a significant correlation between LTA ADP response and clinical outcomes, results similar to some authors.[28,55] Laboratory clopidogrel non-responsiveness has been thought to be a marker of increased risk of MACE in patients undergoing percutaneous coronary intervention (PCI) with stenting.[56] Therefore, the determination of HTPR is conducive to guiding clinical application and reducing the occurrence of MACE. Of course, some authors oppose this view; they think there is no incremental predictive value over common cardiovascular risk factors for MACE recurrence in stable cardiovascular outpatients.[29,57] Therefore, to clarify the relationship between HTPR and clinical outcomes, further research is needed with expanded sample size.
Study limitation
It should be noted that this study has examined only demographic data and partial lifestyle, laboratory results, and therapy regimens. As a matter of fact, HTPR was affected by more confounding factors.[13] Therefore, we should include more candidate factors in the next step.
Conclusion
Notwithstanding its limitation, this study does suggest that advanced age, higher BMI, treatment with regular DAPT, diabetes, and CYP2C19∗2 or CYP2C19∗3 carriers are significantly associated with HTPR to clopidogrel, measured by LTA. The predictive model of HTPR might have useful discrimination and good calibration and may predict the long-term MACE.
Acknowledgements
The authors gratefully acknowledge the Guangdong Geriatrics Institute team group for data collection and verification. The authors gratefully thank the Statistics Office, Information and Statistics Center, Guangdong Provincial People's Hospital (Guangdong Academy of Medical Sciences) for statistical assistance in this work.
Funding
This work was supported by grants from the Science and Technology Plan Projects of Guangdong Province (No. 2017ZC0273); the Major Disease Prevention Action Plan (No. ZX01C2016083); and the Medical Scientific Research Foundation of Guangdong Province (No. C2013028).
Conflicts of interest
None.
Footnotes
How to cite this article: Ma Q, Chen GZ, Zhang YH, Zhang L, Huang LA. Clinical outcomes and predictive model of platelet reactivity to clopidogrel after acute ischemic vascular events. Chin Med J 2019;0:00–00. doi: 10.1097/CM9.0000000000000210
References
- 1.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation 2017; 135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med 2013; 369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol 2007; 6:456–464. doi: 10.1016/S1474-4422(07)70004-2. [DOI] [PubMed] [Google Scholar]
- 4.Kiernan TJ, Yan BP, Jaff MR. Antiplatelet therapy for the primary and secondary prevention of cerebrovascular events in patients with extracranial carotid artery disease. J Vasc Surg 2009; 50:431–439. doi: 10.1016/j.jvs.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Ping Y, Lin H, He P, Li W, Dai H. Antiplatelet strategies and outcomes in patients with noncardioembolic ischemic stroke from a real-world study with a five-year follow-up. Transl Stroke Res 2017; 8:228–233. doi: 10.1007/s12975-016-0516-0. [DOI] [PubMed] [Google Scholar]
- 6.Alberts MJ, Bergman DL, Molner E, Jovanovic BD, Ushiwata I, Teruya J. Antiplatelet effect of aspirin in patients with cerebrovascular disease. Stroke 2004; 35:175–178. doi: 10.1161/01.STR.0000106763.46123.F6. [DOI] [PubMed] [Google Scholar]
- 7.Fiolaki A, Katsanos AH, Kyritsis AP, Papadaki S, Kosmidou M, Moschonas IC, et al. High on treatment platelet reactivity to aspirin and clopidogrel in ischemic stroke: a systematic review and meta-analysis. J Neurol Sci 2017; 376:112–116. doi: 10.1016/j.jns.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol 2007; 49:1505–1516. doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Lai X, Li W, Xiong Z, Xu A, Xu A, et al. VASP phosphorylation and genetic polymorphism for clopidogrel resistance in Chinese patients with non-cardioembolic ischemic stroke. Thromb Res 2014; 134:1272–1277. doi: 10.1016/j.thromres.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Xie X, Wei D, Zhang S, Wu Y, Fu X, et al. Baseline platelet parameters for predicting early platelet response and clinical outcomes in patients with non-cardioembolic ischemic stroke treated with clopidogrel. Oncotarget 2017; 8:93771–93784. doi: 10.18632/oncotarget.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YG, Suh JW, Yoon CH, Oh IY, Cho YS, Youn TJ, et al. Platelet volume indices are associated with high residual platelet reactivity after antiplatelet therapy in patients undergoing percutaneous coronary intervention. J Atheroscler Thromb 2014; 21:445–453. [DOI] [PubMed] [Google Scholar]
- 12.Bitigen A, Tanalp AC, Elonu OH, Karavelioglu Y, Ozdemir N. Mean platelet volume in patients with isolated coronary artery ectasia. J Thromb Thrombolysis 2007; 24:99–103. doi: 10.1007/s11239-006-9028-1. [DOI] [PubMed] [Google Scholar]
- 13.Jiang XL, Samant S, Lesko LJ, Schmidt S. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin Pharmacokinet 2015; 54:147–166. doi: 10.1007/s40262-014-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giusti B, Gori AM, Marcucci R, Abbate R. Relation of CYP2C19 loss-of-function polymorphism to the occurrence of stent thrombosis. Expert Opin Drug Metab Toxicol 2010; 6:393–407. doi: 10.1517/17425251003598878. [DOI] [PubMed] [Google Scholar]
- 15.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 16.Bouman HJ, Harmsze AM, van Werkum JW, Breet NJ, Bergmeijer TO, Ten Cate H, et al. Variability in on-treatment platelet reactivity explained by CYP2C19∗2 genotype is modest in clopidogrel pretreated patients undergoing coronary stenting. Heart 2011; 97:1239–1244. doi: 10.1136/hrt.2010.220509. [DOI] [PubMed] [Google Scholar]
- 17.Wu H, Qian J, Xu J, Sun A, Sun W, Wang Q, et al. Effects of CYP2C19 variant alleles on postclopidogrel platelet reactivity and clinical outcomes in an actual clinical setting in China. Pharmacogenet Genomics 2012; 22:887–890. doi: 10.1097/FPC.0b013e328359253a. [DOI] [PubMed] [Google Scholar]
- 18.Saydam F, Degirmenci I, Birdane A, Ozdemir M, Ulus T, Ozbayer C, et al. The CYP2C19∗2 and CYP2C19∗17 polymorphisms play a vital role in clopidogrel responsiveness after percutaneous coronary intervention: a pharmacogenomics study. Basic Clin Pharmacol Toxicol 2017; 121:29–36. doi: 10.1111/bcpt.12763. [DOI] [PubMed] [Google Scholar]
- 19.Ou W, He Y, Li A, Liu B, Jin L. Genotype frequencies of CYP2C19, P2Y12 and GPIIIa polymorphisms in coronary heart disease patients of Han ethnicity, and their impact on clopidogrel responsiveness. Int Heart J 2016; 57:586–592. doi: 10.1536/ihj.16-006. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Luo Y, Lai Y, Yao Y, Li J, Wang Y, et al. Effect of genetic and coexisting polymorphisms on platelet response to clopidogrel in Chinese Han patients with acute coronary syndrome. J Genet 2016; 95:231–237. [DOI] [PubMed] [Google Scholar]
- 21.Bergmeijer TO, Janssen PW, Schipper JC, Qaderdan K, Ishak M, Ruitenbeek RS, et al. CYP2C19 genotype-guided antiplatelet therapy in ST-segment elevation myocardial infarction patients-Rationale and design of the Patient Outcome after primary PCI (POPular) Genetics study. Am Heart J 2014; 168:16–22. e1. doi: 10.1016/j.ahj.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Verdoia M, Pergolini P, Nardin M, Rolla R, Barbieri L, Schaffer A, et al. Impact of diabetes on immature platelets fraction and its relationship with platelet reactivity in patients receiving dual antiplatelet therapy. J Thromb Thrombolysis 2016; 42:245–253. doi: 10.1007/s11239-016-1348-1. [DOI] [PubMed] [Google Scholar]
- 23.Schuette C, Steffens D, Witkowski M, Stellbaum C, Bobbert P, Schultheiss HP, et al. The effect of clopidogrel on platelet activity in patients with and without type-2 diabetes mellitus: a comparative study. Cardiovasc Diabetol 2015; 14:15.doi: 10.1186/s12933-015-0182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, et al. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol 2010; 55:2427–2434. doi: 10.1016/j.jacc.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 25.Verdoia M, Pergolini P, Rolla R, Nardin M, Barbieri L, Schaffer A, et al. Mean platelet volume and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Expert Opin Pharmacother 2015; 16:1739–1747. doi: 10.1517/14656566.2015.1056151. [DOI] [PubMed] [Google Scholar]
- 26.Gaglia MA, Jr, Torguson R, Pakala R, Xue Z, Sardi G, Mahmoudi M, et al. Relation of body mass index to on-treatment (clopidogrel + aspirin) platelet reactivity. Am J Cardiol 2011; 108:766–771. doi: 10.1016/j.amjcard.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 27.Haberka M, Mizia-Stec K, Lasota B, Kyrcz-Krzemien S, Gasior Z. Obesity and antiplatelet effects of acetylsalicylic acid and clopidogrel in patients with stable angina pectoris after percutaneous coronary intervention. Pol Arch Med Wewn 2015; 125:620–630. doi: 10.20452/pamw.3039. [DOI] [PubMed] [Google Scholar]
- 28.Valenti R, Marcucci R, Capodanno D, De Luca G, Migliorini A, Gori AM, et al. Residual platelet reactivity to predict long-term clinical outcomes after clopidogrel loading in patients with acute coronary syndromes: comparison of different cutoff values by light transmission aggregometry from the responsiveness to clopidogrel and stent thrombosis 2-acute coronary syndrome (RECLOSE 2-ACS) study. J Thromb Thrombolysis 2015; 40:76–82. doi: 10.1007/s11239-014-1159-1. [DOI] [PubMed] [Google Scholar]
- 29.Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016; 388:2015–2022. doi: 10.1016/S0140-6736(16)31323-X. [DOI] [PubMed] [Google Scholar]
- 30.Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther 2013; 94:317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurbel PA, Antonino MJ, Bliden KP, Dichiara J, Suarez TA, Singla A, et al. Platelet reactivity to adenosine diphosphate and long-term ischemic event occurrence following percutaneous coronary intervention: a potential antiplatelet therapeutic target. Platelets 2008; 19:595–604. doi: 10.1080/09537100802351065. [DOI] [PubMed] [Google Scholar]
- 32.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56:919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 33.Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ 2009; 338:b604.doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 34.Joo HJ, Ahn SG, Park JH, Park JY, Hong SJ, Kim SY, et al. Effects of genetic variants on platelet reactivity and one-year clinical outcomes after percutaneous coronary intervention: a prospective multicentre registry study. Sci Rep 2018; 8:1229.doi: 10.1038/s41598-017-18134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomek A, Mat’oska V, Frydmanova A, Magerova H, Sramek M, Paulasova-Schwabova J, et al. Impact of CYP2C19 Polymorphisms on clinical outcomes and antiplatelet potency of clopidogrel in Caucasian poststroke survivors. Am J Ther 2018; 25:e202–e212. doi: 10.1097/MJT.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 36.Amin AM, Sheau Chin L, Azri Mohamed Noor D, Sk Abdul Kader MA, Kah Hay Y, Ibrahim B. The personalization of clopidogrel antiplatelet therapy: the role of integrative pharmacogenetics and pharmacometabolomics. Cardiol Res Pract 2017; 2017:8062796.doi: 10.1155/2017/8062796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fricke-Galindo I, Cespedes-Garro C, Rodrigues-Soares F, Naranjo ME, Delgado A, de Andres F, et al. Interethnic variation of CYP2C19 alleles, ’predicted’ phenotypes and ’measured’ metabolic phenotypes across world populations. Pharmacogenomics J 2016; 16:113–123. doi: 10.1038/tpj.2015.70. [DOI] [PubMed] [Google Scholar]
- 38.Holmes DR, Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA Clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation 2010; 122:537–557. doi: 10.1161/CIR.0b013e3181ee08ed. [DOI] [PubMed] [Google Scholar]
- 39.Golukhova EZ, Grigoryan MV, Ryabinina MN, Bulaeva NI, Serebruany VL. Body mass index and plasma P-selectin before coronary stenting predict high residual platelet reactivity at 6 months on dual antiplatelet therapy. Cardiology 2018; 139:132–136. doi: 10.1159/000485555. [DOI] [PubMed] [Google Scholar]
- 40.Khalaf H, Al Meman AA, Rasool S. Impact of cytochrome P450 2C19∗2 and ∗3 on clopidogrel loading dose in Saudi patients with acute coronary syndrome. Drug Metab Lett 2016; 10:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shilpi K, Potekar RM. A study of platelet indices in type 2 diabetes mellitus patients. Indian J Hematol Blood Transfus 2018; 34:115–120. doi: 10.1007/s12288-017-0825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angiolillo DJ, Bernardo E, Zanoni M, Vivas D, Capranzano P, Malerba G, et al. Impact of insulin receptor substrate-1 genotypes on platelet reactivity and cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 2011; 58:30–39. doi: 10.1016/j.jacc.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 43.Angiolillo DJ, Jakubowski JA, Ferreiro JL, Tello-Montoliu A, Rollini F, Franchi F, et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol 2014; 64:1005–1014. doi: 10.1016/j.jacc.2014.06.1170. [DOI] [PubMed] [Google Scholar]
- 44.Xiao CC, Ren A, Yang J, Ye SD, Xing XN, Li SM, et al. Effects of pioglitazone and glipizide on platelet function in patients with type 2 diabetes. Eur Rev Med Pharmacol Sci 2015; 19:963–970. [PubMed] [Google Scholar]
- 45.Nardin M, Verdoia M, Sartori C, Pergolini P, Rolla R, Barbieri L, et al. Body mass index and platelet reactivity during dual antiplatelet therapy with clopidogrel or ticagrelor. J Cardiovasc Pharmacol 2015; 66:364–370. doi: 10.1097/FJC.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 46.Jakubowski JA, Angiolillo DJ, Zhou C, Small DS, Moser BA, Ten Berg JM, et al. The influence of body size on the pharmacodynamic and pharmacokinetic response to clopidogrel and prasugrel: a retrospective analysis of the FEATHER study. Thromb Res 2014; 134:552–557. doi: 10.1016/j.thromres.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Jiang XL, Samant S, Lewis JP, Horenstein RB, Shuldiner AR, Yerges-Armstrong LM, et al. Development of a physiology-directed population pharmacokinetic and pharmacodynamic model for characterizing the impact of genetic and demographic factors on clopidogrel response in healthy adults. Eur J Pharm Sci 2016; 82:64–78. doi: 10.1016/j.ejps.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner H, Angiolillo DJ, Ten Berg JM, Bergmeijer TO, Jakubowski JA, Small DS, et al. Higher body weight patients on clopidogrel maintenance therapy have lower active metabolite concentrations, lower levels of platelet inhibition, and higher rates of poor responders than low body weight patients. J Thromb Thrombolysis 2014; 38:127–136. doi: 10.1007/s11239-013-0987-8. [DOI] [PubMed] [Google Scholar]
- 49.Verdoia M, Pergolini P, Rolla R, Nardin M, Schaffer A, Barbieri L, et al. Advanced age and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. J Thromb Haemost 2016; 14:57–64. doi: 10.1111/jth.13177. [DOI] [PubMed] [Google Scholar]
- 50.Silvain J, Cayla G, Hulot JS, Finzi J, Kerneis M, O’Connor SA, et al. High on-thienopyridine platelet reactivity in elderly coronary patients: the SENIOR-PLATELET study. Eur Heart J 2012; 33:1241–1249. doi: 10.1093/eurheartj/ehr407. [DOI] [PubMed] [Google Scholar]
- 51.Bebia Z, Buch SC, Wilson JW, Frye RF, Romkes M, Cecchetti A, et al. Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther 2004; 76:618–627. doi: 10.1016/j.clpt.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet 2010; 376:1233–1243. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 53.Fefer P, Beigel R, Rosenberg N, Shechter M, Gannot S, Varon D, et al. Evaluation of platelet response to different clopidogrel dosing regimens in patients with acute coronary syndrome in clinical practice. Platelets 2015; 26:127–131. doi: 10.3109/09537104.2014.888410. [DOI] [PubMed] [Google Scholar]
- 54.Kim YG, Suh JW, Kang SH, Park JJ, Yoon CH, Cho YS, et al. Cigarette smoking does not enhance clopidogrel responsiveness after adjusting VerifyNow P2Y12 reaction unit for the influence of hemoglobin level. JACC Cardiovasc Interv 2016; 9:1680–1690. doi: 10.1016/j.jcin.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 55.Han Y, Lv HH, Liu X, Dong Q, Yang XL, Li SX, et al. Influence of genetic polymorphisms on clopidogrel response and clinical outcomes in patients with acute ischemic stroke CYP2C19 genotype on clopidogrel response. CNS Neurosci Ther 2015; 21:692–697. doi: 10.1111/cns.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J 2007; 154:221–231. doi: 10.1016/j.ahj.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Reny JL, Berdague P, Poncet A, Barazer I, Nolli S, Fabbro-Peray P, et al. Antiplatelet drug response status does not predict recurrent ischemic events in stable cardiovascular patients: results of the Antiplatelet Drug Resistances and Ischemic Events study. Circulation 2012; 125:3201–3210. doi: 10.1161/CIRCULATIONAHA.111.085464. [DOI] [PubMed] [Google Scholar]


