Abstract
Acetyl-CoA carboxylase (ACCase) is the key regulator of fatty acid biosynthesis. In most plants, ACCase exists in two locations (cytosol and plastids) and in two forms (homomeric and heteromeric). Heteromeric ACCase comprises four subunits, three of them (ACCA–C) are nuclear encoded (nr) and the fourth (ACCD) is usually plastid encoded. Homomeric ACCase is encoded by a single nr-gene (ACC). We investigated the ACCase gene evolution in gymnosperms by examining the transcriptomes of newly sequenced Gnetum ula, combined with 75 transcriptomes and 110 plastomes of other gymnosperms. AccD-coding sequences are elongated through the insertion of repetitive DNA in four out of five cupressophyte families (except Sciadopityaceae) and were functionally transferred to the nucleus of gnetophytes and Sciadopitys. We discovered that, among the three genera of gnetophytes, only Gnetum has two copies of nr-accD. Furthermore, using protoplast transient expression assays, we experimentally verified that the nr-accD precursor proteins in Gnetum and Sciadopitys can be delivered to the plastids. Of the two nr-accD copies of Gnetum, one dually targets plastids and mitochondria, whereas the other potentially targets plastoglobuli. The distinct transit peptides, gene architectures, and flanking sequences between the two Gnetum accDs suggest that they have independent origins. Our findings are the first account of two distinctly targeted nr-accDs of any green plants and the most comprehensive analyses of ACCase evolution in gymnosperms to date.
Keywords: accD, acetyl-CoA carboxylase (ACCase), fatty acid biosynthesis, plastid-to-nucleus gene transfer, plastid localization, evolution
Introduction
Fatty acid biosynthesis in plants begins with the conversion of acetyl-CoA into malonyl-CoA, catalyzed by acetyl-CoA carboxylase (ACCase). Malonyl-CoA is an essential substrate for fatty acid formation (see reviews by Brown et al. [2010] and Huerlimann and Heimann [2013]). There are generally two types of ACCases in plants: the multisubunit heteromeric ACCase (ACCA–D) in plastids and the single-polypeptide homomeric ACCase (ACC) in the cytosol (Sasaki and Nagano 2004). The plastid heteromeric ACCase is similar to the prokaryotic ACCase and believed to have originated from cyanobacteria, whereas the cytosol homomeric ACCase is the eukaryotic type ACCase (Nikolau et al. 2003). Heteromeric ACCase consists of four subunits: 1) the alpha-subunit of carboxyltransferase (α-CT; encoded by accA), 2) biotin-carboxyl carrier protein (BCCP; encoded by accB), 3) biotin-carboxylase (BC; encoded by accC), and 4) the beta-subunit of carboxyltransferase (β-CT; encoded by accD). Genes encoding the former three reside in the nuclei, whereas accD resides in the plastids of most plant species. Loss of plastid accD has been reported in some seed plants, including monocots: Poaceae (Konishi and Sasaki 1994) and Acorus (Goremykin et al. 2005); eudicots: Trifolium (Magee et al. 2010), some Silene species (Sloan et al. 2012), Campanulaceae (Rousseau-Gueutin et al. 2013), and a few species of Pelargonium (Röschenbleck et al. 2017); and gymnosperms: gnetophytes (Wu et al. 2009) and Sciadopitys (Hsu, Wu, and Chaw 2016; Li et al. 2016). Homomeric ACCase is encoded by a single gene, ACC (Nikolau et al. 2003).
Although both plastid and cytosol ACCases convert acetyl-CoA into malonyl-CoA, the two compartments produce distinct end products. Malonyl-CoA is mainly converted into free fatty acids in the plastids (Brown et al. 2010), whereas in the cytosol it is used to synthesize flavonoids, anthocyanins, very long-chain fatty acids, and for the malonylation of D-amino acids and ethylene precursors (Sasaki and Nagano 2004). Thus, both ACCases play vital roles in plants. The loss of either of these genes is lethal, as was demonstrated in Arabidopsis (Baud et al. 2003) and tobacco (Kode et al. 2005). The only exceptions were reported in grasses (Poaceae) and Silene noctiflora, in which heteromeric ACCase is absent (supplementary fig. S1, Supplementary Material online; Konishi et al. 1996; Rockenbach et al. 2016), but a copy of homomeric ACCase has acquired plastid-targeting transit peptides (TPs) and replaced the function of heteromeric ACCase in plastids (Gornicki et al. 1997; Podkowinski et al. 2003; Rockenbach et al. 2016). In other cases (supplementary fig. S1, Supplementary Material online), both heteromeric and homomeric ACCase coexist in the plastids of Arabidopsis (Babiychuk et al. 2011) and Geraniaceae (Park et al. 2017).
Little is known about ACCase evolution in gymnosperms. Extant gymnosperms encompass about 1,100 species in 83 genera and 12 families (Christenhusz and Byng 2016). They comprise five groups—ginkgo, cycads, gnetophytes, Pinaceae (conifers I), and cupressophytes (conifers II) (Chaw et al. 2000). The cupressophytes are made up of five families—Cupressaceae, Taxaceae, Sciadopityaceae, Araucariaceae, and Podocarpaceae (Gernandt et al. 2011).
To date, the only characterization of ACCase genes in gymnosperms is limited to the loss of plastid-encoded accD from gnetophytes (Wu et al. 2009) and Sciadopitys (Hsu, Wu, and Chaw 2016; Li et al. 2016). Whether the lost plastid accD has been transferred to the nuclear genomes of these two lineages has not been verified. As a previous study identified a partial ACCD transcript in Sciadopitys (Li et al. 2016), we hypothesized that its accD was transferred to the nucleus. However, no information is available for the gnetophytes; it is unknown whether they retained heteromeric ACCase or if it was replaced with homomeric ACCase (as in grasses). In addition, the plastid accD sequences of cupressophyte genera (Hirao et al. 2008; Yi et al. 2013; Li et al. 2018) and a Pinaceae genus, Tsuga (Sudianto et al. 2016), are much longer than their homologs from other gymnosperms. This elongation may have accelerated accD nucleotide substitution rates in both Tsuga (Sudianto et al. 2016) and cupressophytes (Li et al. 2018). Whether the accelerated rates of ACCD subunit in these taxa influence the other ACCase subunits remains uncertain.
Here, we report the characterization of genes and transcripts encoding heteromeric and homomeric ACCases in Gnetum ula and 75 additional gymnosperm transcriptomes sampled from public repository data. We also assess if both ACCase forms are present in gymnosperms. Furthermore, we investigate the evolutionary fate of accD across the five major groups of gymnosperms, including the accD that was lost from gnetophytes and Sciadopitys plastomes. Finally, we perform a protoplast transient expression assay to identify the localization target of nuclear-encoded accD from gnetophytes and Sciadopitys.
Materials and Methods
Isolation of Nucleic Acids and cDNA Synthesis
We collected G.ula (voucher Chaw 1569) and Sciadopitys verticillata (voucher Chaw 1496) from the living plants in the green house of Academia Sinica and Floriculture Experimenter Center, Taipei, respectively. Both specimens were deposited in the Herbarium of Academia Sinica, Taipei (HAST). Total RNA was extracted from both tissues following the protocol of Hsu, Wu, and Chaw (2016). First strand cDNA was synthesized using RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fischer Scientific, Waltham) using random hexamer primers.
Sequence Retrieval
Coding regions of the plastid accD gene, including three nonvascular plants and 116 species of gymnosperms, were collected from GenBank and transcriptome data (supplementary table S1, Supplementary Material online). For the 76 transcriptomes analyzed in this study, we downloaded 74 assembled gymnosperm transcriptomes from oneKP (Matasci et al. 2014) and NCBI TSA databases (https://www.ncbi.nlm.nih.gov/genbank/tsa/; Last accessed on 10 November 2018), including 54 cupressophytes, 11 Pinaceae, 4 gnetophytes, 4 cycads, and 1 ginkgo (supplementary table S2, Supplementary Material online). We de novo assembled the transcriptomes of the two remaining Gnetum species—G. parvifolium and G. ula. Gnetumparvifolium reads were obtained from NCBI SRA (SRX1133345).
RNA Sequencing, Transcriptome Assembly, and Identification of ACCase Transcripts
RNA-Seq of G. ula was sequenced using an Illumina HiSeq2500 platform, yielding ∼100 million paired-end reads (2× 100-bp length). Trimmomatic (Bolger et al. 2014) was used to remove low-quality reads and adapters from both the newly sequenced G. ula and retrieved G. parvifolium reads. Transcriptomes of both species were de novo assembled using SOAPdenovo-Trans 1.03 (Xie et al. 2014). TransDecoder 4.0.0 (Haas et al. 2013) was used to identify candidate coding regions. We used BlastP (E-value <1e−5) to identify the potential ACCase proteins from the gymnosperm transcriptome assemblies. Protein sequences of four heteromeric ACCase (accA, accB, accC, and accD) and two homologs of homomeric ACCase (ACC1 and ACC2) from Arabidopsis were used as queries.
Tandem Repeats and TP Identification
Tandem repeats (TRs) of the elongated ACCD in cupressophytes were identified using T-REKS (Jorda and Kajava 2009) with default parameters. TP sequences were predicted using a number of prediction tools, including TargetP (Emanuelsson et al. 2007), Predotar (Small et al. 2004), ProteinProwler (Hawkins and Bodén 2006), and LOCALIZER (Sperschneider et al. 2017).
Sequence Alignment and Phylogenetic Tree Reconstruction
ACCase protein sequences were aligned using MAFFT v7 (Katoh and Standley 2013) in Auto setting. Sequences with <50% total protein length and duplicated sequences with 100% similarity were removed from further analyses. ProtTest3 (Darriba et al. 2011) was used to find the best-fit model for phylogenetic tree reconstruction. Maximum likelihood (ML) trees were reconstructed using RAxML 8.2.10 (Stamatakis 2014) in the recommended JTTGAMMAI model with 1,000 bootstrap replications. Tajima’s relative rate tests were performed using MEGA 7 (Kumar et al. 2016).
Identification of ACCase Genes in Nuclear Genomes
BlastN was used to search for ACCase genes in the nuclear genomes of Ginkgo biloba (Guan et al. 2016; http://gigadb.org/dataset/100209; Last accessed on 3 January 2018), Pinus taeda (Zimin et al. 2017; http://pinegenome.org/pinerefseq; Last accessed on 4 January 2018), and draft G. ula using their respective ACCase transcripts as the queries. Splign (Kapustin et al. 2008) and Easyfig (Sullivan et al. 2011) were used to identify exon–intron boundaries and illustrate the gene structures, respectively.
Protoplast Transient Expression of nr-ACCDs TPs
The putative TP sequences from G. ula (both nr-accD1 and nr-accD2) and Sciadopitys were amplified from cDNA of respective species with specific primers (see supplementary table S3, Supplementary Material online, for primers used). Amplified fragments were each cloned into the p326-GFP vector at XbaI and BamHI restriction sites. These constructs were transfected into Arabidopsis protoplasts following polyethylene glycol-mediated transformation described by Lee and Hwang (2011). In brief, Arabidopsis protoplasts were isolated from the leaves of 3-week-old plants as previously described (Wu et al. 2009) and then transformed with 10–20 µg of plasmid DNA from three GFP constructs described above. Plasmid DNA from p326-GFP vector was also used as a control to specify cytosol localization. Protoplasts and plasmids were incubated in the dark for 16 h at room temperature. The transformed protoplast images were captured using a Zeiss LSM780 ELYRA PS1 confocal microscope system.
Molecular Dating and Estimation of Absolute Nucleotide Substitution Rates
We sampled 34 gymnosperm genera in which all 5 ACCase sequences were available from the plastome and transcriptome data. Both synonymous (dS) and nonsynonymous (dN) substitution rates of these ACCase subunits were estimated using PAMLX (Xu and Yang 2013). We used the program CODEML with the following parameters: runmode = 0, seqtype = 1, CodonFreq = 2, estFreq = 0, model = 1, and cleandata = 1. We concatenated the accA–C sequences to calculate the dS and dN of nr-heteromeric ACCase. The constraint tree topology (supplementary fig. S2, Supplementary Material online) was reconstructed using 29 plastid-encoded photosynthetic genes. The relative divergence times were estimated using RelTime (Tamura et al. 2012) in MEGA 7.0. Seven estimated points from TimeTree (Kumar et al. 2017) were used as the calibration points (supplementary fig. S3, Supplementary Material online). The absolute synonymous (RS) and nonsynonymous substitution rates (RN) were derived by dividing the dN and dS branch lengths by their respective divergence times. We compared the absolute branch lengths of 1) accD versus ACC, 2) accD versus concatenated accA–C (nr-heteromeric ACCase), and 3) nr-heteromeric ACCase versus ACC to determine their correlations. Only terminal branches were considered in our analyses.
Results
Gymnosperms Have Both Heteromeric and Homomeric ACCases
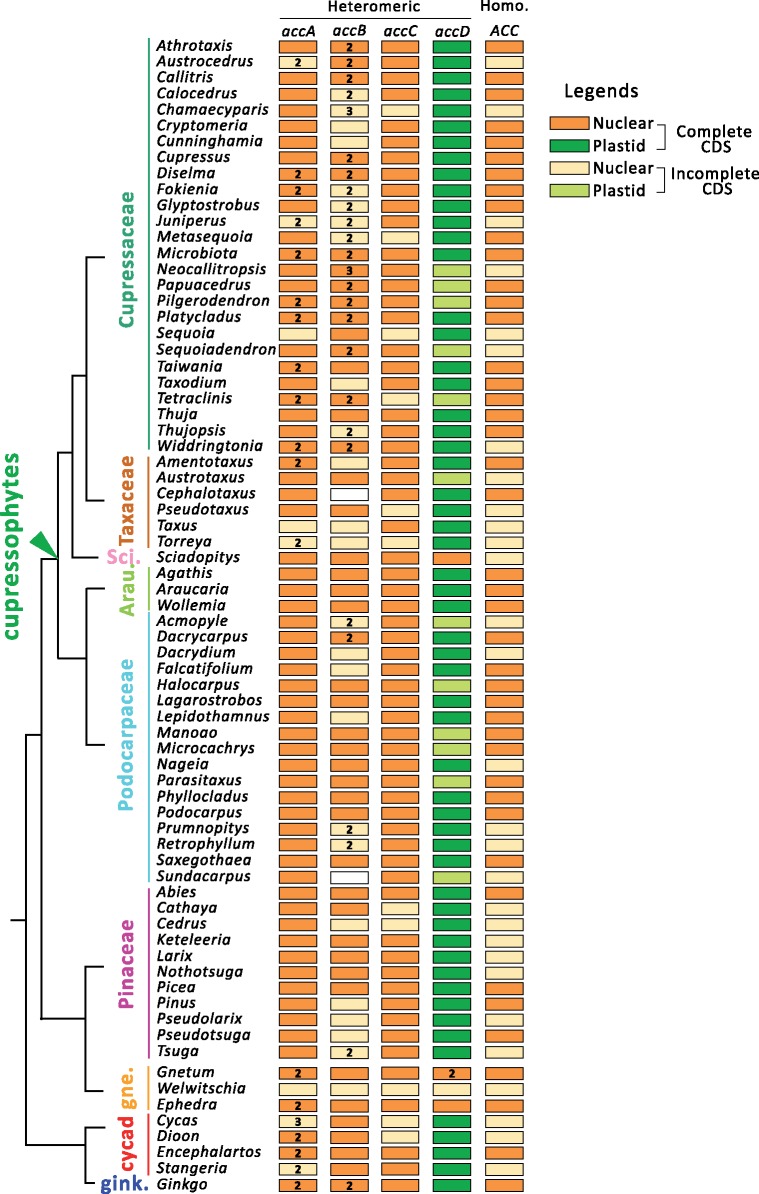
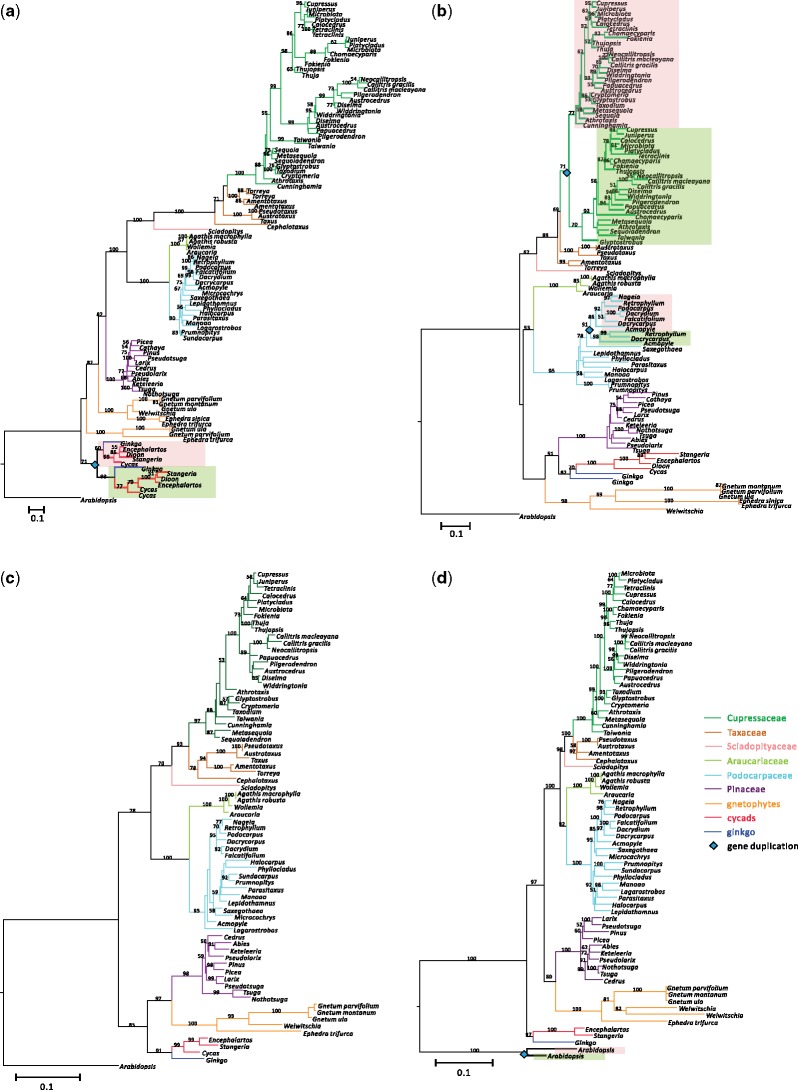
Transcripts encoding heteromeric and homomeric ACCases were identified in most gymnosperms. Transcripts of accA–D were present in all examined species, except Cephalotaxus and Sundacarpus, whose accB transcript was absent, possibly due to fragmented transcriptome assemblies (fig. 1 ). All accA, accB, and accC transcripts carried plastid-targeting TPs (supplementary table S4, Supplementary Material online). Two to three unique transcripts of accA and accB were detected in ginkgo, cycads, gnetophytes, and cupressophytes (fig. 1). In Cupressaceae, some duplicated accA and accB transcripts contained mitochondria-targeting TPs (supplementary table S4, Supplementary Material online). No duplicated accC transcript was detected, suggesting that there is only a single copy of accC in gymnosperms. We detected two copies of accD transcripts in Gnetum and one copy in Ephedra, Welwitschia, and Sciadopitys, though this gene is absent from their plastid genomes (plastomes). A single copy of homomeric ACCase (ACC) transcripts was present in all examined gymnosperms, and no gymnosperms had plastid-targeting TPs (supplementary table S4, Supplementary Material online).
Fig. 1.
—The gymnosperms contain both heteromeric and homomeric ACCase transcripts. The ACCase complex is differentiated into two groups: heteromeric (including accA, accB, accC, and accD) and homomeric (ACC). The color-filled boxes indicate the location and completeness of the transcript. The number inside the boxes indicates the presence of two or three transcript copies in some species. Phylogenetic relationships between the groups were derived from Li et al. (2017). Sci., Sciadopityaceae; gne., gnetophytes; and gink., ginkgo.
TR Insertions Influence ACCD Length in Gymnosperms
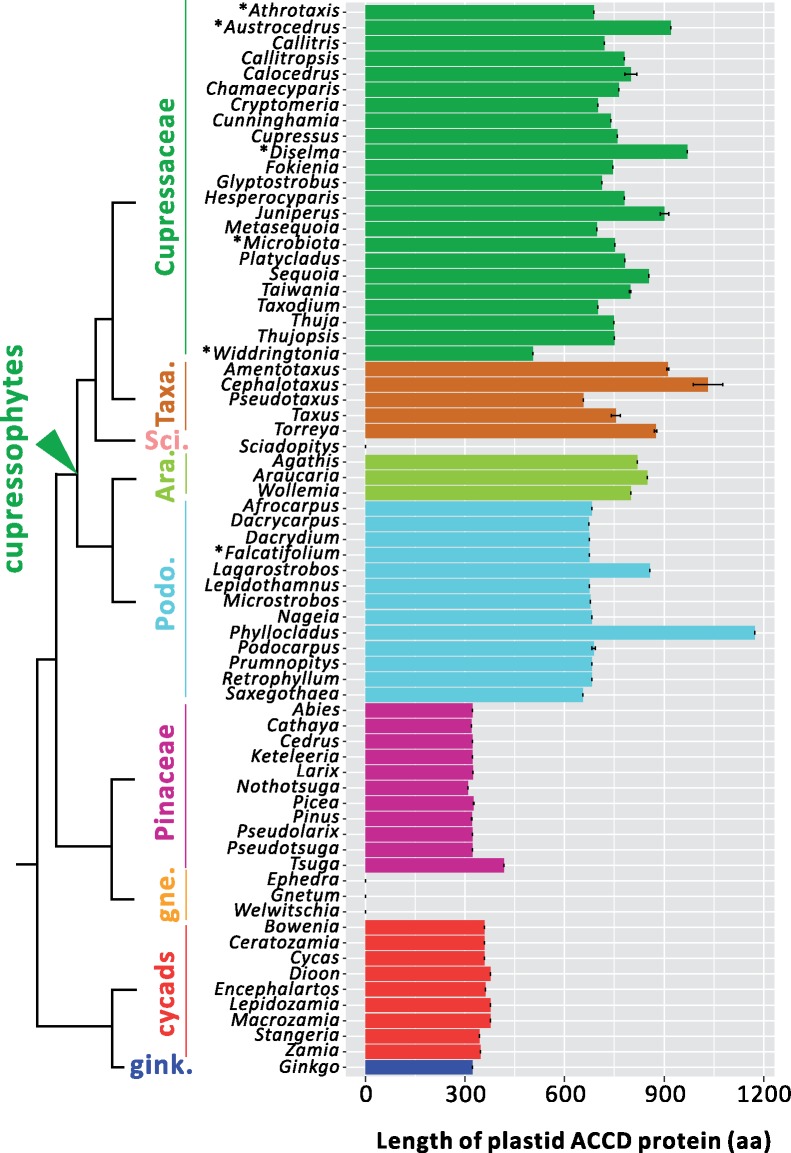
ACCDs in gymnosperms vary in length from 309 to 1,173 amino acids (aa) (fig. 2 and supplementary table S1, Supplementary Material online). Cupressophytes have significantly longer ACCDs than other gymnosperms (Mann–Whitney test, P < 0.001). In cupressophytes, Widdringtonia (504 aa) has the shortest ACCD sequence, whereas the ACCD sequence of Phyllocladus (1,173 aa) is ∼3–4-fold longer than the ACCD sequences of three other major groups: the Pinaceae, ginkgos, and cycads (ranging from 300 to 400 aa). Other Podocarpaceous genera have ACCD sequences 1.3–2-fold shorter than Phyllocladus’ (670–857 aa).
Fig. 2.
—Comparisons of plastid ACCD among the gymnosperms. The lengths of ACCD amino acid sequences were deduced from the plastid genes or transcriptomes of gymnosperms. Each group is coded by a specific color, as depicted in the legend. AccD is absent from the plastomes of Sciadopitys and gnetophytes. See supplementary table S2, Supplementary Material online, for a complete list of sampled species and their accession numbers. Asterisks (*) denote the sequences that were derived only from transcriptomes (i.e., no plastome available). Phylogenetic relationships between the groups were derived from Li et al. (2017). Taxa., Taxaceae; Sci., Sciadopityaceae; Ara., Araucariaceae; Podo., Podocarpaceae; gne., gnetophytes; and gink., ginkgo.
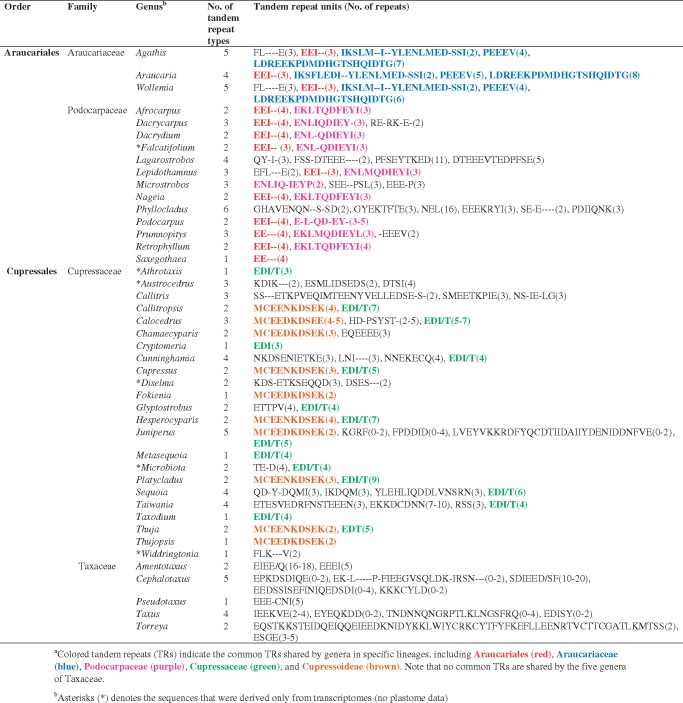
All sampled cupressophyte ACCDs bear insertions with little to no similarities to those of Pinaceae, ginkgo, and cycads (supplementary fig. S4, Supplementary Material online). These insertions contain various types of TRs that differ among closely related lineages. The number of TR types ranges from one to six for each cupressophyte genus. For example, 13 genera in the Araucariales order (including Araucariaceae and Podocarpaceae) share one TR type (see red font in table 1). Other than order-specific TRs, family-specific TRs were also identified in Araucariaceae (3 TRs, blue), Podocarpaceae (1 TR, purple), and Cupressaceae (1 TR, green). In addition, genera of the subfamily Cupressoideae share one subfamily-specific TR (brown). No common TR type was found in Taxaceae.
Table 1.
Tandem Repeat Units Identified in the Plastid accD Protein of 44 Sampled Cupressophytes Generaa
|
The Plastid accDs of Gnetophytes and Sciadopitys Were Transferred to the Nucleus and Gnetum Retains Two Copies of nr-ACCD
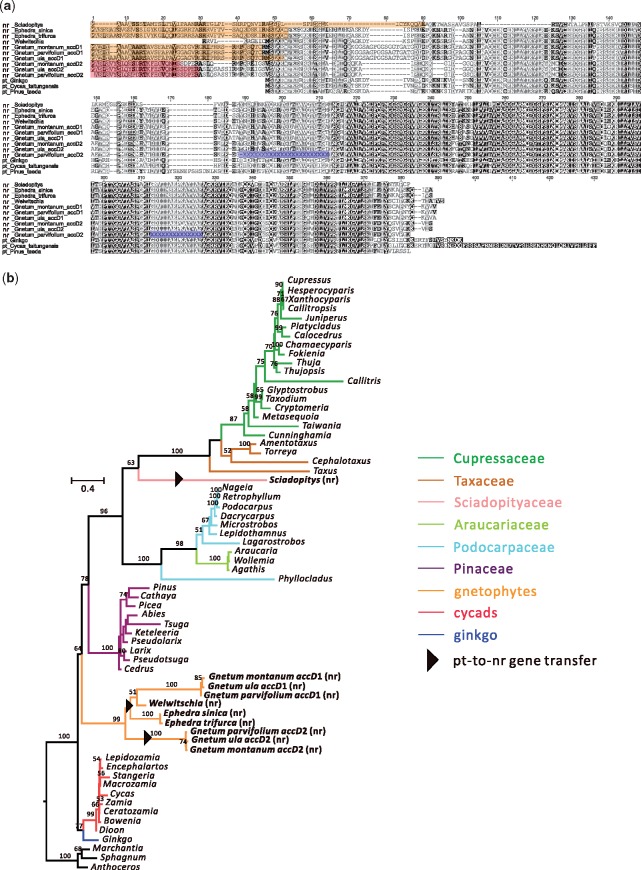
We detected ACCD transcripts in the transcriptome assemblies of gnetophytes and Sciadopitys, with predicted protein sequences ranging from 312 aa (Sciadopitys) to 370 aa (Gnetum) despite the absence of accD from their plastomes. This finding suggests that accD was transferred to the nuclear genomes of both lineages. The nuclear-encoded (nr) ACCD of Sciadopitys discovered in this study is 100 aa longer than previously reported by Li et al. (2016). We identified two homologous ACCD sequences in the three Gnetum transcriptomes (G. ula, G. montanum, and G. parvifolium; fig. 3). By contrast, the two Ephedra species sampled in this study (E. sinica and E. trifurca) and Welwitschia only contain one copy of ACCD (fig. 3). The nr-ACCD of both Sciadopitys and gnetophytes contain 24–51 aa upstream of the usual start codon (fig. 3a), which were predicted to be leader sequences that target plastids (supplementary table S5, Supplementary Material online). The nr-ACCD1 of Gnetum was also predicted to target mitochondria, albeit with lower scores. Because the identified nr-ACCD transcript of Welwitschia only contains partial TP, its localization target was not clearly predicted. TPs in gnetophytes were estimated to be 29 aa (nr-ACCD2 of Gnetum) to 50 aa (Ephedra) in length. Pairwise sequence identity of orthologous TPs is >90% among congeneric species. In contrast, the predicted TPs of nr-ACCD1 and nr-ACCD2 of Gnetum spp. share low sequence similarity (15.6–18.2%).
Fig. 3.
—Sequence comparisons among the ACCD of Sciadopitys, gnetophytes, and other gymnosperm representatives. (a) Identification of predicted nr-ACCD proteins from Sciadopitys and three genera of gnetophytes. The putative nr-ACCDs were aligned with the plastid (pt) ACCDs of three other gymnosperms: Ginkgo, Cycas, and Pinus. Orange shading denotes the TP sequences as predicted by LOCALIZER and TargetP. Red shading denotes the TPs predicted by TargetP only. The gaps in the Gnetum parvifolium transcriptome assembly are highlighted with blue shading. (b) ML tree of ACCD sequences. The nr-ACCD sequences of Sciadopitys and gnetophytes (bold) were aligned with the plastid ACCD sequences of other 53 sampled gymnosperm species. Bootstrapping supports for the node are shown when they are >50%. A black arrow indicates a plastid-to-nuclear accD gene transfer event. Marchantia, Sphagnum, and Anthoceros were designated as the outgroups.
A phylogenetic tree inferred from 66 ACCD sequences places the newly identified nr-ACCD of Sciadopitys within the cupressophytes (fig. 3b), consistent with the species tree. Using Tajima’s relative rate test, we found that the ACCD sequences of cupressophytes evolve faster than those of other gymnosperms except Gnetum (supplementary table S6, Supplementary Material online). The two nr-ACCD sequences from each of the three studied Gnetum species form a clade with nr-ACCD sequences of Ephedra and Welwitschia (fig. 3b). However, the two clades of Gnetum nr-ACCD were not sister to each other. The nr-ACCD2 clade of the three Gnetum species was placed as the outgroup to gnetophytes’ nr-ACCD1 clade (fig. 3b), suggesting that the common ancestor of gnetophytes had two nr-ACCDs.
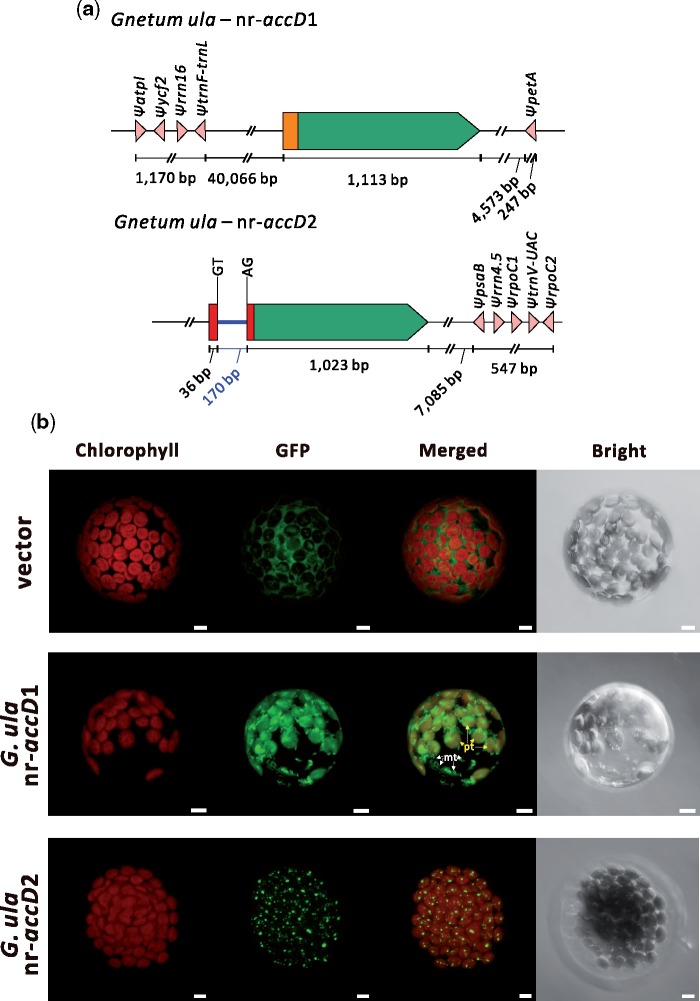
By mapping the two nr-ACCD transcripts of G. ula to its draft genome, we discovered that the gene architectures of nr-accD1 and nr-accD2 are distinct (fig. 4a). The former contains a single exon of 1,113 bp, whereas the latter contains two exons (36 and 1,023 bp) and one intron (170 bp) with a total length of 1,229 bp (fig. 4a). In the nr-accD1 scaffold, two short fragments (1,170 and 247 bp) reside at 40-kb upstream and 4.5-kb downstream of the coding region, respectively, were identified as pseudogenes of plastome origins. Similarly, in nr-accD2 scaffold, a 547-bp fragment at 7-kb downstream of the stop codon is composed of five plastid pseudogenes (fig. 4a). None of these nuclear plastid sequences (NUPTs) contain full-length and/or in-frame coding sequences. Despite their high sequence similarities to their corresponding genes in the plastome (>85% identity), these NUPTs (33–284 bp) are not syntenic with the G. ula plastome (Hsu, Wu, Surveswaran, et al. 2016), implying that they were rearranged either during transfer or subsequently (Hazkani-Covo and Martin 2017).
Fig. 4.
—Two nr-accD genes in Gnetum ula have distinct architectures and targeting sites. (a) The nr-accD gene architectures in the G. ula draft genome. The green box and blue line denote the exon and intron, respectively. Orange and red boxes correspond to the distinct TPs of the two nr-accDs in G. ula. Sequences with high similarity to G. ula plastid genes are designated as pink triangles. The intron donor and splice sites are labeled with GT and AG, respectively. Figures are not drawn to scale. (b) Transient expression assays using nr-accD TPs of G. ula indicate that the nr-accD1 dually targets plastids and putative mitochondria, but nr-accD2 likely targets plastoglobuli. Red and green signals in the first two columns represent chlorophyll autofluorescence and GFP, respectively. “Merged” column shows combined chlorophyll and GFP signals, whereas “bright” indicates bright field picture of the protoplasts. The yellow and white arrows in G. ula nr-accD1 merged column indicate plastids and mitochondria localization, respectively. Scale bar = 5 µm. Pt, plastid; mt, mitochondria.
Our first attempt to perform transient expression using the length of predicted TPs (52–60 aa) failed to detect the plastid localization in either Gnetum or Sciadopitys. By increasing the length of cloned TPs to >80 aa (extending further into the protein sequence), we concluded that the two accDs of Gnetum target distinct compartments. The TP of nr-ACCD1 dually targets plastid stroma and mitochondria, whereas TP of nr-ACCD2 potentially targets plastoglobuli, a microcompartment within the plastids (fig. 4b). Meanwhile, the nr-ACCD of Sciadopitys mainly targets cytosol with some putative plastid localization (supplementary fig. S5, Supplementary Material online). Overall, we experimentally demonstrated that the nr-ACCDs of gnetophytes and Sciadopitys have made up for the loss of accD from the plastomes of both lineages.
Nuclear Genes Encoding Two Heteromeric ACCase Subunits (accA and accB) Are Duplicated in Various Lineages of Gymnosperms
Figure 5a suggests accA duplication occurred in the common ancestor of ginkgo and cycads. Although some gnetophyte and cupressophyte genera have at least two copies of accA (figs. 1 and 5a), we could not infer the time of their duplications in the gene tree. In the accB phylogeny (fig. 5b), however, there are two likely scenarios of gene duplication at the nodes leading to some Podocarpaceous genera and Cupressaceae. We propose that independent duplications have taken place in Cupressaceae and Podocarpaceae, as most genera of the former and some of the latter family contain two accB copies (fig. 5b). By mapping the accA and accB transcripts of Ginkgo and Gnetum to their respective genomes, we confirmed that Ginkgo has two copies of both accA and accB, whereas Gnetum has two copies of accA that are located in different scaffolds (supplementary fig. S6, Supplementary Material online). In contrast, single copies of heteromeric accC and homomeric ACC were found in 76 sampled species (fig. 5c and d). The number of exons in each gene (accA–C and ACC) is identical in Ginkgo, Gnetum, and Pinus, except for accC. The accC in Ginkgo has one more exon than in Gnetum and Pinus (supplementary fig. S6, Supplementary Material online). However, intron lengths are highly variable between species, ranging from 75 bp to >300 kb with Gnetum having shorter introns. The accA1 of Ginkgo and the accB of Pinus contain extremely long introns of 59,205 and 311,403 bp, respectively (supplementary fig. S6, Supplementary Material online). The extremely long intron in the accB of Pinus is close to the longest intron (318,524 bp) ever identified in Pinus taeda, the genome of which contains 108 introns with length of >100 kb (Wegrzyn et al. 2014).
Fig. 5.
—Phylogeny-based scenario of nr-ACCase gene duplications in gymnosperms. ML trees of the four nr-ACCase proteins in gymnosperms. Numbers on the branches are bootstrap values (only >50% are shown). A blue diamond at the nodes indicates where a gene duplication event has occurred. The two duplicated gene copies are highlighted with pink and olive color shadings. The gene trees of heteromeric ACCase gene complex include (a) accA, (b) accB, and (c) accC. The homomeric ACCase (ACC) gene tree is depicted in (d).
Nucleotide Substitution Rates of Plastid accD and nr-Heteromeric ACCase Are Not Coelevated
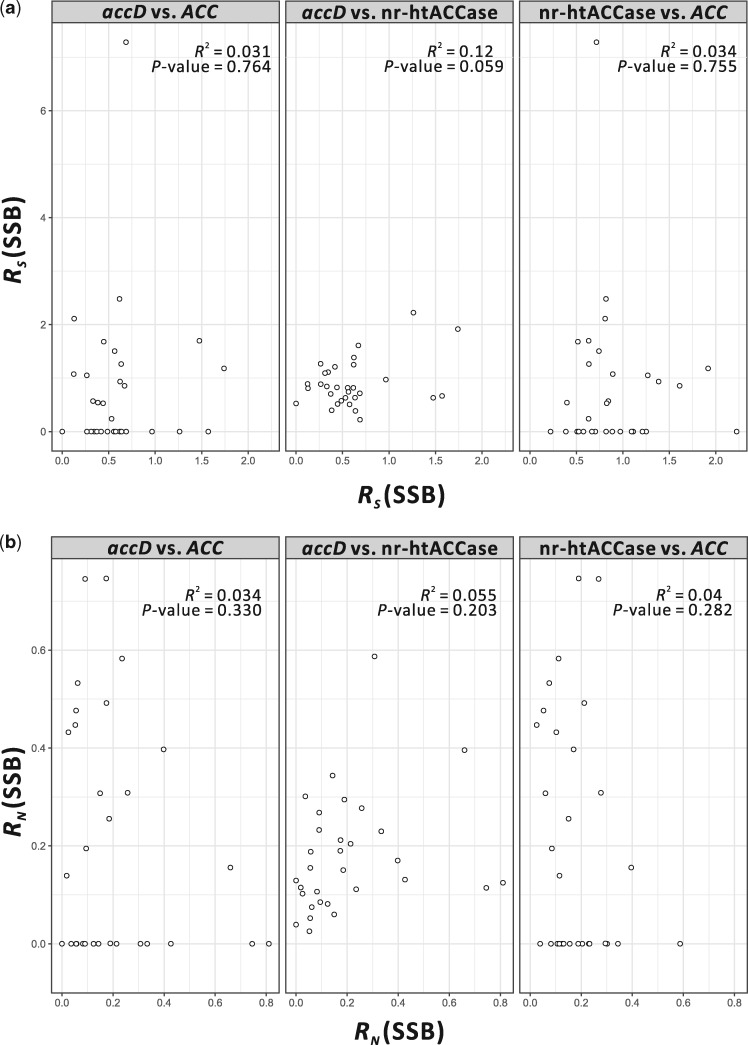
We calculated the nucleotide substitution rates of ACCase genes in the five major gymnosperm groups (cycads, ginkgo, pines, cupressophytes, and gnetophytes). Gnetophytes and Sciadopitys were excluded from our further analyses as their plastid accD genes were transferred to the nucleus and their nr-accDs have much higher substitution rates than others (supplementary fig. S7, Supplementary Material online). We only found weak correlation (R2 = 0.12, P = 0.059) between the RS of plastid accD and the transcripts encoding nr-heteromeric ACCase, but not RN (R2 = 0.055, P = 0.203) (fig. 6). No significant correlation was observed between plastid accD (or nr-heteromeric ACCase) and ACC at both RS (fig. 6a) and RN sites (fig. 6b). These results indicate that in gymnosperms, mutations in the plastid accD sequences have little to no effect on their nr-heteromeric ACCase.
Fig. 6.
—Comparison of absolute nucleotide substitution rates between accD, nr-htACCase, and ACC of gymnosperms, except gnetophytes and Sciadopitys. Scatterplot and regression analyses of accD versus ACC, accD versus nr-htACCase, and nr-htACCase versus ACC for (a) absolute synonymous rates (RS) and (b) absolute nonsynonymous rates (RN), respectively. The points in each plot represent the 31 gymnosperm genera included in this analysis. Nr-htACCase, nuclear-encoded heteromeric ACCase; SSB, substitutions per site per billion years.
Discussion
No Homomeric ACCase Was Found in the Plastids of Any Gymnosperms
Previous studies indicate that the plastids of some angiosperms contain homomeric ACCase, either from substitutions or coexisting with the heteromeric ACCase. For instance, grasses (Poaceae) and Silene noctiflora have completely lost heteromeric ACCase from their plastids (supplementary fig. S1, Supplementary Material online; Konishi et al. 1996; Rockenbach et al. 2016). Meanwhile, in Brassicaceae and Geraniaceae, both ACCase forms coexist in the plastids (supplementary fig. S1, Supplementary Material online; Rousseau-Gueutin et al. 2013; Park et al. 2017). Similarly, in some algal groups, such as Prasinophyceae (green algae), haptophytes, and heterokonts (red algae), the plastid heteromeric ACCase is replaced by plastid-targeted homomeric ACCase (Huerlimann and Heimann 2013; Huerlimann et al. 2015). AccD is absent from the plastomes of chlamydomonadalean algae but present in their nuclear genomes (Smith et al. 2013; Smith and Lee 2014). The presence of homomeric ACCase in angiosperm plastids sometimes coincides with the loss of plastid accD (e.g., grasses) or accD elongation (e.g., Geraniaceae).
This study shows that the accDs of some gymnosperms have been lost (e.g., gnetophytes, Sciadopitys) or elongated (e.g., cupressophytes). However, transcripts encoding homomeric ACCase (ACC) are present as a single copy in all sampled gymnosperms (figs. 1 and 5d) and none of them possessed TPs (supplementary table S4, Supplementary Material online). We also demonstrate that nr-ACCDs of gnetophytes and Sciadopitys are transcribed (figs. 3 and 4). Prediction- and experimental-based assays (fig. 4b and supplementary fig. S5 and supplementary table S5, Supplementary Material online) confirmed that their encoded products can be targeted to plastids to compensate for the loss of plastid-encoded accD in both lineages. Thus, we found no evidence of homomeric ACCase replacing or coexisting with heteromeric ACCase in the plastids of any sampled gymnosperm.
Insertions of TR into Plastid accD Do Not Affect nr-Heteromeric ACCase Evolution
We verified that accDs are elongated by in-frame, lineage-specific TR insertions in four of the five cupressophyte families (excluding Sciadopityaceae) (table 1 and supplementary fig. S4, Supplementary Material online), and they are 2–4-fold longer than those of cycads, ginkgo, or pines (fig. 2). Different lineages have specific TRs (table 1) that likely arose in the four cupressophyte families independently. Insertion of lineage-specific TRs in the accD has been reported in a number of seed plants, including Capsicum annuum (Jo et al. 2011), Medicago truncatula (Gurdon and Maliga 2014), Tsuga chinensis (Sudianto et al. 2016), Geraniaceae (Park et al. 2017), and Passiflora (Rabah et al. 2019). Thus, elongation of accD appears to have occurred repeatedly during seed plant evolution and coincides with elevated nucleotide substitution rates. It has been hypothesized that repetitive elements in the inserted sequences promoted accD sequence variability (Li et al. 2018). Length polymorphism in the accD is likely the result of “replication slippage” as reported in the Oenothera plastomes (Massouh et al. 2016). Recent finding suggests that these length variations may account for the differences in competitiveness among the four plastid genotypes of Oenothera (Sobanski et al. 2019).
Although ACCD subunit has been known to directly interact with the heteromeric ACCA subunit (Sasaki and Nagano 2004), we did not detect significant evidence of coevolution between the plastid and nuclear genes (fig. 6). TR insertions that elongate the accD of cupressophytes (and Tsuga) mostly occur in the middle of the sequence (supplementary fig. S4, Supplementary Material online). However, catalytic sites of ACCD, which interact with the ACCA subunit, are located in the C-terminal region (Lee et al. 2004) and highly conserved among gymnosperms (supplementary fig. S4, Supplementary Material online). Thus, TR insertions do not affect plastid-nuclear interaction in the heteromeric ACCase of gymnosperms. A similar finding was previously reported in Silene species, where protein structural analyses indicate that large insertions in their ACCD subunit did not involve functionally important residues in protein–protein interactions (Rockenbach et al. 2016).
The Two nr-accDs of Gnetum Were the Product of Independent Transfers Rather than Gene Duplication
Plastid accD genes of gnetophytes and Sciadopitys have been transferred to the nucleus, as nr-accD transcripts from the two lineages along with their plastid-targeting TPs (fig. 3a) attest. Based on our dated tree (supplementary fig. S3, Supplementary Material online), the accD transfer in gnetophytes took place after its common ancestor split from Pinaceae (ca. 245 Ma) and before the diversification of three gnetophyte genera (ca. 145 Ma), and the transfer occurred less than 253 Ma in Sciadopitys. Gnetum has two copies of nr-accD (figs. 1, 3, and 4), which is so far unique among green plants. This was validated in the transcriptomes of three sampled Gnetum species (fig. 3) and the draft genome of G. ula (fig. 4). The presence of two nr-accDs in Gnetum could have been caused either by 1) two independent plastid-to-nucleus transfer events or 2) the duplication of nr-accD after transfer to the nuclear genome. The former scenario appears to be favored because the two nr-accDs are distinctive in their TPs (fig. 3a), gene architectures and flanking NUPTs (fig. 4a). We propose that, in the common ancestor of gnetophytes, two copies of accD were independently transferred from the plastid to the nucleus. However, we could not detect any nr-ACCD2 from the transcriptomes of Ephedra and Welwitschia (fig. 3). This absence may be due to the loss of nr-accD2 gene from both genera or, alternatively, the lack of nr-accD2 gene expression in the isolated RNA. The nuclear genomes of Ephedra and Welwitschia need to be sequenced in order to confirm if the nr-accD2 was indeed lost from both genera.
The Two nr-ACCDs of Gnetum Target Different Sites
Using protoplast transient expression assays, we verified that the two nr-accDs of Gnetum are targeted to different subplastidic structures (fig. 4b). The nr-accD1 TP directed GFP to the plastid stroma (and to a lesser extent to mitochondria), whereas nr-accD2 TP likely targeted GFP to the plastoglobuli. The speckled pattern we observed in the nr-accD2 construct (fig. 4b) closely resembles the phytoene synthases (PSYs) of maize and rice that are delivered to the plastoglobuli (Shumskaya et al. 2012). The distinct targeting of the two nr-accD genes potentially suggests neo- and sub-functionalization of nr-ACCD1 and nr-ACCD2, respectively.
We were surprised to observe that nr-accD1 of Gnetum likely targets the mitochondria. Regulation of ACCase in plant mitochondria is less well characterized than its counterparts in plastids or cytosols. ACCase is reportedly absent from eudicot mitochondria, and their malonyl-CoA is synthesized using alternative pathways (Gueguen et al. 2000). However, in grasses, malonyl-CoA is generated by a homomeric ACCase that dually targets plastids and mitochondria (Focke et al. 2003). To date, little is known about ACCase in the gymnosperm mitochondria, especially whether the gymnosperm mitochondria produce malonyl-CoA via ACCase (as in grasses) or malonyl-CoA synthetase (as in eudicots) remains unclear. Nr-accD1 function in the mitochondria of Gnetum requires further investigation, as none of the other heteromeric ACCase subunits (accA–C) were predicted to be localized to mitochondria (supplementary table S4, Supplementary Material online). Mitochondrial localization might also just be a case of evolutionary noise in subcellular targeting evolution (Martin 2010).
Similarly, it is unusual that nr-accD2 targets putative plastoglobuli. Plastoglobuli are plastid lipid microcompartments that aid in plastid metabolism (reviewed in Van Wijk and Kessler [2017]). A number of nr-proteins have been reported to target plastoglobuli (Shumskaya et al. 2012; Delfosse et al. 2015). Although plastoglobuli play important roles in lipid storage and metabolism (Bréhélin and Kessler 2008), no ACCase metabolic pathway has been elucidated in the microcompartment. The only fatty acid–related enzyme identified in plastoglobuli so far is phytol ester synthase (PES; Van Wijk and Kessler 2017).
In summary, our study sheds light on the ACCase complex and its evolutionary history in seed plants. We found little evidence for coevolution between accD and its counterparts in heteromeric ACCase and the possible effects of TR insertions on its enzymatic function remain elusive. To date, we still rely on the bacterial ACCase structure to interpret the function and interaction of plants’ heteromeric ACCase subunits. Elucidation of the plant-specific heteromeric ACCase structure will be critical to decipher the plastid-nuclear subunit interactions in heteromeric ACCase. Moreover, further studies on the functions of the two accDs in Gnetum will be necessary to identify why the genus has two copies of the gene.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
We thank the Plant Tech Core Facility and Advanced Optical Microscope Core Facility of Agricultural Biotechnology Research Center, Academia Sinica, for supplying Arabidopsis plants and assisting with the laser confocal scanning microscope, respectively. We also thank oneKP for making the data publicly available. We are grateful to Dr Chung-Shien Wu and Dr William Martin for their critical reading and comments on our draft. We would like to thank the two anonymous reviewers and Dr Susanne S. Renner for their suggestions and valuable comments, which greatly improve the first version of manuscript. We are indebted to Dr Celicia Koo Botanic Garden for providing fresh leaves of several rare gymnsoperms. This work was supported by research grants from the Ministry of Science and Technology Taiwan (MOST 103-2621-B-001-007-MY3 to S.-M.C.); Biodiversity Research Center’s PI grant (from 2016 to 2018 to S.-M.C.); Central Academic Advisory Committee of Academia Sinica (to S.-M.C.); and the Taiwan International Graduate Program Student Fellowship (to E.S.).
Data deposition: The newly identified acetyl-CoA carboxylase genes of Gnetum ula were deposited at DDBJ under the accession numbers LC425655–LC425656 and LC428529–LC428533.
Literature Cited
- Babiychuk E, et al. 2011. Plastid gene expression and plant development require a plastidic protein of the mitochondrial transcription termination factor family. Proc Natl Acad Sci U S A. 108(16):6674–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, et al. 2003. Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J. 33(1):75–86. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30(15): 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréhélin C, Kessler F.. 2008. The plastoglobule: a bag full of lipid biochemistry tricks. Photochem Photobiol. 84(6):1388–1394. [DOI] [PubMed] [Google Scholar]
- Brown AP, Slabas AR, Rafferty JB.. 2010. Fatty acid biosynthesis in plants—metabolic pathways, structure and organization In: Wada H, Murata N, editors. Advances in photosynthesis and respiration. Lipids in photosynthesis. Dordrecht (the Netherlands: ): Springer; p. 11–34. [Google Scholar]
- Chaw SM, Parkinson CL, Cheng Y, Vincent TM, Palmer JD.. 2000. Seed plant phylogeny inferred from all three plant genomes: monophyly of extant gymnosperms and origin of Gnetales from conifers. Proc Natl Acad Sci U S A. 97(8):4086–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenhusz MJM, Byng JW.. 2016. The number of known plants species in the world and its annual increase. Phytotaxa 261(3):201. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D.. 2011. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27(8):1164–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfosse K, et al. 2015. Fluorescent protein aided insights on plastids and their extensions: a critical appraisal. Front Plant Sci. 6:1253.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H.. 2007. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2(4):953–971. [DOI] [PubMed] [Google Scholar]
- Focke M, et al. 2003. Fatty acid biosynthesis in mitochondria of grasses: malonyl-coenzyme A is generated by a mitochondrial-localized acetyl-coenzyme A carboxylase. Plant Physiol. 133(2):875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernandt DS, Willyard A, Syring J, Liston A.. 2011. The conifers (Pinophyta) In: Kole C, editor. Genetics, genomics and breeding of conifers. Florida (US): CRC Press. p. 1–39. [Google Scholar]
- Goremykin VV, Holland B, Hirsch-Ernst KI, Hellwig FH.. 2005. Analysis of Acorus calamus chloroplast genome and its phylogenetic implications. Mol Biol Evol. 22(9):1813–1822. [DOI] [PubMed] [Google Scholar]
- Gornicki P, et al. 1997. Plastid-localized acetyl-CoA carboxylase of bread wheat is encoded by a single gene on each of the three ancestral chromosome sets. Proc Natl Acad Sci U S A. 94(25):14179–14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, et al. 2016. Draft genome of the living fossil Ginkgo biloba. GigaScience 5(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguen V, Macherel D, Jaquinod M, Douce R, Bourguignon J.. 2000. Fatty acid and lipoic acid biosynthesis in higher plant mitochondria. J Biol Chem. 275(7):5016–5025. [DOI] [PubMed] [Google Scholar]
- Gurdon C, Maliga P.. 2014. Two distinct plastid genome configurations and unprecedented intraspecies length variation in the accD coding region in Medicago truncatula. DNA Res. 21(4):417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, et al. 2013. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 8(8):1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J, Bodén M.. 2006. Detecting and sorting targeting peptides with neural networks and support vector machines. J Bioinf Comput Biol. 4(1):1–18. [DOI] [PubMed] [Google Scholar]
- Hazkani-Covo E, Martin WF.. 2017. Quantifying the number of independent organelle DNA insertions in genome evolution and human health. Genome Biol Evol. 9(5):1190–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao T, Watanabe A, Kurita M, Kondo T, Takata K.. 2008. Complete nucleotide sequence of the Cryptomeria japonica D. Don. chloroplast genome and comparative chloroplast genomics: diversified genomic structure of coniferous species. BMC Plant Biol. 8:70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-Y, Wu C-S, Chaw S-M.. 2016. Birth of four chimeric plastid gene clusters in Japanese umbrella pine. Genome Biol Evol. 8(6):1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-Y, Wu C-S, Surveswaran S, Chaw S-M.. 2016. The complete plastome sequence of Gnetum ula (Gnetales: Gnetaceae). Mitochondrial DNA A DNA Mapp Seq Anal. 27(5):3721–3722. [DOI] [PubMed] [Google Scholar]
- Huerlimann R, Heimann K.. 2013. Comprehensive guide to acetyl-carboxylases in algae. Crit Rev Biotechnol. 33(1):49–65. [DOI] [PubMed] [Google Scholar]
- Huerlimann R, Zenger KR, Jerry DR, Heimann K.. 2015. Phylogenetic analysis of nucleus-encoded acetyl-CoA carboxylases targeted at the cytosol and plastid of algae. PLoS One 10(7):e0131099.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YD, et al. 2011. Complete sequencing and comparative analyses of the pepper (Capsicum annuum L.) plastome revealed high frequency of tandem repeats and large insertion/deletions on pepper plastome. Plant Cell Rep. 30(2):217–229. [DOI] [PubMed] [Google Scholar]
- Jorda J, Kajava AV.. 2009. T-REKS: identification of Tandem REpeats in sequences with a K-meanS based algorithm. Bioinformatics 25(20):2632–2638. [DOI] [PubMed] [Google Scholar]
- Kapustin Y, Souvorov A, Tatusova T, Lipman D.. 2008. Splign: algorithms for computing spliced alignments with identification of paralogs. Biol Direct 3:20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kode V, Mudd EA, Iamtham S, Day A.. 2005. The tobacco plastid accD gene is essential and is required for leaf development. Plant J Cell Mol Biol. 44(2):237–244. [DOI] [PubMed] [Google Scholar]
- Konishi T, Sasaki Y.. 1994. Compartmentalization of two forms of acetyl-CoA carboxylase in plants and the origin of their tolerance toward herbicides. Proc Natl Acad Sci U S A. 91(9):3598–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T, Shinohara K, Yamada K, Sasaki Y.. 1996. Acetyl-CoA carboxylase in higher plants: most plants other than Gramineae have both the prokaryotic and the eukaryotic forms of this enzyme. Plant Cell Physiol. 37(2):117–122. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Suleski M, Paymer M, Hedges SB.. 2017. TimeTree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol. 34(7):1812–1819. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K.. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7 for bigger datasets. Mol Biol Evol. 33(7):1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Hwang I.. 2011. Transient expression and analysis of chloroplast proteins in Arabidopsis protoplasts. Methods Mol Biol. 774:59–71. [DOI] [PubMed] [Google Scholar]
- Lee SS, et al. 2004. Characterization of the plastid-encoded carboxyltransferase subunit (accD) gene of potato. Mol Cells 17(3):422–429. [PubMed] [Google Scholar]
- Li J, Su Y, Wang T.. 2018. The repeat sequences and elevated substitution rates of the chloroplast accD gene in cupressophytes. Front Plant Sci. 9:533.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. 2016. Evolution of short inverted repeat in cupressophytes, transfer of accD to nucleus in Sciadopitys verticillata and phylogenetic position of Sciadopityaceae. Sci Rep. 6:20934.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. 2017. Single-copy genes as molecular markers for phylogenomic studies in seed plants. Genome Biol Evol. 9(5):1130–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee AM, et al. 2010. Localized hypermutation and associated gene losses in legume chloroplast genomes. Genome Res. 20(12):1700–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WF. 2010. Evolutionary origins of metabolic compartmentalization in eukaryotes. Philos Trans R Soc B Biol Sci. 365(1541):847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massouh A, et al. 2016. Spontaneous chloroplast mutants mostly occur by replication slippage and show a biased pattern in the plastome of Oenothera. Plant Cell 28(4):911–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matasci N, et al. 2014. Data access for the 1,000 plants (1KP) project. GigaScience 3:17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolau BJ, Ohlrogge JB, Wurtele ES.. 2003. Plant biotin-containing carboxylases. Arch Biochem Biophys. 414(2):211–222. [DOI] [PubMed] [Google Scholar]
- Park S, et al. 2017. Contrasting patterns of nucleotide substitution rates provide insight into dynamic evolution of plastid and mitochondrial genomes of Geranium. Genome Biol Evol. 9(6):1766–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podkowinski J, et al. 2003. Expression of cytosolic and plastid acetyl-coenzyme A carboxylase genes in young wheat plants. Plant Physiol. 131(2):763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabah SO, et al. 2019. Passiflora plastome sequencing reveals widespread genomic rearrangements. J Syst Evol. 57(1):1–14. [Google Scholar]
- Rockenbach K, et al. 2016. Positive selection in rapidly evolving plastid-nuclear enzyme complexes. Genetics 204(4):1507–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röschenbleck J, Wicke S, Weinl S, Kudla J, Müller KF.. 2017. Genus-wide screening reveals four distinct types of structural plastid genome organization in Pelargonium (Geraniaceae). Genome Biol Evol. 9(1):64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau-Gueutin M, et al. 2013. Potential functional replacement of the plastidic acetyl-CoA carboxylase subunit (accD) gene by recent transfers to the nucleus in some angiosperm lineages. Plant Physiol. 161(4):1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Nagano Y.. 2004. Plant acetyl-CoA carboxylase: structure, biosynthesis, regulation, and gene manipulation for plant breeding. Biosci Biotechnol Biochem. 68(6):1175–1184. [DOI] [PubMed] [Google Scholar]
- Shumskaya M, Bradbury LMT, Monaco RR, Wurtzel ET.. 2012. Plastid localization of the key carotenoid enzyme phytoene synthase is altered by isozyme, allelic variation, and activity. Plant Cell 24(9):3725–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Alverson AJ, Wu M, Palmer JD, Taylor DR.. 2012. Recent acceleration of plastid sequence and structural evolution coincides with extreme mitochondrial divergence in the angiosperm genus Silene. Genome Biol Evol. 4(3):294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I, Peeters N, Legeai F, Lurin C.. 2004. Predotar: a tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4(6):1581–1590. [DOI] [PubMed] [Google Scholar]
- Smith DR, Lee RW.. 2014. A plastid without a genome: evidence from the nonphotosynthetic green algal genus Polytomella. Plant Physiol. 164(4):1812–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, et al. 2013. Organelle genome complexity scales positively with organism size in volvocine green algae. Mol Biol Evol. 30(4):793–797. [DOI] [PubMed] [Google Scholar]
- Sobanski J, et al. 2019. Chloroplast competition is controlled by lipid biosynthesis in evening primroses. Proc Natl Acad Sci U S A 116(12): 5665–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperschneider J, et al. 2017. LOCALIZER: subcellular localization prediction of both plant and effector proteins in the plant cell. Sci Rep. 7:44598.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9):1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudianto E, Wu C-S, Lin C-P, Chaw S-M.. 2016. Revisiting the plastid phylogenomics of Pinaceae with two complete plastomes of Pseudolarix and Tsuga. Genome Biol Evol. 8(6):1804–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJ, Petty NK, Beatson SA.. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27(7):1009–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, et al. 2012. Estimating divergence times in large molecular phylogenies. Proc Natl Acad Sci U S A. 109(47):19333–19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijk KJ, Kessler F.. 2017. Plastoglobuli: plastid microcompartments with integrated functions in metabolism, plastid developmental transitions, and environmental adaptation. Annu Rev Plant Biol. 68:253–289. [DOI] [PubMed] [Google Scholar]
- Wegrzyn JL, et al. 2014. Unique features of the loblolly pine (Pinus taeda L.) megagenome revealed through sequence annotation. Genetics 196(3):891–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-S, Lai Y-T, Lin C-P, Wang Y-N, Chaw S-M.. 2009. Evolution of reduced and compact chloroplast genomes (cpDNAs) in gnetophytes: selection toward a lower-cost strategy. Mol Phylogenet Evol. 52(1):115–124. [DOI] [PubMed] [Google Scholar]
- Wu F-H, et al. 2009. Tape-Arabidopsis sandwich—a simpler Arabidopsis protoplast isolation method. Plant Methods. 5:16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, et al. 2014. SOAPdenovo-Trans: de novo transcriptome assembly with short RNA-Seq reads. Bioinformatics 30(12):1660–1666. [DOI] [PubMed] [Google Scholar]
- Xu B, Yang Z.. 2013. PAMLX: a graphical user interface for PAML. Mol Biol Evol. 30(12):2723–2724. [DOI] [PubMed] [Google Scholar]
- Yi X, Gao L, Wang B, Su Y-J, Wang T.. 2013. The complete chloroplast genome sequence of Cephalotaxus oliveri (Cephalotaxaceae): evolutionary comparison of Cephalotaxus chloroplast DNAs and insights into the loss of inverted repeat copies in gymnosperms. Genome Biol Evol. 5(4):688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin AV, et al. 2017. An improved assembly of the loblolly pine mega-genome using long-read single-molecule sequencing. GigaScience 6:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.