Abstract
Background
Anastomotic biliary strictures (ABS) following liver transplantation (LT) are one of the most common complications, occurring in 4.5-32% of patients. Multiple plastic stenting (MPS) requires multiple sessions, with the associated risk, cost and patient discomfort. Fully covered self-expandable metal stents (FC-SEMS) have increasingly been used in this setting. We performed a systematic review and meta-analysis of randomized controlled trials (RCTs), comparing the role of FC-SEMS and MPS in the treatment of ABS post-LT.
Methods
We conducted a bibliographic search using PubMed and EMBASE, aimed at identifying available RCTs that compared MPS to FC-SEMS in patients with ABS post LT from January 2000 to October 2017. Primary outcomes were ABS resolution and recurrence, while secondary outcomes were adverse events and number of procedures performed. Pooled estimates were calculated using random-effects models.
Results
Four RCTs (205 patients) were included. ABS resolution and recurrence did not differ significantly between the groups (odds ratio [OR] 1.05, 95% confidence interval [CI] 0.43-2.56, P=0.92; and OR 2.37, 95%CI 0.54-10.38, P=0.25). The same was true for adverse events (OR 0.91, 95%CI 0.84-3.48, P=0.86) and migration rate (OR 1.31, 95%CI 0.46-3.71, P=0.61). The mean number of endoscopic retrograde cholangiopancreatography procedures was lower for FC-SEMS (mean difference [MD] -2.08).
Conclusions
FC-SEMS and MPS had equal ABS resolution and recurrence, although there was a trend towards a higher recurrence rate in FC-SEMS that disappeared when trials with a shorter stent indwelling time were excluding. No difference was found in overall adverse events or migration rate.
Keywords: Randomized controlled trials, meta-analysis, biliary tract disease, stent, self-expandable metal stent, plastic stent, liver transplantation, anastomotic biliary stricture
Introduction
Anastomotic biliary strictures (ABS) are one of the most common complications following liver transplantation (LT), occurring in approximately 4.5-15% of cases after deceased donor LT and 8-32% after living donor-related LT (LDLT) [1-7]. ABS usually develop within the first year after transplant [1,8,9] and their formation is often due to surgical technical issues, a fibroproliferative response to local ischemia, or both [10].
Endoscopic treatment has become the standard of care for the management of ABS and several techniques have been described, including balloon dilation, multiple plastic stent (MPS) insertion and fully covered self-expandable metal stent (FC-SEMS) insertion. Balloon dilation as monotherapy has been abandoned because of its low success rate and high rate of ABS recurrence [11].
Currently, the standard of care is the placement of plastic stents with or without balloon dilation, with variable timing of subsequent endoscopic retrograde cholangiopancreatography (ERCP); this approach has proven to be effective and safe, with a low rate of ABS recurrence [12-15]. In order to minimize the issues associated with the placement of multiple plastic stents, such as stent occlusion, suboptimal long-term efficacy and the need for multiple endoscopic sessions, several studies have assessed the validity of single FC-SEMS as an alternative to MPS, with heterogeneous results [16-19].
A previous randomized controlled trial (RCT) comparing different types of FC-SEMS showed a clinical success rate of 70%, which was below the reported success rate for MPS [20]. This data was confirmed by 3 previous meta-analyses [15,21,22], comparing the efficacy and safety of FC-SEMS vs. MPS in patients with ABS post-LT and LDLT. These meta-analysis, including only observational studies with low quality, showed that, although FC-SEMS was a promising option in this setting, there was no clear advantage of FC-SEMS over MPS. Recently, 2 new RCTs have been published comparing the efficacy and safety of FC-SEMS vs. MPS [23,24].
The aim of this study was to perform an updated systematic review of the current literature comparing the safety and efficacy of FC-SEMS vs. MPS insertion in the management of post-LT ABS.
Patients and methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [25]. The methods of analysis and inclusion criteria were specified in advance and documented in a protocol according to the Cochrane guidelines. A PRISMA checklist is provided in the supplementary materials (S1).
Search strategy
Studies were identified by searching, with the assistance of a research librarian, in PubMed, EMBASE, Google Scholar and the Cochrane Library. The following MESH and keyword search terms were used: “liver transplantation”, “anastomotic biliary stricture”, “biliary strictures”, “self-expandable metal stents”, “plastic stents”. Any duplicate citation was removed.
Inclusion criteria
Types of studies: RCTs comparing the efficacy and safety of fully covered SEMS vs. MPS.
Types of participants: patients older than 18 years old with ABS following orthotopic LT without previous stent placement.
Types of interventions: MPS vs. FC-SEMS placement.
Types of outcome measures: ABS resolution and recurrence, cause of stent dysfunction and adverse events.
Exclusion criteria
Non-randomized studies, non-anastomotic strictures, and other benign biliary strictures were excluded from the meta-analysis. The keywords “biliary tract disease”, “biliary obstruction”, “biliary stricture”, “anastomotic stricture”, “liver transplantation”, “randomized controlled trial”, “meta-analysis”, “endoprosthesis”, “metal stent”, and “systematic review” were associated in different combinations using the Boolean terms AND/OR. Queries were limited to those involving human subjects. Manual searches of reference lists of relevant literature reviews were used to complement the computer searches. A search strategy is provided in the Supplementary Material (S2). Each article was read and analyzed by at least 2 members of the research team (AT & MM) and eligibility assessment was performed independently in a non-blinded standardized manner.
Definitions
Only one trial [24] reported definitions of measured outcomes that would allow an objective assessment of the results. ABS was defined as a dominant stricture at the anastomotic site without effective passage of contrast medium, as shown by cholangiographic imaging. ABS resolution was defined as cholangiographic resolution of stricture, assessed by easy passage of an 8.5 mm extraction balloon through the anastomosis site, and no need for further interventional procedure. ABS recurrence was defined as relapse characterized by the onset of new clinical symptoms and/or increase in cholestatic enzymes or total bilirubin with cholangiographic evidence of an ABS that requires a subsequent interventional procedure. Adverse events were defined as the occurrence of complications after the procedure and graded according to the Cotton criteria [26].
Data collection
Two investigators (AT & MC) extracted data from the eligible publications independently. The following data were retrieved into a standardized database:
-Descriptive data: first author, year and type of publication, country of origin, study setting, number of patients, age and sex of patients, reason for LT, time to stricture, treatment time, number of ERCPs, stent type and covering material, MPS protocols, length of follow up, adverse events, and procedural related costs.
-Qualitative data: random sequence generation, allocation concealment, blinding of participant and personnel, blinding outcome assessment, incomplete outcome data, selective reporting, and lost to follow up.
-Outcome data: For primary outcomes we extracted the odds ratio (OR) with 95% confidence interval (CI), when reported in the original publication, or we collected additional information in order to apply statistical methods to compute them. For secondary outcomes, we extracted the number of patients and events in each arm.
-Quality appraisal: Each included study was appraised for quality by 2 independent evaluators (AT & BG). Quality appraisal was performed using the risk-of-bias tool, as recommended by the Cochrane collaboration [27].
Outcome measures
The primary outcome measures were the rates of ABS resolution and recurrence. Secondary outcomes included overall adverse events, pancreatitis, cholangitis and bleeding rate, stent migration and the median number of procedures (ERCP). No RCTs reported cost-effectiveness analysis so that these data were not available for statistical analysis.
Statistical analysis
Dichotomous outcomes were evaluated in terms of ORs with their 95%CIs and summarized across studies through a random-effects model. If no between-study heterogeneity was evident, the pooled estimate from the random-effects model would be equal to the one derived from a fixed-effects model. Procedure time was analyzed using the Hedges’ Standardized Mean Difference estimator. When studies used median and range, results were converted to mean and standard deviation using the formula of Hozo et al [28]. The hazard ratios (HR) for time to achieve resolution and time to re-obstruction could not be obtained because they were not reported in the trials included in the analysis. Finally, we tried to derive the estimation of an indirect measure of the HRs from log-rank P-values or Kaplan-Meier curves, as previously reported [29], but it was only possible to extract the time to reobstruction for 2 trials [23,24] and the time to achieve ABS resolution for one trial [30], not enough to allow the calculation of relevant outcomes. We performed a cost-analysis converting data from one study from Australian to US dollars and calculating median, range and standard deviation comparing using the Student’s t-test. Between-studies heterogeneity was assessed using the Q test based on the chi-squared statistics, and inconsistency was quantified in terms of the I2 statistic [31]. In order to assess potential sources of heterogeneity, we firstly performed a sensitivity analysis by removing each study in turn (leave-one-out-method) to evaluate its influence on the final pooled estimate. Publication bias was assessed by visual inspection of funnel plots for asymmetry and through Egger’s test for asymmetry [32].
Data were synthesized using Review Manager software (version 5.1 for Windows, the Cochrane Collaboration, Oxford, UK). Finally, we graded the quality of evidence using Grade system software—GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.)—according to the GRADE recommendation [33].
Results
Study selection
Eight hundred fifty unique studies were identified through the systematic review of the literature. Following the screening of abstracts and titles, we identified 103 potentially eligible studies for which full-text reading was required. Finally, 4 studies [23,24,30,34] were included, as shown in the PRISMA flow diagram in Fig. 1.
Figure 1.
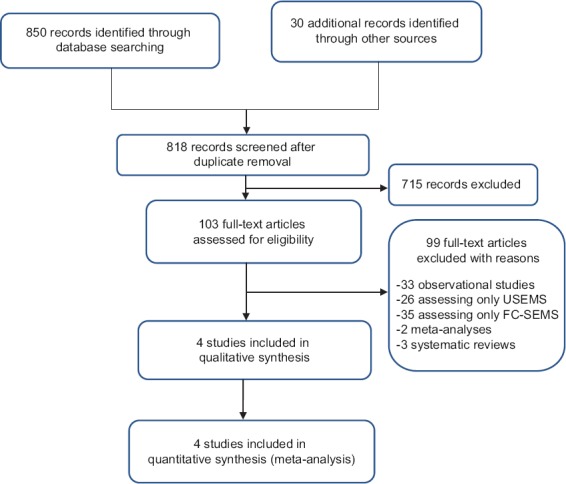
Prisma flow diagram
USEMS, uncovered self-expanding metal stents; FC-SEMS, fully covered self-expandable metal stent.
Characteristics of the included studies
The main characteristics of the 4 included studies are reported in Table 1. The studies were published between 1995 and 2017 and included a total of 205 patients, randomized to FC-SEMS (n=103) or MPS (n=102).
Table 1.
Baseline characteristics of included studies
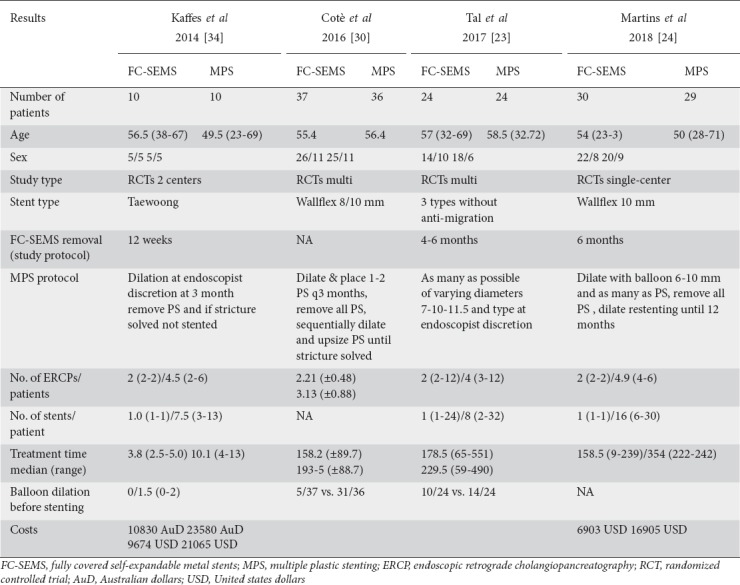
Two studies [23,30] reported the rate of balloon dilation in FC-SEMS and MPS, showing that FC-SEMS group had a lower rate of balloon dilation before stent placement (OR 0.14, 95%CI 0.06-0.30). One trial [30] did not report the primary and secondary outcomes in the setting of LT because it was designed to compare FC-SEMS and MPS for all benign biliary strictures. We obtained the extracted data from the authors.
In the MPS stent group, the 4 RCTs included in the analysis followed different protocols, as shown in Table 1. No RCTs in the MPS group reported how many patients had ABS resolution at 3-6 or 12 months, preventing a direct comparison between the 2 groups. In the FC-SEMS group, there was different time to stent removal protocols, as shown in Table 1. The evaluation using the Cochrane risk-of-bias tool did not show significant bias (Fig. 2).
Figure 2.
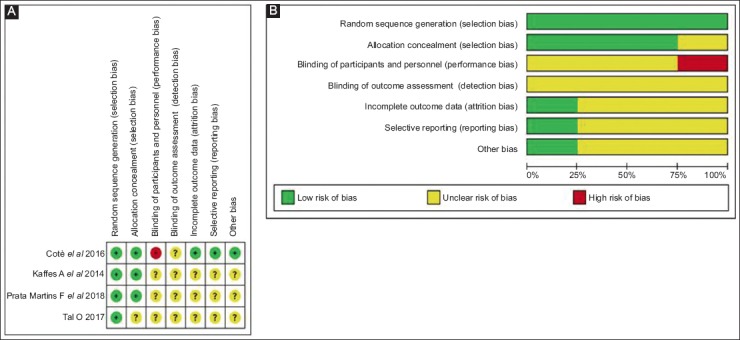
Cochrane risk of bias. (A) Risk of bias summary. (B) risk of bias in the individual study
Primary outcomes
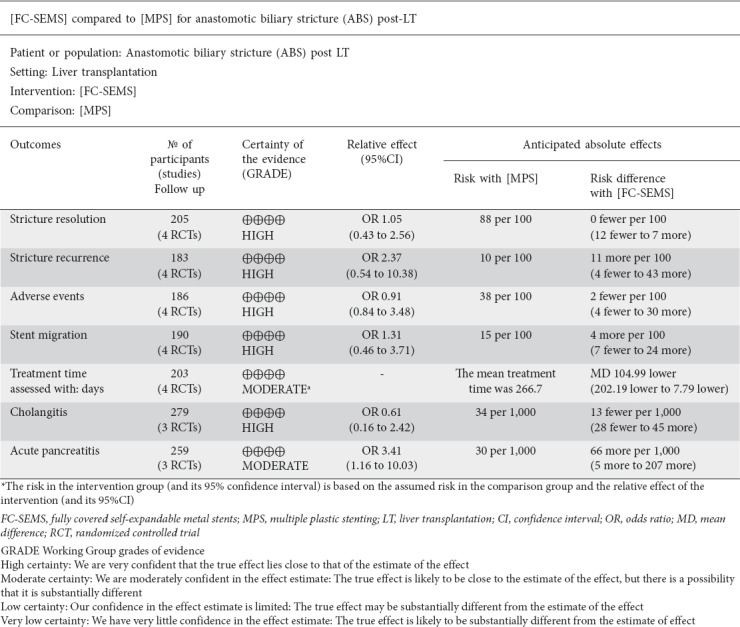
Four studies [23,24,30,34], including 103 FC-SEMS patients and 102 MPS patients, reported the ABS resolution rate. There was no statistically significant difference between FC-SEMS and MPS (OR 1.05, 95%CI 0.43-2.56) (Fig. 3). FC-SEMS showed a trend towards a higher recurrence rate, but the difference was not statistically significant (OR 2.37, 95%CI 0.54-10.38, P=0.25; I2 53%) (Fig. 4).
Figure 3.
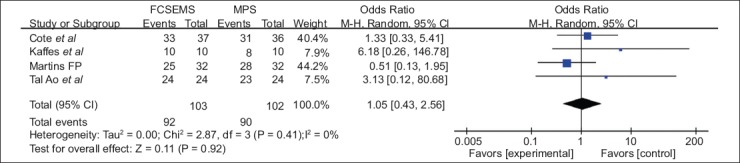
Forest plots showing the results of a meta-analysis comparing stricture resolution between FC-SEMS and MPS
CI, confidence interval; FC-SEMS, fully covered self-expandable metal stents; MPS, multiple plastic stenting.
Figure 4.
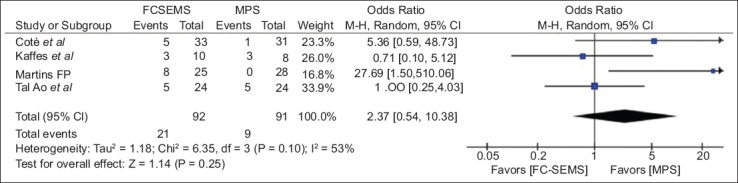
Forest-plots showing the results of meta-analysis comparing stricture recurrence between FC-SEMS vs. MPS
CI, confidence interval; FC-SEMS, fully covered self-expandable metal stents; MPS, multiple plastic stenting.
The sensitivity analysis was performed by removing each trial analysis in turn to assess the influence of each individual study on the global analysis. We noted that excluding the trials in which FC-SEMS were removed later (6 months) [24,30], the trend towards a higher recurrence rate in the FC-SEMS group became more evident (OR 3.90, 95%CI 0.56-27.25, P=0.17), while excluding studies that removed FC-SEMS earlier (12 weeks to 4 months) [23,34] eliminated the difference between the 2 groups (OR 0.89, 95%CI 0.29-2.79, P=0.85). After exclusion of the trial by Martins et al [24], the only one to show a higher recurrence rate for FC-SEMS, no statistically significant difference was noted (OR 1.31, 95%CI 0.46-3.74, P=0.61), without heterogeneity (I2 5%).
Secondary outcomes
The results of the secondary outcomes are provided in Table 2. There were 4 studies, involving 97 FC-SEMS and 89 MPS patients, that reported overall adverse events. The pooled OR was 0.91 (95%CI 0.32-2.62), showing that there was no statistical difference between FC-SEMS and MPS groups. There was also no statistically significant difference between FC-SEMS and MPS regarding cholangitis rate (OR 0.61, 95%CI 0.16-2.42, P=0.49), bleeding rate, perforation rate or migration rate.
Table 2.
Primary and secondary outcome
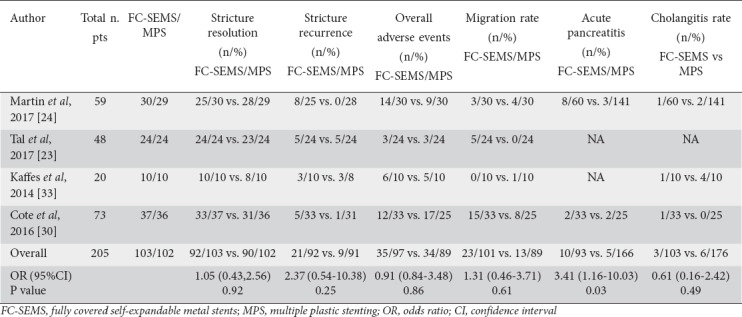
Acute pancreatitis was assessed in 2 RCTs [24,30] that showed a higher rate of pancreatitis in the FC-SEMS group (OR 3.41, 95%CI 1.16-10.03, P=0.03). An RCT by Martins et al [24] had a bias due to the fact that the author did not perform endoscopic sphincterotomy before FC-SEMS placement, as highlighted in the interim analysis. When endoscopic sphincterotomy was performed the rate of pancreatitis was equal in both groups.
The median number of ERCP procedures was lower in the FC-SEMS group compared with MPS group (MD -2.08, 95%CI -3.29 to -0.86) [23,24,30,34]. No RCT reported data to allow a cost-effectiveness analysis. Two RCTs [24,34] reported the total costs for both procedures, showing that FC-SEMS allowed a cost saving of between 9,800 and 10,000 US dollars, favoring FC-SEMS as a less expensive procedure (P<0.001).
A subgroup analysis could not be performed because of the small sample size, insufficient to power the results, and the low number of RCTs published to date. The risk-of-bias analysis of individual studies showed a high risk of performance and detection bias, probably related to the need for ABS assessment, and stent placing and removal by the physicians. The Grade summary of evidence is reported in the Supplementary material (S3).
Publication bias
Visual inspection of funnel plots (Fig. 5) showed no evidence of asymmetry. As a confirmation, Egger’s test for funnel plot asymmetry gave a P-value of 0.13, showing no potential publication bias for the outcomes considered.
Figure 5.
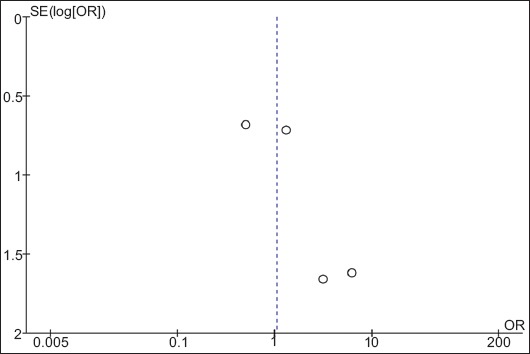
Funnel plot showing no publication bias
SE, standard error; OR, odds ratio.
Discussion
We performed a systematic review and meta-analysis of RCTs specifically designed to assess the efficacy and safety of placement of single FC-SEMS vs. MPS in the management of ABS following LT. In our study, there was no statistically significant difference in the rates of ABS resolution and recurrence between FC-SEMS and MPS in patients with ABS post-LT, although the pooled ORs show that FC-SEMS had a trend for a higher recurrence rate compared with MPS, with between-study heterogeneity (I2 53%). This trend was especially apparent when stent removal was performed earlier, but the small sample size was not enough to provide robust data that would allow a definitive conclusion. ABS dilation before stent deployment was performed more frequently in the MPS group than in the FC-SEMS group, but it was not possible to assess the effect of biliary dilation on the ABS recurrence rate.
A crucial issue that remains unsolved is ABS recurrence, because more information would be needed. The study by Martins was the only one to show a significantly higher rate of recurrence in the FC-SEMS group, and the authors suggested that the shorter indwelling time (6 months) in the FC-SEMS group was responsible for this result. We suggest according to our data that should be prolonged the indwelling time in the FC-SEMS group and that future RCTs should take this issue into account.
Another critical point to be stressed is the difference between the 2 procedures (FC-SEMS vs. MPS) because, while FC-SEMS is associated with 2 steps (placement and removal after a specified time), the MPS technique involves 5 or 6 procedures (placement and removal). Furthermore, the MPS protocol could be different across different centers and protocols, depending on personal technique, for example, stent removal and replacement, or adding one or more stents without stent removal. In the 4 RCTs included in our meta-analysis, the MPS protocols differed significantly, leading to confounding factors that produced a bias.
One unresolved point that should be analyzed in future trials is the method of FC-SEMS placement (transpapillary vs. intraductal), which would be likely to further influence the performance of FC-SEMS, reducing the migration rate and SEMS dysfunction from sludge. Another point that should be mentioned is the ABS location, because no data was available from the primary studies. This could be a factor that limits the use of FC-SEMS placement, if closeness to the bifurcation causes closure of the duct, resulting in cholangitis.
A meta-analysis by Kao et al including only observational studies, assessed the role of MPS in 8 studies (446 patients) in CDLT, 3 studies (120 patients) using MPS in LDLT, and 10 studies (200 patients) using SEMSs without a direct comparing of the two treatments. The authors concluded that ABS resolution and recurrence rate were higher in cases with a longer stent indwell duration (>12 months) compared with a shorter duration (<12 months); this is similar to our own findings.
In the meta-analysis by Kao et al [15] available data were in the form of case series. Each study had small numbers of patients, with 148 being the highest number of participants, and none fulfilled all the criteria for high-quality studies. Significant heterogeneity existed among the studies with respect to primary outcome, patient selection, stent protocol, stent duration, types of SEMS and follow-up periods; this made it difficult to compare FC-SEMS with MPS.
A meta-analysis by Aparicio et al [21] that included 10 studies (1 RCT, 6 non-randomized prospective studies and 3 cohort studies) showed an equal ABS resolution with FC-SEMS and MPS, but only the single RCT assessed the ABS resolution rate between FC-SEMS and MPS. A recent meta-analysis was published by Landi et al [22], including 3 RCTs and 1 observational study with a total of 119 patients receiving FC-SEMS and 179 MPS. The results showed that FC-SEMS were superior in terms of the number of ERCP procedures and days of treatment, whereas ABS resolution and recurrence rate showed equal efficacy in both groups. This meta-analysis is underpowered to draw definitive conclusions and the consistent heterogeneity observed in the included studies suggest caution for interpreting the results. The authors did not consider the results of previous meta-analyses, while the findings regarding ABS resolution and recurrence from the randomized trials by Cote et al [30], designed for all benign biliary strictures, included in their meta-analysis, did not report the real results for the subgroup of ABS post-LT.
Only 2 RCTs, those by Kaffes et al [34] and Martins et al [24], reported the cost-analysis, which included the cost of the procedure and the health personnel involved, and both showed a cost-saving favoring FC-SEMS. Furthermore, an economic analysis published in abstract form found that FC-SEMS were overwhelming favored as the more economical strategy, offering an overall less expensive hospital stay and fewer lost days of work compared to MPS, even with the more expensive implant cost.
The limitations of our meta-analysis are based on the missing relevant data from the primary studies, such as risk factors for biliary complications, different protocols, need for balloon dilation, SEMS type, type of covering, different axial and radial force, and the different protocols used in the RCTs for the MPS group. Another limitation is the absence of cost-analysis and cost-benefit studies that could allow a choice of better treatment approach, taking into account the costs to the health system. The small sample size (205 patients) of the RCTs included could have affected various results and limited the conclusions; it did not allow us to determine the reasons for the trend noted towards a higher recurrence rate in the FC-SEMS group. Finally, we assessed ABS resolution and recurrence as a dichotomous variable and not as a time to event, because, unfortunately, the HRs of the 2 primary outcomes were often unavailable or not obtainable.
In conclusion, our systematic review shows that FC-SEMS had equal stricture resolution, stricture recurrence and overall adverse events compared with MPS, although there was a trend toward a higher recurrence rate in the FC-SEMS groups when the stents were removed early. Our meta-analysis is the first systematic review including only RCTs that have conducted to a more robust conclusion. FC-SEMS are associated with reduced number of procedures overall a cost sparing procedure. According to our data and cost-analysis data, the use of new FC-SEMS with an antimigration system for a longer dwell time could be the best approach. Further RCTs with larger sample size and longer follow up focusing on risk factors of recurrence rate and using FC-SEMS with anti-migration features with appropriate dwelling time are warranted.
Summary Box.
What is already known:
Multiple plastic stents (MPS) are the gold standard for the treatment of anastomotic biliary strictures post-liver transplantation
Fully covered self-expandable metal stents (FC-SEMS) are increasingly used as rescue treatment in case of refractory strictures or as primary treatment
Many trials and meta-analyses addressed the comparison between MPS and FC-SEMS without finding any relevant difference, but those studies had many flaws
What the new findings are:
Our study for the first time analyzed only randomized controlled trials (RCTs) and showed that MPS and FC-SEMS are equally effective and safe procedures
We stressed the difference between the 2 procedures because, while FC-SEMS is associated with 2 steps (placement and removal after a specific time), the MPS technique involves 5 or 6 steps (placement and removal)
The study highlights the missing relevant data from the primary studies, such as risk factors for biliary complications, different protocols, need for balloon dilation, SEMS type, type of covering, different axial and radial forces, the different protocols used in the RCTs, as well as the absence of cost-analysis that could allow a choice of better treatment approach considering health system costs
Acknowledgment
We thank the data providers, Stuart Sherman, MD, Indiana University School of Medicine, Gastroenterology, & Leslie J. Pfeffer, BS, CHP University HIPAA Privacy, for permission to use de-identified data (“Summary of the data collected for this study from patients with post-liver transplantation biliary stricture”) for the purposes of this article.
Biography
Ospedale Ca’ Granda Niguarda, Milan, Italy; IRCCS ISMETT, Palermo, Italy; Nuovo Regina Margherita Hospital, Rome, Italy; University of Utah, Salt Lake City, Utah, USA
Supplementary Table 1.
PRISMA check list PRISMA 2009 Checklist to be included with meta-analyses
Search strategy
Supplementary material 1
Search strategy PubMed
Biliary stricture (exp)
Bile duct disease
Cholestasis
Common bile duct disease
Jaundice, obstructive
Or/ 1-5
Liver transplantation
OLT
Or/6-7
Stent
Plastic stent
Or/ 9-10
Metallic stent
SEMS
Self-expandable metal stent
Or/12-14
Randomized controlled trial (pt)
Controlled clinical trial
Randomized (tiab)
Randomly (tiab)
Trial (tiab)
Groups (tiab)
Or/16-21
5 and 8 and 11 and 15 and 22
Grade summary of evidence
Footnotes
Conflict of Interest: none
References
- 1.Greif F, Bronsther OL, Van Thiel DH, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40–45. doi: 10.1097/00000658-199401000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thethy S, Thomson BN, Pleass H, et al. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647–653. doi: 10.1111/j.1399-0012.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 3.Rerknimitr R, Sherman S, Fogel EL, et al. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis:endoscopic findings and results of therapy. Gastrointest Endosc. 2002;55:224–231. doi: 10.1067/mge.2002.120813. [DOI] [PubMed] [Google Scholar]
- 4.Gondolesi GE, Varotti G, Florman SS, et al. Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation. 2004;77:1842–1848. doi: 10.1097/01.tp.0000123077.78702.0c. [DOI] [PubMed] [Google Scholar]
- 5.Soejima Y, Taketomi A, Yoshizumi T, et al. Biliary strictures in living donor liver transplantation:incidence, management, and technical evolution. Liver Transpl. 2006;12:979–986. doi: 10.1002/lt.20740. [DOI] [PubMed] [Google Scholar]
- 6.Chang JH, Lee I, Choi MG, Han SW. Current diagnosis and treatment of benign biliary strictures after living donor liver transplantation. World J Gastroenterol. 2016;22:1593–1606. doi: 10.3748/wjg.v22.i4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation:past, present and preventive strategies. Liver Transpl. 2008;14:759–769. doi: 10.1002/lt.21509. [DOI] [PubMed] [Google Scholar]
- 8.Albert JG, Filmann N, Elsner J, et al. Long-term follow-up of endoscopic therapy for stenosis of the biliobiliary anastomosis associated with orthotopic liver transplantation. Liver Transpl. 2013;19:586–593. doi: 10.1002/lt.23643. [DOI] [PubMed] [Google Scholar]
- 9.Verdonk RC, Buis CI, Porte RJ, et al. Anastomotic biliary strictures after liver transplantation:causes and consequences. Liver Transpl. 2006;12:726–735. doi: 10.1002/lt.20714. [DOI] [PubMed] [Google Scholar]
- 10.Koksal AS, Eminler AT, Parlak E, Gurakar A. Management of biliary anastomotic strictures after liver transplantation. Transplant Rev (Orlando) 2017;31:207–217. doi: 10.1016/j.trre.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Zoepf T, Maldonado-Lopez EJ, Hilgard P, et al. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl. 2006;12:88–94. doi: 10.1002/lt.20548. [DOI] [PubMed] [Google Scholar]
- 12.Tringali A, Barbaro F, Pizzicannella M, et al. Endoscopic management with multiple plastic stents of anastomotic biliary stricture following liver transplantation:long-term results. Endoscopy. 2016;48:546–551. doi: 10.1055/s-0042-100277. [DOI] [PubMed] [Google Scholar]
- 13.Tabibian JH, Asham EH, Han S, et al. Endoscopic treatment of postorthotopic liver transplantation anastomotic biliary strictures with maximal stent therapy (with video) Gastrointest Endosc. 2010;71:505–512. doi: 10.1016/j.gie.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Costamagna G, Pandolfi M, Mutignani M, Spada C, Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54:162–168. doi: 10.1067/mge.2001.116876. [DOI] [PubMed] [Google Scholar]
- 15.Kao D, Zepeda-Gomez S, Tandon P, Bain VG. Managing the post-liver transplantation anastomotic biliary stricture:multiple plastic versus metal stents:a systematic review. Gastrointest Endosc. 2013;77:679–691. doi: 10.1016/j.gie.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Chaput U, Scatton O, Bichard P, et al. Temporary placement of partially covered self-expandable metal stents for anastomotic biliary strictures after liver transplantation:a prospective, multicenter study. Gastrointest Endosc. 2010;72:1167–1174. doi: 10.1016/j.gie.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Tarantino I, Traina M, Mocciaro F, et al. Fully covered metallic stents in biliary stenosis after orthotopic liver transplantation. Endoscopy. 2012;44:246–250. doi: 10.1055/s-0031-1291465. [DOI] [PubMed] [Google Scholar]
- 18.Sauer P, Chahoud F, Gotthardt D, et al. Temporary placement of fully covered self-expandable metal stents in biliary complications after liver transplantation. Endoscopy. 2012;44:536–538. doi: 10.1055/s-0031-1291714. [DOI] [PubMed] [Google Scholar]
- 19.Hu B, Gao DJ, Yu FH, Wang TT, Pan YM, Yang XM. Endoscopic stenting for post-transplant biliary stricture:usefulness of a novel removable covered metal stent. J Hepatobiliary Pancreat Sci. 2011;18:640–645. doi: 10.1007/s00534-011-0408-3. [DOI] [PubMed] [Google Scholar]
- 20.Cerecedo-Rodriguez J, Phillips M, Figueroa-Barojas P, et al. Self expandable metal stents for anastomotic stricture following liver transplant. Dig Dis Sci. 2013;58:2661–2666. doi: 10.1007/s10620-013-2703-0. [DOI] [PubMed] [Google Scholar]
- 21.Aparicio DPDS, Otoch JP, Montero EFS, Khan MA, Artifon ELA. Endoscopic approach for management of biliary strictures in liver transplant recipients:A systematic review and meta-analysis. United European Gastroenterol J. 2017;5:827–845. doi: 10.1177/2050640616681909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landi F, de'Angelis N, Sepulveda A, et al. Endoscopic treatment of anastomotic biliary stricture after adult deceased donor liver transplantation with multiple plastic stents versus self-expandable metal stents:a systematic review and meta-analysis. Transpl Int. 2018;31:131–151. doi: 10.1111/tri.13089. [DOI] [PubMed] [Google Scholar]
- 23.Tal AO, Finkelmeier F, Filmann N, et al. Multiple plastic stents versus covered metal stent for treatment of anastomotic biliary strictures after liver transplantation:a prospective, randomized, multicenter trial. Gastrointest Endosc. 2017;86:1038–1045. doi: 10.1016/j.gie.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Martins FP, De Paulo GA, Contini MLC, Ferrari AP. Metal versus plastic stents for anastomotic biliary strictures after liver transplantation:a randomized controlled trial. Gastrointest Endosc. 2018;87:131.e1–131.e13. doi: 10.1016/j.gie.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management:an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coté GA, Slivka A, Tarnasky P, et al. Effect of covered metallic stents compared with plastic stents on benign biliary stricture resolution:A randomized clinical trial. JAMA. 2016;315:1250–1257. doi: 10.1001/jama.2016.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schünemann H, Brożek J, Guyatt GOA. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. 2013;2013 [Google Scholar]
- 34.Kaffes A, Griffin S, Vaughan R, et al. A randomized trial of a fully covered self-expandable metallic stent versus plastic stents in anastomotic biliary strictures after liver transplantation. Therap Adv Gastroenterol. 2014;7:64–71. doi: 10.1177/1756283X13503614. [DOI] [PMC free article] [PubMed] [Google Scholar]


