Alzheimer's disease (AD) is one of the most prevalent neurodegenerative disorders, and it greatly reduces quality of life in the affected population (Anand et al., 2014). Extracellular accumulation of amyloid-β (Aβ) plaques and intracellular aggregation of neurofibrillary tangles are the most obvious pathological hallmarks of AD (Selkoe, 2004; Ballatore et al., 2007), but neural and synaptic loss has been suggested as key players in the development of the disease, particularly in initial stages (Ingelsson et al., 2004). It should be held in mind that AD does not result from a single pathologic mechanism, but from a combination of several converging events. Even so, among the suggested pathologic mechanisms, synaptic dysfunction has received copious attention. In this regard, evidence suggests that Aβ oligomers are the primary cause of disrupted structure and function of cortical synapses (Walsh and Selkoe, 2007). Furthermore, AD-related cognitive impairments are most strongly associated with disruptions in the structure and function of cortical synapses (DeKosky and Scheff, 1990; Scheff et al., 2007).
Membranous protrusions known as dendritic spines are the site of the majority of excitatory synaptic input to cortical neurons, and it is postulated that loss of dendritic spines is responsible for excessive synaptic demise in AD (Walsh et al., 2005). Dendritic spine morphology, plasticity, and maintenance are heavily dependent on the underlying actin cytoskeleton. Actin filaments (F-actin) are built from monomers of globular (G) actin, and some of the actin in dendritic spines can be best conceived of as a dynamic equilibrium between F- and G-actin (Kasai et al., 2003). Actin filaments are polar structures depolymerizing from one pole (pointed end) and polymerizing at the other pole (barbed end), in a process known as treadmilling. This dynamic equilibrium is essential for activity-dependent remodeling of spine structure and, therefore, synaptic plasticity (Matus, 2005; Penzes and VanLeeuwen, 2011). At the same time, the backbone of dendritic spine structure is maintained by a pool of actin filaments with different lifetimes, ranging from more stable fractions, such as cross-linked filaments, to less stable fractions, such as branched filaments (Star et al., 2002; Blanchoin et al., 2014). Therefore, the loss of synapses in AD might stem from disruption of actin dynamics in dendritic spines. Indeed, previous work (Krucker et al., 2000; Blanpied and Ehlers, 2004; Szabó et al., 2016) has shown that actin dynamics are important for both LTD and LTP, which are compromised in AD. However, it is not clear whether F-actin disassembly precedes disruption of synapses or the elimination of synapses impairs F-actin assembly. Moreover, the association between the loss of dendritic spines and AD-related cognitive impairments is not well established.
In a recent study, Kommaddi et al. (2018) scrutinized the role of F-actin disassembly in Aβ-induced dendritic spine loss and cognitive impairment. Using mice harboring AD-linked mutations (APPswe/PS1E9 mice), the authors investigated G-/F-actin equilibrium as an indicator of F-actin nanoarchitecture in dendritic spines. Synaptic terminals (synaptosomes) were extracted from the cortex of 1-month-old male AD mice. Importantly, APPswe/PS1E9 mice show no signs of AD-related pathology at this age. Nonetheless, a significant shift from F-actin to G-actin with no alteration in total actin levels was observed in APP/PS1 mice compared with controls. Thus, F-actin disassembly preceded the accumulation of Aβ plaques and neurofibrillary tangles in this AD model. The disequilibrium in G-/F-actin was also present in 9-month-old AD mice, in which substantial Aβ plaques were present, suggesting that F-actin disassembly is a continuous and possibly progressive event in the pathological development of AD. Similar to the findings in the mouse model of AD, immunoblot analysis of postmortem tissues from people with mild cognitive impairment or AD revealed a significant reduction in levels of synaptosomal F-actin. In addition to demonstrating that F-actin disassembly precedes plaque formation, this result suggests that assessment of synaptic F-actin might be of value in diagnosing and, thus, treating AD patients at a much earlier stage of the disease.
One of the most important findings of the study by Kommaddi et al. (2018) regards the possible role of disrupted F-actin nanoarchitecture in AD-related behavioral impairments. The authors used fear conditioning to study learning in AD mice. Specifically, an aversive stimulus (shock) was repeatedly delivered in an otherwise neutral context or in conjunction with a neutral sensory cue. This causes normal mice to express fear responses to the neutral context or cue (Maren, 2001). A previous study (Huang et al., 2013) showed that F-actin disassembly impaired cued and contextual fear memory. In the study by Kommaddi et al. (2018), APP/PS1 mice showed decreased freezing responses compared with control mice in the shock-paired context, suggesting that fear learning was impaired. An actin-polymerizing agent, jasplakinolide, and an actin-depolymerizing agent, latrunculin, were used to determine the role of F-actin disassembly in the observed impairment of fear learning. Jasplakinolide led to elevated freezing responses in 2-month-old APP/PS1 mice, whereas latrunculin led to decreased freezing responses in 4-month-old control mice, suggesting that intact nanoarchitecture and dynamics of F-actin are required for normal learning and memory. Consistent with this, F-actin reduction in postmortem tissue from AD patients was correlated with defects in episodic memory and working memory, providing further evidence for a role of F-actin disassembly in AD-induced cognitive impairments. Even more notably, the results from mice indicate that synaptic loss seen in AD is reversible and can be reversed with actin-polymerizing agents.
To further investigate the relationship between F-actin disruption and spine loss in cortical neurons, Kommaddi et al. (2018) analyzed cultured primary cortical neurons from APP/PS1 mice. After 10 DIV, dendritic spines were minimally altered, whereas F-actin structure was significantly diminished. By 16 DIV, both the number and morphology of dendritic spines and F-actin were altered. This suggests that F-actin disassembly preceded and likely caused dendritic spine loss in the AD mice. In addition to cortical neurons, a considerable portion of AD-related cognitive impairments are suggested to result from synaptic dysfunction in the hippocampus (Scheibel, 1979). However, Kommaddi et al. (2018) focused only on neurons from cortical regions. Hence, further studies are required to investigate possible cytoskeletal deficits in hippocampal synaptosomes, as well.
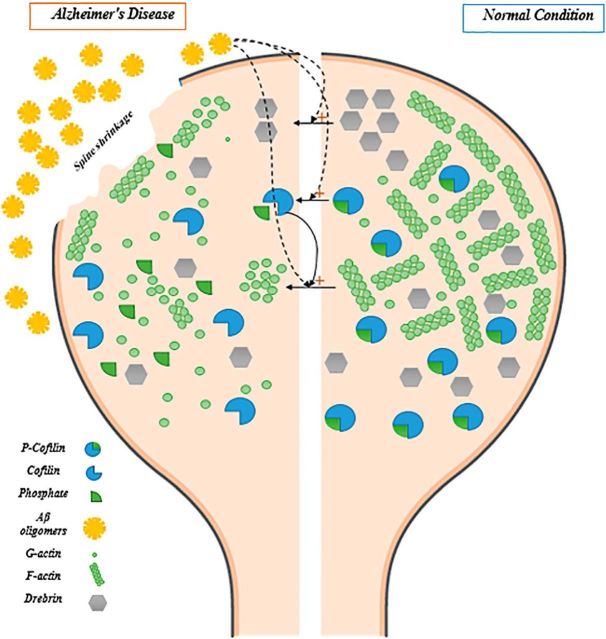
In the last part of their study, Kommaddi et al. (2018) scrutinized the interaction between actin and its major intracellular regulators, including ADF/cofilin, a negative regulator of actin polymerization, and drebrin, a positive regulator of actin polymerization (Bamburg, 1999; Bamburg and Bernstein, 2016). Dephosphorylated (active) cofilin causes a shift in G-/F-actin equilibrium by prompting the transformation of F-actin to G-actin (Bamburg and Bernstein, 2016). Phosphorylated (inactive) cofilin, p-cofilin/cofilin ratio, and drebrin were reduced in synaptosomes extracted from 1-month-old and 9-month-old AD mice, suggesting that ADF/cofilin and drebrin, as well as F-actin, are disrupted in early and later stages of AD. It is conceivable that Aβ-induced actin disassembly results from alternations in the levels and function of ADF/cofilin family members (Fig. 1). However, validation of cofilin as one of the mediators of Aβ-induced actin disassembly is missing in this study. It also remains possible that upstream proteins alter F-actin dynamics and nanoarchitecture indirectly (Penzes and VanLeeuwen, 2011).
Figure 1.
F-actin nanoarchitecture and two of its regulators (cofilin and drebrin) in dendritic spines. Under normal conditions, F-actin nanoarchitecture provides structural and functional support for dendritic spines (right). In Alzheimer's disease, Aβ oligomers disrupt the equilibrium between G- and F-actin, which will eventually lead to spine shrinkage and disappearance (left). The detrimental effects of Aβ oligomers on G-/F-actin equilibrium might occur through direct effects on actin monomers, or through its effects on P-cofilin/cofilin ratio and decreased levels of drebrin. Activated (dephosphorylated) cofilin catalyzes the conversion of F-actin to G-actin, whereas drebrin contributes to F-actin polymerization.
Synaptic dysfunction, particularly loss of dendritic spines, is currently recognized as a predominant pathologic event in the initial stages of AD, and it is closely correlated with AD-related cognitive impairments. The findings from cellular studies, animal models, and human postmortem brain tissue discussed herein provide strong evidence that dynamics of F-actin nanoarchitecture, specifically G-/F-actin equilibrium, along with its cellular regulators, including ADF/cofilin and drebrin, are impaired, even in early stages of AD. F-actin cytoskeleton contributes to the maintenance of dendritic spine structure and supports the scaffolding of several important synaptic proteins. Thus, Aβ-medicated F-actin disassembly likely leads to spine collapse and synaptic dysfunction. The maintenance of F-actin stability could be a promising therapeutic target in the future treatment of AD.
There are some major questions regarding Aβ-mediated F-actin disassembly that should be addressed in future studies. First and foremost, how does Aβ cause the F-actin disassembly in synaptosomes and, thus, cause spine loss? It remains to be investigated whether Aβ exerts its effects directly on F-actin nanoarchitecture or whether there are mediators linking Aβ toxicity to loss of dendritic spines. Second, in addition to Aβ, tau protein has been proposed to contribute to AD pathology. However, the connection between F-actin disassembly and tau protein pathology is unclear. Finally, the animal model of AD revealed that disruptions in F-actin nanoarchitecture could be reversed through actin polymerization agents. Actin-polymerizing agents could be potentially translated to be applicable in clinical settings. However, such agents are likely to produce severe side effects given the ubiquitous importance of the actin cytoskeleton throughout the body. Therefore, targeted treatment strategies, such as actin-polymerizing agents conjugated with antibodies directed against AD-affected neurons, could be considered in future studies to maximize the effect of actin-polymerizing agents and minimize the likelihood of possible side effects.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/preparing-manuscript#journalclub.
The authors declare no competing financial interests.
References
- Anand R, Gill KD, Mahdi AA (2014) Therapeutics of Alzheimer's disease: past, present and future. Neuropharmacology 76:27–50. 10.1016/j.neuropharm.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci 8:663–672. 10.1038/nrn2194 [DOI] [PubMed] [Google Scholar]
- Bamburg JR. (1999) Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 15:185–230. 10.1146/annurev.cellbio.15.1.185 [DOI] [PubMed] [Google Scholar]
- Bamburg JR, Bernstein BW (2016) Actin dynamics and cofilin-actin rods in Alzheimer disease. Cytoskeleton (Hoboken) 73:477–497. 10.1002/cm.21282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J (2014) Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev 94:235–263. 10.1152/physrev.00018.2013 [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Ehlers MD (2004) Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry 55:1121–1127. 10.1016/j.biopsych.2003.10.006 [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol 27:457–464. 10.1002/ana.410270502 [DOI] [PubMed] [Google Scholar]
- Huang W, Zhu PJ, Zhang S, Zhou H, Stoica L, Galiano M, Krnjević K, Roman G, Costa-Mattioli M (2013) mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci 16:441–448. 10.1038/nn.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, Albert MS, Hyman BT, Irizarry MC (2004) Early abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 62:925–931. 10.1212/01.WNL.0000115115.98960.37 [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H (2003) Structure-stability-function relationships of dendritic spines. Trends Neurosci 26:360–368. 10.1016/S0166-2236(03)00162-0 [DOI] [PubMed] [Google Scholar]
- Kommaddi RP, Das D, Karunakaran S, Nanguneri S, Bapat D, Ray A, Shaw E, Bennett DA, Nair D, Ravindranath V (2018) Abeta mediates F-actin disassembly in dendritic spines leading to cognitive deficits in Alzheimer's disease. J Neurosci 38:1085–1099. 10.1523/JNEUROSCI.2127-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S (2000) Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci U S A 97:6856–6861. 10.1073/pnas.100139797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. (2001) Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24:897–931. 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- Matus A. (2005) Growth of dendritic spines: a continuing story. Curr Opin Neurobiol 15:67–72. 10.1016/j.conb.2005.01.015 [DOI] [PubMed] [Google Scholar]
- Penzes P, VanLeeuwen JE (2011) Impaired regulation of synaptic actin cytoskeleton in Alzheimer's disease. Brain Res Rev 67:184–192. 10.1016/j.brainresrev.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ (2007) Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68:1501–1508. 10.1212/01.wnl.0000260698.46517.8f [DOI] [PubMed] [Google Scholar]
- Scheibel AB. (1979) The hippocampus: organizational patterns in health and senescence. Mech Ageing Dev 9:89–102. 10.1016/0047-6374(79)90123-4 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. (2004) Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med 140:627–638. 10.7326/0003-4819-140-8-200404200-00047 [DOI] [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN (2002) Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci 5:239–246. 10.1038/nn811 [DOI] [PubMed] [Google Scholar]
- Szabó EC, Manguinhas R, Fonseca R (2016) The interplay between neuronal activity and actin dynamics mimic the setting of an LTD synaptic tag. Sci Rep 6:33685. 10.1038/srep33685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ (2007) A beta oligomers: a decade of discovery. J Neurochem 101:1172–1184. 10.1111/j.1471-4159.2006.04426.x [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Shankar GM, Townsend M, Fadeeva JV, Betts V, Podlisny MB, Cleary JP, Ashe KH, Rowan MJ, Selkoe DJ (2005) The role of cell-derived oligomers of abeta in Alzheimer's disease and avenues for therapeutic intervention. Biochem Soc Trans 33:1087–1090. 10.1042/BST20051087 [DOI] [PubMed] [Google Scholar]