Abstract
Aging adults typically show reduced ability to ignore task-irrelevant information, an essential skill for optimal performance in many cognitive operations, including those requiring working memory (WM) resources. In a first experiment, young and elderly human participants of both genders performed an established WM paradigm probing inhibitory abilities by means of valid, invalid, and neutral retro-cues. Elderly participants showed an overall cost, especially in performing invalid trials, whereas younger participants' general performance was comparatively higher, as expected.
Inhibitory abilities have been linked to alpha brain oscillations but it is yet unknown whether in aging these oscillations (also typically impoverished) and inhibitory abilities are causally linked. To probe this possible causal link in aging, we compared in a second experiment parietal alpha-transcranial alternating current stimulation (tACS) with either no stimulation (Sham) or with two control stimulation frequencies (theta- and gamma-tACS) in the elderly group while performing the same WM paradigm. Alpha- (but not theta- or gamma-) tACS selectively and significantly improved performance (now comparable to younger adults' performance in the first experiment), particularly for invalid cues where initially elderly showed the highest costs. Alpha oscillations are therefore causally linked to inhibitory abilities and frequency-tuned alpha-tACS interventions can selectively change these abilities in the elderly.
SIGNIFICANCE STATEMENT Ignoring task-irrelevant information, an ability associated to rhythmic brain activity in the alpha frequency band, is fundamental for optimal performance. Indeed, impoverished inhibitory abilities contribute to age-related decline in cognitive functions like working memory (WM), the capacity to briefly hold information in mind. Whether in aging adults alpha oscillations and inhibitory abilities are causally linked is yet unknown. We experimentally manipulated frequency-tuned brain activity using transcranial alternating current stimulation (tACS), combined with a retro-cue paradigm assessing WM and inhibition. We found that alpha-tACS induced a significant improvement in target responses and misbinding errors, two indexes of inhibition. We concluded that in aging alpha oscillations are causally linked to inhibitory abilities, and that despite being impoverished, these abilities are still malleable.
Keywords: ageing, alpha oscillation, inhibition, tACS, working memory
Introduction
The ability to ignore information that is irrelevant for a given cognitive activity is fundamental for optimal performance (Hasher and Zacks, 1988; Gazzaley and Nobre, 2012). Importantly, impoverished inhibitory abilities are a contributing factor to age-related decline in several cognitive functions including working memory (WM), the capacity to hold information in mind for brief periods of time (Hasher and Zacks, 1988; Salthouse and Meinz, 1995; Gazzaley et al., 2005; Hasher et al., 2007).
Inhibitory abilities have been linked to brain oscillations, i.e., rhythmic brain activity, in the alpha frequency band (8–13 Hz) in the occipitoparietal areas among others (Klimesch et al., 2007; Rihs et al., 2007; Tuladhar et al., 2007; Jensen and Mazaheri, 2010; Gazzaley and Nobre, 2012; Herrmann et al., 2013; Constantinidis and Klingberg, 2016). Alpha oscillations have been associated to functional inhibition as alpha amplitude has been shown to decrease in task-relevant brain areas and increase in task-irrelevant ones (Kelly et al., 2006; Thut et al., 2006; Rihs et al., 2009; Sauseng et al., 2009; Zanto and Gazzaley, 2009; Jensen and Mazaheri, 2010; Romei et al., 2010; Foxe and Snyder, 2011; Hanslmayr et al., 2011). This is supported by correlational evidence from EEG studies showing that when ongoing alpha amplitude is high, young participants successfully inhibit task-irrelevant stimuli in WM tasks (Klimesch, 1999; Sauseng et al., 2009). Recently, alpha frequency-related physiological and behavioral effects have been induced by experimentally manipulating frequency-tuned brain stimulation in the form of transcranial alternating current stimulation (tACS). This safe technique allows us targeting specific brain oscillations (Sauseng and Klimesch, 2008; Thut et al., 2011; Antal and Paulus, 2013; Herrmann et al., 2013; Marshall and Binder, 2013; Parkin et al., 2015), and possibly modulating cognitive functions relying on these oscillations (Başar et al., 2001; Engel et al., 2001; Herrmann et al., 2004; Helfrich et al., 2014; Cecere et al., 2015). For instance, in young participants alpha-tACS increases endogenous alpha activity in parieto-occipital areas (Zaehle et al., 2010), and modulates target detection performance (Helfrich et al., 2014).
The age-related reduction of alpha amplitude (Klimesch, 1999; Klimesch et al., 2007; Vaden et al., 2012) and the age-associated weakening of inhibitory abilities purportedly associated with such oscillations (Hasher and Zacks, 1988; Salthouse and Meinz, 1995; Craik and Salthouse, 2000; McEvoy et al., 2001; Gazzaley et al., 2005, 2008; Hasher et al., 2007) may suggest a causal link between inhibitory processes and alpha oscillations. To test whether such a link exists, we adapted an established WM paradigm that provides an index of WM precision, and of the source of error in performance including indexes of inhibitory abilities (Bays and Husain, 2008; Bays et al., 2011; Gorgoraptis et al., 2011; Pertzov et al., 2013; Ma et al., 2014). During the WM maintenance interval, we used probabilistic retrospective cues (retro-cues) triggering top-down biasing mechanisms, which prioritize a maintained stimulus in WM (Griffin and Nobre, 2003; Landman et al., 2003; Makovski and Jiang, 2007; Matsukura et al., 2007; Makovski et al., 2008; Berryhill et al., 2012; Gazzaley and Nobre, 2012; Tanoue and Berryhill, 2012; Pertzov et al., 2013; Rerko and Oberauer, 2013; Gözenman et al., 2014; Mok et al., 2016). Crucially, some of these retro-cues (invalid retro-cues) prioritize information that will not subsequently be recalled, and therefore need a stronger suppression of task-irrelevant information at retrieval stage, a process that in younger adults typically results in reduced accuracy relative to other retro-cues (Griffin and Nobre, 2003; Matsukura et al., 2007; Astle et al., 2012; Pertzov et al., 2013). By combining this retro-cue paradigm with parietal alpha-tACS we reasoned that if inhibitory abilities are linked to alpha oscillations in the aging brain, then alpha-tACS may modulate aging participants' impoverished endogenous alpha amplitude, which may in turn impact on inhibitory abilities and ameliorate WM performance. Alternatively, no changes in inhibitory abilities by alpha-tACS may suggest that any link between alpha oscillations and declined inhibitory abilities is just mediated by age.
Materials and Methods
Participants.
Fifty right-handed, stimulation-compatible (Antal and Paulus, 2013; Tavakoli and Yun, 2017) subjects with normal or corrected vision provided written consent to participate in our double-blind experiment that was approved by the local ethics committee. Twenty-five of them were elderly (14 males; mean age = 69.1 ± 4.5 years; age range = 62–78; education: mean = 16.2 ± 4 years; range = 13–22) and 25 were younger (11 males; mean age = 24.8 ± 4.3 years; age range = 18–33; education: mean = 15.2 ± 5 years; range = 14–20).
Younger adults performed the same experimental task as the elderly adults in the absence of tAC stimulation for comparative purposes (Experiment 1, Experimental design and task). A future study will investigate young participants' performance with an equivalent tACS-based study.
All participants were assessed for color blindness and none of them showed impairment in color perception. Moreover, none of the participants had past history of neurological or psychiatric disorders, was under regular medication, or showed major cognitive impairments assessed with the Mini-Mental State Examination (MMSE; Folstein et al., 1975; for elderly participants only). Subjects received a monetary compensation to complete the experiment. The same elderly participants took part in Experiments 1 and 2, and a subgroup of them took part in Experiment 3.
Experimental design and task.
The same retro-cue WM paradigm was used in the first and second experiments, where we tested, respectively, for any differential performance between young and elderly adults, and for the possible causal link between alpha oscillations and inhibitory abilities in our aging sample only. Our paradigm is more complex than simpler WM tasks, such as the digit or letter span, but was purposely chosen because: (1) it provides a continuous rather than a binary measure of WM performance, which allows better measuring of the fidelity or quality of WM representations (Alvarez and Cavanagh, 2004; Bays and Husain, 2008; Bays et al., 2009; Ma et al., 2014); (2) it measures WM accuracy as well as the source of errors; and (3) some of these errors, specifically the probability of target responses and misbinding, provide a measure of inhibitory processes, which is the focus of our investigation.
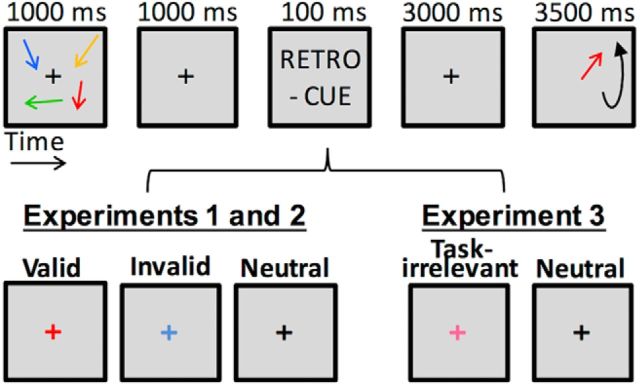
Participants memorized a 1000 ms display of four arrow stimuli (visual angle: 2° × 0.3°) differing in color and orientation. The arrows were simultaneously presented to the left and right (2 for each side) of a black, 0.8° diameter fixation cross. Within a trial, they appeared in four of five randomly selected and easily distinguishable colors (yellow, red, blue, green, and white), and were arbitrarily oriented with a minimum of 10° difference between the stimuli. Participants were asked to keep in mind both the orientation and color of these arrows. Memory array was followed by a 1000 ms delay during which a retro-cue may or may not have been presented (100 ms). A 3000 ms delay period preceded the presentation of one of the four colored arrows (the probe), which reappeared in a random orientation. Participants used a continuous, analog response to match it as closely as possible to the original orientation (Pertzov et al., 2013; Fig. 1).
Figure 1.
The WM retro-cueing task. Participants memorized a display of four arrow stimuli differing in orientation and color. Following a delay period, one of the four colored arrows reappeared in a random orientation and participants matched it as closely as possible to the original orientation. In 70% of the trials during the delay in Experiments 1 and 2, a colored cue was presented, which highlights an item that was more likely to be later probed. In these trials, the probe either matched the cued items (validly cued trials, n = 56) or it did not (invalidly cued trials, n = 28). In the remaining 30% of the trials (n = 42), no cue was present during the delay. In Experiment 3, which aimed to test whether retro-cues may act as distracters, 30% of the trials (n = 42) consisted of a neutral condition equivalent to the one used in Experiments 1 and 2. In the remaining 70% of the trials (n = 84) the color of the fixation changed into a color that was never part of the stimulus display, i.e., pink (task-irrelevant cueing condition). ms = milliseconds.
In Experiments 1 and 2, 30% of trials (n = 42) had the memory delay filled by a fixation cross that remained black, i.e., a neutral condition. In the remaining 70% of trials, a 100 ms retro-cue was presented 1000 ms after the presentation of the memory array. The retro-cue indicated the color of the stimulus arrow most likely to be later probed. In 70% of these retro-cue trials (n = 56), the cue corresponded to the item that was subsequently probed (valid condition). The remaining 30% of the retro-cue trials (n = 28) cued an item that was not subsequently probed (invalid condition). In Experiment 3, which aimed to test whether retro-cues may act as distracters, 30% of the trials (n = 42) consisted of a neutral condition equivalent to the one used in Experiments 1 and 2. In the remaining 70% of the trials (n = 84) the color of the fixation changed into a color that was never part of the stimulus display, i.e., pink (task-irrelevant cueing condition).
In all experiments, participants were seated in front of a 21 inch CRT monitor at a viewing distance of 60 cm. Testing sessions were conducted in a darkened and soundproof room; to maintain a stable visual field, a chin rest supported participants' heads. Tasks were programmed in MATLAB 7.0 using the Cogent toolbox (http://www.mathworks.co.uk). Each testing session lasted ∼1 h.
Using an established probabilistic model, the retro-cue WM paradigm assesses general WM precision, and quantifies the contribution of separate sources of error to performance (Bays and Husain, 2008; Bays et al., 2011; Gorgoraptis et al., 2011; Pertzov et al., 2013; Ma et al., 2014). Specifically, errors were in terms of the noisiness of memory for the target item, the probability of responding to the target, to a non-target, and of responding at random (guessing). Noisiness of memory or increase in variability of memory for the target orientation is an indication of how well the memory trace was “protected” during the retention period. Increase probability to respond to the target orientation is a measure of maintained inhibitory abilities and selective attention. Increase probability to respond to the non-target orientation (non-probed item) is used as a measure of impaired inhibition and selective attention, because observers misbind the color of the probed item to the orientation of one of the other items in memory. Finally, an increase in random responses, i.e., independent of any orientation in memory, can also contribute to error in performance due to factors such as inattention, distraction, or lack of compliance with the task.
Stimulation design.
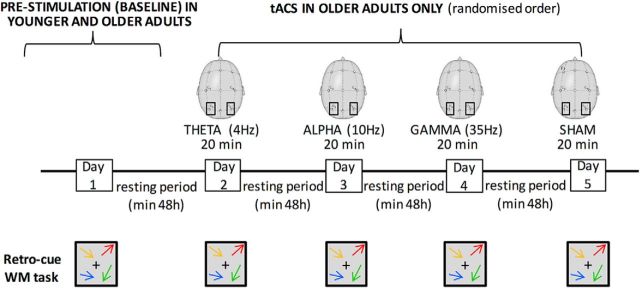
In Experiment 2, we used the same WM retro-cueing task and data analysis as Experiment 1 to probe the possible causal link between alpha oscillations and inhibitory abilities in our aging sample. Aging adults only underwent four experimental sessions at least 2 d apart; in each session, while performing the same task, they received bilateral parietal tACS stimulation at either 4 Hz (θ band), 10 Hz (α band), 35 Hz (γ band), or Sham. The order of the stimulation conditions was counterbalanced and pseudorandomized across participants (Fig. 2). Parietal regions were targeted: (1) because they are known for being involved in inhibitory processes, which are at the core of our investigation (Kelly et al., 2006; Gazzaley and Nobre, 2012; Klimesch, 2012; Constantinidis and Klingberg, 2016); and (2) because they have also been systematically targeted as the main alpha generator, as shown in several M/EEG (Fu et al., 2001; Rihs et al., 2009; Capotosto et al., 2017) and brain stimulation studies (Romei et al., 2010, 2012; Thut et al., 2012).
Figure 2.
Experimental design. In Experiment 1, younger and older participants performed the WM retro-cueing task in a prestimulation session with no tACS (baseline). In Experiment 2, older participants only performed the same task while receiving 20 min of bilateral parietal (P3 and P4 on 10–20 EEG system) tACS stimulation at either 4 Hz (θ band), 10 Hz (α band), 35 Hz (γ band), or Sham. The order of the stimulation conditions was counterbalanced and pseudo-randomized across participants, with testing sessions at least 48 h apart.
We reasoned that if alpha oscillations are causally linked to inhibitory processes, then relative to Sham or to another stimulation frequency (see below) alpha-tACS may enhance inhibitory abilities and attenuate both the interstimuli competition at encoding and the effect of the retro-cue. Attenuating this competition may increase interstimuli interference (Desimone and Duncan, 1995; Berryhill et al., 2012; Bonnefond and Jensen, 2013). This may in turn impoverish memory recall and increase misbinding errors more strongly in neutral cues where no retro-cue is available to offset the interstimuli interference. Moreover, any alpha-tACS induced change in performance may be larger or specific to invalidly-cued trials; in our first experiment these trials led to the largest cost in performance in aging adults because they require suppressing information that had been invalidly prioritized (Results, Experiment 1; Pertzov et al., 2013), hence reducing the effect of retro-cue with alpha-tACS may result in the largest improvements in these invalid trials.
To exclude any generic learning or fatigue effects, Sham stimulation was used in the same aging participants in a different testing session. Theta-tACS was used as an active stimulation condition, with no significant changes in performance predicted following it. This is because theta oscillations are known to more strongly reflect maintenance of items presented sequentially and with progressively increasing load (Jensen, 2006), two factors that we did not manipulate in our design. We also tested whether gamma-tACS may modulate the precision of memory recall because gamma oscillations are known to reflect changes in encoding or maintaining items in WM, as well as in redirecting attention to internal WM representations (Tallon-Baudry et al., 1999; Jensen et al., 2007; Buzsáki and Wang, 2012; Roux et al., 2012; Poch et al., 2014; Ray and Maunsell, 2015).
In Experiment 3, some of the same aging participants underwent two experimental sessions each, at least 2 d apart, based on the same task and procedures as in Experiment 2. However, a variant of the retro-cueing condition described above was used, consisting of including task-irrelevant and neutral cues. Because the results of Experiment 2 indicated significant changes in performance following alpha stimulation relative to Sham (Results, Experiment 2), Experiment 3 focused on alpha and Sham parietal stimulation only, which were applied to aging adults in alternated order.
A sinusoidal stimulation was applied with a Magstim stimulator and delivered through two 35 cm2 (5 × 7 cm) rubber electrodes, each covered with a sponge pad soaked in saline solution and positioned over the subject's scalp. In each session, participants were stimulated at a specific frequency (4, 10, or 35 Hz in Experiment 2; 10 Hz only in Experiment 3, in addition to Sham in all studies) for 20 min with a current strength of 1500 μA (average current density was ∼42.9 μA/cm2) and a fade in/out period of 20 s. To allow successful blinding of participants, during Sham stimulation the same setting was maintained compared with active stimulation, but the current settled at the lowest frequency (i.e., 4 Hz) was turned off after 20 s, so that any cutaneous sensation was the same during active and Sham stimulation (Gandiga et al., 2006; Fertonani et al., 2011).
Based on the standard 10–20 EEG system (Oostenveld and Praamstra, 2001), parietal and Sham stimulation electrodes were placed on the target parietal areas corresponding to P3 and P4, and held in place by means of an elastic band. During the whole time course of the experiment, participants as well as the experimenter placing the electrodes and running the experimental protocol were not told whether active or Sham stimulation was used in any given session. All aging participants performed all experiments, and a subgroup of them performed Experiment 3 during parietal alpha-tACS. Given the posterior electrode montage none of the participants reported seeing phosphenes.
Statistical analysis.
Recall precision (P) was used as an overall measure of performance, obtained by calculating the angular deviation between the orientation reported by the subject and the orientation of the target arrow in the initial memory display. For each retro-cueing condition, recall precision was defined as the reciprocal of the circular SD of error in response.
Moreover, by applying an established probabilistic model (Bays and Husain, 2008; Bays et al., 2011), the sources of error underlying performance in the WM retro-cueing task could be deconstructed, and their effect on performance estimated separately. This model is described as follows:
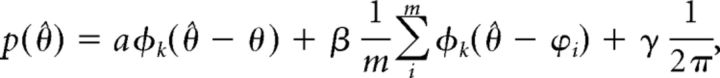 |
where θ is the true orientation of the target item, ∧ the orientation reported by the subject, and Φκ is the von Mises distribution (the circular analog of the Gaussian distribution) with mean of 0 and concentration parameter κ. Concentration parameter κ reflects the variability of recall of the target feature, whereby higher κ corresponds to lower variability. The probability of reporting the correct target item (pT) is given by α. The probability of misreporting a non-target item (pNT) is given by β, and {ϕ1, ϕ2, … ϕm} are the orientations of the non-target items. The probability of responding randomly (pU) is given by γ = 1 − α − β. Maximum likelihood estimates (Myung et al., 2013) of the parameters κ, α, β, and γ were obtained separately for each subject, stimulation condition, and retro-cue type using an expectation–maximization algorithm.
Performance in absence of stimulation was investigated by fitting repeated measures regressions, using as predictors retro-cueing type (valid, invalid, and neutral) and either age group (young and older) for Experiment 1, or stimulation condition (Sham, Alpha, and Gamma) in Experiment 2. For this experiment, the same analysis was repeated also including performance of the subgroup of participants who received theta-tACS.
The generalized estimating equations (GEE) procedure legitimates the analysis of data violating the normality assumption, as in the case of the current data. Accuracy (precision) and each index of error (pT, pNT, κ, pU) were separately modeled through gamma regression with a loglog link function. Significant main effects or interactions were followed by GEE-based t tests with the least-significant difference test correction for multiple comparisons (for a similar approach, Santarnecchi et al., 2013).
Across all performance indexes and stimulation conditions, nine data points for the aging adults (0.6%), and six for the younger sample (1.6%) were disregarded because of poor model fitting. An additional nine (0.6%) and three (0.8%) data points, which were >3 SD from the group mean, were excluded from the analyses of the older and younger adults' performance, respectively.
Results
Experiment 1
Working memory retro-cueing task in younger and aging adults
Overall performance: recall precision.
The fidelity with which the probe was recalled was significantly more precise in younger than older adults across cueing conditions (χ2 = 14.5, p < 0.001), a large difference (Cohen's d = 1.05) based on Cohen's criteria (Cohen, 1988). Moreover, in the two age groups, precision was differently modulated by cue type (significant interaction of retro-cue type and age group, χ2 = 6.6, p = 0.036), because it was significantly greater in valid trials in younger relative to older adults (mean difference = −0.27, p < 0.001 corrected), a large between-group difference (Cohen's d = 1.12). In aging compared with younger participants precision was also significantly lower in invalid and neutral trials (respectively, mean difference = −0.22, p = 0.03, and −0.18, p < 0.01 corrected; Cohen's d = 0.82 and 0.82; Table 1).
Table 1.
Experiment 1 (no stimulation): accuracy (precision, P) and source of error (κ, pT, pNT, pU) in younger and older participants
| Cueing condition | Younger participants |
Older participants |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | κ | pT | PNT | pU | P | κ | pT | pNT | pU | |
| All retro-cues | 1.23 | 2.62 | 0.81 | 0.14 | 0.06 | 1.0* | 2.27 | 0.68* | 0.24* | 0.08 |
| 0.06 | 0.25 | 0.02 | 0.03 | 0.01 | 0.02 | 0.20 | 0.03 | 0.02 | 0.02 | |
| Valid | 1.27 | 2.63 | 0.87 | 0.09 | 0.04 | 1.01* | 2.01 | 0.74* | 0.21* | 0.05 |
| 0.06 | 0.24 | 0.02 | 0.02 | 0.01 | 0.03 | 0.24 | 0.04 | 0.04 | 0.03 | |
| Invalid | 1.19 | 3.42 | 0.76 | 0.18 | 0.10 | 0.97* | 3.18 | 0.55* | 0.30* | 0.15 |
| 0.07 | 0.5 | 0.05 | 0.05 | 0.04 | 0.02 | 0.56 | 0.05 | 0.04 | 0.02 | |
| Neutral | 1.21 | 2.88 | 0.77 | 0.17 | 0.03 | 1.04* | 1.82 | 0.78 | 0.20* | 0.05 |
| 0.06 | 0.3 | 0.04 | 0.03 | 0.01 | 0.03 | 0.15 | 0.04 | 0.04 | 0.02 | |
Mean with SE in italics.
*Indicates significant group difference (p < 0.05).
In younger adults, recall precision was significantly greater for valid relative to neutral trials (mean difference = 0.06, p = 0.04 corrected; Cohen's d = 0.24). No other effects reached significance.
Inhibitory abilities—probability to respond to the target orientation (pT).
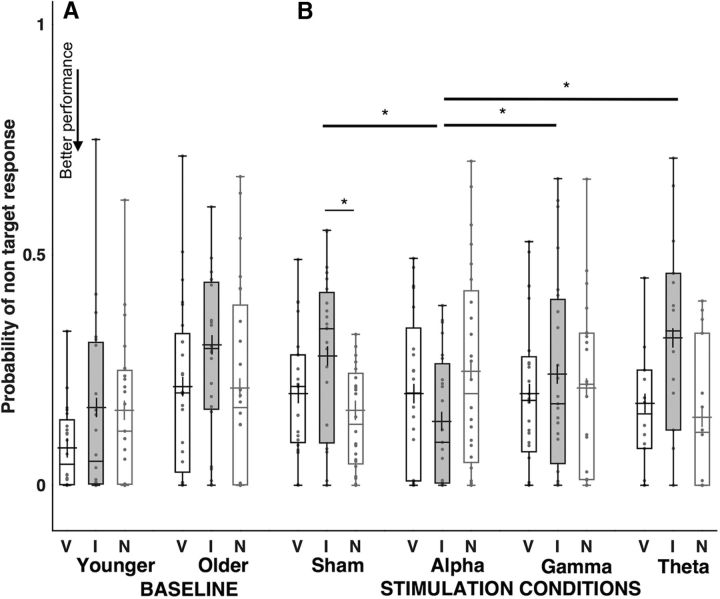
Regardless of cue type, the probability of responding to the target orientation was higher in younger relative to older participants (χ2 = 9.5, p = 0.002), a large group difference (Cohen's d = 0.92). Moreover, target responses were influenced by cue type in both younger and older participants (χ2 = 13.2, p = 0.001), because performance was worse in invalid trials (0.65, SE: 0.04) relative to valid (0.80, SE: 0.02; mean difference = −0.155, p < 0.001 corrected) and to neutral ones (0.78, SE: 0.03; mean difference = −0.13, p = 0.002 corrected).
As well as by cue type, target responses were also significantly influenced by their combination with age group (interaction retro-cue and group, χ2 = 8.05, p < 0.02), because there was an advantage for younger relative to older participants in valid trials (mean difference = 0.14, p = 0.002 corrected; Cohen's d = 0.86), and a larger cost for older relative to younger participants in invalid ones (mean difference = −0.21, p = 0.002 corrected; Cohen's d = 0.86). Remarkably, in absence of retro-cue (neutral cue), performance was equivalent in the two age groups (p = 0.9). This is supported by the Bayesian factor (BF01 = 0.35), obtained by running a Bayesian analysis on the JASP platform (v0.8.2, JASP Team; Wagenmakers et al., 2018) with default (Cauchy) prior, which suggested strong evidence (Rafery, 1995) for the similarity of target responses in the two age groups in trials with neutral retro-cues; Fig. 3A; Table 1).
Figure 3.
Probability of target responses in Experiments 1 and 2. A, Performance at prestimulation (baseline) in younger and older adults, and (B) changes following alpha-, gamma-, theta-tACS, and Sham in older participants only in valid, invalid, and neutral retro-cues. Each dot indicates a participant's performance in each condition. Cross symbols refer to the group mean, and bold lines to the comparisons most relevant for the study's hypotheses. Asterisks denote statistically significant differences (p < 0.05).
In the aging group, the cost associated with invalid trials was significantly higher relative to valid (mean difference = −0.18, p = 0.005 corrected; Cohen's d = 0.78) and neutral (i.e., un-cued) trials (mean difference = −0.23, p < 0.001 corrected; Cohen's d = 0.90), whereas in younger adults the advantage associated to valid trials was significantly higher than in neutral (mean difference = 0.10, p < 0.03 corrected, Cohen's d = 0.9) and invalid ones (mean difference = −0.12, p < 0.02 corrected, Cohen's d = 0.67; Fig. 3A; Table 1).
Inhibitory abilities—misbinding: probability of responding to non-target orientations (pNT).
Older adults made significantly more misbinding errors compared with younger participants, regardless of the retro-cueing condition (χ2 = 7.9, p = 0.005; Cohen's d = 0.79). Across age groups, the occurrence of these errors was also modulated by cue type (χ2 = 9.8, p = 0.007), because there were significantly more misbinding errors (worse performance) in invalid relative to valid trials (mean difference = 0.09, p = 0.005 corrected; Cohen's d = 0.6; Fig. 4A; Table 1). The interaction of cue type and age group did not reach significance.
Figure 4.
Misbinding errors in Experiments 1 and 2. A, Performance at prestimulation (baseline) in younger and older adults, and (B) changes following alpha-, gamma-, theta-tACS, and Sham in older participants only in valid, invalid, and neutral retro-cues. Each dot indicates a participant's performance in each condition. Cross symbols refer to the group mean, and bold lines to the comparisons most relevant for the study's hypotheses. Asterisks denote statistically significant differences (p < 0.05).
Noisiness of memory for the target item (κ).
The noisiness of memory for the target item did not change in any retro-cueing conditions across age groups (p values >0.1; Table 1).
Random error (pU).
No changes in the proportion of random responses were observed in any retro-cueing conditions across age groups (p values >0.2; Table 1).
Experiment 1 showed that age modulates memory precision as well as two indexes of inhibitory abilities, target responses and misbinding errors. Specifically, memory recall was significantly less precise, the probability of target responses lower and of non-target responses (misbinding errors) higher in aging adults compared with younger across all retro-cued trials. Moreover, in older relative to younger adults there was also a significantly higher cost in performing invalid trials and a reduced benefit of valid trials in memory recall and target response. Strikingly, in absence of retro-cue (neutral cue), target responses did not differ in the two age groups.
This pattern of results first indicates that when not influenced by the retro-cue, aging adults were as good as younger at responding to targets. Second, despite a cost in performance, aging adults' memory was still modulated by retro-cues, with a significant difference between cue types, which suggests that elderly's memory retained some flexibility. Using parietal tACS in our aging sample, Experiment 2 tested whether inhibitory abilities, measured in terms of target responses and misbinding errors, as well as memory precision, may be causally linked to specific brain oscillations.
Experiment 2
Working memory retro-cueing task with tACS in aging adults
Values reflecting accuracy (precision) and the sources of error (pT, pNT, κ, pU) in all stimulation conditions are presented in Table 2.
Table 2.
Experiments 2 and 3: accuracy (precision, P) and source of error (κ, pT, pNT, pU) in older participants in each stimulation and retro-cueing conditions
| Cueing condition | Stimulation condition (Older participants) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham |
Alpha |
Theta |
Gamma |
|||||||||||||||||
| P | κ | pT | pNT | pU | P | κ | pT | pNT | pU | P | κ | pT | pNT | pU | P | κ | pT | pNT | pU | |
| Exp 2 | ||||||||||||||||||||
| All retro-cues | 1.11 | 2.79 | 0.72 | 0.22 | 0.07 | 1.12 | 2.52 | 0.77 | 0.19 | 0.04 | 1.08 | 2.33 | 0.75 | 0.21 | 0.03 | 1.11 | 2.50 | 0.70 | 0.21 | 0.06 |
| 0.05 | 0.25 | 0.03 | 0.02 | 0.02 | 0.05 | 0.26 | 0.03 | 0.02 | 0.01 | 0.05 | 0.21 | 0.04 | 0.03 | 0.02 | 0.05 | 0.19 | 0.04 | 0.03 | 0.02 | |
| Valid | 1.12 | 2.86 | 0.77 | 0.20 | 0.07 | 1.11 | 2.30 | 0.75 | 0.20 | 0.05 | 1.15 | 2.35 | 0.81 | 0.17 | 0.02 | 1.11 | 2.54 | 0.68 | 0.20 | 0.07 |
| 0.04 | 0.46 | 0.03 | 0.03 | 0.03 | 0.05 | 0.19 | 0.04 | 0.03 | 0.02 | 0.06 | 0.28 | 0.03 | 0.04 | 0.02 | 0.05 | 0.24 | 0.05 | 0.04 | 0.03 | |
| Invalid | 1.07 | 2.85 | 0.59 | 0.29 | 0.06 | 1.11 | 2.50 | 0.84* | 0.14* | 0.04 | 1.01 | 2.52 | 0.61 | 0.33 | 0.07 | 1.10 | 2.50 | 0.68 | 0.23 | 0.06 |
| 0.05 | 0.35 | 0.05 | 0.04 | 0.03 | 0.05 | 0.32 | 0.03 | 0.03 | 0.02 | 0.06 | 0.34 | 0.06 | 0.06 | 0.05 | 0.04 | 0.32 | 0.05 | 0.04 | 0.03 | |
| Neutral | 1.14 | 2.67 | 0.82 | 0.16 | 0.08 | 1.13 | 2.47 | 0.71 | 0.24* | 0.04 | 1.13 | 2.11 | 0.85 | 0.14 | 0.01 | 1.12 | 2.47 | 0.73 | 0.21 | 0.05 |
| 0.05 | 0.30 | 0.04 | 0.03 | 0.03 | 0.05 | 0.35 | 0.05 | 0.04 | 0.02 | 0.05 | 0.22 | 0.04 | 0.04 | 0.008 | 0.05 | 0.35 | 0.04 | 0.04 | 0.03 | |
| Exp 3 | ||||||||||||||||||||
| All retro-cues | 1.09 | 2.67 | 0.73 | 0.26 | 0.11 | 1.16 | 2.86 | 0.72 | 0.22 | 0.14 | nt | |||||||||
| 0.05 | 0.23 | 0.03 | 0.02 | 0.02 | 0.06 | 0.26 | 0.04 | 0.03 | 0.04 | |||||||||||
| Task-irrelevant | 1.06 | 2.41 | 0.67 | 0.29 | 0.12 | 1.14 | 2.92 | 0.73 | 0.22 | 0.13 | ||||||||||
| 0.05 | 0.15 | 0.04 | 0.03 | 0.03 | 0.06 | 0.38 | 0.04 | 0.03 | 0.07 | |||||||||||
| Neutral | 1.11 | 2.93 | 0.78 | 0.22 | 0.09 | 1.18 | 2.79 | 0.71 | 0.22 | 0.15 | ||||||||||
| 0.05 | 0.41 | 0.03 | 0.03 | 0.03 | 0.07 | 0.27 | 0.05 | 0.04 | 0.04 | |||||||||||
Mean with SE in italics. Exp, Experiment; nt, not tested as not part of the planned investigation.
*Indicates significant difference from Sham (p < 0.05).
Overall performance: recall precision.
Precision values can be influenced by changes in any of the three sources of error, and we did not observe any significant effect of retro-cue type, stimulation condition or their combination on precision at the group level in our regression analysis of the precision data (all p values >0.1). Similar results were obtained when theta-tACS performance was considered (p values >0.4; Table 2). However, a closer inspection of the data showed a large variability in performance such that ∼2/3 of the participants showed that the fidelity with which the probe was recalled improved following alpha- and gamma-tACS, whereas in the remaining 1/3 recall precision did not improve following stimulation. These results therefore suggest that following alpha- and gamma-tACS, lack of significant group changes in precision may be because of the large individual variability in elderly's performance in this index.
Inhibitory abilities: probability to respond to the target orientation (pT).
A further GEE-based regression analysis of the modeling data with tACS conditions and cue type as predictors, shows that, however, stimulation had a marginal significant impact on target responses overall (χ2 = 5.5, p = 0.06). This is because regardless of retro-cue type, the probability of responding to the target stimulus was higher following alpha-tACS relative to Gamma (mean difference = 0.066, p < 0.03 corrected, Cohen's d = 0.51), and marginally to Sham (mean difference = 0.046, p = 0.07 corrected, Cohen's d = 0.46).
Target responses also depended on the specific cueing condition (significant interaction of stimulation and cue type, χ2 = 31.1, p < 0.001). In all but two aging adults there was a large (Cohen, 1988) and significant difference in target responses between alpha-tACS and both Sham (mean difference = 0.25, p < 0.001 corrected, Cohen's d = 1.24) and gamma-tACS (mean difference = 0.16, p = 0.001 corrected, Cohen's d = 0.82) in trials with invalid retro-cues (Table 2; Fig. 3B). Similar results were observed when theta-tACS performance was considered (χ2 = 27.2, p < 0.001), with invalid trials significantly better performed during alpha compared with theta-tACS (mean difference = 0.24, p < 0.001 corrected, Cohen's d = 0.98). Remarkably, in aging participants target responses in invalid trials following alpha-tACS did not differ from target responses in the same trials in younger participants in the prestimulation (baseline) condition. The Bayesian factor (BF01 = 0.25) obtained by running a Bayesian analysis on the JASP platform (v0.8.2, JASP Team; Wagenmakers et al., 2018) with default (Cauchy) prior, strongly (Rafery, 1995) supported this observation (Table 2; Fig. 3B). Alpha stimulation therefore successfully restored performance in the elderly. Aging adults' alpha-tACS target responses in neutral trials also did not differ from target responses in invalid trials in younger adults at baseline. Again, the Bayesian factor (BF01 = 0.31) based on a Bayesian analysis on the JASP platform (v0.8.2, JASP Team; Wagenmakers et al., 2018) with default (Cauchy) prior, strongly (Rafery, 1995) maintained this conclusion (Table 2; Fig. 3B).
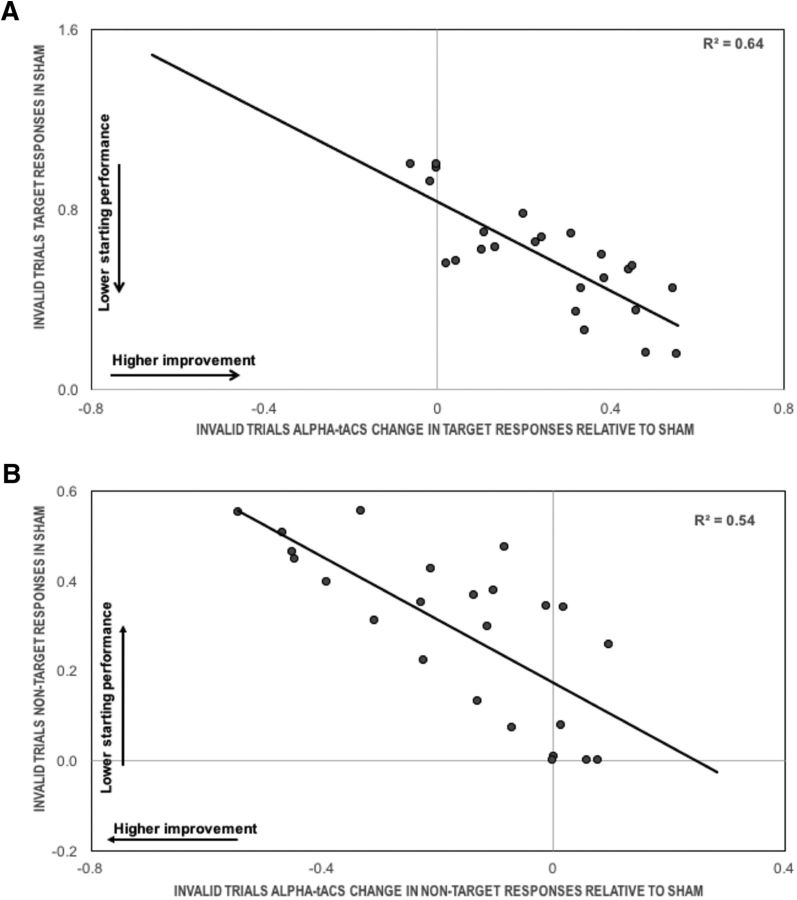
To examine the extent to which alpha-based improvement in invalid trials was modulated by individual differences in the Sham condition, which served as baseline performance, a correlation analysis was run. This showed that alpha-tACS induced changes depended on the baseline performance (Spearman's ρ correlation, r = −0.82, p < 0.001), because older participants with lower probability of target responses at the start improved the most following alpha-tACS (Fig. 5A). There was also a significant correlation between gamma-tACS target responses and baseline (r = −0.57, p = 0.003), and a trend towards significance between theta-tACS target responses and baseline (p > 0.08). We note however, that because gamma- and theta-tACS changes were not significantly different from Sham, interpreting these correlations is difficult.
Figure 5.
Alpha-tACS effects in Experiment 2 relative to baseline performance. Performance in (A) target (pT) and (B) non-target responses (pNT) in invalid retro-cues following alpha-tACS as a function of baseline performance (here Sham) in older adults.
We also observed marginally significant decreased target responses in neutral trials during alpha-tACS relative to Sham (mean difference = −0.11, p = 0.06 corrected, Cohen's d = 0.49; Fig. 3B; Table 2). Alpha-driven changes in performing these neutral trials did not significantly correlate with baseline performance. During Sham tACS, the probability of responding to the target stimuli was significantly higher in valid relative to invalid trials (mean difference = 0.17, p < 0.001, Cohen's d = 0.88), and showed a trend toward significance relative to neutral trials (mean difference = −0.05, p = 0.08, Cohen's d = 0.29). No significant changes in performance were observed following other stimulation or cueing conditions.
Inhibitory abilities—misbinding: probability of responding to non-target orientations (pNT).
Further GEE analyses of the modeling data show that stimulation significantly modulated non-target responses depending on the type of retro-cue (interaction of retro-cue and stimulation condition, χ2 = 13.8, p < 0.01). Specifically, there was a significant change in misbinding errors following alpha-tACS: relative to Sham and gamma stimulation, pNT decreased in invalid trials (mean difference = −0.17, p < 0.001, Cohen's d = 0.93, and −0.11, p < 0.02, Cohen's d = 0.5 corrected, respectively) and showed a tendency to increase in neutral ones when compared with Sham (mean difference = 0.1, p = 0.06 corrected, Cohen's d = 0.4), but not gamma-tACS (mean difference = 0.03, p = 0.6; Fig. 4B; Table 2). During Sham tACS, the probability to respond to non-target stimuli (misbinding errors, pNT) was significantly higher in invalid relative to valid trials (mean difference = −0.11, p = 0.002, Cohen's d = 0.28). No other effects reached significance (all p values >0.1). Similar results were observed when theta-tACS performance was considered (χ2 = 30.5, p < 0.001), with a significant difference between alpha- and theta-tACS performance in misbinding errors (mean difference = 0.14, p < 0.02 corrected, Cohen's d = 0.52).
To examine the extent to which these alpha-based changes were influenced by baseline performance (Sham condition), a correlation analysis was run. This showed that misbinding errors following alpha-tACS in invalidly-cued trials depended from participants' baseline performance (Spearman's ρ correlation, r24 = −0.73, p < 0.001), because older adults with higher probability of non-target responses in Sham improved the most following alpha-tACS (Fig. 5B). There was also a significant correlation between gamma-tACS misbinding errors and baseline (r24 = 0.45, p = 0.03); we note however, that because gamma-tACS changes were not significant, definite interpretations of this correlation are difficult. No significant correlation emerged between theta-tACS misbinding errors and baseline (p > 0.2).
Noisiness of memory for the target item (κ).
No significant changes in performance were observed in any stimulation or retro-cueing conditions (all p values >0.1) in aging adults. The same was found when considering theta-tACS (all p values >0.2).
Random error (pU).
No significant changes were observed in the proportion of random responses in any stimulation or retro-cueing conditions in aging participants (all p values >0.3). The same results were found when theta-tACS was taken into account (all p values >0.2).
Experiment 2 indicated for the first time that alpha-tACS successfully restored performance in aging adults who showed significantly higher probability to respond to target stimuli (pT) and lower probability of misbinding errors (pNT) relative to Sham, gamma and theta stimulation. These changes may be because alpha-tACS reduced the stimuli competition for attentional and memory resources at encoding, and attenuated the impact of the retro-cue on the probe. Reducing stimuli competition is likely to have increased the interference among the stimuli, which was especially detrimental for performing neutral trials (i.e., no retro-cue). This is because stimuli interference was not counteracted by any retro-cue, such that no item was prioritized relative to the others, and misbinding errors (pNT) therefore increased. Alpha also attenuated the effect of the retro-cue such that responses were less corrupted by the cued item, an advantage that was particularly strong in invalid trials since prestimulation they corresponded to the largest cost in performance and therefore allowed larger improvements. Strikingly, post-alpha the probability of target responses in aging adults' in invalid trials did not differ from baseline performance in younger adults.
Experiment 3
WM retro-cueing task with task-irrelevant retro-cues in aging adults
The alpha-driven improvement in target responses in aging participants may be due to reduced stimuli competition and to the attenuation of the retro-cue effect, which especially reduced the impact on performance of the invalidly prioritized item. In aging, however, retro-cues also act as distractors (Duarte et al., 2013; Newsome et al., 2015), leaving unclear whether alpha may have had a role in suppressing distractors. To assess this, we designed a third experiment using the identical experimental paradigm as Experiments 1 and 2, and with an equally distracting but task-irrelevant retro-cueing condition. This consisted of a retro-cue of a color that never belonged to the arrow stimuli (pink), and therefore was not intended to modulate response to the target orientation.
The same parameters as in Experiments 1 and 2 reflecting accuracy (precision) and the sources of error (pT, pNT, κ, pU) in both stimulation conditions are presented in Table 2. As for the previous analyses, independent regression analyses were run for each value, based on the GEE with gamma regression and loglog link, with tACS conditions (Sham, Alpha) and retro-cue type (task-irrelevant and neutral) as predictors.
We found no significant change in participants' recall precision in any of the retro-cueing or stimulation conditions (no main effects or interactions, all p values >0.09). Likewise, there was no significant change in κ (all p values >0.27), in the proportion of non-target (pNT, all p values >0.2), and random responses (pU, all p values >0.36). We also observed no changes in target responses (pT, all p values >0.09).
These results showed that in absence of stimulation, task-irrelevant retro-cues did not significantly affect aging adults' performance as task-relevant retro-cues (Experiments 1 and 2) did. We note, however, that there was a nonsignificant tendency for lower probability of responding to target stimuli (pT), and higher to non-target ones (pNT) in trials with task-irrelevant retro-cues compared with neutral ones during Sham stimulation (Fig. 6A,B; Table 2). This suggests that task-irrelevant retro-cues may have had a marginal distracting effect. However, the data exclude that retro-cues effects could be solely attributed to distractibility, and do not completely rule out the possibility that participants processed the task-irrelevant retro-cue as if it was a neutral one. Because there was no strong effect of these task-irrelevant cues to start with (i.e., in Sham), the effect of alpha-tACS was negligible.
Figure 6.
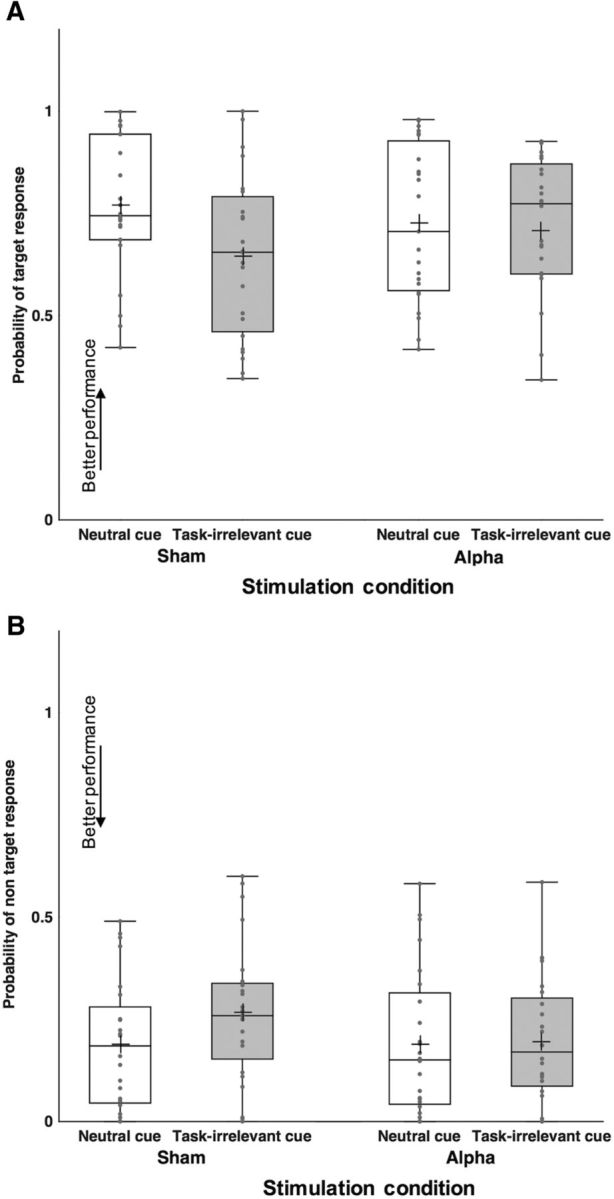
Results of Experiment 3. Performance changes in (A) target (pT) and (B) non-target responses (pNT) in task-irrelevant and neutral cues following alpha-tACS and Sham in aging participants. Each dot indicates a participant's performance in each condition. Cross symbols refer to the group mean.
To find out whether testing the same group of older adults may have led to significant learning effects in the results, performance (target responses, pT) in valid trials was compared across experimental sessions in chronological order, regardless of the stimulation received. Valid trials were used because they were not significantly modulated by any stimulation condition, therefore allowing identifying any learning effect more clearly. A Kruskal–Wallis H test showed that there was no significant difference in performance across testing sessions (χ22 = 1.562, p = 0.458), therefore excluding significant learning effects in aging participants' performance.
Discussion
Using brain stimulation coupled with a WM retro-cueing paradigm, we investigated whether in the aging brain alpha oscillations may be causally linked to inhibitory abilities, namely the capacity of ignoring task-irrelevant information. We purposefully choose to experiment this causal link in the aging brain because both these oscillations and abilities are known to deteriorate with aging (Hasher and Zacks, 1988; Salthouse and Meinz, 1995; Gazzaley et al., 2005; Hasher et al., 2007; Klimesch et al., 2007; Vaden et al., 2012). We therefore reasoned that any alpha-tACS-induced changes in inhibition may more clearly reveal a causal link between alpha oscillations and these abilities. In a prestimulation condition, we first established that inhibitory abilities were significantly poorer in aging than in younger adults. To probe the possible causal link between alpha and inhibition in aging adults, we subsequently compared parietal alpha-tAC stimulation with either no stimulations (Sham) or with two control stimulation conditions (theta- and gamma-tACS). Stimulation was combined with a WM retro-cueing paradigm that measures overall WM recall precision as well as the source of error in performance including indexes of inhibitory abilities, i.e., probability of target and of non-target responses (Gazzaley and Nobre, 2012; Pertzov et al., 2013).
Our analyses yielded several new findings. First, we showed that during prestimulation there was an advantage in younger but not older participants for valid retro-cued trials. There was also an overall cost in performing invalid retro-cued trials, which was significantly higher in older than younger participants. Specifically, older adults showed a lower probability to respond to target stimuli (smaller pT) in invalid relative to valid and neutral retro-cues, and more misbinding errors (larger probability to respond to non-target stimuli, pNT) regardless of the retro-cue, similar to previous reports (Pertzov et al., 2012; Peich et al., 2013). Remarkably, however, in absence of retro-cue (i.e., in neutral trials) aging participants' target responses were equivalent to younger adults. Together these results indicate that aging adults were still susceptible to the effect of retro-cues, suggesting that their memory retained some flexibility, similar to younger adults (Zokaei et al., 2014). Second, we found that relative to Sham, parietal alpha-tACS in aging participants resulted in an increased probability of target responses, specifically in trials with invalid retro-cues. Therefore, the cost in performance associated with invalid retro-cues was significantly ameliorated following alpha parietal-tACS, and no longer differed from younger adults. In the context of these invalid retro-cues, we also observed fewer misbinding errors after stimulation. Last, recall precision was significantly decreased relative to younger adults similar to previous reports (Peich et al., 2013), and changed in most of our older adults following alpha and gamma stimulation, but because of the high variability within the sample this change did not reach significance at a group level.
Older adults do not benefit from visual retro-cue
Prestimulation, older adults were influenced by the retro-cue but differently from younger, there was no advantage for valid trials and an enlarged cost in invalid trials (fewer target responses and increased misbinding errors, respectively). Weaker performance in invalid trials may be due to impoverished redirection of attention and reduced ability to suppress task-irrelevant information held in WM, which are known to decline with aging (Hasher and Zacks, 1988; Craik and Salthouse, 2000; Gazzaley et al., 2005; Hasher et al., 2007). In contrast, our younger participants showed a significant advantage, i.e., higher probability of target responses, in performing valid retro-cues consistent with previous studies (Pertzov et al., 2013). This advantage may reflect a combination of factors, such as facilitated recall, which can be initiated with the item prioritized in WM by the retro-cue, as well as an increased robustness to interference from task-irrelevant stimuli, and extra protection of the retro-cued item from temporal decay (Pertzov et al., 2013).
Research on the role of retro-cue in aging adults showed that this may either improve performance (Mok et al., 2016) or not (Duarte et al., 2013; Newsome et al., 2015). Methodological differences may account for this inconsistency, such as the use of binary relative to continuous, analog responses as in our task, or the use of only valid retro-cues compared with a combination of valid and invalid retro-cues as in our experiment. Paradigms with only valid retro-cues may induce participants to “trust” the information provided by the cue, and they also do not tax on inhibitory abilities as much as our paradigm did (Gazzaley and Nobre, 2012; Pertzov et al., 2013). These factors may explain our aging adults' lack of advantage for valid trials and the cost associated with the invalid ones. This cost in performance represents an important novelty in our study because it revealed residual memory functioning and the flexibility of older adults' memory because elderly performed significantly different in the valid and invalid retro-cues. Retro-cues may have also lead to weaker performance because they act as distractors, and consequently diverted participants' attention (Healey et al., 2008; Duarte et al., 2013; Newsome et al., 2015). However, we specifically explored this issue in Experiment 3 and found that distractibility alone did not account for significant costs in performance.
Cognitive and physiological changes in inhibitory abilities associated with parietal alpha-tACS
Parietal alpha stimulation resulted in improved target responses, specifically in the context of invalid retro-cues, and in a change in misbinding errors that increased in neutral retro-cue (no retro-cue) and decreased in invalid ones. Alpha oscillations are linked to inhibitory processes, such that, for instance, in young adults strong alpha modulation corresponds to less interference from distractors (Klimesch et al., 2007; Scheeringa et al., 2009; Jensen and Mazaheri, 2010; Bonnefond and Jensen, 2012). We therefore suggest that the inhibitory nature of alpha-tACS may have suppressed the interstimuli conflict at encoding, whereby stimuli typically compete with each other for WM resources (Desimone and Duncan, 1995; Berryhill et al., 2012; Bonnefond and Jensen, 2012), and it also attenuated the retro-cue effect. The reduction of the interstimuli conflict at encoding may have increased the interstimuli interference, a change that was not offset in neutral retro-cues trials. Hence this resulted in impoverished performance, i.e., fewer target responses and more misbinding errors in our aging participants. Moreover, the diminished effect of the retro-cue resulted in increased target responses especially in invalid retro-cues because prestimulation these retro-cues were associated with the largest cost in performance. Therefore, weakening the effect of these cues resulted in the largest increase in target responses.
Evidence of the link between alpha oscillations and inhibitory abilities has so far been mainly correlational and based on EEG studies (Jensen and Mazaheri, 2010; Klimesch, 2012). Recently, evidence of the causal role of alpha in inhibition came from a tACS experiment in younger participants (Helfrich et al., 2014). Older adults allowed us to further establish the role of alpha oscillations in inhibitory abilities by showing that these oscillations continue to be causally linked to inhibition later in life. tACS has been implemented to either entrain (Ozen et al., 2010; Zaehle et al., 2010; but see Vossen et al., 2015) or desynchronize oscillatory activity (Strüber et al., 2014; Guerra et al., 2016). We suggest that in our aging participants improved inhibitory abilities following stimulation may be due to alpha-tACS amplifying neuronal activity in the frontoparietal network based on the phenomenon of resonance (Buzsaki, 2006). Resonance entails that matching the endogenous oscillation of brain networks supporting a particular cognitive task with the frequency of tAC stimulation may result in augmenting the activity of these networks and their coherence, i.e., neuronal synchronization (Herrmann et al., 2013). This is because tACS is thought to promote a wider recruitment of neurons specific for a cognitive function into rhythmically firing networks (Herrmann et al., 2013; Battleday et al., 2014), which in turns is likely to result in behavioral changes in activities subserved by these neurons.
In our aging adults, parietal alpha-tACS may have helped recruiting a larger population of neurons engaged in inhibition. By triggering a top-down mechanism that inhibited the processing of task-irrelevant information (Sauseng et al., 2009; Zanto and Gazzaley, 2009; Jensen and Mazaheri, 2010; Hanslmayr et al., 2011), alpha stimulation may in turn have significantly increased target responses and reduced misbinding errors especially in trials associated with the largest cost in performance prestimulation. These improvements were particularly noticeable in an aging sample because both alpha oscillations and inhibitory abilities are impoverished (Hasher and Zacks, 1988; Salthouse and Meinz, 1995; Gazzaley et al., 2005; Hasher et al., 2007; Klimesch et al., 2007). Our data provide evidence that alpha oscillations represent a potentially viable inhibitory mechanism in the elderly, which could be reinforced via non-invasive neurostimulation. However, alternative explanations cannot be excluded, for instance that older people may compensate the alteration of alpha oscillations with other mechanisms during memory retention, which allow significant residual WM performance (Leenders et al., 2016).
Our results also showed that recall precision did not change significantly following any stimulation conditions at a group level. However, precision did improve in most aging adults following alpha and gamma stimulation, suggesting that lack of significant group changes in this index may be due to the large individual variability in our aging sample.
In sum, we studied whether in aging adults alpha oscillations and inhibitory abilities are causally linked. We combined tACS with a retro-cue paradigm assessing WM and inhibitory abilities and found alpha-tACS induced improvement in both target responses and misbinding errors, two indexes of inhibitory abilities. Most aging adults also showed a tendency to improved memory recall following alpha and gamma-tACS. We therefore concluded that in aging alpha oscillations are causally linked to inhibitory abilities and that, despite being impoverished, these abilities can still be changed.
Footnotes
This work was supported by a Royal Society Dorothy Hodgkin Fellowship (502008.K518/SLB) and a British Academy Grant (SG090611). We thank Giulia Grande for help with data collection and two anonymous reviewers for their helpful comments.
The authors declare no competing financial interests.
References
- Alvarez GA, Cavanagh P (2004) The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychol Sci 15:106–111. 10.1111/j.0963-7214.2004.01502006.x [DOI] [PubMed] [Google Scholar]
- Antal A, Paulus W (2013) Transcranial alternating current stimulation (tACS). Front Hum Neurosci 7:317. 10.3389/fnhum.2013.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle DE, Nobre AC, Scerif G (2012) Attentional control constrains visual short-term memory: insights from developmental and individual differences. Q J Exp Psychol 65:277–294. 10.1080/17470218.2010.492622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar E, Başar Eroglu C, Karakaş S, Schürmann M (2001) Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 39:241–248. 10.1016/S0167-8760(00)00145-8 [DOI] [PubMed] [Google Scholar]
- Battleday RM, Muller T, Clayton MS, Cohen Kadosh R (2014) Mapping the mechanisms of transcranial alternating current stimulation: a pathway from network effects to cognition. Front Psychiatry 5:162. 10.3389/fpsyt.2014.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays PM, Husain M (2008) Dynamic shifts of limited working memory resources in human vision. Science 321:851–854. 10.1126/science.1158023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays PM, Catalao RF, Husain M (2009) The precision of visual working memory is set by allocation of a shared resource. J Vis 9(10):7 1–11. 10.1167/9.10.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays PM, Wu EY, Husain M (2011) Storage and binding of object features in visual working memory. Neuropsychologia 49:1622–1631. 10.1016/j.neuropsychologia.2010.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Richmond LL, Shay CS, Olson IR (2012) Shifting attention among working memory representations: testing cue types, awareness, and strategic control. Q J Exp Psychol 65:426–438. 10.1080/17470218.2011.604786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O (2012) Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr Biol 22:1969–1974. 10.1016/j.cub.2012.08.029 [DOI] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O (2013) The role of gamma and alpha oscillations for blocking out distraction. Commun Integr Biol 6:e22702. 10.4161/cib.22702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. (2006) Rhythms of the brain. Oxford, UK: Oxford UP. [Google Scholar]
- Buzsáki G, Wang XJ (2012) Mechanisms of gamma oscillations. Annu Rev Neurosci 35:203–225. 10.1146/annurev-neuro-062111-150444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Baldassarre A, Sestieri C, Spadone S, Romani GL, Corbetta M (2017) Task and regions specific top-down modulation of alpha rhythms in parietal cortex. Cereb Cortex 27:4815–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecere R, Rees G, Romei V (2015) Individual differences in alpha frequency drive crossmodal illusory perception. Curr Biol 25:231–235. 10.1016/j.cub.2014.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioral sciences. New York, NY: Routledge Academic. [Google Scholar]
- Constantinidis C, Klingberg T (2016) The neuroscience of working memory capacity and training. Nat Rev Neurosci 17:438–449. 10.1038/nrn.2016.43 [DOI] [PubMed] [Google Scholar]
- Craik FIM, Salthouse TA (2000) The handbook of aging and cognition, Ed 2 Mahwah, NJ: Erlbaum. [Google Scholar]
- Desimone R, Duncan J (1995) Neural mechanisms of selective visual attention. Annu Rev Neurosci 18:193–222. 10.1146/annurev.ne.18.030195.001205 [DOI] [PubMed] [Google Scholar]
- Duarte A, Hearons P, Jiang Y, Delvin MC, Newsome RN, Verhaeghen P (2013) Retrospective attention enhances visual working memory in the young but not the old: an ERP experiment. Psychophysiology 50:465–476. 10.1111/psyp.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W (2001) Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2:704–716. 10.1038/35094565 [DOI] [PubMed] [Google Scholar]
- Fertonani A, Pirulli C, Miniussi C (2011) Random noise stimulation improves neuroplasticity in perceptual learning. J Neurosci 31:15416–15423. 10.1523/JNEUROSCI.2002-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC (2011) The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol 2:154. 10.3389/fpsyg.2011.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu KM, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE (2001) Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Brain Res Cogn Brain Res 12:145–152. 10.1016/S0926-6410(01)00034-9 [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG (2006) Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117:845–850. 10.1016/j.clinph.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC (2012) Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci 16:129–135. 10.1016/j.tics.2011.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M (2005) Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci 8:1298–1300. 10.1038/nn1543 [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D'Esposito M (2008) Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A 105:13122–13126. 10.1073/pnas.0806074105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoraptis N, Catalao RF, Bays PM, Husain M (2011) Dynamic updating of working memory resources for visual objects. J Neurosci 31:8502–8511. 10.1523/JNEUROSCI.0208-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gözenman F, Tanoue RT, Metoyer T, Berryhill ME (2014) Invalid retro-cues can eliminate the retro-cue benefit: evidence for a hybridized account. J Exp Psychol Hum Percept Perform 40:1748–1754. 10.1037/a0037474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin IC, Nobre AC (2003) Orienting attention to locations in internal representations. J Cogn Neurosci 15:1176–1194. 10.1162/089892903322598139 [DOI] [PubMed] [Google Scholar]
- Guerra A, Pogosyan A, Nowak M, Tan H, Ferreri F, Di Lazzaro V, Brown P (2016) Phase dependency of the human primary motor cortex and cholinergic inhibition cancelation during beta tACS. Cereb Cortex 26:3977–3990. 10.1093/cercor/bhw245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Gross J, Klimesch W, Shapiro KL (2011) The role of alpha oscillations in temporal attention. Brain Res Rev 67:331–343. 10.1016/j.brainresrev.2011.04.002 [DOI] [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks RT (2007) Inhibitory mechanisms and the control of attention. In: Variation in working memory (Conway A, Jarrold C, Kane M, Miyake A, Townse J, eds), pp 227–249. New York, NY: Oxford UP. [Google Scholar]
- Hasher L, Zacks RT (1988) Working memory, comprehension, and aging: a review and a new view. In: The psychology of learning and motivation (Bower GH, ed), pp 193–225. San Diego, CA: Academic. [Google Scholar]
- Healey MK, Campbell KL, Hasher L (2008) Cognitive aging and increased distractibility: costs and potential benefits. Prog Brain Res 169:353–363. 10.1016/S0079-6123(07)00022-2 [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS (2014) Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol 24:333–339. 10.1016/j.cub.2013.12.041 [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Munk MH, Engel AK (2004) Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci 8:347–355. 10.1016/j.tics.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Rach S, Neuling T, Strüber D (2013) Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front Hum Neurosci 7:279. 10.3389/fnhum.2013.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O. (2006) Maintenance of multiple working memory items by temporal segmentation. Neuroscience 139:237–249. 10.1016/j.neuroscience.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP (2007) Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30:317–324. 10.1016/j.tins.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A (2010) Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4:186. 10.3389/fnhum.2010.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ (2006) Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol 95:3844–3851. 10.1152/jn.01234.2005 [DOI] [PubMed] [Google Scholar]
- Klimesch W. (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev 29:169–195. 10.1016/S0165-0173(98)00056-3 [DOI] [PubMed] [Google Scholar]
- Klimesch W. (2012) Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 16:606–617. 10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S (2007) EEG alpha oscillations: the inhibition–timing hypothesis. Brain Res Rev 53:63–88. 10.1016/j.brainresrev.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Landman R, Spekreijse H, Lamme VA (2003) Large capacity storage of integrated objects before change blindness. Vis Res 43:149–164. 10.1016/S0042-6989(02)00402-9 [DOI] [PubMed] [Google Scholar]
- Leenders MP, Lozano-Soldevilla D, Roberts MJ (2016) Diminished alpha lateralization during working memory but not during attentional cueing in older adults. Cerebral Cortex 28:21–32. 10.1093/cercor/bhw345 [DOI] [PubMed] [Google Scholar]
- Ma WJ, Husain M, Bays PM (2014) Changing concepts of working memory (2014). Nat Neurosci 17:347–356. 10.1038/nn.3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovski T, Jiang YV (2007) Distributing versus focusing attention in visual short-term memory. Psychonom Bull Rev 14:1072–1078. 10.3758/BF03193093 [DOI] [PubMed] [Google Scholar]
- Makovski T, Sussman R, Jiang YV (2008) Orienting attention in visual working memory reduces interference from memory probes. J Exp Psychol Learn Mem Cogn 34:369–380. 10.1037/0278-7393.34.2.369 [DOI] [PubMed] [Google Scholar]
- Marshall L, Binder S (2013) Contribution of transcranial oscillatory stimulation to research on neural networks: an emphasis on hippocampo-neocortical rhythms. Front Hum Neurosci 7:614. 10.3389/fnhum.2013.00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M, Luck SJ, Vecera SP (2007) Attention effects during visual short-term memory maintenance: protection or prioritization? Percept Psychophys 69:1422–1434. 10.3758/BF03192957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Pellouchoud E, Smith ME, Gevins A (2001) Neurophysiological signals of working memory in normal aging. Brain Res Cogn Brain Res 11:363–376. 10.1016/S0926-6410(01)00009-X [DOI] [PubMed] [Google Scholar]
- Mok RM, Myers NE, Wallis G, Nobre AC (2016) Behavioral and neural markers of flexible attention over working memory in aging. Cereb Cortex 26:1831–1842. 10.1093/cercor/bhw011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung JI, Cavagnaro DR, Pitt MA (2013) A tutorial on adaptive design optimization. J Math Psychol 57:53–67. 10.1016/j.jmp.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome RN, Duarte A, Pun C, Smith VM, Ferber S, Barense MD (2015) A retroactive spatial cue improved VSTM capacity in mild cognitive impairment and medial temporal lobe amnesia but not in healthy older adults. Neuropsychologia 77:148–157. 10.1016/j.neuropsychologia.2015.08.017 [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Praamstra P (2001) The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol 112:713–719. 10.1016/S1388-2457(00)00527-7 [DOI] [PubMed] [Google Scholar]
- Ozen S, Sirota A, Belluscio MA, Anastassiou CA, Stark E, Koch C, Buzsáki G (2010) Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci 30:11476–11485. 10.1523/JNEUROSCI.5252-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin BL, Ekhtiari H, Walsh VF (2015) Non-invasive human brain stimulation in cognitive neuroscience: a primer. Neuron 87:932–945. 10.1016/j.neuron.2015.07.032 [DOI] [PubMed] [Google Scholar]
- Peich MC, Husain M, Bays PM (2013) Age-related decline of precision and binding in visual working memory. Psychol Aging 28:729–743. 10.1037/a0033236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertzov Y, Bays PM, Joseph S, Husain M (2013) Rapid forgetting prevented by retrospective attention cues. J Exp Psychol Hum Percept Perform 39:1224–1231. 10.1037/a0030947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertzov Y, Dong MY, Peich MC, Husain M (2012) Forgetting what was where: the fragility of object–location binding. PLoS One 7:e48214. 10.1371/journal.pone.0048214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch C, Campo P, Barnes GR (2014) Modulation of alpha and gamma oscillations related to retrospectively orienting attention within working memory. Eur J Neurosci 40:2399–2405. 10.1111/ejn.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafery AE. (1995) Bayesian model selection in social research. In Sociological methodology (Marsden PV, ed), pp 111–196. Cambridge, MA: Blackwell. [Google Scholar]
- Ray S, Maunsell JH (2015) Do gamma oscillations play a role in cerebral cortex? Trends Cogn Sci 19:78–85. 10.1016/j.tics.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerko L, Oberauer K (2013) Focused, unfocused, and defocused information in working memory. J Exp Psychol Learn Mem Cogn 39:1075–1096. 10.1037/a0031172 [DOI] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G (2007) Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci 25:603–610. 10.1111/j.1460-9568.2007.05278.x [DOI] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G (2009) A bias for posterior a-band power suppression versus enhancement during shifting versus maintenance of spatial attention. Neuroimage 44:190–199. 10.1016/j.neuroimage.2008.08.022 [DOI] [PubMed] [Google Scholar]
- Romei V, Gross J, Thut G (2010) On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci 30:8692–8697. 10.1523/JNEUROSCI.0160-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Thut G, Mok RM, Schyns PG, Driver J (2012) Causal implication by rhythmic transcranial magnetic stimulation of alpha frequency in feature-based local vs. global attention. Eur J Neurosci 35:968–974. 10.1111/j.1460-9568.2012.08020.x [DOI] [PubMed] [Google Scholar]
- Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ (2012) Gamma-band activity in human prefrontal cortex codes for the number of items maintained during working memory. J Neurosci 32:12411–12420. 10.1523/JNEUROSCI.0421-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Meinz EJ (1995) Aging, inhibition, working memory, and speed. J Gerontol B Psychol Sci Soc Sci 50:P297–306. [DOI] [PubMed] [Google Scholar]
- Santarnecchi E, Polizzotto NR, Godone M, Giovannelli F, Feurra M, Matzen L, Rossi A, Rossi S (2013) Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr Biol 23:1449–1453. 10.1016/j.cub.2013.06.022 [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W (2008) What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci Biobehav Rev 32:1001–1013. 10.1016/j.neubiorev.2008.03.014 [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC (2009) Brain oscillatory substrates of visual short-term memory capacity. Curr Biol 19:1846–1852. 10.1016/j.cub.2009.08.062 [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Petersson KM, Oostenveld R, Norris DG, Hagoort P, Bastiaansen MC (2009) Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage 44:1224–1238. 10.1016/j.neuroimage.2008.08.041 [DOI] [PubMed] [Google Scholar]
- Strüber D, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS (2014) Antiphasic 40 hz oscillatory current stimulation affects bistable motion perception. Brain Topogr 27:158–171. 10.1007/s10548-013-0294-x [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Kreiter A, Bertrand O (1999) Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis Neurosci 16:449–459. [DOI] [PubMed] [Google Scholar]
- Tanoue RT, Berryhill ME (2012) The mental wormhole: internal attention shifts without regard for distance. Atten Percept Psychophyss 74:1199–1215. 10.3758/s13414-012-0305-0 [DOI] [PubMed] [Google Scholar]
- Tavakoli AV, Yun K (2017) Transcranial alternating current stimulation (tACS) mechanisms and protocols. Front Cell Neurosci 11:214. 10.3389/fncel.2017.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A (2006) Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26:9494–9502. 10.1523/JNEUROSCI.0875-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Schyns PG, Gross J (2011) Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front Psychol 2:170. 10.3389/fpsyg.2011.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Miniussi C, Gross J (2012) The functional importance of rhythmic activity in the brain. Curr Biol 22:R658–R663. 10.1016/j.cub.2012.06.061 [DOI] [PubMed] [Google Scholar]
- Tuladhar AM, ter Huurne N, Schoffelen JM, Maris E, Oostenveld R, Jensen O (2007) Parieto-occipital sources account for the increase in alpha activity with working memory load. Hum Brain Mapp 28:785–792. 10.1002/hbm.20306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaden RJ, Hutcheson NL, McCollum LA, Kentros J, Visscher KM (2012) Older adults, unlike younger adults, do not modulate alpha power to suppress irrelevant information. Neuroimage 63:1127–1133. 10.1016/j.neuroimage.2012.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen A, Gross J, Thut G (2015) Alpha power increase after transcranial alternating current stimulation at alpha frequency (α-tACS) reflects plastic changes rather than entrainment. Brain Stimul 8:499–508. 10.1016/j.brs.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers EJ, Love J, Marsman M, Jamil T, Ly A, Verhagen J, Selker R, Gronau QF, Dropmann D, Boutin B, Meerhoff F, Knight P, Raj A, van Kesteren EJ, van Doorn J, Šmíra M, Epskamp S, Etz A, Matzke D, de Jong T, et al. (2018) Bayesian inference for psychology. Part II: Example applications with JASP. Psychon Bull Rev 25:58–76. 10.3758/s13423-017-1323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle T, Rach S, Herrmann CS (2010) Transcranial alternating current stimulation enhances individual alpha activity in human EEG. Plos One 5:e13766. 10.1371/journal.pone.0013766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A (2009) Neural suppression of irrelevant information underlies optimal working memory performance. J Neurosci 29:3059–3066. 10.1523/JNEUROSCI.4621-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zokaei N, Ning S, Manohar S, Feredoes E, Husain M (2014) Flexibility of representational states in working memory. Front Hum Neurosci 8:853. 10.3389/fnhum.2014.00853 [DOI] [PMC free article] [PubMed] [Google Scholar]