Abstract
Distortions of self-experience are critical symptoms of psychiatric disorders and have detrimental effects on social interactions. In light of the immense need for improved and targeted interventions for social impairments, it is important to better understand the neurochemical substrates of social interaction abilities. We therefore investigated the pharmacological and neural correlates of self- and other-initiated social interaction. In a double-blind, randomized, counterbalanced, crossover study 24 healthy human participants (18 males and 6 females) received either (1) placebo + placebo, (2) placebo + lysergic acid diethylamide (LSD; 100 μg, p.o.), or (3) ketanserin (40 mg, p.o.) + LSD (100 μg, p.o.) on three different occasions. Participants took part in an interactive task using eye-tracking and functional magnetic resonance imaging completing trials of self- and other-initiated joint and non-joint attention. Results demonstrate first, that LSD reduced activity in brain areas important for self-processing, but also social cognition; second, that change in brain activity was linked to subjective experience; and third, that LSD decreased the efficiency of establishing joint attention. Furthermore, LSD-induced effects were blocked by the serotonin 2A receptor (5-HT2AR) antagonist ketanserin, indicating that effects of LSD are attributable to 5-HT2AR stimulation. The current results demonstrate that activity in areas of the “social brain” can be modulated via the 5-HT2AR thereby pointing toward this system as a potential target for the treatment of social impairments associated with psychiatric disorders.
SIGNIFICANCE STATEMENT Distortions of self-representation and, potentially related to this, dysfunctional social cognition are central hallmarks of various psychiatric disorders and critically impact disease development, progression, treatment, as well as real-world functioning. However, these deficits are insufficiently targeted by current treatment approaches. The administration of lysergic acid diethylamide (LSD) in combination with functional magnetic resonance imaging and real-time eye-tracking offers the unique opportunity to study alterations in self-experience, their relation to social cognition, and the underlying neuropharmacology. Results demonstrate that LSD alters self-experience as well as basic social cognition processing in areas of the “social brain”. Furthermore, these alterations are attributable to 5-HT2A receptor stimulation, thereby pinpointing toward this receptor system in the development of pharmacotherapies for sociocognitive deficits in psychiatric disorders.
Keywords: eye-tracking, hallucinogens, joint attention, psychedelics, serotonin, social cognition
Introduction
The coherent experience and sense of our “self” is a critical feature of human waking consciousness (Northoff, 2013; Carhart-Harris et al., 2014). The so-called “minimal self” incorporates immediate aspects of self-experience such as senses of ownership, agency, and embodiment, and gives rise to the “narrative self” involving cognition and conceptual thought about memories of the past and intentions toward the future (Gallagher, 2000). Distortions of self-experience, particularly of the fundamental, minimal self, are a critical symptom of major psychiatric disorders such as depression, personality disorder, and schizophrenia (Grimm et al., 2009; Moutoussis et al., 2014b; Nordgaard and Parnas, 2014; Picard and Friston, 2014; Gerrans, 2015).
Furthermore, the concept of the self is closely intertwined with the concept of the other (Decety and Sommerville, 2003; Metzinger and Gallese, 2003). The ability to distinguish between self and other plays a crucial role in establishing a coherent self-representation (Moutoussis et al., 2014a,b). Moreover, important dimensions of the self, such as awareness of individuality, are only meaningful when considering the self in the context of the other (Decety and Sommerville, 2003). In light of the immense need for improved and targeted interventions for transdiagnostic social impairments, it is important to better understand the neurochemical substrates of our sense of self and social interaction abilities, and the importance of coherence of self-experience for social interactions (Crockett and Fehr, 2014; Schilbach, 2016).
Pharmacological neuroimaging provides the opportunity to study specific neurotransmitter systems that are involved in psychiatric disorders and lead to a mechanistic understanding of clinically relevant processes underlying our sense of self and social cognition (Anticevic et al., 2013; Crockett and Fehr, 2014). A unique opportunity to study the self is to identify the neuronal correlates of pharmacologically induced altered states of consciousness in which the experience of the self is temporarily altered. Lysergic acid diethylamide (LSD) is a classic hallucinogen that transiently produces, dependent on dose, discrete to profound changes in the sense of self, such as a loosening of self boundaries, a state of oneness with the external world, and changes in meaning processing and self-relevance (Geyer and Vollenweider, 2008; Schmid et al., 2015; Preller and Vollenweider, 2016; Preller et al., 2017). LSD has predominantly agonist activity at 5-HT2A/C,-1A/B,-6, and -7, and dopamine (D2 and D1) receptors (Rs; Marona-Lewicka et al., 2002; Nichols, 2004; De Gregorio et al., 2016). Pretreatment with the selective 5-HT2A and α-adreno R antagonist ketanserin has been shown to block the overall subjective effects of LSD and therefore offers the possibility to investigate the specific contribution of the 5-HT2A R system to self-processing and its relationship to social interaction (Preller et al., 2017).
Therefore, the present study set out to investigate the role of the 5-HT2A R system in self- and other-initiated social interaction by combining neuroimaging with pharmacological manipulations (LSD with and without ketanserin pretreatment, and placebo). The applied task was specifically designed to capture the reciprocal and interactive nature of social encounters where participants engage in a gaze-based interaction with an anthropomorphic virtual character in real time (Schilbach et al., 2010; Preller et al., 2014) and furthermore giving the opportunity to investigate self- versus other-initiated interaction, as well as joint- and non-joint attention processing. Engagement in joint attention is considered to reflect our understanding of another person's point of view and a perquisite of advanced social skills such as theory of mind (Charman et al., 2000; Shepherd, 2010). Impaired joint attention processing is found in neurodevelopmental and psychiatric disorders (Charman, 2003; Preller et al., 2014; Timmermans and Schilbach, 2014). Furthermore, the 5-HT system is suggested to play an important role in illnesses, such as autism spectrum disorders, which are characterized by deficits in joint attention execution and processing (Muller et al., 2016; Bolis and Schilbach, 2017). We therefore hypothesized that LSD, at the dose tested, leads to alterations in self-experience, in particular, experiences of loosening of self-boundaries accompanied by decreased differentiation between self and other, and thus modulates the processing of self- versus other-initiated social interaction. Joint attention, i.e., an integration of perspectives while maintaining a robust sense of self can be disturbed by the loosening of self-boundaries as for instance in schizophrenia (Fuchs, 2015). Based on this line of thought, we furthermore hypothesized that LSD would affect measures of joint attention both behaviorally as well as at the neural level in brain areas relevant for self-processing as well as social cognition such as the precuneus and posterior cingulate cortex (PCC), temporal cortex, and medial prefrontal cortex (Schilbach et al., 2012). Additionally, we hypothesized that LSD-induced alterations of self- and social-interaction processing would be at least partially blocked by ketanserin.
Materials and Methods
Participants
Participants were recruited through advertisements placed in local universities. Before inclusion in the study, participants underwent a screening visit 1 week before the first test day. All included subjects were healthy according to medical history, physical examination, blood analysis, and electrocardiography and had normal or corrected-to-normal vision. The Mini-International Neuropsychiatric Interview (Sheehan et al., 1998), the DSM-IV self-rating questionnaire for Axis-II personality disorders (SCID-II; Fydrich et al., 1997), and the Hopkins Symptom Checklist (SCL-90-R; Franke, 1995) were used to exclude subjects with present or previous psychiatric disorders or a history of major psychiatric disorders in first-degree relatives. Further exclusion criteria included left-handedness, poor knowledge of the German language, cardiovascular disease, history of head injury or neurological disorder, history of alcohol or illicit drug dependence, magnetic resonance imaging (MRI) exclusion criteria including claustrophobia, and previous significant adverse reactions to a hallucinogenic drug. Participants were asked to abstain from the use of any prescription or illicit drugs for a minimum of 2 weeks before the first test day and for the duration of the entire study, and to abstain from drinking alcohol for at least 24 h before test days. Urine tests and self-report questionnaires were used to verify the absence of drug and alcohol use. Urine tests of all participants included in the study were negative on screening and each test day. Urine tests were also used to exclude pregnancy.
The initial study population consisted of 25 participants. One participant was not able to complete the task due to technical problems with the eye-tracker. Therefore, the data of 24 participants were included in the statistical analyses (n = 18 males and 6 females; mean age = 25.42 years; SD = 3.69 years; range = 20–34 years). Subjects received written and oral descriptions of the study procedures, as well as details regarding the effects and possible risks of LSD and ketanserin treatment. All participants provided written informed consent statements in accordance with the Declaration of Helsinki before participation in the study. The Swiss Federal Office of Public Health, Bern, Switzerland, authorized the use of LSD in humans, and the study was approved by the Cantonal Ethics Committee of Zurich. No substantial side effects were recorded. Four participants reported transient mild headaches after drug effects had worn off. One participant reported transient sleep disturbances for the first 2 nights after drug administration. Participants were contacted again 3 months after the last drug administration. No further side effects were recorded.
Experimental design
In a double-blind, randomized, crossover design, subjects received either (1) placebo + placebo (Pla) condition: placebo (179 mg mannitol and 1 mg Aerosil, p.o.) after pretreatment with placebo (179 mg mannitol and 1 mg Aerosil, p.o.), (2) placebo + LSD (LSD) condition: LSD (100 μg, p.o.) after pretreatment with placebo (179 mg mannitol and 1 mg Aerosil, p.o.), or 3) ketanserin + LSD (Ket+LSD) condition: LSD (100 μg, p.o.) after pretreatment with the 5-HT2A antagonist ketanserin (40 mg, p.o.) at three different occasions 2 weeks apart. Pretreatment with placebo or ketanserin occurred 60 min before treatment with placebo or LSD. The task was conducted 310 min after treatment administration. The 5D-ASC (a retrospective self-report questionnaire; Dittrich, 1998) was administered to participants 720 min after drug treatment to assess subjective experience after drug intake. Mood state was assessed using the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988). Participants completed the PANAS 10 min before pretreatment with placebo or ketanserin and 720 min after treatment with placebo or LSD. At the latter time point, participants were instructed to retrospectively rate mood state at the time of peak subjective effects. Participants were required to abstain from smoking for at least 60 min before MRI assessment and from drinking caffeine during the test day.
Social interaction task
The social interaction task was based on the procedure described in previous publications (Schilbach et al., 2010; Preller et al., 2014). Joint attention is a central and basic element of social interaction and is established when one person follows another person's gaze and both attend to the same object (Materna et al., 2008; Nummenmaa and Calder, 2009). All participants received standardized instructions before drug administration and before the task. They were told that they would engage in an interactive game with another participant located in another room but because of anonymity constraints, they would not be able to meet the other participant in person. Further, they were instructed that they would see male and female anthropomorphic virtual characters on the screen to keep the sex of the other participant concealed. During the game, they would be instructed to either lead or follow the gaze of the virtual character to one of two objects on the screen located on the left and right of the avatar's face. Their gaze behavior would be tracked and transferred to the other participant's computer. Likewise, they would be able to see the other participant's gaze behavior, allowing for real-time interaction.
The experiment comprised five experimental conditions: selfjoint, selfnonjoint, otherjoint, othernonjoint, and a baseline condition. Each condition was presented in blocks lasting 5 s. Each block was preceded by an instruction screen lasting 2 s. In each condition, participants were told to first establish “eye contact” with the other participant. During the self condition (selfjoint and selfnonjoint), participants had to choose one of the two objects on the screen and gaze at it. Upon fixation, the object turned from gray to blue. Participants were instructed to maintain their gaze on the object until the color changed back to gray (after 1500 ms). During this time, participants were able to peripherally observe the gaze direction of the other participant, who would either follow the gaze (joint attention) or look at another object (nonjoint attention). The reaction of the avatar varied on a block-by-block basis.
During the other condition (otherjoint and othernonjoint), subjects were instructed that the other participant had to choose one of the objects and that they had to react by either looking at the same object [joint attention (JA)] or choose another object [nonjoint attention (NJA)]. The instruction to establish joint or non-joint attention varied on a block-by-block basis analogous to the self-condition. If participants reacted according to the instructions, the object's color changed from gray to blue. In the baseline condition the participant was instructed to look at one of the objects and the avatar closed his eyes after eye contact was established.
Only one interaction (establishment of eye contact, fixation of participant and avatar on the object according to the condition, object turning from gray to blue) per trial was possible in each condition, according to a fast event-related design. If establishment of eye contact and fixation on the object did not occur, the trial was counted as “error trail”. Furthermore, two measures of latency for eye-movement were extracted: Latency to establish eye-contact (all conditions: time of eye contact–beginning of trial) and latency to establish JA (otherjoint condition: time of established JA by the participant–beginning of trial). Each of the five conditions was repeated 20 times, resulting in 100 trials presented in a pseudorandomized order divided into two runs with 50 trials each. The presentation of male and female virtual characters was balanced across conditions. A fixation cross was presented between trials with a jittered duration of 3–7 s (mean 5 s). The stimuli were presented using Presentation v14.1 (Neurobehavioral Systems).
Eye tracking
MRI-compliant resonance technology eye-tracking system (NordicNeuroLab VisualSystem, http://www.nordicneurolab.com/) was used to record gaze directions from the right eye. The system produced real-time output of gaze positions, which was transferred via a fast network connection to another computer controlling the visual stimulation. Saccades and fixations were detected online. The eye-tracker's sampling rate was 60 Hz. Eye-tracking calibration was performed before data acquisition. Gaze positions were transformed to stimulus screen coordinates using Presentation software. Fixations were subsequently tested to see whether they occurred in one of the fields-of-interest (FOIs): the face of the avatar and the objects. If this was not the case, the algorithm searched for another fixation. By entering the FOIs for the online analysis, the avatar's gaze behavior was made contingent to the participant's fixations (Schilbach et al., 2010; Preller et al., 2014).
MRI data acquisition and preprocessing
Magnetic resonance data were acquired on a Philips Achieva 3.0T whole-body scanner (Best). A 32-channel receive head coil and MultiTransmit parallel radio frequency transmission was used. Images were acquired using a whole-brain gradient-echoplanar imaging (EPI) sequence (repetition time, 2500 ms; echo time, 27 ms; slice thickness, 3 mm; 45 axial slices; no slice gap; field-of-view, 240 × 240 mm2; in-plane resolution, 3 × 3 mm; sensitivity-encoding reduction factor, 2.0). Additionally, high-resolution anatomical images (voxel size, 0.7 × 0.7 × 0.7 mm) were acquired using a standard T1-weighted 3D magnetization prepared rapid-acquisition with gradient echo sequence. Images were analyzed using SPM12 (www.fil.ion.ucl.ac.uk; RRID:SCR_007037). Preprocessing consisted of slice time correction, realignment, spatial normalization to the standard EPI template of the Montreal Neurological Institute (MNI), and spatial smoothing using a Gaussian kernel of 8 mm FWHM to meet the statistical requirements of the general linear model. Head movement did not exceed 3 mm in any participant.
Statistical analysis
Subjective effects, number of errors, and latency.
The 5D-ASC comprises 94 items that are answered on visual analog scales (Dittrich et al., 2006). Scores were calculated for 11 recently validated scales (Studerus et al., 2010): experience of unity, spiritual experience, blissful state, insightfulness, disembodiment, impaired control and cognition, anxiety, complex imagery, elementary imagery, audio-visual synesthesia, and changed meaning of percepts. 5D-ASC score was analyzed using a repeated-measures ANOVA with treatment condition (Pla, LSD, and Ket+LSD) and scale as within-subject factors. PANAS score was calculated for the positive and negative affect scales. PANAS score was analyzed using a repeated-measures ANOVA with treatment condition (Pla, LSD, and Ket+LSD), time (pre-drug administration and post-drug administration), and scale (positive affect and negative affect) as within-subject factors. Number of errors (error trials) and latency measures (latency to establish eye-contact, latency to establish JA) were analyzed using repeated-measures ANOVAs with treatment condition (Pla, LSD, and Ket+LSD) as within-subject factor. Significant main effects and interactions were followed by Bonferroni-corrected pairwise comparisons and simple main effects analyses, respectively. Results did not change using Tukey–Kramer correction instead of Bonferroni. The confirmatory statistical comparisons of all data were performed with a significance level of p < 0.05 (two-tailed test). Analyses were conducted using SPSS Statistics 21 software (IBM).
fMRI data.
The experimental conditions (self-initiated joint attention, self-initiated non-joint attention, other-initiated joint attention, other-initiated non-joint attention, baseline) were modeled as 5 s blocks convolved with a canonical hemodynamic response function in the first-level analysis for each subject. Trials without a successful object fixation were modeled using a separate regressor of no interest. Low-frequency signal drifts were filtered using a 128 s high-pass filter. Furthermore, six head motion regressors (3 translation, 3 rotation) were included in each participant's first-level model. According to a summary statistic approach (Worsley et al., 2002), the following contrasts were computed for each participant: (1) self>other, (2) JA > NJA, (3) self-initiated JA > self-initiated NJA, and (4) other-initiated JA > other-initiated NJA. The individual contrasts were entered into a second-level random-effects group analysis using a paired t test for the comparison between drug treatment conditions and a one-sample t test for the main effects. Effects were then analyzed using small-volume correction (SVC). Because we were particularly interested in areas related to self-processing and social cognition due to our a priori hypothesis, four ROIs were defined based on a previous meta-analysis (Schilbach et al., 2012): left precuneus/PCC (x = −6, y = −54, z = 24), left superior medial gyrus/medial prefrontal cortex (x = −2, y = 52, z = 14), right middle temporal gyrus (x = 52, y = −62, z = 16), and left middle temporal gyrus (x = −46, y = −66, z = 18). Search volumes were defined as spheres with a 10 mm radius centered on previously reported MNI coordinates (Schilbach et al., 2012). Peak-level familywise error corrections (FWE) were used in all SVC ROI analyses at a threshold of p < 0.05. All reported effects represent significant differences between drug sessions as evidenced by direct comparisons of drug condition. All brain coordinates are reported in the MNI atlas space.
Correlational analysis.
Correlation analyses were conducted to further investigate the relationship between LSD-induced differences in BOLD signal and subjective effects. In case of significant differences between conditions, BOLD signal responses (first eigenvariate) were extracted from the predefined ROIs applied for SVC. Pearson's product–moment correlations were conducted between the extracted BOLD responses in these ROIs (Pla − LSD and Ket+LSD − LSD change scores) and the 5D-ASC scale scores (LSD condition, because 5D-ASC scores represent percentage deviations from the normal waking state) “changed meaning of percepts” and “experience of unity” because these scales have previously been shown to be related to alterations in social cognition (Preller et al., 2016; Pokorny et al., 2017) and capture alterations in self-experience such as altered relationships between the observer and the observed objects or persons, and include the experience of oneness and connectedness with the surroundings as well as a loss of sense of the past and future (Studerus et al., 2010). These results were replicated by using 5D-ASC scores as covariates of interest and regressing them against the BOLD within the selected ROIs. Furthermore, to assess the influence of mood, changes in mood states [Pla-LSD; (Ket+LSD)-LSD] after substance administration were additionally correlated with the extracted BOLD responses in the above described ROIs.
Results
Subjective effects
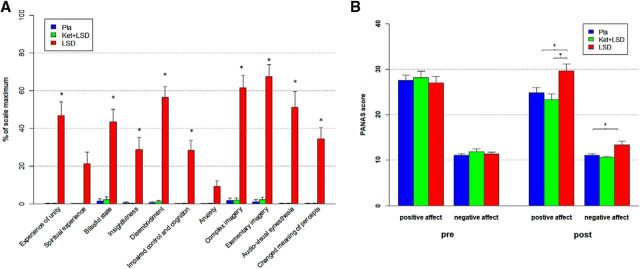
A repeated-measures ANOVA (treatment × scale) was conducted for the retrospectively administered 5D-ASC questionnaire, and revealed significant main effects for treatment (F(2,46) = 77.40, p < 0.001) and scale (F(10,230) = 16.65, p < 0.001), and a significant interaction of treatment × scale (F(20,460) = 15.06, p < 0.001). Bonferroni corrected simple main effect analyses showed increased ratings on all 5D-ASC scales in the LSD condition compared with Pla and Ket+LSD conditions (all p < 0.05) except for the scales spiritual experience and anxiety (all p > 0.20). Pla and LSD+Ket scores did not differ on any scale (all p > 0.90; Fig. 1A).
Figure 1.
Subjective drug effects. A, Retrospectively assessed 5D-ASC scores in the Pla, Ket+LSD, and LSD treatment conditions. Scores are expressed as a percentage of the scale maximum. Scores in the LSD treatment condition differed significantly from Pla and Ket+LSD treatment conditions on each scale except for spiritual experience and anxiety (*p < 0.05, Bonferroni-corrected). B, Effect of drug on mood state. Ratings on the PANAS in the Pla, Ket+LSD, and LSD treatment conditions. PANAS was completed 10 min before each pretreatment (pre) to assess current mood and 720 min after each drug treatment (post) to retrospectively assess peak drug effects. Data are expressed as mean + SEM. *p < 0.05, Bonferroni corrected. n = 24 participants.
To investigate treatment effects on mood state, a repeated-measures (time × treatment × scale) ANOVA for the PANAS revealed a significant main effect for scale (F(1,23) = 232.98, p < 0.001), indicating a higher score on the positive affect scale than on the negative affect scale, a significant main effect for treatment (F(2,46) = 6.10, p < 0.01), and significant interactions for treatment × time (F(2,46) = 20.14, p < 0.001), time × scale (F(1,23) = 11.77, p < 0.01), and treatment × time × scale (F(2,46) = 3.95, p < 0.05). Bonferroni-corrected simple main effect analyses revealed that scores on the positive and negative affect scales did not differ between treatments before drug administration (all p > 0.9). After drug administration, score on the positive affect scale was significantly greater in the LSD treatment condition than in both the Pla and Ket+LSD treatment conditions (all p < 0.05), and score on the negative affect scale was greater in the LSD treatment condition than in the Pla treatment condition (p < 0.05). Scores did not differ between the Pla and Ket+LSD treatment conditions for either the positive or negative affect scale (all p > 0.9; Fig. 1B).
Social interaction task
Main effects
Significant effects were found for the self > other contrast in the right (x = 48, y = −64, z = 7, cluster size = 97, T = 5.71) and left (x = −48, y = 70, z = 10, cluster size = 14, T = 3.51) middle temporal gyrus (p < 0.05 FWE-corrected after SVC).
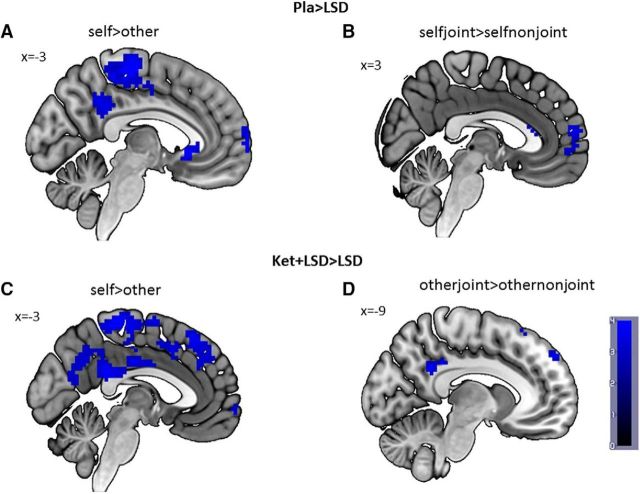
Pla versus LSD
Comparing the contrast self > other between Pla and LSD treatment conditions revealed a greater BOLD signal in the left PCC (x = −3, y = −46, z = 28, cluster size = 40, T = 3.50) in the Pla condition compared with LSD (p < 0.05 FWE-corrected after SVC; Fig. 2A). For the selfjoint > selfnonjoint contrast, the analysis revealed significantly higher BOLD signal in the medial prefrontal cortex (x = 3, y = 59, z = 13, cluster size = 19, T = 3.66) in the Pla condition (p < 0.05 FWE-corrected after SVC; Fig. 2B). No suprathreshold voxels were found for LSD > Pla in any contrast.
Figure 2.
fMRI data. A, Self > other contrast for Pla > LSD at peak PCC voxel (x = −3, y = −46, z = 28); (B) selfjoint > selfnonjoint contrast for Pla > LSD at peak superior medial frontal gyrus voxel (x = 3, y = 59, z = 13); (C) self > other contrast for Ket+LSD > LSD at peak PCC voxel (x = −3, y = −46, z = 28); (D) otherjoint > othernonjoint contrast for Ket+LSD > LSD at peak PCC voxel (x = −9, y = −49, z = 31). Data displayed at p < 0.005 (uncorrected and unmasked) for illustration purposes only. Inferences are based on p < 0.05, FWE-corrected after SVC. n = 24.
Ket+LSD versus LSD
Comparison of the self > other contrast between Ket+LSD and LSD treatment conditions revealed a significant higher BOLD signal in the left PCC (x = −3, y = −46, z = 28, clister size = 30, T = 3.31) and the right middle temporal gyrus (x = 51, y = −55, z = 22, cluster size = 42, T = 3.50) after Ket+LSD treatment (p < 0.05 FWE-corrected after SVC; Fig. 2C). Furthermore, comparison of the otherjoint > othernonjoint contrast revealed a significant increase in BOLD signal in the left PCC (x = −9, y = −49, z = 31, cluster size = 67, T = 3.94) and the left middle temporal gyrus (x = −48, y = −67, z = 28, cluster size = 7, T = 3.35) after Ket+LSD treatment (p < 0.05 FWE-corrected after SVC; Fig. 2D). No suprathreshold voxels were found for LSD > Ket+LSD in any contrast.
Ket+LSD versus Pla
No significant differences in any contrast were found when comparing Ket+LSD and placebo treatment conditions.
Number of errors
There was no significant difference between drug conditions regarding the number of errors during the task [F(2,46) = 2.36, p > 0.1; mean (SD): Pla: 11.71 (11.80); LSD: 16.89 (13.31); Ket+LSD: 10.29 (12.39)].
Latency
Latency to establish eye contact was not different between drug conditions [F(2,44) = 1.30, p > 0.2; mean in seconds (SD): Pla: 0.44 (0.17); LSD: 0.50 (0.18); Ket+LSD: 0.43 (0.14)]. A significant effect of drug condition was found for latency to establish JA [F(2,44) = 6.90, p < 0.01] with significantly (all p < 0.05) longer latency in the LSD condition [mean in seconds (SD): 1.82 (0.27)] than in both the Pla condition [mean in seconds (SD): 1.65 (0.17)] and the Ket+LSD condition [mean in seconds (SD): 1.67 (0.17)].
Correlations
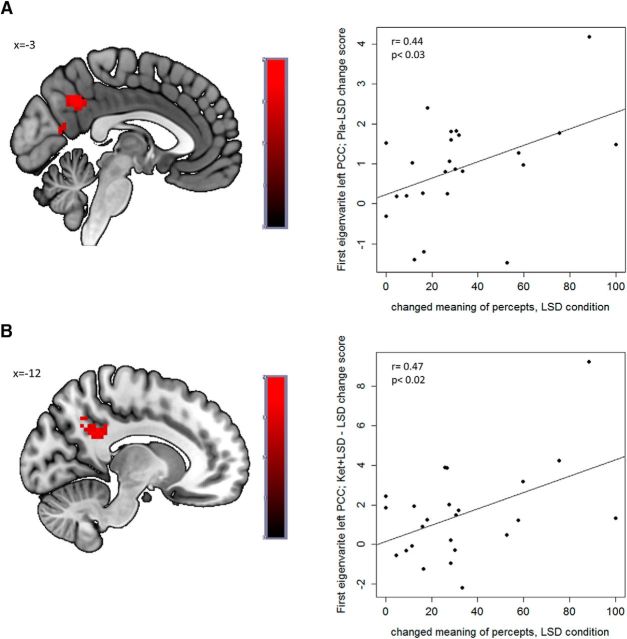
A significant positive Pearson correlation was found between the LSD-induced decrease in BOLD signal in the PCC in the self > other contrast compared with both Pla (r = 0.44, p < 0.03; Fig. 3A) and LSD+ Ket (r = 0.47, p < 0.02; Fig. 3B) and the 5D-ASC scale “changed meaning of percepts” in the LSD condition. These results were replicated using Spearman correlations, except for the relationship between LSD-induced decrease compared with LSD+Ket and the 5D-ASC scale “changed meaning of percepts” did not reach significance. Changes in mood were not significantly correlated with changes in BOLD signal (all p > 0.1, uncorrected). By using subjective scores as covariates of interest and regressing them against the BOLD within the selected ROIs, these results were replicated showing a significant (p < 0.05, FWE-corrected) correlation between “changed meaning of percepts” and the LSD-induced decrease in BOLD signal in the PCC in the self > other contrast compared with the Pla condition (peak voxel: x = −12, y = −49, z = 28, cluster size = 11, T = 3.50) and the Ket+LSD condition (peak voxel: x = −3, y = −55, z = 34, cluster size = 25, T = 3.52; Fig. 3).
Figure 3.
Correlations. Positive association between changed meaning of percepts in the LSD condition assessed by the 5D-ASC questionnaire and (A) left PCC activation in the self > other contrast in the Placebo versus LSD condition (peak voxel: x = −12, y = −49, z = 28; r = 0.44, p < 0.03; first eigenvariate; Pla–LSD change score) and (B) the Ket+LSD versus LSD condition (peak voxel: x = −3, y = −55, z = 34,r = 0.47; p < 0.02; first eigenvariate; Ket+LSD−LSD change score). fMRI data displayed at p < 0.005 (uncorrected and unmasked) for illustration purposes only. Inferences are based on p < 0.05, FWE-corrected after SVC. n = 24.
Discussion
The present study investigated the role of the 5-HT2A R system in social interaction by combining pharmacological manipulations with neuroimaging and real-time eye-tracking. Our results show that LSD decreased the response to participation in self-initiated compared with other-initiated social interaction in the PCC and the temporal gyrus, more precisely the angular gyrus. The results are well in line with previous studies showing that the angular gyrus is part of the default-mode network and implicated in self-referential processing (Blanke et al., 2002; Kim, 2010). Moreover, the results are in line with a recent study showing that self-related processes are driven via PCC activity (Davey et al., 2016). The PCC has been associated with experiential self-reflection (Johnson et al., 2006), self-referential processing and the integration of self-referential stimuli in the context of one's own person (Northoff et al., 2006), and autobiographical memory retrieval (Maddock et al., 2001). Additionally, the PCC is part of the so called cortical midline structures, which have been identified as a component in generating a model of the self (Northoff and Bermpohl, 2004) and has been shown to be involved in disturbances of self-experience in schizophrenia patients (Ebisch et al., 2014; Leech and Sharp, 2014). The current results are consistent with previous studies showing that altered resting-state connectivity and density in the angular gyrus and the retrosplenial cortex, a part of the PCC, after LSD administration was related to the dissolution of self or “ego” (Carhart-Harris et al., 2016; Tagliazucchi et al., 2016). The current study extends these findings by demonstrating that LSD also alters self-processing during social interaction. Furthermore, the LSD-induced decrease in BOLD signal in the PCC compared with both Pla and Ket+LSD in the self > other contrast was significantly correlated with the 5D-ASC scale score “changed meaning of percepts”. This is in line with a previous study showing that this scale was associated with psilocybin-induced alterations in social cognition (Pokorny et al., 2017). This scale captures the relationship between the observer and the observed objects or the environment and therefore corroborates that the sense of self is intertwined with the sense of our surroundings and in particular other persons around us. Therefore, the LSD-induced reduction of self-related processing seems to decrease the differentiation between the self and other during social interaction as indicated by a blunted differential response to self- versus other-initiated social interaction in the PCC and the temporal cortex. Furthermore, our results show that the 5-HT2A R plays a key role in these processes, because the LSD-induced reduction of self-other differentiation was normalized by ketanserin and no significant differences were found between Ket+LSD and placebo conditions.
When comparing selfjoint > selfnonjoint, greater BOLD signal in the medial prefrontal cortex was found in the Pla condition compared with LSD. The medial prefrontal cortex has been shown to be involved in social cognition tasks related to joint attention, self-knowledge, mentalizing, and “the meeting of minds”, and may be related to monitoring one's own emotions during social interaction (Amodio and Frith, 2006; Schilbach et al., 2010). However, increased activity and hyperconnectivity of the medial prefrontal cortex with networks related to social and self-referential cognition has been reported to be clinically relevant in depressed patients and was related to an increased self-focus in these patients (Lemogne et al., 2012; Schilbach et al., 2014). Furthermore, comparing otherjoint > othernonjoint greater BOLD signal was found in the PCC and the temporal cortex, e.g., the angular gyrus, in the Ket+LSD condition compared with LSD. Both the PCC and the angular gyrus have repeatedly been implicated in social cognitive abilities, in particular in “theory of mind” (Saxe and Kanwisher, 2003; Mar, 2011) pointing to the interconnected nature of self- and other-processing. This is also reflected in the increased latency and therefore reduced efficiency to establish joint attention with others under LSD. In sum, the current results suggest that LSD-induced alterations in self-experience, particularly the decreased differentiation between the self and the other, influence the processing of fundamental aspects of social interaction. However, it has to be acknowledged that LSD-induced effects on joint attention processing were more subtle that its effects on self-processing and experience.
In parallel to the blocking effect of ketanserin in the self > other contrast, no significant differences were found between Ket+LSD and Pla in the selfjoint > selfnonjoint and otherjoint > othernonjoint contrasts. Furthermore, the decreased efficiency in establishing joint attention observed in the LSD condition was also blocked by ketanserin. These findings are in line with the observation that the subjective effects of LSD have been fully blocked by ketanserin in this study as well as reported previously (Preller et al., 2017). This suggests that the 5-HT2A R plays a key role in the processing and execution of fundamental aspects of social interaction such as joint attention. Our findings also point to the importance of this receptor system in psychiatric disorders such as depression, where recovered-depressed participants on the one hand respond appropriately to joint attention, but on the other hand seem to not use joint attention to form trust appraisals of others, suggesting normal social attention but impaired cognition about the self and trusted other (Bayliss et al., 2017).
However, because other receptors stimulated by LSD have not been investigated in this study, it cannot be excluded that other receptors mediate LSD-induced effects as well. Furthermore, considering that the number of errors was not different between drug conditions, it is unlikely that the LSD-induced alterations in self and joint attention processing are influenced by attention. Yet, one limitation of this study is the unequal gender distribution of 18 males and 6 females.
In summary, LSD induced a feeling of loosening of self-boundaries that was reflected in a reduced neural response to self- versus other-initiated real-time social interaction. In particular, the current data show that the PCC and the angular gyrus are involved in providing a coherent self-representation during social interaction. Moreover, LSD altered the processing of joint attention, a basic and vital component of social cognition, in the medial prefrontal cortex. Additionally, using pretreatment with ketanserin, the current study was able to identify that aberrant 5-HT2A R-mediated signaling underlies these interdependent changes in self-processing and social cognition. On the one hand, not being able to generate a stable self-representation, as for instance in patients suffering from an incoherent self-experience such as seen in schizophrenia, may lead to social withdrawal (Schilbach et al., 2016). Therefore, these patients might benefit from treatment with 5-HT2A R antagonists. On the other hand, an increased self-focus or self-referential bias, for instance in patients with depression (Pyszczynski and Greenberg, 1987), can also lead to social interaction difficulties (Schilbach et al., 2014), emphasizing the potential use of 5-HT2A R agonists in the treatment of patients with increased self-focus. Future research should therefore explore the potential of 5-HT2A agonists and antagonists respectively to improve social abilities in different patient groups.
Footnotes
This study was financially supported by Grants from the Heffter Research Institute (1-190413), the Swiss Neuromatrix Foundation (2015-0103), the Usona Institute (2015-2056), and the Swiss National Science Foundation (SNSF; P2ZHP1_161626). We thank Dr. Rainer Kraehenmann, Dr. Philipp Stämpfli, and Dr. Matthias Liechti for supporting this study.
The authors declare no competing financial interests.
References
- Amodio DM, Frith CD (2006) Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 7:268–277. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Savic A, Driesen NR, Yang G, Cho YT, Murray JD, Glahn DC, Wang XJ, Krystal JH (2013) Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry 4:169. 10.3389/fpsyt.2013.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss AP, Tipper SP, Wakeley J, Cowen PJ, Rogers RD (2017) Vulnerability to depression is associated with a failure to acquire implicit social appraisals. Cogn Emot 31:825–833. 10.1080/02699931.2016.1160869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M (2002) Stimulating illusory own-body perceptions. Nature 419:269–270. 10.1038/419269a [DOI] [PubMed] [Google Scholar]
- Bolis D, Schilbach L (2017) Observing and participating in social interactions: action perception and action control across the autistic spectrum. Dev Cogn Neurosci. Advance online publication. Accessed on March, 2018. 10.1016/j.dcn.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, Tagliazucchi E, Schenberg EE, Nest T, Orban C, Leech R, Williams LT, Williams TM, Bolstridge M, Sessa B, McGonigle J, Sereno MI, Nichols D, Hellyer PJ, Hobden P, et al. (2016) Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A 113:4853–4858. 10.1073/pnas.1518377113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, Chialvo DR, Nutt D (2014) The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 8:20. 10.3389/fnhum.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T. (2003) Why is joint attention a pivotal skill in autism? Philos Trans R Soc Lond B Biol Sci 358:315–324. 10.1098/rstb.2002.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A, Drew A (2000) Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cogn Dev 15:481–498. 10.1016/S0885-2014(01)00037-5 [DOI] [Google Scholar]
- Crockett MJ, Fehr E (2014) Social brains on drugs: tools for neuromodulation in social neuroscience. Soc Cogn Affect Neurosci 9:250–254. 10.1093/scan/nst113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Pujol J, Harrison BJ (2016) Mapping the self in the brain's default mode network. Neuroimage 132:390–397. 10.1016/j.neuroimage.2016.02.022 [DOI] [PubMed] [Google Scholar]
- Decety J, Sommerville JA (2003) Shared representations between self and other: a social cognitive neuroscience view. Trends Cogn Sci 7:527–533. 10.1016/j.tics.2003.10.004 [DOI] [PubMed] [Google Scholar]
- De Gregorio D, Posa L, Ochoa-Sanchez R, McLaughlin R, Maione S, Comai S, Gobbi G (2016) The hallucinogen d-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT1A, D2 and TAAR1 receptors. Pharmacol Res 113:81–91. 10.1016/j.phrs.2016.08.022 [DOI] [PubMed] [Google Scholar]
- Dittrich A. (1998) The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31:80–84. 10.1055/s-2007-979351 [DOI] [PubMed] [Google Scholar]
- Dittrich A, Lamparter D, Maurer M (2006) 5D-ABZ: fragebogen zur erfassung aussergewöhnlicher bewusstseinszustände. Eine kurze Einführung. [5D-ASC: Questionnaire for the assessment of altered states of consciousness: a short introduction]. PSIN Plus Publications. [Google Scholar]
- Ebisch SJ, Mantini D, Northoff G, Salone A, De Berardis D, Ferri F, Ferro FM, Di Giannantonio M, Romani GL, Gallese V (2014) Altered brain long-range functional interactions underlying the link between aberrant self-experience and self-other relationship in first-episode schizophrenia. Schizophr Bull 40:1072–1082. 10.1093/schbul/sbt153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke G. (1995) Die symptom-check-liste von Derogatis; Deutsche version. Göttingen, Germany: Beltz Test Gesellschaft. [Google Scholar]
- Fuchs T. (2015) Pathologies of intersubjectivity in autism and schizophrenia. J Consciousness Stud 22:191–214. [Google Scholar]
- Fydrich T, Renneberg B, Schmitz B, Wittchen HU (1997) SKID-II Strukturiertes Klinisches interview für DSM-IV, Achse II: persönlichkeitsstörungen. [SCID-II Structured Clinical Interview for DSM-IV, Axis II: Personality disorders.] Goettingen, Germany: Hogrefe. [Google Scholar]
- Gallagher S. (2000) Philosophical conceptions of the self: implications for cognitive science. Trends Cogn Sci 4:14–21. 10.1016/S1364-6613(99)01417-5 [DOI] [PubMed] [Google Scholar]
- Gerrans P. (2015) All the self we need. In: Open mind (Metzinger T, Windt JM, eds). Frankfurt am Main: MIND Group. [Google Scholar]
- Geyer MA, Vollenweider FX (2008) Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci 29:445–453. 10.1016/j.tips.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G (2009) Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum Brain Mapp 30:2617–2627. 10.1002/hbm.20693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S (2006) Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc Cogn Affect Neurosci 1:56–64. 10.1093/scan/nsl004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. (2010) Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage 50:1648–1657. 10.1016/j.neuroimage.2010.01.051 [DOI] [PubMed] [Google Scholar]
- Leech R, Sharp DJ (2014) The role of the posterior cingulate cortex in cognition and disease. Brain 137:12–32. 10.1093/brain/awt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P (2012) Medial prefrontal cortex and the self in major depression. J Affect Disord 136:e1–e11. 10.1016/j.jad.2010.11.034 [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2001) Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104:667–676. 10.1016/S0306-4522(01)00108-7 [DOI] [PubMed] [Google Scholar]
- Mar RA. (2011) The neural bases of social cognition and story comprehension. Annu Rev Psychol 62:103–134. 10.1146/annurev-psych-120709-145406 [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Kurrasch-Orbaugh DM, Selken JR, Cumbay MG, Lisnicchia JG, Nichols DE (2002) Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats. Psychopharmacology (Berl) 164:93–107. 10.1007/s00213-002-1141-z [DOI] [PubMed] [Google Scholar]
- Materna S, Dicke PW, Thier P (2008) Dissociable roles of the superior temporal sulcus and the intraparietal sulcus in joint attention: a functional magnetic resonance imaging study. J Cogn Neurosci 20:108–119. 10.1162/jocn.2008.20008 [DOI] [PubMed] [Google Scholar]
- Metzinger T, Gallese V (2003) The emergence of a shared action ontology: building blocks for a theory. Conscious Cogn 12:549–571. 10.1016/S1053-8100(03)00072-2 [DOI] [PubMed] [Google Scholar]
- Moutoussis M, Trujillo-Barreto NJ, El-Deredy W, Dolan RJ, Friston KJ (2014a) A formal model of interpersonal inference. Front Hum Neurosci 8:160. 10.3389/fnhum.2014.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutoussis M, Fearon P, El-Deredy W, Dolan RJ, Friston KJ (2014b) Bayesian inferences about the self (and others): a review. Conscious Cogn 25:67–76. 10.1016/j.concog.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CL, Anacker AMJ, Veenstra-VanderWeele J (2016) The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience 321:24–41. 10.1016/j.neuroscience.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. (2004) Hallucinogens. Pharmacol Ther 101:131–181. 10.1016/j.pharmthera.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Nordgaard J, Parnas J (2014) Self-disorders and the schizophrenia spectrum: a study of 100 first hospital admissions. Schizophr Bull 40:1300–1307. 10.1093/schbul/sbt239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. (2013) Brain and self: a neurophilosophical account. Child Adolesc Psychiatry Ment Health 7:28. 10.1186/1753-2000-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F (2004) Cortical midline structures and the self. Trends Cogn Sci 8:102–107. 10.1016/j.tics.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J (2006) Self-referential processing in our brain: a meta-analysis of imaging studies on the self. Neuroimage 31:440–457. 10.1016/j.neuroimage.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Calder AJ (2009) Neural mechanisms of social attention. Trends Cogn Sci 13:135–143. 10.1016/j.tics.2008.12.006 [DOI] [PubMed] [Google Scholar]
- Picard F, Friston K (2014) Predictions, perception, and a sense of self. Neurology 83:1112–1118. 10.1212/WNL.0000000000000798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny T, Preller KH, Kometer M, Dziobek I, Vollenweider FX (2017) Effect of psilocybin on empathy and moral decision-making. Int J Neuropsychopharmacol 20:747–757. 10.1093/ijnp/pyx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Vollenweider FX (2016) Phenomenology, structure, and dynamic of psychedelic states. Curr Top Behav Neurosci. Advance online publication. Accessed on March, 2018 10.1007/7854_2016_459 [DOI] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Schilbach L, Stämpfli P, Hulka LM, Vonmoos M, Ingold N, Vogeley K, Tobler PN, Seifritz E, Quednow BB (2014) Functional changes of the reward system underlie blunted response to social gaze in cocaine users. Proc Natl Acad Sci U S A 111:2842–2847. 10.1073/pnas.1317090111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Pokorny T, Hock A, Kraehenmann R, Stämpfli P, Seifritz E, Scheidegger M, Vollenweider FX (2016) Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U S A 113:5119–5124. 10.1073/pnas.1524187113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stämpfli P, Liechti ME, Seifritz E, Vollenweider FX (2017) The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Curr Biol 27:451–457. 10.1016/j.cub.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Pyszczynski T, Greenberg J (1987) Self-regulatory perseveration and the depressive self-focusing style: a self-awareness theory of reactive depression. Psychol Bull 102:122–138. 10.1037/0033-2909.102.1.122 [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N (2003) People thinking about thinking people. the role of the temporo-parietal junction in “theory of mind”. Neuroimage 19:1835–1842. 10.1016/S1053-8119(03)00230-1 [DOI] [PubMed] [Google Scholar]
- Schilbach L. (2016) Towards a second-person neuropsychiatry. Philos Trans R Soc Lond B Biol Sci 371:20150081. 10.1098/rstb.2015.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, Shah NJ, Fink GR, Vogeley K (2010) Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J Cogn Neurosci 22:2702–2715. 10.1162/jocn.2009.21401 [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB (2012) Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social and unconstrained cognition. PLoS One 7:e30920. 10.1371/journal.pone.0030920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Müller VI, Hoffstaedter F, Clos M, Goya-Maldonado R, Gruber O, Eickhoff SB (2014) Meta-analytically informed network analysis of resting state FMRI reveals hyperconnectivity in an introspective socio-affective network in depression. PLoS One 9:e94973. 10.1371/journal.pone.0094973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Derntl B, Aleman A, Caspers S, Clos M, Diederen KM, Gruber O, Kogler L, Liemburg EJ, Sommer IE, Müller VI, Cieslik EC, Eickhoff SB (2016) Differential patterns of dysconnectivity in mirror neuron and mentalizing networks in schizophrenia. Schizophr Bull 42:1135–1148. 10.1093/schbul/sbw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Müller F, Borgwardt S, Liechti ME (2015) Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry 78:544–553. 10.1016/j.biopsych.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:22–33;quiz 34–57. [PubMed] [Google Scholar]
- Shepherd SV. (2010) Following gaze: gaze-following behavior as a window into social cognition. Front Integr Neurosci 4:5. 10.3389/fnint.2010.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX (2010) Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5:e12412. 10.1371/journal.pone.0012412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Roseman L, Kaelen M, Orban C, Muthukumaraswamy SD, Murphy K, Laufs H, Leech R, McGonigle J, Crossley N, Bullmore E, Williams T, Bolstridge M, Feilding A, Nutt DJ, Carhart-Harris R (2016) Increased global functional connectivity correlates with LSD-induced ego dissolution. Curr Biol 26:1043–1050. 10.1016/j.cub.2016.02.010 [DOI] [PubMed] [Google Scholar]
- Timmermans B, Schilbach L (2014) Investigating alterations of social interaction in psychiatric disorders with dual interactive eye tracking and virtual faces. Front Hum Neurosci 8:758. 10.3389/fnhum.2014.00758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54:1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC (2002) A general statistical analysis for fMRI data. Neuroimage 15:1–15. 10.1006/nimg.2001.0933 [DOI] [PubMed] [Google Scholar]