Abstract
The neural mechanism responsible for migraine remains unclear. While an external trigger has been proposed to initiate a migraine, it has also been proposed that changes in brainstem function are critical for migraine headache initiation and maintenance. Although the idea of altered brainstem function has some indirect support, no study has directly measured brainstem pain modulation circuitry function in migraineurs particularly immediately before a migraine. In male and female humans, we performed fMRI in 31 controls and 31 migraineurs at various times in their migraine cycle. We measured brainstem function during noxious orofacial stimulation and assessed resting-state functional connectivity. First, we found that, in individual migraineurs, pain sensitivity increased over the interictal period but then dramatically decreased immediately before a migraine. Second, despite overall similar pain intensity ratings between groups, in the period immediately before a migraine, compared with controls and other migraine phases, migraineurs displayed greater activation in the spinal trigeminal nucleus during noxious orofacial stimulation and reduced functional connectivity of this region with the rostral ventromedial medulla. Additionally, during the interictal phase, migraineurs displayed reduced activation of the midbrain periaqueductal gray matter and enhanced periaqueductal gray connectivity with the rostral ventromedial medulla. These data support the hypothesis that brainstem sensitivity fluctuates throughout the migraine cycle. However, in contrast to the prevailing hypothesis, our data suggest that, immediately before a migraine attack, endogenous analgesic mechanisms are enhanced and incoming noxious inputs are less likely to reach higher brain centers.
SIGNIFICANCE STATEMENT It has been hypothesized that alterations in brainstem function are critical for the generation of migraine. In particular, modulation of orofacial pain pathways by brainstem circuits alters the propensity of external triggers or ongoing spontaneous activity to evoke a migraine attack. We sought to obtain empirical evidence to support this theory. Contrary to our hypothesis, we found that pain sensitivity decreased immediately before a migraine, and this was coupled with increased sensitivity of the spinal trigeminal nucleus to noxious stimuli. We also found that resting connectivity within endogenous pain modulation circuitry alters across the migraine cycle. These changes may reflect enhanced and diminished neural tone states proposed to be critical for the generation of a migraine and underlie cyclic fluctuations in migraine brainstem sensitivity.
Keywords: brainstem pain modulation, functional connectivity, migraine, orofacial pain, periaqueductal gray matter, spinal trigeminal nucleus
Introduction
Migraine is a common, distressing disorder characterized by headaches often accompanied by aura, nausea, and sensitivity to light and sound. While the exact neural mechanisms surrounding migraine head pain are still debated, human brain imaging investigations have shown that, during a migraine attack, activity increases in a number of cortical areas, such as the cingulate cortex, insula, thalamus, and hypothalamus, as well as brainstem nuclei, such as the spinal trigeminal nucleus (SpV), dorsal pons, and midbrain periaqueductal gray matter (PAG) (Bahra et al., 2001; Denuelle et al., 2007; Tajti et al., 2012; Borsook et al., 2016; Coppola et al., 2016). These sites are particularly important in pain processing because the SpV is the site of orofacial nociceptor afferent termination and the PAG is involved in the modulation of noxious inputs and generation of autonomic and behavioral consequences of pain (Sessle, 2000; Keay and Bandler, 2002). Additionally, several studies have shown that, even between attacks, migraineurs display neural changes, such as decreased gray matter volume density, altered sensitivity to somatosensory stimuli, and changes in brainstem, thalamic, and cortical oscillatory activity (Mathur et al., 2016; Chong et al., 2017; Porcaro et al., 2017; Marciszewski et al., 2018; Meylakh et al., 2018).
A recent review has proposed that these observed changes are not permanent, but dynamic in nature (May, 2017). Building on the current focus of migraine research in identifying a structure in the brainstem pain-modulation system that may be associated with the initiation of a migraine attack (Akerman et al., 2011; Schulte and May, 2017), this review suggests that the initiation and maintenance of migraine attacks are unlikely to be caused by one area of the brainstem. It is far more likely that spontaneous fluctuations of complex networks involving the hypothalamus, brainstem pain-modulation circuitry, and possibly higher cortical areas lead to the initiation and termination of headache attacks. While several independent functional studies have identified activation of brainstem sites thought to be involved in endogenous pain-modulatory function, both during and between attacks (Weiller et al., 1995; Stankewitz et al., 2011), few studies have tracked changes in brain sensitivity, activity, and volume throughout all stages of the migraine cycle. Importantly, few have explored the critical 24 h period preceding a migraine, which is essential if we are to understand how migraines are initiated.
Using fMRI, we recently reported increased resting infra-slow oscillatory activity (0.03–0.06 Hz) and altered hypothalamic-brainstem functional connectivity in migraineurs only in the period immediately before a migraine attack, when individuals were not in pain (Meylakh et al., 2018). Importantly, these changes did not occur immediately after the migraine when individuals were recovering from an attack, or during the interictal phase. These data are consistent with the idea that the initiation of a migraine is associated with changes in brain function, in particular, changes within the brainstem. Indeed, it has been hypothesized that changes in sensitivity of brainstem regions to noxious orofacial inputs are critical for the initiation of a migraine. More specifically, in migraineurs, brainstem function oscillates between (1) an “enhanced” neural tone state during which the effectiveness of endogenous analgesic mechanisms is too great to allow incoming noxious inputs to evoke head pain and (2) a “diminished” state during which endogenous analgesic mechanisms are limited and incoming noxious inputs can evoke head pain (Burstein et al., 2015). Currently, there is little neural evidence to support the idea of altered brainstem endogenous modulation of SpV immediately before a migraine.
The aim of this investigation is to determine whether functional connectivity within the brainstem endogenous pain-modulating circuitry is altered throughout the migraine cycle. Furthermore, we aim to determine whether individuals with migraine show altered sensitivity and neural activity to noxious stimuli applied to the trigeminal nerve distribution in different stages of the migraine cycle. We hypothesize that migraineurs will show increased sensitivity and SpV activation to noxious stimuli and reduced functional connectivity within the brainstem pain modulation circuitry immediately before a migraine attack.
Materials and Methods
Subjects.
Thirty-one subjects with migraine (6 males, mean ± SEM age: 29.6 ± 1.7 years, range 19–55 years) and 60 pain-free controls (20 males; mean ± SEM age: 26.2 ± 0.9 years, range 19–56 years) were recruited from the general population using an advertisement. Migraine subjects were diagnosed according to the criteria set by the International Headache Classification Committee of the International Headache Society (IHS, 2013) (Ed 3, Sections 1.1 and 1.2). Seven migraineurs reported experiencing aura with their migraines, and the remaining 24 reported no aura. Of the 31 migraineurs, 28 were scanned during the interictal period (6 males, mean ± SEM age: 29.6 ± 1.8 years); that is, between 72 h after and 24 h before a migraine attack; 10 during the 24 h period immediately before a migraine (3 males, age 29.1 ± 3.4 years) and 10 within the 72 h period following a migraine (1 male, age 31 ± 3.1 years). For subjects scanned before an attack, there was no predicting factor that they were within 24 h of a migraine. Six migraineurs were scanned during all 3 phases, and another 5 migraineurs were scanned during 2 of 3 phases.
All migraine subjects indicated the pain intensity (6 point visual analog scale; 0 = no pain, 5 = most intense imaginable pain) and facial distribution (drawing) of pain they commonly experience during a migraine attack. Each subject described the qualities of their migraines and indicated any current treatments used to prevent or abort a migraine once started. Exclusion criteria for controls were the presence of any current pain or chronic pain condition, current use of analgesics, and any neurological disorder. Exclusion criteria for migraineurs were any pain condition other than migraine, and any other neurological disorder. Informed written consent was obtained for all procedures according to the Declaration of Helsinki seventh revision and local Institutional Human Research Ethics Committees approved the study. Data from 25 of the 29 migraineurs were used in a previous investigation (Marciszewski et al., 2018).
MRI acquisition.
In all subjects, before entering the MRI scanner, a 3 × 3 cm MRI-compatible thermode (Medoc, Ramat Yishai, Israel) was placed on the right side of the mouth covering the upper and lower lips for each subject. In migraineurs, this was done on the side most commonly experiencing headaches (5 left-sided, 23 right-sided). Care was taken to secure the thermode in the same location in each individual subject and to ensure it did not cross the midline. A temperature that evoked moderate pain ratings was determined for each individual subject with a Thermal Sensory Analyzer (TSA-II, Medoc), from a resting temperature of 32°C to temperatures at 0.5°C intervals between 44°C and 49°C. Temperatures were randomly applied in 15 s intervals for 10 s during which each subject rated the pain intensity using a 10 point Computerised Visual Analog Scale (CoVAS, Medoc; 0 = no pain, 10 = worst imaginable pain). The temperature at which individuals indicated a pain intensity rating of ∼6 of 10, was used for the remainder of the experiment.
All subjects then lay supine on the bed of a 3T MRI scanner (Philips, Achieva) with their head immobilized in a 32-channel head coil. With each subject relaxed and at rest, a high-resolution 3D T1-weighted anatomical image set covering the entire brain was collected (turbo field echo; FOV 250 × 250 mm, matrix size = 288 × 288, slice thickness = 0.87 mm, repetition time = 5600 ms, echo time = 2.5 ms, flip angle = 8°). A series of 180 gradient-echo echo planar resting-state fMRI volumes, using BOLD contrast, was then collected. Each image volume contained 35 axial slices covering the entire brain (FOV = 240 × 240 mm, matrix size = 80 × 78, slice thickness = 4 mm, repetition time = 2000 ms; echo time = 30 ms, flip angle = 90°). Following this resting state fMRI series, a series of 140 gradient-echo echo planar fMRI image volumes with BOLD contrast was collected with each image volume covering the entire brain (38 axial slices, repetition time = 2500 ms, raw voxel size 1.5 × 1.5 × 4.0 mm thick). Following a 30-volume baseline period, 8 noxious thermal stimuli were delivered (see Fig. 1A). Each noxious stimulus was delivered for 15 s (including ramp up and down periods of 2.5 s each), followed by a 6 volume baseline (32°C) period. During each period of noxious stimulation, the subject was asked to rate the pain intensity online using the CoVAS.
Figure 1.
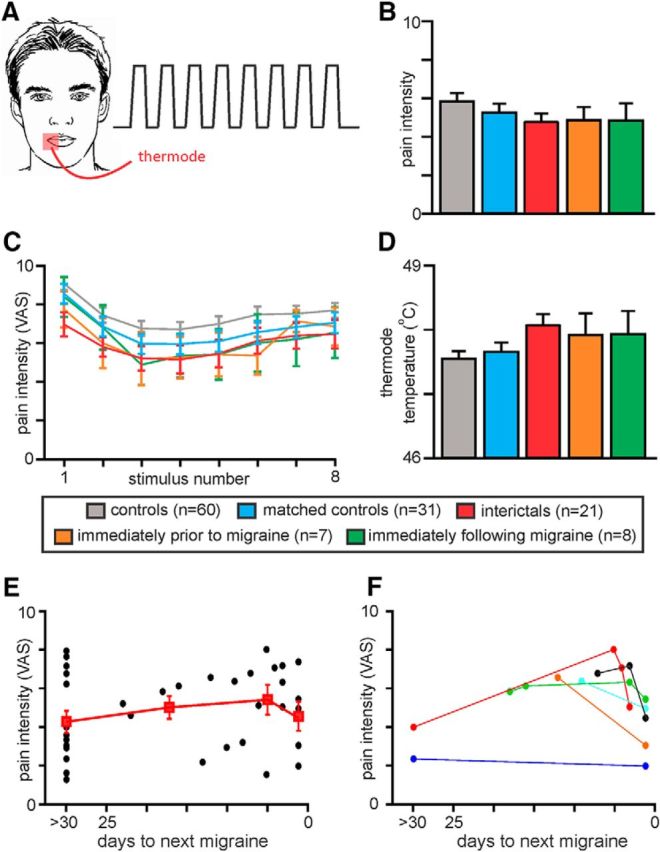
A, In each participant, a thermode was placed on the corner of the mouth and 8 noxious heat stimuli were delivered. Each participant rated the pain intensity during each noxious stimulus on a CoVAS, where 0 = no pain and 10 = most pain imaginable. B, Plots of mean ± SEM pain intensity ratings for each subject group. In each subject, pain intensity for each of the 8 noxious stimuli was averaged and these were then averaged across subjects for each group. The control group (n = 60) was reduced to 31 subjects so that the average pain intensity was not significantly different between controls and each of the three migraine groups. C, Plots of mean ± SEM pain intensity ratings for each of the 8 noxious stimuli for each subject group. D, Plots of mean ± SEM stimulus temperatures for each subject group. E, Plots of pain intensity ratings for individual migraineurs with respect to their next migraine (black circles). In addition, mean ± SEM pain intensity ratings for the following periods: >30 d until next migraine (n = 12), 30 to 10 d until next migraine (n = 4), 9 to 2 d until next migraine (n = 5), and 1 d until next migraine (n = 7) are also plotted (red filled squares). F, Plots of pain intensity ratings for 6 individual migraineurs with respect to their next migraine. Perceived pain intensity increases over the interictal period and then dramatically decreases immediately before their next migraine.
Pain rating analysis.
For each subject, the mean pain intensity ratings during each of the 8 noxious stimulus periods were calculated and plotted. Our aim was to explore changes in brain activation patterns over the migraine cycle compared with controls. Because we found that overall the control group rated the pain intensity higher than the migraineurs, we removed those controls with higher pain ratings to match pain intensities across all control and migraine groups (see Fig. 1B,C). In addition, for the pain activation protocol, we removed 7 migraineurs scanned during the interictal, 3 scanned immediately before migraine phase and 2 scanned immediately following migraine phase, due to excessive head motion (>1 mm volume-to-volume movement in the X, Y, and Z planes and 0.05 radians in the pitch, roll, and yaw directions). There was no significant difference in applied thermode temperature (°C) between the groups after removal of these subjects (see Fig. 1D).
The thermal stimulation analysis was conducted on a control group of 31 subjects (10 males, mean ± SEM age: 26.5 ± 1.2 years), interictal migraine group of 21 subjects (4 males, mean age: 29.8 ± 2.1 years), immediately before migraine group of 7 subjects (2 males, mean age: 30.4 ± 4.7 years), and immediately following migraine group of 8 subjects (1 male, mean age: 29.4 ± 1.9 years). There was no significant difference in age (t test; p > 0.05), gender composition (χ2 test, p > 0.05), pain rating (t test; p > 0.05), or stimulus temperature (t test; p > 0.05). To explore changes throughout the migraine cycle, we plotted the mean ± SEM pain intensity ratings for the following periods: >30 d until next migraine (n = 12), 30 to 10 d until next migraine (n = 4), 9 to 2 d until next migraine (n = 5), 1 d until next migraine (n = 7), and 1 to 3 d following a migraine (n = 8). In addition, in 5 subjects, thermal stimulation testing was performed during both the interictal and immediately before migraine phases, and in another subject 4 sessions including one 2 d before a migraine were collected. For these subjects, their pain intensity ratings during each session were plotted individually. Finally, we used the same subjects to run the resting state connectivity analysis but only needed to remove 3 control subjects due to excessive head movement (28 controls, 28 interictal migraineurs, 10 immediately before a migraine, 10 immediately following migraine; no significant differences in age or gender).
MRI analysis.
Using SPM12 (Friston et al., 1994) and custom software, all fMRI images in the resting-state and the thermal stimuli protocol were motion corrected, and subjects with excessive head movement were removed as described above. Five migraineurs experienced migraines most commonly on the left side, and the thermode was placed on the left side of the mouth; therefore, their images were reflected in the X plane (“flipped”) so that fMRI signals could be assessed ipsilateral and contralateral to the most common side of migraine. The effect of movement on signal intensity was modeled and removed, and physiological (i.e., cardiovascular and respiratory) noise was modeled and removed using the DRIFTER toolbox (Särkkä et al., 2012). The fMRI images were linear detrended to remove global signal intensity changes, and each subject's fMRI image set was coregistered to their own T1-weighted anatomical image set so that the T1-weighted and fMRI images were in the same locations in 3D space. Using brainstem-specific isolation software (SUIT toolbox) (Diedrichsen, 2006), a mask of the brainstem was created individually for each subject for both the T1 and fMRI image sets. Using these masks, the brainstem of the T1 and fMRI image sets was isolated and then spatially normalized to a brainstem-specific template in MNI space and spatially smoothed using a 3 mm FWHM Gaussian filter.
Noxious thermal stimuli, experimental design, and statistical analysis.
Significant changes in signal intensity during the 8 test stimuli were determined using a repeated box-car model convolved with a canonical hemodynamic response function and time dispersions. First, we assessed regional signal intensity increases and decreases across all four subject groups, primarily to verify that orofacial noxious stimuli activate the region of the SpV (random effects conjunction ANOVA, p < 0.05, family-wise error corrected for multiple comparisons). Following this, significant differences in brainstem activation patterns were determined between (1) controls and interictal migraineurs, (2) controls and migraineurs during the phase immediately before a migraine, and (3) controls and migraineurs during the phase immediately following a migraine (two-group random-effects analysis, p < 0.001 uncorrected for multiple comparisons, minimum cluster size 2 contiguous voxels, age and gender included as nuisance variables). Given we hypothesized that noxious thermal stimuli would be associated with activation in brainstem regions such as the SpV and PAG, we created ROIs comprising 3 mm-radius spheres in these brainstem sites based on the atlas by Paxinos and Huang (1995). Following the initial uncorrected threshold of p < 0.001, we applied small volume corrections using these ROIs (p < 0.05) to reduce the likelihood of Type II errors.
Significant clusters were overlaid onto a standard brainstem template in MNI space. For each significant cluster, the percentage change in signal intensity was extracted by comparing the signal intensity of the 30 baseline volumes with both the signal intensities during the 8 noxious thermal stimuli periods (“ON” periods) and signal intensities during the intervening rest periods (“OFF” periods). These signal intensity changes were extracted for all four groups and significant differences between groups determined (p < 0.05, two-tailed, two-sample t test, Bonferroni corrected for multiple comparisons). Significant differences between controls and the group from which the cluster was derived during the original voxel-by-voxel analysis were not determined, to avoid “double-dipping.” In addition, to explore changes throughout the migraine cycle, we plotted the mean ± SEM signal changes for the following periods: >30 d until next migraine (n = 12), 30 to 10 d until next migraine (n = 4), 9 to 2 d until next migraine (n = 5), 1 d until next migraine (n = 7), and 1 to 3 d following a migraine (n = 8).
Functional connectivity: experimental design and statistical analysis.
We performed brainstem-only functional connectivity analyses using a seeding region encompassing the rostral ventromedial medulla (RVM) to determine resting connectivity strengths in the well-described PAG-RVM-SpV pain-modulating pathway (Basbaum and Fields, 1984; Heinricher et al., 2009; Ossipov et al., 2010). The RVM seeding region comprised 6 contiguous voxels: 2 voxels each at 3 rostrocaudal levels from z coordinate −53 to −49 in MNI space (see Fig. 4). In each subject, signal intensity changes were extracted from the RVM seed and voxel-by-voxel analyses were performed to determine which brainstem areas displayed significant signal intensity covariations with this region. The connectivity maps were placed into second level, random-effects procedures to determine significant differences in RVM connectivity strength between controls and each of the migraine groups. Following an initial uncorrected threshold of p < 0.001, small volume corrections were applied on the midbrain PAG, dorsolateral pons (dlPons), and SpV using 40 mm3 hyper-rectangles centered at the location of each region based on the Duvernoy's Brainstem Atlas (Naidich et al., 2009) and the atlas by Paxinos and Huang (1995).
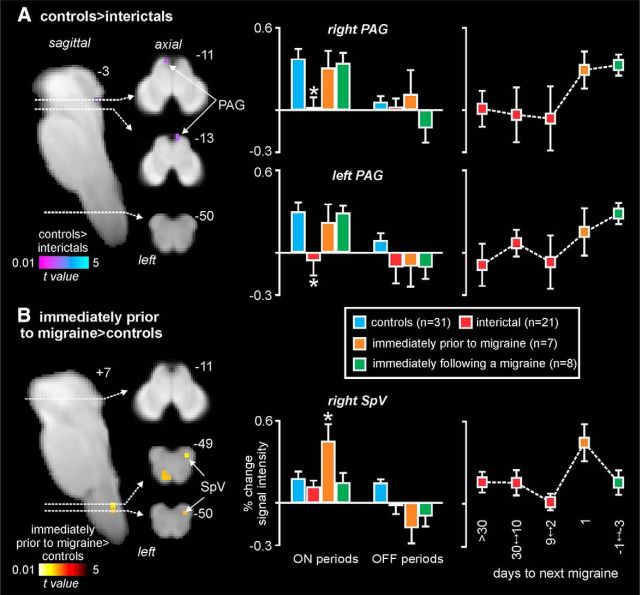
Figure 4.
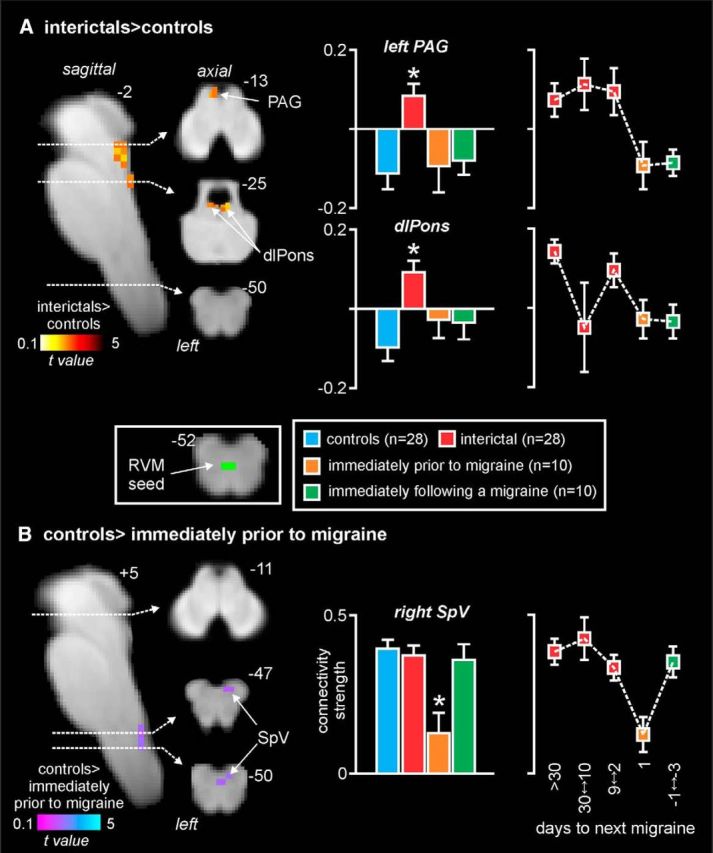
Resting-state connectivity. Significant differences in RVM resting connectivity in migraineurs compared with controls. A, Significantly greater connectivity (hot color scale) in migraineurs during the interictal phase compared with controls overlaid onto a sagittal and axial slices of a T1-weighted brainstem template. Top right, Location of each slice in MNI space (dashed horizontal lines). Plots of mean ± SEM connectivity strengths (arbitrary units) for the left midbrain PAG and dlPons clusters for each of the four subject groups are also shown. Right, Plots of connectivity changes over the migraine cycle. There is stability during the interictal period and the dramatic change immediately before a migraine. B, Significantly reduced connectivity strengths (cool color scale) in migraineurs during the phase immediately before a migraine compared with controls. Right, Connectivity strengths in the SpV in all four subject groups. *p < 0.05, derived from voxel-by-voxel analyses. Middle inset, RVM seed used for the analysis. Right, Plots of connectivity changes over the migraine cycle. There is stability during the interictal period and the dramatic change immediately before a migraine.
The resulting clusters of significant difference were used to extract connectivity strength values in each subject, and the mean ± SEM values were plotted to provide a measure of connectivity direction. Additionally, connectivity strength values were extracted for all four groups and significant differences between groups determined (p < 0.05, two-tailed, two-sample t test, Bonferroni corrected for multiple comparisons). Significant differences between controls and the group from which the cluster was derived during the original voxel-by-voxel analysis were not determined, to avoid “double-dipping.” To explore changes throughout the migraine cycle, we plotted the mean ± SEM connectivity strengths for the following periods: >30 d until next migraine (n = 16), 30 to 10 d until next migraine (n = 4), 9 to 2 d until next migraine (n = 8), 1 d until next migraine (n = 10), and 1 to 3 d following a migraine (n = 10). Finally, we assessed whether there were any areas that displayed both altered activation during noxious thermal stimuli and altered functional connectivity, by determining the intersection of significant brainstem maps. For regions of overlap, linear relationships between percentage changes in signal intensity and resting RVM connectivity were determined (Pearson's correlation, p < 0.05).
Results
Migraine characteristics
In the 31 migraineurs, 12 reported that their headaches were more common on the right side, while 5 reported more on the left and the remaining 14 reported that they were mostly bilateral. Migraine subjects most frequently described their migraine pain as “throbbing,” “sharp,” and/or “pulsating” in nature and indicated that “stress,” “lack of sleep,” and/or “dehydration” most often triggered their migraine attacks. The mean estimated frequency of migraine attacks was 22.2 ± 2.1 per year, mean length of time since the onset of migraine attacks (years suffering) 14.1 ± 1.8 years, and mean pain intensity of migraines 3.7 ± 0.2 on a 6 point visual analog scale. Although 19 of the 31 migraineurs were taking some form of daily medication (mostly the oral contraceptive pill; 12 migraineurs), none of the migraine subjects was taking prophylactic medication prescribed for migraine.
Activation during noxious thermal stimuli
The overall pain intensity ratings during the 8 brief noxious heat stimuli were similar in all four groups (mean ± SEM VAS: controls, 5.3 ± 0.4; interictal, 4.7 ± 0.5; immediately before migraine, 4.9 ± 0.7; immediately following migraine, 4.9 ± 0.8; two-tailed t test, all p > 0.05). There was also no significant difference in the applied thermode temperature used to evoke these pain levels between groups (mean temperature: controls, 47.7 ± 0.1°C; interictal, 48 ± 0.2°C; immediately before migraine, 47.9 ± 0.3°C; immediately following migraine, 47.9 ± 0.4°C; Fig. 1B–D). While pain intensity ratings remained constant over the three migraine periods, when intensity ratings were plotted relative to the next migraine, there was a gradual increase in pain intensity as the next migraine approached (Fig. 1E). However, strikingly, these ratings did not continue to increase but instead decreased in the period immediately before a migraine. This change was clear at an individual level with dramatic decreases in perceived pain intensities in the period immediately before a migraine attack despite subjects receiving the same stimuli temperatures during each testing period (Fig. 1F).
In all subjects, noxious thermal stimuli evoked increases in signal intensity in various brainstem regions, including the regions of the left and right SpV, left and right dlPons, and in the medullary raphe (Fig. 2). Comparison of signal intensity changes evoked by noxious thermal stimuli revealed regional differences over the migraine cycle. While no significant difference occurred between controls and migraineurs during the phase immediately following a migraine, there were significant differences during the interictal and the phase immediately before a migraine. During the interictal phase of migraine, acute orofacial pain was associated with significantly reduced activation in the region of the left and right PAG compared with controls (Fig. 3A; Table 1). Extraction of signal intensity changes revealed that this decrease in activation was specific to the interictal phase and did not occur during the phases immediately before or following a migraine for both the right PAG (mean percentage change: controls, 0.32 ± 0.08; interictal, 0.04 ± 0.09; immediately before migraine, 0.35 ± 0.11; immediately following migraine, 0.30 ± 0.07) and left PAG (controls, 0.26 ± 0.07; interictal, −0.04 ± 0.11; immediately before migraine, 0.22 ± 0.15; immediately following migraine, 0.25 ± 0.06). Plots of signal intensity changes throughout the migraine cycle revealed that, in migraineurs, signal intensity changes in both the left and right PAG remained stable at ∼0 throughout the interictal period and then increased dramatically to control levels in the period immediately before a migraine. Furthermore, there were no significant differences between signal intensity changes during the intervening “OFF” periods for both the right PAG (controls, 0.07 ± 0.06; interictal, −0.01 ± 0.06; immediately before migraine, 0.06 ± 0.18; immediately following migraine, −0.12 ± 0.11, all p > 0.05) and left PAG (controls, 0.09 ± 0.06; interictal, −0.07 ± 0.11; immediately before migraine, −0.08 ± 0.14; immediately following migraine, −0.09 ± 0.10, all p > 0.05).
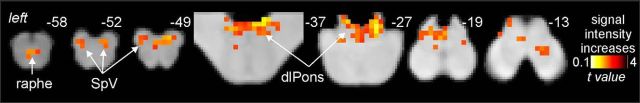
Figure 2.
fMRI response to pain. Brainstem activation common to the four subject groups during 8 brief noxious stimuli overlaid onto a series of axial slices of a brainstem T1-weighted anatomical template. Top right, Location of each sagittal and axial slice in MNI space. Noxious stimuli applied to the right side of the mouth evoked signal intensity increases (hot color scale) in the region of the SpV bilaterally, the region of the medullary raphe, and in the dlPons.
Figure 3.
fMRI response to pain by group. Significant differences in brainstem activation during noxious orofacial stimuli in migraineurs compared with controls. A, Significantly reduced activation (cool color scale) in migraineurs during the interictal phase compared with controls overlaid onto a sagittal and axial slices of a T1-weighted brainstem template. Top right, Location of each slice in MNI space (dashed horizontal lines). Plots of mean ± SEM percentage signal intensity changes during noxious orofacial stimulation for the left and right midbrain PAG clusters during the stimulus periods (ON) and baseline periods (OFF) for each of the four subject groups are shown. Right, Plots of mean ± SEM signal changes for the following periods: >30 d until next migraine (n = 12), 30 to 10 d until next migraine (n = 4), 9 to 2 d until next migraine (n = 5), 1 d until next migraine (n = 7), and 1 to 3 d following a migraine (n = 8). Note the stability of the signal changes during the interictal period and the dramatic increases during the phase immediately before a migraine. B, Significantly greater activation (hot color scale) in migraineurs during the phase immediately before a migraine compared with controls. Signal intensity changes during noxious stimuli in the SpV in all four subject groups during the ON and OFF periods are shown. Right, Plots of signal changes over the migraine cycle. Note the stability during the interictal period and the dramatic change immediately before a migraine. *p < 0.05, derived from voxel-by-voxel analyses.
Table 1.
MNI coordinates, cluster size, and t score for regions in which activation during noxious stimuli or resting RVM connectivity was significantly different between controls and migraineurs
| Brain region | MNI coordinate |
Cluster size | t score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Noxious stimuli activation | |||||
| Controls > interictals | |||||
| Left midbrain PAG | −2 | −36 | −11 | 4 | 3.28 |
| Right midbrain PAG | 4 | −38 | −13 | 3 | 3.32 |
| Immediately prior to migraine > controls | |||||
| Right spinal trigeminal nucleus | 8 | −42 | −48 | 3 | 3.67 |
| Ventral medial medulla | −2 | −32 | −49 | 4 | 3.31 |
| Resting rostral ventromedial medulla connectivity | |||||
| Interictals > controls | |||||
| Left midbrain PAG | −2 | −36 | −17 | 5 | 3.42 |
| Dorsolateral pons | 4 | −38 | −25 | 9 | 3.41 |
| Controls > immediately prior to migraine | |||||
| Right spinal trigeminal nucleus | 4 | −42 | −47 | 8 | 3.24 |
In striking contrast, only during the phase immediately before a migraine was signal intensity significantly greater during noxious stimulation in migraineurs than in controls, with this increase encompassing the region of the right SpV (Fig. 3B; Table 1). Extraction of signal intensity changes revealed a significant increase in signal intensity within the right SpV only during the phase that immediately preceded a migraine (controls: 0.17 ± 0.05; interictal: 0.11 ± 0.05; immediately before migraine: 0.42 ± 0.12; immediately following migraine: 0.15 ± 0.08). In addition, during the “off” periods, there was no significant difference between controls and the interictal phase, although there were significantly greater signal intensity reductions during the phases immediately before and after a migraine (controls: 0.13 ± 0.03; interictal: −0.02 ± 0.07; immediately before migraine: −0.17 ± 0.12, p < 0.05; immediately following migraine: −0.08 ± 0.05, p < 0.05). Plots of signal intensity changes throughout the migraine cycle revealed that, in migraineurs, signal intensity changes in the SpV remained stable at approximately control levels throughout the interictal period and then increased dramatically to well above control levels in the period immediately before a migraine.
Functional connectivity
In addition to brainstem activation patterns during noxious thermal stimuli, we assessed resting functional connectivity of the endogenous pain modulation circuitry. That is, we assessed the connectivity of the well-described PAG-RVM-SpV circuitry by using an RVM seed. Remarkably, we found a very similar pattern of difference in migraineurs compared with controls to the above-mentioned activation patterns evoked by noxious stimuli, albeit in the opposite direction. That is, during the interictal phase, migraineurs displayed significantly greater connectivity strength between the left PAG and RVM (Fig. 4A; Table 1). Again, extraction of connectivity strength values revealed that this difference in PAG-RVM connectivity occurred during the interictal phase only and was in the opposite direction to that of the controls and other migraine phases (mean connectivity strength: controls, −0.11 ± 0.04; interictal, 0.09 ± 0.03; immediately before migraine, −0.09 ± 0.07; immediately following migraine, −0.09 ± 0.03). Furthermore, the connectivity strength increase remained relatively stable over the interictal phase and then decreased dramatically in the period immediately before a migraine. As well as the PAG, connectivity strength was also significantly greater during the interictal phase in the region of the dlPons bilaterally (controls: −0.10 ± 0.04; interictal: 0.09 ± 0.03; immediately before migraine: −0.03 ± 0.05; immediately following migraine: −0.04 ± 0.05), although the connectivity strength between this region and the RVM was less stable over the interictal phase.
In contrast, during the phase immediately before a migraine, RVM connectivity strength was significantly reduced in the region of the right SpV (Fig. 4B; Table 1). Extraction of connectivity strength values confirmed the specificity of this change during the phase immediately before migraine only (controls: 0.40 ± 0.03; interictal: 0.38 ± 0.03; immediately before migraine: 0.13 ± 0.06; immediately following migraine: 0.36 ± 0.05). Furthermore, SpV-RVM connectivity strength remained stable during the interictal phase and then decreased dramatically during the 24 h period immediately before a migraine.
Noxious stimuli activation and functional connectivity overlap
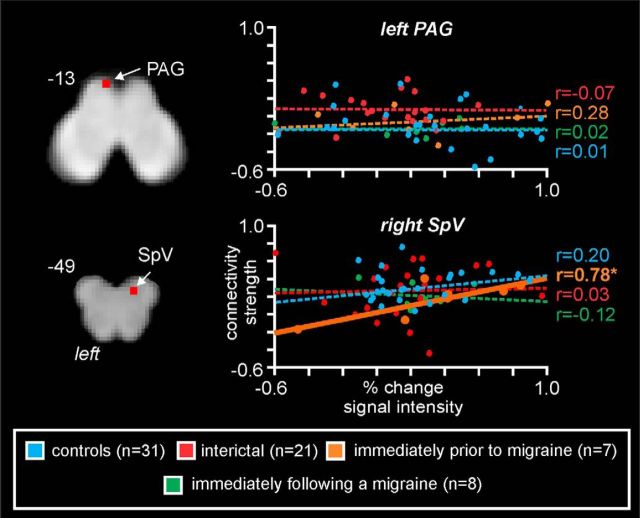
The PAG and SpV displayed significant differences in activation during noxious stimuli and resting state functional connectivity. That is, the PAG displayed reduced activation and enhanced RVM connectivity during the interictal phase, whereas the SpV displayed enhanced activation and reduced RVM connectivity during the phase immediately before a migraine (Fig. 5). Plots of RVM connectivity strengths against PAG signal intensity changes revealed no significant linear relationship in any of the four subject groups (controls: r = 0.01, p = 0.94; interictal: r = −0.07, p = 0.77; immediately before migraine: r = 0.28, p = 0.53; immediately following a migraine: r = 0.02, p = 0.96). In contrast, plots of RVM connectivity strengths against SpV signal revealed a significant positive relationship during the phase immediately before migraine only (controls: r = 0.20, p = 0.30; interictal: r = 0.03, p = 0.90; immediately before migraine: r = 0.78, p = 0.03; immediately following a migraine: r = −0.12, p = 0.78).
Figure 5.
Overlap between alterations in noxious stimulus evoked signal intensity changes and resting connectivity (red shading) overlaid onto axial slices of a T1-weighted brainstem template. Top left, Location of each slice in MNI space. Right, Plots of connectivity strengths (arbitrary units) against percentage signal intensity changes during noxious stimuli for the midbrain PAG and SpV in individual subjects for each of the four subject groups. The only significant linear relationship occurred in the SpV in the period immediately before a migraine. That is, the greater the connectivity to the rostral ventromedial medulla, the greater the noxious stimulus-evoked signal intensity. *p < 0.05, two-tailed t test.
Discussion
This study demonstrates that, although sensitivity to applied noxious stimuli increases over the interictal period, in the 24 h period before a migraine this sensitivity decreases dramatically. This decrease in noxious input sensitivity is coupled with altered function in the brainstem pain modulation circuitry. In the period immediately before a migraine attack, migraineurs displayed greater SpV activation to noxious orofacial stimuli and reduced functional connectivity between the RVM and SpV, which may underlie a change in the modulation of trigeminal noxious input to SpV by the RVM. These functional changes occurred despite the same applied stimuli and an overall similar perceived intensity level between controls and migraineurs during different phases of the migraine cycle. Furthermore, during the interictal phase, migraineurs displayed significantly enhanced resting functional connectivity between the PAG and RVM. These data support the hypothesis that brainstem sensitivity fluctuates across the migraine cycle. However, in combination with individual subjects' pain intensity changes, these data suggest that, in contrast to the idea that immediately before a migraine attack the brainstem displays diminished “tone,” our data suggest that, during this period, endogenous analgesic mechanisms are enhanced and incoming noxious inputs are less likely to evoke head pain.
An important finding in this investigation is that, at an individual level, pain sensitivity in migraineurs increases during the interictal period but then dramatically decreases in the 24 h period before a migraine attack. This finding appears at odds with the idea that, immediately before a migraine attack, brainstem endogenous analgesic pathways are in a state as to allow the easy passage of incoming noxious inputs to reach higher brain centers, although it is possible that sensitivity may increase during an actual migraine attack. In concert with the changes in pain sensitivity, we found that, during the period immediately before a migraine, noxious orofacial stimuli evoked greater SpV activation despite similar pain intensity ratings between groups. It is important to note that this increase in SpV sensitivity occurred in the same location as the signal intensity increases evoked by orofacial noxious stimulation in all subjects and was not a separate part of the relatively long and complex SpV nucleus. Furthermore, we did not find a linear relationship between SpV signal intensity and pain perception. While this may appear at odds with some expectations, BOLD signal intensity changes likely represent summed synaptic activity driven by total oxygen demand (Logothetis, 2003) and thus SpV signal intensity changes would represent a combination of afferent drive from the periphery and feedback from brainstem descending circuitry, including that arising in regions such as the RVM and subnucleus reticularis dorsalis.
Although no other investigation has explored brainstem activation during orofacial noxious stimuli in the phase immediately before a migraine, Stankewitz et al. (2011) reported that orofacial noxious stimuli evoked greater SpV signal intensity increases the closer the migraineur was to their next migraine attack. Although in this previous study, individuals were only examined as close as 4 d before their next migraine, our data reveal that SpV activation increases most dramatically in the 24 h period before a migraine. Our finding that pain perception and SpV processing of noxious stimuli are dynamic raises the prospect that orofacial pain processing pathways may also change at the onset or during a migraine itself. Alternatively, because preclinical studies have reported convergence of dural-sensitive and facial cutaneous afferents in SpV (Burstein et al., 1998; Ellrich et al., 1999), it is possible that a decrease in noxious cutaneous afferent drive onto second-order convergent SpV neurons results in an overall increase in dural afferent drive and a change in dural sensitivity. Although this is speculative, it is unlikely that the changes in SpV function reported here are involved in other functions in addition to the processing of orofacial noxious afferents.
The increase in SpV sensitivity immediately before a migraine was associated with a significant reduction in RVM-SpV connectivity. It is possible that, during the phase immediately before migraine, RVM inputs to the SpV are reduced, resulting in an increase in SpV inhibition or reduction in SpV excitation and a reduction in the propensity for incoming noxious inputs to activate higher brain centers. Interestingly, we found that the greater the reduction in RVM-SpV connectivity, the smaller the increase in SpV sensitivity to noxious inputs. There is considerable evidence that the RVM contains “ON” and “OFF” cells that can increase and decrease the excitability of neurons in the SpV/dorsal horn, respectively (Fields et al., 1983; Fields, 2004; Hellman and Mason, 2012; Salas et al., 2016). Indeed, it has been suggested that opposing RVM inhibitory and facilitatory effects are finely balanced at rest in pain-free individuals (Fields and Heinricher, 1985; Heinricher et al., 2009; Ossipov et al., 2010), but that the system can switch between inhibitory and facilitating processes depending on the state of the individual (Fields, 2004). If the inhibitory effects of RVM on SpV during the interictal phase of migraine were increased as a migraine becomes imminent, one would expect that the greater the reduction in connectivity, the greater the reduction in SpV sensitivity, a situation consistent with our findings.
Previous investigations have explored brainstem activation in migraineurs during the interictal phase and reported reduced dlPons activation during noxious thermal face and hand stimuli (Moulton et al., 2008). While we did not find altered noxious stimulus-evoked dlPons activity in this study during the interictal phase, we did find reduced activation in the PAG. Furthermore, this reduced activation was associated with an increase in resting connectivity between the RVM and both the PAG and dlPons. The PAG region that exhibited altered activation and resting functional connectivity was located in the region of the ventrolateral PAG column, an area that upon activation produces opiate-mediated analgesia, receives primarily noxious inputs from deep structures and is thought to mediate the behavioral responses to pain deemed inescapable (e.g., migraine) (Keay and Bandler, 2002). Furthermore, preclinical studies have revealed that superior sagittal sinus stimulation, a key source of noxious trigeminal input in migraine, activates the ventrolateral PAG and that ventrolateral PAG stimulation can inhibit SpV activity evoked by superior sagittal sinus stimulation (Hoskin et al., 2001; Knight and Goadsby, 2001).
Our data suggest that PAG activity and connectivity set the RVM-SpV into a state similar to nonmigraine controls because noxious orofacial activation evoked similar SpV signal intensity changes and pain intensity ratings in both controls and in migraineurs during the interictal phase. The precise roles of descending and ascending PAG inputs in altering the functional state of the PAG during the interictal phase of migraine are yet to be determined. We have recently shown increased PAG-hypothalamic connectivity only in the phase immediately before a migraine (Meylakh et al., 2018), and a recent case study reported that the hypothalamus displayed increased noxious stimuli sensitivity and increased functional coupling with the dorsomedial pons and SpV also during the phase immediately before an attack (Schulte and May, 2016). Indeed, there is a hypothesis that activity changes within the hypothalamus initiate migraine attacks, which is not inconsistent with the data presented here (Schulte et al., 2017).
Previous human brain imaging studies have also reported altered resting PAG connectivity with higher brain regions implicated in pain modulation other than the hypothalamus, such as the amygdala, prefrontal cortex, and cingulate cortex (Mainero et al., 2011). Furthermore, there is evidence that, during the interictal phase, migraineurs displayed significantly reduced endogenous analgesic ability as assessed by diffuse noxious inhibitory control (Sandrini et al., 2006). While these results suggest that, during the interictal phase of migraine, pain sensitivity should be increased, we found no significant difference in pain intensity; indeed, we found that the temperature needed to evoke moderate pain were, if anything, greater in migraineurs than controls. Furthermore, our data suggest that endogenous pain-modulating circuits are enhanced immediately before a migraine, given that pain sensitivities dramatically decreased during this period.
While we are confident in the robustness of our results, there are some limitations that need noting. First, it is possible that our interpretation of the signal intensity changes to noxious stimuli and RVM connectivity is not related to alterations in endogenous pain-modulatory circuitries. For example, it has been shown that RVM cells that process noxious information are also involved in micturition and micturition-related neurons have also been identified in the PAG and hypothalamus (Baez et al., 2005; Numata et al., 2008). Given that, in many migraineurs, increased urination occurs immediately before a migraine, it is possible that the changes reported here relate to symptoms other than the processing of incoming noxious inputs. Second, given the relatively low spatial resolution of fMRI scans, it is difficult to accurately localize each cluster to a specific brainstem nucleus or region. However, we used brainstem atlases to determine the location of each significant cluster, and our brainstem clusters overlap with regions that have been shown to be involved in nociceptive transmission. Third, the brainstem is prone to physiological noise and movement-related artifacts. To limit the influence of these factors, we applied a physiological noise correction to account for potential cardiac and respiratory noise, and we modeled and removed effects of movement on signal intensity. Finally, with mounting evidence of migraine being a “cycling” brain disorder, scans in individual migraineurs over the course of a few weeks while measuring pain sensitivity and brain activity would provide evidence to support such a hypothesis.
In conclusion, it is becoming increasingly clear that the brainstem pain-modulating circuitry is altered in migraineurs throughout the migraine cycle. While there is a suggestion that pain sensitivity increases the closer one is to a migraine, our data show that indeed pain sensitivity falls dramatically, immediately before a migraine. While data are consistent with the idea that brainstem circuits fluctuate from “enhanced” and “diminished” neural tone states, in light of our data, the timing of these fluctuations needs to be reexamined (Burstein et al., 2015; May, 2017). We speculate that, during the interictal phase of migraine, the PAG influences the RVM-SpV circuit to produce a balance of ON and OFF cell function that is similar to that of controls and thereby results in similar SpV sensitivities to noxious inputs. In contrast, as a migraine approaches, the balance of ON and OFF influences on the SpV shifts to a state dominated by OFF cell inputs; and, as a result, the SpV becomes less sensitive to incoming noxious inputs. Whether this brainstem state shifts again as a migraine attack develops is yet to be determined.
Footnotes
This work was supported by the National Health and Medical Research Council of Australia Grant 1143547. We thank the many volunteers in this study.
The authors declare no competing financial interests.
References
- Akerman S, Holland PR, Goadsby PJ (2011) Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 12:570–584. 10.1038/nrn3057 [DOI] [PubMed] [Google Scholar]
- Baez MA, Brink TS, Mason P (2005) Roles for pain-modulatory cells during micturition and continence. J Neurosci 25:384–394. 10.1523/JNEUROSCI.3536-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ (2001) Brainstem activation specific to migraine headache. Lancet 357:1016–1017. 10.1016/S0140-6736(00)04250-1 [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL (1984) Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7:309–338. 10.1146/annurev.ne.07.030184.001521 [DOI] [PubMed] [Google Scholar]
- Borsook D, Veggeberg R, Erpelding N, Borra R, Linnman C, Burstein R, Becerra L (2016) The insula: a “Hub of Activity” in migraine. Neuroscientist 22:632–652. 10.1177/1073858415601369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Yamamura H, Malick A, Strassman AM (1998) Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 79:964–982. 10.1152/jn.1998.79.2.964 [DOI] [PubMed] [Google Scholar]
- Burstein R, Noseda R, Borsook D (2015) Migraine: multiple processes, complex pathophysiology. J Neurosci 35:6619–6629. 10.1523/JNEUROSCI.0373-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong CD, Plasencia JD, Frakes DH, Schwedt TJ (2017) Structural alterations of the brainstem in migraine. Neuroimage Clin 13:223–227. 10.1016/j.nicl.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G, Di Renzo A, Tinelli E, Di Lorenzo C, Di Lorenzo G, Parisi V, Serrao M, Schoenen J, Pierelli F (2016) Thalamo-cortical network activity during spontaneous migraine attacks. Neurology 87:2154–2160. 10.1212/WNL.0000000000003327 [DOI] [PubMed] [Google Scholar]
- Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G (2007) Hypothalamic activation in spontaneous migraine attacks. Headache 47:1418–1426. 10.1111/j.1526-4610.2007.00776.x [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. (2006) A spatially unbiased atlas template of the human cerebellum. Neuroimage 33:127–138. 10.1016/j.neuroimage.2006.05.056 [DOI] [PubMed] [Google Scholar]
- Ellrich J, Andersen OK, Messlinger K, Arendt-Nielsen L (1999) Convergence of meningeal and facial afferents onto trigeminal brainstem neurons: an electrophysiological study in rat and man. Pain 82:229–237. 10.1016/S0304-3959(99)00063-9 [DOI] [PubMed] [Google Scholar]
- Fields H. (2004) State-dependent opioid control of pain. Nat Rev Neurosci 5:565–575. 10.1038/nrn1431 [DOI] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM (1985) Anatomy and physiology of a nociceptive modulatory system. Philos Trans R Soc Lond B Biol Sci 308:361–374. 10.1098/rstb.1985.0037 [DOI] [PubMed] [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G (1983) The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci 3:2545–2552. 10.1523/JNEUROSCI.03-12-02545.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS (1994) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210. 10.1002/hbm.460020402 [DOI] [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS) (2013) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33:629–808. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM (2009) Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev 60:214–225. 10.1016/j.brainresrev.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman KM, Mason P (2012) Opioids disrupt pro-nociceptive modulation mediated by raphe magnus. J Neurosci 32:13668–13678. 10.1523/JNEUROSCI.1551-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin KL, Bulmer DC, Lasalandra M, Jonkman A, Goadsby PJ (2001) Fos expression in the midbrain periaqueductal grey after trigeminovascular stimulation. J Anat 198:29–35. 10.1046/j.1469-7580.2001.19810029.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay KA, Bandler R (2002) Distinct central representations of inescapable and escapable pain: observations and speculation. Exp Physiol 87:275–279. 10.1113/eph8702355 [DOI] [PubMed] [Google Scholar]
- Knight YE, Goadsby PJ (2001) The periaqueductal grey matter modulates trigeminovascular input: a role in migraine? Neuroscience 106:793–800. 10.1016/S0306-4522(01)00303-7 [DOI] [PubMed] [Google Scholar]
- Logothetis NK. (2003) The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci 23:3963–3971. 10.1523/JNEUROSCI.23-10-03963.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainero C, Boshyan J, Hadjikhani N (2011) Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 70:838–845. 10.1002/ana.22537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciszewski KK, Meylakh N, Di Pietro F, Macefield VG, Macey PM, Henderson LA (2018) Altered brainstem anatomy in migraine. Cephalalgia 38:476–486. 10.1177/0333102417694884 [DOI] [PubMed] [Google Scholar]
- Mathur VA, Moayedi M, Keaser ML, Khan SA, Hubbard CS, Goyal M, Seminowicz DA (2016) High-frequency migraine is associated with lower acute pain sensitivity and abnormal insula activity related to migraine pain intensity, attack frequency, and pain catastrophizing. Front Hum Neurosci 10:489. 10.3389/fnhum.2016.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. (2017) Understanding migraine as a cycling brain syndrome: reviewing the evidence from functional imaging. Neurol Sci 38:125–130. 10.1007/s10072-017-2866-0 [DOI] [PubMed] [Google Scholar]
- Meylakh N, Marciszewski KK, Di Pietro F, Macefield VG, Macey PM, Henderson LA (2018) Deep in the brain: changes in subcortical function immediately preceding a migraine attack. Hum Brain Mapp 39:2651–2663. 10.1002/hbm.24030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D (2008) Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One 3:e3799. 10.1371/journal.pone.0003799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidich TP, Duvernoy HM, Delman BN, Sorensen AG, Kollias SS, Haacke EM (2009) Duvernoy's atlas of the human brain stem and cerebellum. Vienna: Springer. [Google Scholar]
- Numata A, Iwata T, Iuchi H, Taniguchi N, Kita M, Wada N, Kato Y, Kakizaki H (2008) Micturition-suppressing region in the periaqueductal gray of the mesencephalon of the cat. Am J Physiol Regul Integr Comp Physiol 294:R1996–R2000. 10.1152/ajpregu.00393.2006 [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F (2010) Central modulation of pain. J Clin Invest 120:3779–3787. 10.1172/JCI43766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF (1995) Atlas of the human brainstem. Sydney: Academic. [Google Scholar]
- Porcaro C, Di Lorenzo G, Seri S, Pierelli F, Tecchio F, Coppola G (2017) Impaired brainstem and thalamic high-frequency oscillatory EEG activity in migraine between attacks. Cephalalgia 37:915–926. 10.1177/0333102416657146 [DOI] [PubMed] [Google Scholar]
- Salas R, Ramirez K, Vanegas H, Vazquez E (2016) Activity correlations between on-like and off-like cells of the rostral ventromedial medulla and simultaneously recorded wide-dynamic-range neurons of the spinal dorsal horn in rats. Brain Res 1652:103–110. 10.1016/j.brainres.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Sandrini G, Rossi P, Milanov I, Serrao M, Cecchini AP, Nappi G (2006) Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia 26:782–789. 10.1111/j.1468-2982.2006.01130.x [DOI] [PubMed] [Google Scholar]
- Särkkä S, Solin A, Nummenmaa A, Vehtari A, Auranen T, Vanni S, Lin FH (2012) Dynamic retrospective filtering of physiological noise in BOLD fMRI: DRIFTER. Neuroimage 60:1517–1527. 10.1016/j.neuroimage.2012.01.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte LH, May A (2016) The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 139:1987–1993. 10.1093/brain/aww097 [DOI] [PubMed] [Google Scholar]
- Schulte LH, May A (2017) Of generators, networks and migraine attacks. Curr Opin Neurol 30:241–245. 10.1097/WCO.0000000000000441 [DOI] [PubMed] [Google Scholar]
- Schulte LH, Allers A, May A (2017) Hypothalamus as a mediator of chronic migraine: evidence from high-resolution fMRI. Neurology 88:2011–2016. 10.1212/WNL.0000000000003963 [DOI] [PubMed] [Google Scholar]
- Sessle BJ. (2000) Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med 11:57–91. 10.1177/10454411000110010401 [DOI] [PubMed] [Google Scholar]
- Stankewitz A, Aderjan D, Eippert F, May A (2011) Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci 31:1937–1943. 10.1523/JNEUROSCI.4496-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajti J, Szok D, Pardutz A, Tuka B, Csati A, Kuris A, Toldi J, Vecsei L (2012) Where does a migraine attack originate? In the brainstem. J Neural Transm (Vienna) 119:557–568. 10.1007/s00702-012-0788-9 [DOI] [PubMed] [Google Scholar]
- Weiller C, May A, Limmroth V, Jüptner M, Kaube H, Schayck RV, Coenen HH, Diener HC (1995) Brain stem activation in spontaneous human migraine attacks. Nat Med 1:658–660. 10.1038/nm0795-658 [DOI] [PubMed] [Google Scholar]