Alzheimer's disease (AD), the most prevalent form of dementia, affects 1 in 9 individuals >65 years old (Alzheimer's Association, 2016). Cognitive decline is the most distinctive symptom of AD, and it strongly correlates with synapse loss (Masliah et al., 1990; Terry et al., 1991). Currently, there is no effective strategy to halt or revert AD progression in patients; this can be partially attributed to the yet incipient knowledge of pathophysiological processes underlying disease progression.
The two major histopathological markers of AD are intracellular neurofibrillary tangles, formed by tau protein in its hyperphosphorylated form and extracellular plaques, composed of amyloid-β (Aβ) peptides. Aβ peptide assembles into aggregates of various sizes, ranging from oligomers to fibrils, but soluble oligomers (AβOs) are most strongly correlated with disease severity (Bjorklund et al., 2012; Bilousova et al., 2016). In the last couple of decades, AβOs have consistently been found to be associated with synapse failure and loss, as well as with the memory decline germane to AD pathology (for review, see Ferreira et al., 2015). More recently, AβOs were shown to induce neuroinflammatory processes in AD brains, thereby influencing synaptic pruning and cognition (Hong et al., 2016; for review, see Santos and Ferreira, 2017). Importantly, pharmacological alleviation of AβO-induced inflammation is sufficient to prevent cognitive impairment in murine models of AD, indicating that inflammation is central to pathological processes (Ledo et al., 2016).
An intriguing aspect of AβOs is their capacity to bind to synaptic terminals and trigger neurotoxic signaling that leads to synaptic failure. On the quest to find potential “AβO receptors” at synapses, more than a dozen molecules have been shown to interact with AβOs (for review, see Ferreira et al., 2015). Notably, the cellular prion protein (PrPC) has high affinity for AβOs (Laurén et al., 2009). PrPC is a glycosylphosphatidylinositol-anchored protein localized to the plasma membrane, and it is expressed in most cell types in mammals, but particularly enriched in the nervous system (for review, see Linden et al., 2008). Although known to turn into a misfolded version that causes neurodegeneration in transmissible spongiform encephalopathies, PrPC is thought to be involved in several normal physiological processes, such as multiprotein complex formation on the cell surface (for review, see Castle and Gill, 2017). However, the role of PrPC in synaptic plasticity remains controversial. An early report from Collinge et al. (1994) showed that hippocampal LTP was impaired in PrPC-null mice. Consistent with this, another report indicated that PrPC deletion alters neuronal excitability in hippocampal CA1 (Mallucci et al., 2002). However, Lledo et al. (1996) reported that PrPC deletion had no effects on hippocampal LTP formation.
Although AβOs may lead to memory failure through multiple mechanisms (Balducci et al., 2010), their interactions with PrPC have been shown to mediate aberrant signaling pathways, synapse loss, and cognitive decline in AD models (for review, see Salazar and Strittmatter, 2017). Binding of AβOs to PrPC recruits Type 5 metabotopic gluatamate receptors (mGluR5) to abnormally activate Fyn kinase and impair synapse function (Um et al., 2012; Haas and Strittmatter, 2016). These results have raised the important question of whether interfering with AβO-PrPC interactions could mitigate AD phenotypes and rescue memory. Interestingly, endogenous or synthetic ligands of PrPC interrupt AβO-mediated signaling and prevent neurotoxicity in neurons (Haas et al., 2014; Beraldo et al., 2016). Nonetheless, therapeutic implications and detailed mechanisms linking PrPC to AD progression still remain to be determined.
A recent report published in The Journal of Neuroscience has investigated the effects of PrPC ablation in advanced stages of AD (Salazar et al., 2017). Salazar et al. (2017) crossed mice that express AD-linked mutated genes (APP/PS1) with a strain in which Prnp, the gene encoding to PrPC, could be conditionally knocked out by administering tamoxifen. Using these mice enabled the authors to isolate the role of PrPC in disease progression without disrupting any possible function during normal development or the onset of pathology.
Salazar et al. (2017) investigated the effects of Prnp deletion in mice at 12 and 16 months of age by measuring performance in a water maze test before and after treating mice with tamoxifen to delete Prnp. Before treatment with tamoxifen, 12-month-old APP/PS1 showed greater latency to find the hidden platform than WT mice. Remarkably, the tamoxifen administration rescued impaired memory of both 12- and 16-month-old APP/PS1 mice in cognitive tests (Salazar et al., 2017). This indicates that blocking the action of PrPC may be a promising strategy to rescue cognition in late-onset AD. Furthermore, conditional deletion of Prnp rescued synapse loss in 12- and 16-month-old APP/PS1 mice, as measured by levels of the synaptic proteins PSD-95 and SV2A (Salazar et al., 2017). Therefore, the interaction between PrPC and AβOs appears to be involved in maintaining cognitive impairment in later stages of AD, making it an attractive therapeutic target.
The interaction between PrPC and mGluR5 has previously been shown to play a key role in the persistence of LTD in AD models (Hu et al., 2014). The PrPC-mGluR5 complex, triggered by AβOs, promotes phosphorylation of eukaryotic elongation factor 2 (eEF2). This results in impaired protein synthesis and preferential translation of so-called “LTD proteins” that orchestrate synaptic weakening and loss (Um et al., 2013). Importantly, Salazar et al. (2017) showed that ablation of PrPC in APP/PS1 mice blocks increased phosphorylation of eEF2, which might result in restoration of protein synthesis, thereby restoring neuronal activity to a basal state. Preclinical evidence indicates positive effects of modulating mGluR5-Fyn-eEF2 signaling pathways in AD models (Kaufman et al., 2015; Haas et al., 2017); thus, the development of pharmacological modulators is expected to test the clinical relevance of these findings.
Notably, the late removal of Prnp gene at 12 months altered neither soluble nor insoluble Aβ species in APP/PS1 mouse brains (Salazar et al., 2017). This corroborates previous findings from the same group showing that Prnp knock-out did not affect Aβ levels (Gimbel et al., 2010) and suggests that PrPC does not contribute to AD pathology by altering amyloid burden. Nevertheless, it is possible that PrPC deletion influences tau hyperphosphorylation because Fyn has been linked to somatodendritic accumulation of Tau (Li and Götz, 2017). Data showing a positive effect of PrPC deletion on tau hyperphosphorylation may reinforce the potential of a therapeutic strategy that targets AβO-PrPC-mGlur5 interaction.
In line with previous findings (Gimbel et al., 2010), Salazar et al. (2017) observed no changes in either astrogliosis or microgliosis after PrPC deletion in aged APP/PS1 mice. Therefore, PrPC appears not to be involved in the neuroinflammatory process in AD brains. Notably, Haas et al. (2017) reported that pharmacological modulation of the interaction between mGluR5 and PrPC did not alleviate astrocytosis and microgliosis of APP/PS1 mice, although it rescued cognitive impairment in these mice. These data point toward the possibility that neuroinflammation and PrPC-mGluR5 comprise parallel pathways downstream of Aβ accumulation converging on synapse failure and cognitive decline (Fig. 1). Importantly, it was recently observed that treatment with ibuprofen, a nonsteroidal anti-inflammatory drug, prevents cognitive decline in APP/PS1 mice independently of reduction of inflammatory markers: instead, it changed the expression of synaptic plasticity-related genes (Woodling et al., 2016). The possibility that inflammatory and mGluR5-PrPC processes act in synergy suggests that simultaneously targeting these processes would be beneficial, opening a novel approach to halt AD progression.
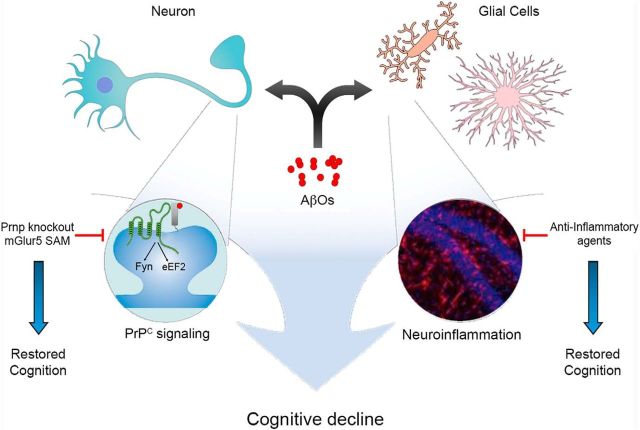
Figure 1.
AβO-induced PrPC signaling and neuroinflammation converge to cause cognitive decline. AβOs can bind to and trigger abnormal signaling cascade in both neurons and glial cells. At synapses, AβOs bind to PrPC and recruit mGluR5, forming a multiprotein complex. This complex signals to increase activation of Fyn and inactivation of eEF2, resulting in reduced protein synthesis. Depletion of PrPC, pharmacological modulation of mGluR5 by using Silent Allosteric Modulation (SAM) or inhibition of Fyn restores cognition. AβOs also induce neuroinflammation, which also leads to cognitive decline. Anti-inflammatory agents, on the other hand, can rescue cognition. Thus, neuroinflammation and PrPC may be convergent or parallel pathways leading to cognitive decline in AD. Neurons and glial cells were adapted from the software Mind The Graph. Left Inset, Expanded view of the synapse, showing AβO-PrPC-mGluR5 activity leading to Fyn activation and eEF2 inactivation. Right Inset, Reprinted with permission (Ledo et al., 2016). Hippocampal slice treated with AβOs and immunostained for Iba-1 and DAPI, showing pronounced microgliosis.
In conclusion, evidence provided by Salazar et al. (2017) indicates that late depletion of PrPC rescues cognition in APP/PS1 mice. Importantly, the results show that ablation of PrPC after disease onset has this beneficial effect on cognition, without changing major disease hallmarks. The collection of preclinical findings regarding the importance of the PrPC-mGluR5 pathway in AD positions PrPC as an attractive therapeutic target and should encourage further steps toward clinical trials.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/preparing-manuscript#journalclub.
R.A.S.L.-F. and M.M.O. were supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro and Conselho Nacional de Desenvolvimento Científico e Tecnológico predoctoral fellowships.
The authors declare no competing financial interests.
References
- Alzheimer's Association (2016) 2016 Alzheimer's disease facts and figures. Alzheimers Dement 12:459–509. 10.1016/j.jalz.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, Chiesa R, Gobbi M, Salmona M, Forloni G (2010) Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci U S A 107:2295–2300. 10.1073/pnas.0911829107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraldo FH, Ostapchenko VG, Caetano FA, Guimaraes AL, Ferretti GD, Daude N, Bertram L, Nogueira KO, Silva JL, Westaway D, Cashman NR, Martins VR, Prado VF, Prado MA (2016) Regulation of amyloid β oligomer binding to neurons and neurotoxicity by the prion protein-mGluR5 complex. J Biol Chem 291:21945–21955. 10.1074/jbc.M116.738286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilousova T, Miller CA, Poon WW, Vinters HV, Corrada M, Kawas C, Hayden EY, Teplow DB, Glabe C, Albay R 3rd, Cole GM, Teng E, Gylys KH (2016) Synaptic amyloid-β oligomers precede p-tau and differentiate high pathology control cases. Am J Pathol 186:185–198. 10.1016/j.ajpath.2015.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund NL, Reese LC, Sadagoparamanujam VM, Ghirardi V, Woltjer RL, Taglialatela G (2012) Absence of amyloid β oligomers at the postsynapse and regulated synaptic Zn2+ in cognitively intact aged individuals with Alzheimer's disease neuropathology. Mol Neurodegener 7:23. 10.1186/1750-1326-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle AR, Gill AC (2017) Physiological functions of the cellular prion protein. Front Mol Biosci 4:19. 10.3389/fmolb.2017.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Whittington MA, Sidle KC, Smith CJ, Palmer MS, Clarke AR, Jefferys JG (1994) Prion protein is necessary for normal synaptic function. Nature 370:295–297. 10.1038/370295a0 [DOI] [PubMed] [Google Scholar]
- Ferreira ST, Lourenco MV, Oliveira MM, De Felice FG (2015) Soluble amyloid-beta oligomers as synaptotoxins leading to cognitive impairment in Alzheimer's disease. Front Cell Neurosci 9:191. 10.3389/fncel.2015.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Laurén J, Gimbel ZA, Strittmatter SM (2010) Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci 30:6367–6374. 10.1523/JNEUROSCI.0395-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas LT, Strittmatter SM (2016) Oligomers of amyloid prevent physiological activation of the cellular prion protein-metabotropic glutamate receptor 5 complex by glutamate in Alzheimer disease. J Biol Chem 291:17112–17121. 10.1074/jbc.M116.720664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas LT, Kostylev MA, Strittmatter SM (2014) Therapeutic molecules and endogenous ligands regulate the interaction between brain cellular prion protein (PrPC) and metabotropic glutamate receptor 5 (mGluR5). J Biol Chem 289:28460–28477. 10.1074/jbc.M114.584342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas LT, Salazar SV, Smith LM, Zhao HR, Cox TO, Herber CS, Degnan AP, Balakrishnan A, Macor JE, Albright CF, Strittmatter SM (2017) Silent allosteric modulation of mGluR5 maintains glutamate signaling while rescuing Alzheimer's mouse phenotypes. Cell Rep 20:76–88. 10.1016/j.celrep.2017.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352:712–716. 10.1126/science.aad8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu NW, Nicoll AJ, Zhang D, Mably AJ, O'Malley T, Purro SA, Terry C, Collinge J, Walsh DM, Rowan MJ (2014) mGlu5 receptors and cellular prion protein mediate amyloid-β-facilitated synaptic long-term depression in vivo. Nat Commun 5:3374. 10.1038/ncomms4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AC, Salazar SV, Haas LT, Yang J, Kostylev MA, Jeng AT, Robinson SA, Gunther EC, van Dyck CH, Nygaard HB, Strittmatter SM (2015) Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann Neurol 77:953–971. 10.1002/ana.24394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457:1128–1132. 10.1038/nature07761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledo JH, Azevedo EP, Beckman D, Ribeiro FC, Santos LE, Razolli DS, Kincheski GC, Melo HM, Bellio M, Teixeira AL, Velloso LA, Foguel D, De Felice FG, Ferreira ST (2016) Cross talk between brain innate immunity and serotonin signaling underlies depressive-like behavior induced by Alzheimer's amyloid- oligomers in mice. J Neurosci 36:12106–12116. 10.1523/JNEUROSCI.1269-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Götz J (2017) Somatodendritic accumulation of Tau in Alzheimer's disease is promoted by Fyn-mediated local protein translation. EMBO J 36:3120–3138. 10.15252/embj.201797724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR (2008) Physiology of the prion protein. Physiol Rev 88:673–728. 10.1152/physrev.00007.2007 [DOI] [PubMed] [Google Scholar]
- Lledo PM, Tremblay P, DeArmond SJ, Prusiner SB, Nicoll RA (1996) Mice deficient for prion protein exhibit normal neuronal excitability and synaptic transmission in the hippocampus. Proc Natl Acad Sci U S A 93:2403–2407. 10.1073/pnas.93.6.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallucci GR, Ratté S, Asante EA, Linehan J, Gowland I, Jefferys JG, Collinge J (2002) Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J 21:202–210. 10.1093/emboj/21.3.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Terry RD, Mallory M, Alford M, Hansen LA (1990) Diffuse plaques do not accentuate synapse loss in Alzheimer's disease. Am J Pathol 137:1293–1297. [PMC free article] [PubMed] [Google Scholar]
- Salazar SV, Gallardo C, Kaufman AC, Herber CS, Haas LT, Robinson S, Manson JC, Lee MK, Strittmatter SM (2017) Conditional deletion of Prnp rescues behavioral and synaptic deficits after disease onset in transgenic Alzheimer's disease. J Neurosci 37:9207–9221. 10.1523/JNEUROSCI.0722-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar SV, Strittmatter SM (2017) Cellular prion protein as a receptor for amyloid-β oligomers in Alzheimer's disease. Biochem Biophys Res Commun 483:1143–1147. 10.1016/j.bbrc.2016.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos LE, Ferreira ST (2017) Crosstalk between endoplasmic reticulum stress and brain inflammation in Alzheimer's disease. Neuropharmacology. Advance online publication. Retrieved Nov. 10, 2017. doi: 10.1016/j.neuropharm.2017.11.016. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572–580. 10.1002/ana.410300410 [DOI] [PubMed] [Google Scholar]
- Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, Wisniewski T, Gunther EC, Strittmatter SM (2012) Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates fyn to impair neurons. Nat Neurosci 15:1227–1235. 10.1038/nn.3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, Kerrisk ME, Vortmeyer A, Wisniewski T, Koleske AJ, Gunther EC, Nygaard HB, Strittmatter SM (2013) Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron 79:887–902. 10.1016/j.neuron.2013.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodling NS, Colas D, Wang Q, Minhas P, Panchal M, Liang X, Mhatre SD, Brown H, Ko N, Zagol-Ikapitte I, van der Hart M, Khroyan TV, Chuluun B, Priyam PG, Milne GL, Rassoulpour A, Boutaud O, Manning-Boğ AB, Heller HC, Andreasson KI (2016) Cyclooxygenase inhibition targets neurons to prevent early behavioural decline in Alzheimer's disease model mice. Brain 139:2063–2081. 10.1093/brain/aww117 [DOI] [PMC free article] [PubMed] [Google Scholar]