Abstract
Background
Leukapheresis is often performed in cancer patients to harvest stem cells, manufacture therapeutic vaccines, or follow immunologic response to therapy. We have recently described the minimal impact of leukapheresis on normal donors. Here we provide additional immunologic data from patients with advanced cancer who underwent leukapheresis.
Methods
Using data from cancer patients on clinical trials who had leukapheresis (n = 64) or peripheral blood draws only (n = 90) as controls for immune analysis, we evaluated the impact of leukapheresis on number and function of lymphocytes.
Results
In the leukapheresis group, median age was 63.5 (range 38–82); 87.5 % were male. Comparing pre- and post-leukapheresis values within the groups, with each patient as its own control, there was no significant difference in enzyme-linked immunosorbent spot (ELISPOT), antivector humoral response, absolute lymphocyte count (ALC), or T cell number. Twelve patients completed three leukaphereses with subsequent ELISPOT analysis; seven had increased responses to flu (1.1- to 2.3-fold) with an even distribution around no change. Nineteen patients had matched ALC values after completing three leukaphereses with no significant change from baseline.
Conclusions
These data provide evidence that leukapheresis has no detectable effects on a cancer patient’s immune system in terms of number or function. These results contribute to a growing body of evidence refuting the hypothesis that a patient’s immune competence is meaningfully affected by the procedure. Limitations include a restriction to 2-L leukapheresis procedure and small sample size.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1738-9) contains supplementary material, which is available to authorized users.
Keywords: Apheresis, Vaccine, Immunotherapy, Clinical trial
Introduction
In 1981, the US Food and Drug Administration (FDA) issued guidance on leukocyte collection, stating that since there was a potential risk associated with the collection of large numbers of lymphocytes from a donor, it was not known how frequently such donations could be made. In 1988, the FDA issued further guidelines on apheresis, suggesting that the number of procedures should not exceed 24 per year. This guidance was updated in 2007 [1]. Professional societies have also issued guidelines limiting apheresis to no more than 2 per week or 24 per year [2, 3].
Leukapheresis removes lymphocytes within the blood. However, circulating lymphocytes represent only a fraction of the total number of lymphocytes in the human body (approximately 2 %) [4]. Furthermore, lymphocytes can readily traffic from the organs into the blood. While there are recognized risks of lymphodepletion in individuals losing over 1 × 1011 lymphocytes over a brief period, or with baseline lymphocyte counts of under 0.5 × 109/L [5], a more limited 1.5–2 blood volume leukapheresis (about 7 × 109 lymphocytes) is not associated with any clinically significant immunodepletion. A large study of over 400 healthy volunteers (median age 34, range 17–70) who underwent 4957 leukapheresis procedures (mean volume processed 6.8 L, range 2–10) at the National Institutes of Health (NIH) has been presented [6, 7]. The vast majority of leukapheresis procedures completed (3370) were within 56 days of a previous leukapheresis (median 23 days). Patients undergoing serial leukapheresis at appropriate intervals had a clinically insignificant 3.6 % decrease in circulating absolute lymphocyte count (ALC) between leukapheresis time points. The estimated cumulative lymphocyte loss after 2–9 leukapheresis procedures was 9.7 % in the peripheral blood, which is <0.2 % of total body lymphocytes. Most importantly, history and physical exam data were reviewed and revealed no increased susceptibility to infectious diseases or cancer.
While this provides an excellent baseline dataset for the impact of leukapheresis in healthy young patients (median age was 34), investigating the effects of the procedure in advanced cancer patients would provide a more clinically relevant context. It would also explore a population in which apheresis may be more likely to have a negative immune impact, as cancer patients are already considered to have impaired immune function at baseline and are likely to be older, particularly in the case of prostate cancer. This may be important, as reports have suggested a gradual decrease in immune repertoire over time [8]. Indeed, the effects of aging on the immune system have been used to suggest that leukapheresis may actually be deleterious to older patients. In addition, the previous dataset only addressed a quantitative measure of blood-cell subsets without any assessment of function. To address these limitations, we explored data from 12 clinical studies (nine at NIH, three collaborations with Georgetown University) [9–20] in cancer patients who had immunologic analysis before and after either a phlebotomy procedure or leukapheresis for evidence of the impact of leukapheresis on immune-cell subset number and function.
Methods
Patients and procedures
We evaluated 154 patients with solid tumors enrolled in IRB-approved studies [21–23] using experimental off-the-shelf immunotherapy approaches. Study details are available in Supplemental Table 1. All patients had a history, physical exam, and clinical laboratories prior to leukapheresis, and leukapheresis was only performed if there was no evidence of infection. All patients had immune analysis at baseline and approximately 3 months later using cells obtained via either leukapheresis (n = 64) or simple phlebotomy as a control (n = 90). Patients who underwent leukapheresis typically had a 2.0-L leukapheresis at baseline and a second procedure about 85 days later, using intermittent flow centrifugation, with an average flow rate of 50–60 mL/min, depending on patient body size and venous access. Approximately 1.4 × 109 leukocytes per liter processed are typically harvested by such procedures; about 75 % of the cells in the apheresis product are lymphocytes, and about 25 % are monocytes [6]. The lymphocytes obtained are about 50–60 % T cells and 15–20 % B cells. Fifty-two patients underwent two and 12 patients underwent three leukapheresis procedures within the 85-day period of the studies.
Assays
To determine functional immune responses, the IFN-γ enzyme-linked immunosorbent spot (ELISPOT) was performed in our laboratory, which has extensive experience with this assay, as previously described [24]. ELISPOT is expressed as the number of antigen-specific T cells (producing IFN-γ in response to antigen) out of 1,000,000 peripheral blood mononuclear cells (PBMCs). In addition, cell numbers (ALC, CD3, CD4, and CD8) were obtained through the NIH Clinical Center Laboratory, a Clinical Laboratory Improvement Act (CLIA)—and College of American Pathologists (CAP)—certified clinical laboratory, using a Beckman Coulter instrument for lymphocyte phenotyping. Humoral responses [antivaccinia virus immunoglobulin G (IgG)] were detected by enzyme-linked immunosorbent assay (ELISA), as previously described [14]. All of the immunotherapy studies had defined prospective collections of PBMCs and serum for these assays, and raw data were used for the current analysis.
Statistical analysis
For all parameters, baseline (pre) values were compared to post-treatment (post) values using a Wilcoxon signed-rank test. Continuous parameters were compared between patients with and without leukapheresis using a form of an exact Wilcoxon rank-sum test. Fractions with positive assays (responses) were compared between the two groups using Fisher’s exact test. p values are two-tailed and presented without any formal adjustment for the number of tests performed. However, in view of the number of comparisons made, only p < 0.01 should be interpreted as a statistically significant effect; 0.01 < p < 0.05 would be considered a strong trend.
Results
Among patients who underwent leukapheresis (n = 64), the median age was 63.5 (range 38–82); 87.5 % were male. Additional baseline characteristics are found in Supplemental Table 2. All patients had adequate bone marrow function at baseline, as required for eligibility on the immunotherapy protocols. Details of the baseline hematologic parameters for the leukapheresis patients are available in Supplemental Table 3.
Impact of leukapheresis on cell counts
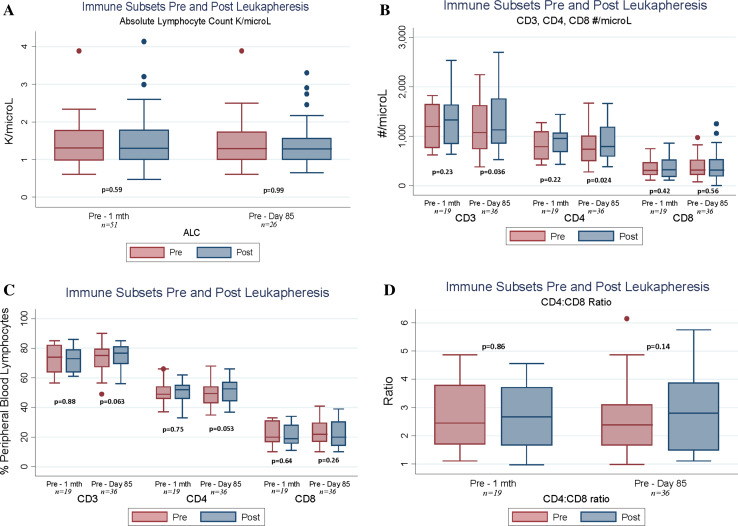
Of the 64 patients tested, 51 had paired samples of ALC pre- versus 7–36 days post-leukapheresis and 19 had immune-cell subsets analyzed 7–36 days after leukapheresis (Fig. 1a–d). There was no change in the proportion of T cells present in the blood (relative values; Fig. 1c) or in the composition of the T cell compartment with respect to the ratio of CD4 to CD8 T cells (Fig. 1d). There was no significant change in ALC, CD3+, CD4+, or CD8+ cells following leukapheresis in those patients, with a trend toward slightly increased numbers in all subsets. These trends persisted in samples taken approximately 85 days post-leukapheresis, with 26 paired ALC samples and 36 immune subsets (Fig. 1a–d). The only strong trends toward significant changes were increases in CD3+ and CD4+ numbers.
Fig. 1.
a–d Impact of 2-L leukapheresis on immune-cell subsets in cancer patients, comparing baseline to ≤1 month post-leukapheresis (day 7–36) and baseline to approximately day 85 post-leukapheresis. In all panels, p values are the result of a paired Wilcoxon signed-rank test of the difference between pre-treatment values and values at 1 month or day 85. a Impact of leukapheresis on absolute lymphocyte counts. b Impact of leukapheresis on CD3, CD4, and CD8 subsets, expressed in cells/µL of whole blood. c Impact of leukapheresis on CD3, CD4, and CD8 subsets, expressed as percentage of peripheral blood lymphocytes. d Impact of leukapheresis on CD4/CD8 ratio
Impact of leukapheresis on T cell function
To examine the function of immune cells, we looked for evidence of the ability of flu-specific T cells to secrete IFN-γ. We tested PBMCs from patients who had either leukapheresis (n = 64) or simple phlebotomy (n = 90) (Table 1). There was no quantitative or qualitative decrease in the ability of flu-specific T cells to secrete IFN-γ in response to flu matrix protein with either serial blood draws or leukapheresis. ELISPOT responses were evaluated in 12 patients following a third leukapheresis. Responses to flu were generally unchanged following leukapheresis, with seven patients demonstrating improved responses relative to baseline (1.1- to 2.3-fold increases). Statistical analysis identified no consistently positive or negative changes in cell subsets in patients who underwent leukapheresis.
Table 1.
Impact of leukapheresis versus simple peripheral blood collection on the proportion of flu-specific T cells identified via IFN-γ ELISPOT in cancer patients (number of antigen-specific cells per 106 PBMCs), comparing baseline values with those at approximately day 85 post-leukapheresis
| Leukapheresis group n = 64 | Nonleukapheresis group n = 90 | |||
|---|---|---|---|---|
| Baseline | Day 85 | Baseline | Day 85 | |
| Mean | 77.43 | 74.05 | 47.99 | 51.30 |
| Median | 45.83 | 48.33 | 30.00 | 29.17 |
| Minimum–maximum | 5.00–503.27 | 5.00–794.28 | 5.00–495.05 | 5.00–416.67 |
| p value: absolute difference pre-/post-within groups | 0.83 | 0.76 | ||
| p value: absolute difference pre-/post-between groups | 0.85 | |||
| p value: ratio pre-/post-within groups | 0.35 | 0.32 | ||
| p value: ratio pre-/post-between groups | 0.94 | |||
p values within groups calculated using two-tailed Wilcoxon signed-rank test. p values for comparisons between groups calculated using an exact two-tailed Wilcoxon rank-sum test
Impact of leukapheresis on humoral response
We also evaluated humoral responses to vaccinia (n = 74), which was given to all patients as the initial vaccine vector in the setting of therapeutic vaccine clinical trials (Table 2). There was no significant change in the proportion of responders (p = 0.0237) or the titer of response (p = 0.0179) in patients who underwent leukapheresis compared with those who underwent simple phlebotomy, though there was a trend toward increased responses in the leukapheresis group. This trend is likely a function of small patient numbers, but the lack of a decrease or a significant difference suggests that there was no functional impairment of B cell responses as a result of leukapheresis.
Table 2.
Impact of leukapheresis versus simple peripheral blood collection on the proportion of post-vaccine viral vector-specific antibody responses in cancer patients
| Leukapheresis group | Nonleukapheresis group | |
|---|---|---|
| Proportion of subjects with positive assay—# positive/total (%) | 30/33 (90.91) | 28/41 (68.29) |
| Median titer post-vaccinia | 1/5000 | 1/1100 |
| Range of titer post-vaccinia | 1/50–>1/>6250 | 1/<50–>1/>8000 |
| Median fold change in titer | 8.33 | 5.00 |
| p value for proportion of responders | 0.0237 | |
| p value: fold change from baseline | 0.0179 | |
p value for comparison of proportion of responders between two leukapheresis groups calculated using Fisher’s exact test; p value for comparing fold changes calculated using Wilcoxon rank-sum test
Impact of age on immunity following leukapheresis
If leukapheresis were disproportionately harmful to older subjects, one might expect to observe a post-procedure deterioration in laboratory measurements of immune parameters that increase with age. To determine the impact of leukapheresis on immune function, flu-specific T cells were quantified using ELISPOT. A comparison of pre- and post-leukapheresis values within the groups, with each patient as their own control, revealed no significant difference by ELISPOT (Table 3). Importantly, when ELISPOT responses were examined in subjects above and below the median age or by age quartiles, no significant changes were observed, nor were changes significant when age was considered as a continuous variable.
Table 3.
Impact of leukapheresis versus simple peripheral blood collection on ratio of flu-specific T cell pre-treatment to approximately day 85 by age quartile of subject (n = 154)
| Age quartile | 20–54 | 55–63 | 64–69 | 70–87 | ||||
|---|---|---|---|---|---|---|---|---|
| Apheresis | No | Yes | No | Yes | No | Yes | No | Yes |
| N | 26 | 14 | 26 | 18 | 19 | 14 | 19 | 18 |
| Mean | 1.08 | 1.53 | 1.15 | 0.98 | 0.96 | 1.38 | 1.17 | 1.76 |
| Median | 1.00 | 1.00 | 1.00 | 0.93 | 0.90 | 1.26 | 1.06 | 1.01 |
| Range | 0.21–2.10 | 0.50–4.80 | 0.35–2.95 | 0.21–1.95 | 0.46–2.09 | 0.22–3.25 | 0.50–2.81 | 0.21–12.5 |
| p value: relative change, ratio | 0.86 | 0.43 | 0.20 | 0.52 | ||||
Discussion
In 2010, sipuleucel-T became the first therapeutic cancer vaccine to be approved by the FDA, based on a 4.1-month improvement in median overall survival (HR 0.77, p = 0.02) in men with metastatic castration-resistant prostate cancer receiving sipuleucel-T versus control infusions. It has been suggested that leukapheresis, used to produce the vaccine sipuleucel-T, may negatively affect the overall survival of cancer patients by removing circulating lymphocytes and diminishing host immunity, especially in the control arm where patients receive only 1/3 of the harvested cells back, with the remainder stored for subsequent vaccine manufacture at crossover [25]. In the published subgroup analysis, all subgroups, including those above and below the median age, showed evidence of benefit from sipuleucel-T versus control. However, a separate subgroup analysis performed to meet Center for Medicare and Medicaid Services requirements showed that in a minority of patients less than 65 years old, there appeared to be no difference in overall survival. However, it should be noted that only 66 control patients (9.0 %) were under the age of 65 out of 737 total patients analyzed and that the inherent risk of a subset analysis is further magnified by the small number of patients in this subset. Huber et al. hypothesized that the difference in overall survival seen in the trial as a whole and in the men over 65 was due to a greater removal of immune cells in the control arm, causing immune suppression that was magnified in older men [25].
In addition to other academic arguments against the immune depletion hypothesis [26, 27], the difference in the proportion of total body lymphocytes removed between control patients and patients treated with sipuleucel-T is <1 %, with no evidence of increased infections [28]. In addition, patients getting active product were able to mount significant immune responses that correlated with overall survival [29].
Our analysis suggests that a 2-L leukapheresis has no measurable impact on the number or function of T cells and no negative impact on the ability to mount a humoral immune response to vector. It should also be noted that leukapheresis (baseline) immediately preceded vaccine (typically on the same day), further suggesting that the procedure did not interfere with patients’ ability to mount a de novo immune response. Furthermore, there is no suggestion of an age-based differential effect post-leukapheresis on measured immune outcomes.
This study had several limitations. First, we only had data from 154 patients, 64 of whom underwent leukaphereses, treated on 12 different immunotherapy trials. Although raw data were analyzed for the purposes of this study, not all analyses were performed for all patients due to several factors, including HLA status (for ELISPOT analysis), lack of availability of post-leukapheresis samples at comparable time points, and variations in protocol design. However, all patients served as their own controls, mitigating the potential impact of the variability between protocols. It is possible that small differences would emerge in an analysis of larger numbers of patients. It should also be noted that the leukaphereses in this study were 2-L procedures, rather than the 1.5–2.0 times the patient’s estimated blood volume (approximately 7–10 L) used in the sipuleucel-T study. Furthermore, the leukaphereses in our study were typically performed 2–3 months apart, which differs from the biweekly schedule for three procedures used in the manufacture of sipuleucel-T. However, in terms of immune depletion, while our 2-L leukapheresis procedures resulted in less estimated loss of peripheral leukocytes (3 × 109), none of these cells were reinfused into patients, as they were in the sipuleucel-T studies, so the overall impact is likely similar.
Our analysis found no discernible decline in the number or function of immune cells in cancer patients undergoing small-volume leukapheresis. Thus, the hypothesis that immune competence is significantly compromised by the procedure is inconsistent with available data. However, in patients with bone marrow insufficiency or those undergoing larger-volume or more frequent leukapheresis procedures, further studies would need to be done to rule out any significant change in immune parameters following leukapheresis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors acknowledge the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI), Center for Cancer Research for their support of this study. We also express appreciation to the professionals at the NIH Clinical Center Department of Transfusion Medicine for their part in performing apheresis procedures in study patients and to the medical oncology fellows at the NCI for their attention to patient care. Finally, we thank Bonnie L. Casey and Debra Weingarten for their editorial assistance in the preparation of this manuscript. This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Abbreviations
- ALC
Absolute lymphocyte count
- CAP
College of American Pathologists
- CLIA
Clinical Laboratory Improvement Act
- ELISA
Enzyme-linked immunosorbent assay
- ELISPOT
Enzyme-linked immunosorbent spot
- FDA
US Food and Drug Administration
- IFN-γ
Interferon-gamma
- IgG
Immunoglobulin G
- NIH
National Institutes of Health
- OS
Overall survival
- PBMCs
Peripheral blood mononuclear cells
Compliance with Ethical Standards
Conflict of interest
No potential conflicts of interest were disclosed.
References
- 1.Guidance for industry and FDA review staff: collection of platelets by automated methods. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm062946.pdf. Accessed 22 Apr 2014
- 2.European Directorate for the Quality of Medicines and Healthcare (2010) Guide to the preparation, use and quality assurance of blood components, 16th edn. http://www.centronazionalesangue.it/sites/default/files/guida_edqm_16_edizione.pdf. Accessed 22 Apr 2014
- 3.Standards for blood banks and transfusion services, 26th edn., Summary of significant changes. http://www.aabb.org/sa/standards/documents/sigchngstds26.pdf. Accessed 22 Apr 2014
- 4.Blum KS, Pabst R. Lymphocyte numbers and subsets in the human blood. Do they mirror the situation in all organs? Immunol Lett. 2007;108(1):45–51. doi: 10.1016/j.imlet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Strauss RG. Effects on donors of repeated leukocyte losses during plateletpheresis. J Clin Apher. 1994;9(2):130–134. doi: 10.1002/jca.2920090208. [DOI] [PubMed] [Google Scholar]
- 6.Kolf C, Bolan C, Wesley R, Browning J, Leitman SF. Sustained decreases in lymphocyte counts in serial long-term leukapheresis donors. Transfusion. 2003;43(9s):28a. [Google Scholar]
- 7.Gulley JL, Leitman SF, Dahut W, Schlom J. Re: interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst. 2012;104(14):1106. doi: 10.1093/jnci/djs280. [DOI] [PubMed] [Google Scholar]
- 8.Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014;30(1):16–22. doi: 10.3109/09513590.2013.852531. [DOI] [PubMed] [Google Scholar]
- 9.Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, Bastian A, Marte J, Tsang KY, Beetham P, Grosenbach DW, Schlom J, Dahut W. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174(2):539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 10.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, Camphausen K, Coleman CN, Sullivan F, Steinberg SM, Schlom J, Dahut W. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 11.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12(4):1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arlen PM, Skarupa L, Pazdur M, Seetharam M, Tsang KY, Grosenbach DW, Feldman J, Poole DJ, Litzinger M, Steinberg SM, Jones E, Chen C, Marte J, Parnes H, Wright J, Dahut W, Schlom J, Gulley JL. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178(4 Pt 1):1515–1520. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 13.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW, Cereda V, Vergati M, Steinberg SM, Halabi S, Jones E, Chen C, Parnes H, Wright JJ, Dahut WL, Schlom J. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59(5):663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, Remondo C, Cereda V, Jones JL, Pazdur MP, Higgins JP, Hodge JW, Steinberg SM, Kotz H, Dahut WL, Schlom J. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14(10):3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, Dahut WL, Schlom J, Gulley JL. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madan RA, Tsang KY, Bilusic M, Vergati M, Poole DJ, Jochems C, Tucker JA, Schlom J, Giaccone G, Gulley JL. Effect of talactoferrin alfa on the immune system in adults with non-small cell lung cancer. Oncologist. 2013;18(7):821–822. doi: 10.1634/theoncologist.2013-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilusic M, Heery CR, Arlen PM, Rauckhorst M, Apelian D, Tsang KY, Tucker JA, Jochems C, Schlom J, Gulley JL, Madan RA. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother. 2014;63(3):225–234. doi: 10.1007/s00262-013-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, Hodge JW, Doren S, Grosenbach DW, Hwang J, Fox E, Odogwu L, Park S, Panicali D, Schlom J. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23(4):720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 19.von Mehren M, Arlen P, Gulley J, Rogatko A, Cooper HS, Meropol NJ, Alpaugh RK, Davey M, McLaughlin S, Beard MT, Tsang KY, Schlom J, Weiner LM. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7(5):1181–1191. [PubMed] [Google Scholar]
- 20.von Mehren M, Arlen P, Tsang KY, Rogatko A, Meropol N, Cooper HS, Davey M, McLaughlin S, Schlom J, Weiner LM. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res. 2000;6(6):2219–2228. [PubMed] [Google Scholar]
- 21.Effect of talactoferrin in adults with non-small cell lung cancer. http://clinicaltrials.gov/show/NCT00923741. Accessed 22 Apr 2014
- 22.An open label phase I study to eval the safety and tolerability of a vaccine (GI-6207) consisting of whole, heat-killed recombinant saccharomyces cerevisiae (Yeast) genetically modified to express CEA protein in adults with metastatic CEA-expressing carcinoma. http://clinicaltrials.gov/show/NCT00924092. Accessed 22 Apr 2014
- 23.Vaccine therapy with PROSTVAC/TRICOM and flutamide versus flutamide alone to treat prostate cancer. http://clinicaltrials.gov/show/NCT00450463. Accessed 22 Apr 2014
- 24.Gulley J, Chen AP, Dahut W, Arlen PM, Bastian A, Steinberg SM, Tsang K, Panicali D, Poole D, Schlom J, Michael Hamilton J. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53(2):109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 25.Huber ML, Haynes L, Parker C, Iversen P. Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst. 2012;104(4):273–279. doi: 10.1093/jnci/djr514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantoff PW, Higano CS, Small EJ, Whitmore JB, Frohlich MW, Schellhammer PF. Re: interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst. 2012;104(14):1107–1109. doi: 10.1093/jnci/djs279. [DOI] [PubMed] [Google Scholar]
- 27.Drake CG. Re: interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst. 2012;104(18):1422. doi: 10.1093/jnci/djs340. [DOI] [PubMed] [Google Scholar]
- 28.Mark D, Samson D, Bonnell C, Ziegler K, Aronson N (2014) Outcomes of sipuleucel-T therapy: technology assessment report. http://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id77TA.pdf. Accessed 22 Apr 2014
- 29.Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY, Whitmore JB, Trager JB, Poehlein CH, Frohlich MW, Urdal DL. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62(1):137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.