Abstract
Emerging immunotherapeutic approaches have revolutionized the treatment of multiple malignancies. Ime-mune checkpoint blockers (ICBs) have enabled never-before-seen success rates in durable tumor control and enhanced survival benefit in patients with advanced cancers. However, this effect is not universal, resulting in responder and nonresponder populations not only between, but also within solid tumor types. Although ICBs are thought to be most effective against tumors with more genetic mutations and higher antigen loads, this is not always the case for all cancers or for all patients within a cancer subtype. Furthermore, debilitating and sometimes deadly immune-related adverse events (irAEs) have resulted from aberrant activation of T-cell responses following immunotherapy. Thus, we must identify new ways to overcome resistance to ICB-based immunotherapies and limit irAEs. In fact, preclinical and clinical data have identified abnormalities in the tumor microenvironment (TME) that can thwart the efficacy of immunotherapies such as ICBs. Here, we will discuss how reprogramming various facets of the TME (blood vessels, myeloid cells, and regulatory T cells [Tregs]) may overcome TME-instigated resistance mechanisms to immunotherapy. We will discuss clinical applications of this strategic approach, including the recent successful phase III trial combining bevacizumab with atezolizumaband chemotherapy for metastatic nonsquamous non-small cell lung cancer that led to rapid approval by the U.S. Food and Drug Administration of this regimen for first-line treatment. Given the accelerated testing and approval of ICBs combined with various targeted therapies in larger numbers of patients with cancer, we will discuss how these concepts and approaches can be incorporated into clinical practice to improve immunotherapy outcomes.
INTRODUCTION
ICBs that revitalize exhausted cytotoxic T cells (CTLs), including antibodies against PD-1 and CTLA-4, have transformed therapeutic modalities and outcomes for some solid tumors such as melanoma, lung cancer, kidney cancer, head and neck cancers, Hodgkin lymphoma, Merkel cell carcinoma, gastric cancer, hepatocellular carcinoma, cervical cancer, colorectal cancer, and bladder cancer. However, these therapies do not benefit the majority of patients with cancer and have failed to produce universal durable responses. Additionally, serious and sometimes life-threatening irAEs, including rash, colitis, and pneumonitis, have resulted following immune activation.1 Although cancers with lower mutational burdens and antigen loads are generally less likely to respond to immunotherapies, other inherent and adaptive resistance mechanisms may be responsible for mediating the response to ICBs.1,2 We posit that the successes and failures of ICBs in solid tumors are considerably dictated by the abnormal and immunosuppressive TME, which comprises stromal and immune cells, extracellular matrix molecules, and blood and lymphatic vessels (Fig. 1).3–5 This complex, interactive, and highly dynamic tissue assembly cooperates to thwart antitumor immunity and immunotherapy efficacy by a variety of mechanisms. These include a dense stromal network with increased mechanical forces, and leaky and compressed blood and lymphatic vessels, which taken together promote hypoperfusion.3 The resulting hypoxic and acidic TME supports resident and infiltrating immunosuppressive cells, induces immune checkpoint expression, and facilitates the exclusion and exhaustion (dysfunction) of CTLs.3 The TME also releases factors into circulation that promote systemic immunosuppression and further inhibit antitumor immunity.1 Therefore, reprogramming these components may normalize the TME and sensitize solid tumors to ICBs.
FIGURE 1. The Tumor-Immune Microenvironment Mediates Tumor Progression and TreatmentResponse.
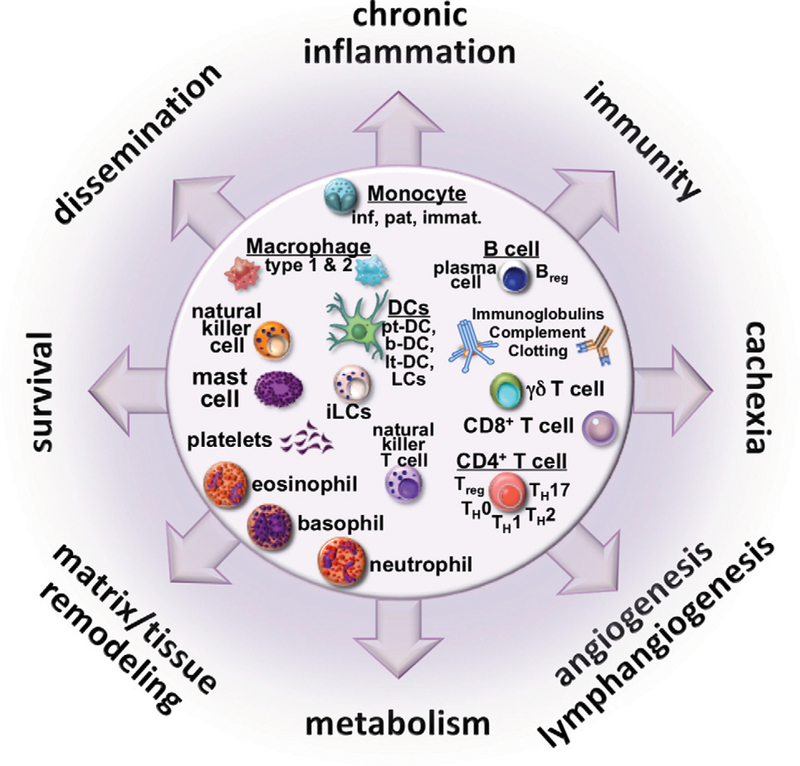
The tumor-immune landscape features a collection of protumor and antitumor immune cells that promote and cooperate with other pathophysiologic features to promote the major hallmarks of cancer progression, immunosuppression, and treatment resistance. Immunotherapeutic strategies, especially involving combination therapies, must be carefully orchestrated to promote antitumor immunity for efficacious outcomes. Abbreviation: DCs, dentritic cells.
In the following sections, we summarize approaches to reprogramming three different facets of the TME that promote immunosuppression—abnormal blood vessels, myeloid cells, and Tregs—and how these emerging strategies can be incorporated into clinical approaches to overcome microenvironment-driven resistance mechanisms to immunotherapy in patients. Finally, we discuss the recent phase III trial combining bevacizumab with atezolizumab and chemotherapy for metastatic nonsquamous non-small cell lung cancer6 as an example of a successful TME-reprogramming strategy.
NORMALIZING THE TUMOR VASCULATURE TO IMPROVE IMMUNOTHERAPY
An abnormal vasculature is a major and consistent hallmark of solid tumors, with irregular morphology and suboptimal function resulting from (1) overexpression of proangiogenic molecules such as VEGF, which promotes a leaky and immature vessel network, and (2) compression of these abnormal vessels via physical forces exerted by overabundant cells (e.g., cancer cells, fibroblasts) and the extracellular matrix molecules they produce (e.g., collagen, hyaluronan).3 These abnormal vessels facilitate immune evasion and reduce immunotherapy efficacy by reducing delivery of drugs, oxygen, and CTLs.3 The resulting hypoxia preferentially supports upregulation of immune checkpoints and infiltration of immunosuppressive cells (such as Tregs and myeloid cells harboring T-cell suppressive activities including neutrophils, macrophages, and the assemblage of monocytes referred to as myeloid-derived suppressor cells [MDSCs]).3 Beyond these indirect effects, VEGF can even directly modulate antitumor immunity to promote immunosuppression in the TME (Fig. 2). Thus, Jain3 put forth two complimentary approaches to improve the function of tumor vessels: (1) normalizing tumor vessels by pruning inefficient vessels and fortifying those remaining, and (2) decompressing tumor vessels by reprogramming stromal cells and extracellular matrix deposition. Both approaches resulted in improved responses to chemo-, radio-, and immunotherapies in multiple studies.3,7–11
FIGURE 2. VEGF Modulates Immune Cells to Promote an Immunosuppressive Tumor Microenvironment.
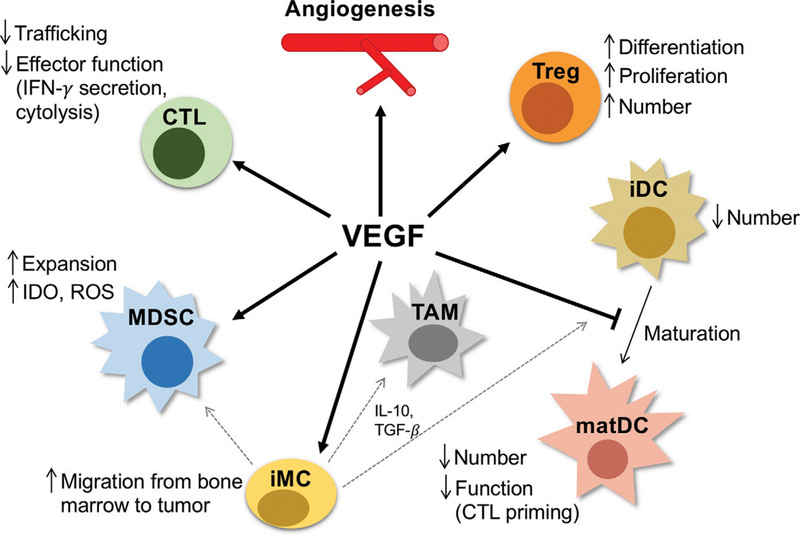
Beyond its indirect ability to promote immunosuppression via an abnormal tumor vasculature, VEGF can directly influence immune cells in the tumor microenvironment and systemically by promoting immunosuppressive cells such as Tregs, MDSCs, and protumor TAMs, while inhibiting antigenpresenting cells (such as DCs and antitumor TAMs) and CTLs. Dotted gray lines indicate differentiation from iMCs to TAMs, iDCs, and MDSCs. Abbreviations: IFN, interferon; CTL, cytotoxic T lymphocyte; Treg, regulatory T cell; iDC, immature dendritic cell; MDSC, myeloid-derived suppressor cell; TAM, tumor-associated macrophage; iMC, immature myeloid cell; IL, interleukin; TGF, transforming growth factor; matDC, mature dendritic cell; DC, dendritic cell.
The most widely used approach for vascular normalization is the blockade of VEGF or its receptors via antiangiogenic agents, such as bevacizumab. However, high and prolonged doses of antiangiogenics can actually excessively prune the vasculature and further compound hypoxia, immunosuppression, tumor progression, and treatment resistance. Indeed, as monotherapies or sometimes in combination with chemotherapy, standard doses of antiangiogenic agents have failed to produce durable survival benefits in patients with cancer.3 Thus, judicious doses of anti-VEGF or similar agents should be administered to achieve optimal vascular normali-zation.3 The result of such an approach is the restoration of the tumor vasculature to a normalized phenotype with more mature vessels fortified with perivascular cell coverage, and a more organized and uniform distribution of the vasculature throughout the tumor tissue. The resulting normalized vessel function enhances tumor perfusion, thus supporting more homogeneous delivery of drugs, oxygen, and immune cells.3 By promoting the accumulation, penetration, and antitumor activity of immune effector cells, and reducing hypoxia and the presence and function of suppressive cells, vascular normalization can convert the immunosuppressive TME into an immunostimulatory one (Fig. 3). This hypothesis has led to dozens of clinical trials currently ongoing to test combinations of antiangiogenics with ICB-based immunotherapies (discussed further in the last section).1
FIGURE 3. Vascular Normalization Can Reprogram the Immunosuppressive Tumor Microenvironment.
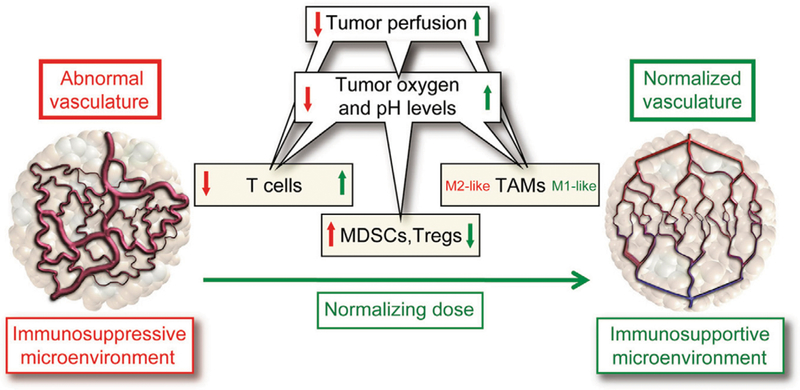
The abnormal tumor vasculature, induced by overexpression of VEGF, can inhibit effector T cells, preferentially recruit immunosuppressive immune cells (e.g., myeloid-derived suppressor cells [MDSCs], regulatory T cells [Tregs], and “M2-like” protumor tumor-associated macrophages [TAMs]), and promote hypoxia, thus establishing an immunosuppressive tumor microenvironment. Judicious dosing of antiangiogenic therapies (such as anti-VEGF antibodies) can reprogram the tumor microenvironment to an immunostimulatory milieu by normalizing the vasculature to facilitate T effector cell infiltration and antitumor function, reduce MDSC and Treg accumulation, and alleviate hypoxia, which can induce conversion of TAMs to an “M1-like” antitumor phenotype. Reprinted with permission from Jain.3
Of particular interest, an emerging field is beginning to investigate the reciprocal regulation of immune cells and tumor vasculature under combined antiangiogenic (including low-dose) and immunotherapeutic approaches.12 In addition to demonstrating tumor control, reduced metastasis, and enhanced survival, preclinical studies have revealed encouraging results with these combinations achieved via both vascular and immune cell-mediated mechanisms that induce vascular normalization. These include (1) enhanced CTL infiltration and activity (e.g., via formation of high-endothelial venules), (2) reduced CTL dysfunction, (3) reduced presence and suppressive function of Tregs and MDSCs, and (4) increased presence and activity of antigen-presenting cells (e.g., dendritic cells [DCs]; see Table 1 in Fukumura et al1). Thus, there is evidence that these modalities synergize to promote an antitumor effect via complementary immune-vascular interactions.
Even in the face of low-dose antiangiogenics, vessels that are normalized remain vulnerable to vascular collapse because of physical forces exerted by growing tumors. This phenomenon may account in part for the failure of antiangiogenic agents to achieve substantial benefits in the clinic as monotherapies in desmoplastic tumors, which feature higher vessel collapse, such as pancreatic ductal adenocarcinoma.3,7–11 Therefore, strategies that can decompress vessels may sensitize tumors not only to antiangiogenic agents but also to combinations with immunotherapies. It has been found that targeting the renin-angiotensin system with antihypertensive agents can reduce stromal cell activity and matrix deposition, thereby decompressing vessels, improving oxygen and drug delivery, and enhancing treatment outcome in preclinical and clinical studies.3,13 This indirect approach to restoring vessel function by “normalizing” tumor stroma may prove highly efficacious and translatable in clinical trials as these commonly prescribed antihypertensive drugs are approved, affordable, and safe. More recently, a preclinical study in metastatic breast cancer has reported that blocking CXCR4—a chemokine receptor linked to immune, vascular, and stromal signaling pathways—reduced tumor stroma, increased CTL infiltration, and improved ICB outcome.14
REPROGRAMMING PROTUMORAL MYELOID CELLS IN THE TME TO ENHANCE ANTITUMOR IMMUNITY
Although most immunotherapeutic approaches are specifically designed to enhance the antitumor activity of CTLs, the concomitant response of myeloid cells, including MDSCs, granulocytes, monocytes, neutrophils, DCs, and tumor-associated macrophages (TAMs), can substantially influence treatment outcome.4 Innate and adaptive immune cell activities are closely intertwined, both during tumor progression and in response to cytotoxic treatments.4 Indeed, depending on the state of the adaptive milieu and cues from the TME, the response of myeloid cells can swing between pro- and antitumor phenotypes because of theirfunctional plasticity (e.g., by regulating processes related to angiogenesis, tissue remodeling, and immune response; Fig. 4).15 Furthermore, the hypoxic and acidic TME can co-opt myeloid cells to promote a protumorigenic and immunosuppressive phenotype capable of recruiting Tregs while blocking CTL proliferation, activation, and infiltration via release of suppressive cytokines and immune checkpoint expression.3–5 Ablative strategies for certain myeloid populations are nonideal because many are required for immune homeostasis. Further, DCs and antitumor TAMs play a key role in mounting an effective immune response against tumors, either directly or by enabling CTL function.4,16 Thus, reprogramming myeloid cell subsets to an immunostimulatory state is an attractive approach to augmenting immunotherapies, beyond their direct effects on adaptive cells.
FIGURE 4. Adaptive Immune Responses Dictate Myeloid Cell Activity to Influence Tumor Progression.
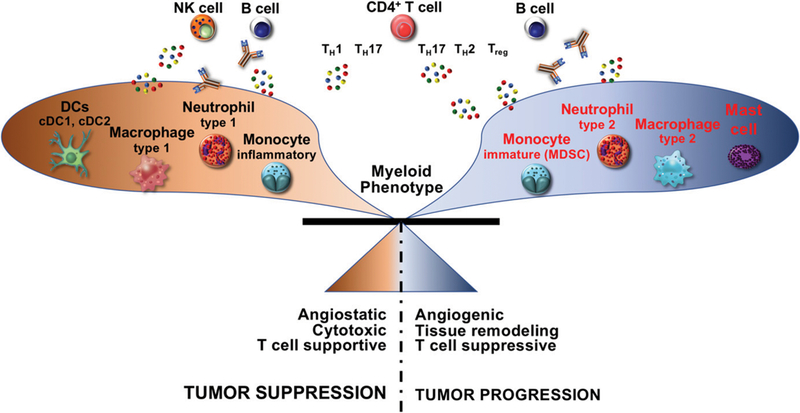
Although regulation of adaptive and innate immune responses is likely bidirectional and can be altered by treatment modalities, different adaptive cells can also promote either pro- or antitumor myeloid cell phenotype and function and together influence tumor progression. In cases where the immune response to cancer results in increased TH1 cytokines by adaptive cells (e.g., CD4+ T cells and natural killer [NK] cells), this induces myeloid cell bioactivity that promotes tumor stabilization or regression. On the other hand, when the responding adaptive response includes chronic B cell, TH2, and regulatory T-cell activation, myeloid cells upregulate programs that promote tumor progression, including angiogenesis and immunosuppression. Abbreviations: DCs, dendritic cells; MDSCs, myeloid-derived suppressor cells.
The reciprocal regulation between innate and adaptive immune cells can be exploited to promote antitumor immunity in the TME by targeting key signaling pathways expressed by myeloid cells. One such pathway is the colony-stimulating factor-1 ligand-receptor pair (CSF-1/R), which regulates the infiltration, phenotypic and functional differentiation, and survival of myeloid cells such as TAMs. The CSF-1/R axis is often upregulated in human tumors and associated with poor outcome.5 Clinical data indicate that high CSF-1/R+ myeloid cell presence as compared with CD8+ T cells, and lower proportions of DCs in the TME are associated with poor clinical responses to cytotoxic therapy and reduced overall survival.5,15 Indeed, preclinical studies have revealed that CSF-1/R antibody therapy sensitizes some tumor types to cancer vaccines and ICBs by reducing the number of infiltrating immunosuppressive myeloid cells and shifting the balance of remaining myeloid cells to T-cell stimulatory phenotypes that thereby support the infiltration and reactivation of previously exhausted CTLs.5,15,17 Thus, reprogramming the myeloid compartment via CSF-1/R blockade can promote an immunostimulatory TME. Clinical trials testing the efficacy of TAM reprogramming in patients with cancer by targeting a variety of signaling pathways (e.g., CSF-1/R, CCL2, interleukin [IL]-6, and CD40) are currently under investigation (see Table 1 in Ruffell and Coussens5).
As described earlier, VEGF expression in the TME and systemically can promote MDSCs and protumor TAMs, while inhibiting DCs and antitumor TAMs.18 VEGF inhibition has been reported to inhibit the infiltration of MDSCs and protumor TAMs, while increasing mature DCs.19 However, the ability of vascular- and myeloid-reprogramming to be synergistically combined may depend on the tumor type and the pretreatment myeloid composition in the TME. For example, preclinical studies in glioblastoma found that targeting the CSF-1/R axis abrogated the benefit of vascular normalization by depleting antitumor TAMs.20 Therefore, combination therapies will have to be carefully designed in a context-dependent manner to promote antitumor immunity and response to immunotherapy.
REPROGRAMMING REGULATORY T CELLS IN THE TME TO OVERCOME IMMUNOSUPPRESSION
Tregs are a specialized subset of CD4 T cells that are required in normal physiologic conditions to balance effector immune responses and maintain immune homeostasis and self-tolerance.21,22 In the TME, however, Tregs are co-opted to suppress antitumor immune responses and promote tumor progression.23–25 Bolstered by the hypoxic TME, Tregs inhibit antigen-presenting cell and CTL function, thereby crippling their ability to mount an effective attack on cancer cells (Fig. 5).3‘19‘24 Because Tregs may be better at recognizing tumor antigens than CTLs, immunotherapeutic approaches with cancer vaccines may, paradoxically, further promote immunosuppression via heightened antigen-specific Treg activity over that of CTLs.24,26 Thus, Tregs are emerging as a desirable target in cancer immunotherapy.24 Systemic Treg ablation is nonideal because it must be present to curtail overactive CTLs in the face of immunotherapy; otherwise, its absence may enhance irAEs.19,24 Accordingly, current explorations are aimed at inhibiting Treg-mediated suppression through two unique approaches: (1) selective depletion of intratumoral Tregs,19,23 and (2) reprogramming intratumoral Tregs to an antitumor effector phenotype.27,28 These strategies aim to inhibit the suppressive function of Tregs to augment immunotherapies, while maintaining systemic Tregs that are required for physiologic function.19,23 Although Tregs, like other immune cells, may exist across heterogeneous phenotypic and functional states, there are key suppressive mechanisms that may be targeted to boost antitumor immunity.23
FIGURE 5. Mechanisms of Regulatory T-Cell Suppression in the Tumor Microenvironment.

Regulatory T cells (Tregs) are able to inhibit the function of antigen-presenting cells (APCs) and T effector cells by three main mechanisms. First, Tregs support their own immunosuppressive function by consuming interleukin (IL)-2 (via the IL-2 receptor CD25). Second, Tregs inhibit APCs (via CTLA-4 binding to CD80/CD86) to downregulate costimulatory signals to T effector cells. Third, Tregs directly inhibit T effector cells and APCs with suppressive cytokines (IL-10, IL-35, and transforming growth factor [TGF]-β) or by inducing apoptosis (perforin and granzyme). Alternatively, Tregs can induce suppression by catabolizing adenosine (ADO) from ATP released by apoptotic Tregs under oxidative stress (in the hypoxic and acidic TME) via CD39 and CD79, which binds to ADO A2A receptors (A2AR) on CTLs and APCs to inhibit their function. Abbreviations: TCR, T-cell receptor; MHC, major histocompatibility complex.
ICBs against CTLA-4 were originally designed to block inhibitory signals on CTLs to reactivate their cytotoxic effects; however, it was recently realized that the majority of benefit was actually achieved by concomitant inhibition of CTLA-4-expressing Tregs.19,24 Conversely, PD-1 blockade can activate the immunosuppressive function of Tregs, which often exhibit comparable expression levels of PD-1 with CTLs.29 Indeed, in human gastric cancer, anti-PD-1 therapy augmented Treg-mediated immunosuppression in some patients, which contributed to disease hyperprogression during therapy.30 Although decreased Treg infiltration in tumors following ICB treatment correlates with clinical benefit, these populations are often unchanged or increased in tumors following immunotherapy.31 Tregs within the TME can depend on alternative signaling pathways more than peripheral Tregs for infiltration and to maintain their suppressive function (e.g., Helios, and neuropilin-123,25,28,32) and typically feature higher expression of suppressive molecules (e.g., CTLA-4, TIGIT, 0X40, and GITR).23,24 Thus, TME-specific Treg-targeting strategies are needed for effective combination therapies and may be able to selectively reprogram these suppressive cells to an immunostimulatory state.19,27,28,33,34
Clinical studies attempting to deplete Tregs (e.g., via targeting cell surface molecules such as CD25 and CCR4) have been able to reduce Tregs in the TME but have shown mixed effects on CTL activity and treatment outcome.19 On the other hand, targeted strategies with the ability to disrupt the suppressive function of Tregs in the TME (e.g., by exploiting the CD25, 0X40, GITR, 4–1BB, and IDO signaling axes) have seen successful inhibition of immunosuppression by these cells in preclinical studies and feasibility in early clinical trials.19,24,35,37 Indeed, preclinical studies have found that reprogrammed Tregs adopt an immunostimulatory T effector cell phenotype and produce inflammatory cytokines including interferon gamma and IL-17, which may translate in patients.19,26,33,34
As described earlier, VEGF signaling and overexpression promotes Treg proliferation and activation.19,38 VEGF pathway inhibition is associated with reduced immune checkpoint expression and Treg infiltration in both pre-clinical and clinical studies.1,3,19,39 Furthermore, protumor TAMs and MDSCs can produce Treg-recruiting chemo-kines.19 As with antiangiogenic approaches, the timing and dosage of antibodies against/agonizing TME-Treg-specific pathways will be essential for tumor- and Treg-selective control.24 Thus, combinations with either myeloid- or vascular-targeting approaches may enhance Treg functional inhibition to improve immunotherapeutic outcome.
COMBINING ANTIANGIOGENESIS AND IMMUNE CHECKPOINT BLOCKADE IN THE CLINIC
Approaches for combining antiangiogenic agents with im-munotherapeutics have gained traction and are being tested in dozens of ongoing clinical trials (see Table 2 in Fukumura et al1). The majority of work investigating these combinations in patients has been conducted in metastatic melanoma, but new explorations in other solid tumors are ongoing.1,18 Most recently, a phase III clinical trial demonstrated that patients with metastatic nonsquamous non-small cell lung cancer receiving atezolizumab (an ICB against the ligand PD-L1) with chemotherapy had improved progression-free survival and overall survival when they were concomitantly treated with the antiangiogenic agent bevacizumab.6 Importantly, this result was independent of mutational status and checkpoint expression in these tumors, and the success of this trial led to rapid approval by the U.S. Food and Drug Administration of this regimen for first-line treatment of these patients (without EGFR or ALK mutations). Specifically chosen for its immunomodulatory effects on the TME, bevacizumab combined with atezolizumab in patients with hepatocellular carcinoma has had encouraging response rates in interim results from a phase Ib study.40 As a result, the combination of atezolizumab and bevacizumab has been granted breakthrough designation by the U.S. Food and Drug Administration for first-line treatment of hepatocellular carcinoma. Combining pem-brolizumab (anti-PD-1) with axitinib (a tyrosine kinase inhibitor of VEGF receptors) improved overall survival, progression-free survival, and objective response rates over the standard of care as first-line treatment of patients with advanced or metastatic renal cell carcinoma in a phase III trial.41,42 Similarly, combining avelumab (anti-PD-L1) with axitinib improved progression-free survival and objective response rates over the standard of care as first-line treatment of patients with advanced or metastatic renal cell carcinoma in another phase III trial.43 Furthermore, bevacizumab was found to potentiate ICB treatment by increasing antitumor immune activation systemi-cally and in the TME in (1) patients with metastatic renal cell carcinoma (in combination with atezolizumab) in a phase Ib trial, and (2) patients with melanoma (in combination with ipilimu-mab, an anti-CTLA-4 antibody) in a phase I trial.44–46
These early successes may represent only the tip of the iceberg in efficacious combination of immunotherapies with TME-targeting agents. However, care must be taken in the rational design of these approaches to ensure optimal response, which may be determined through careful pre-clinical and clinical studies. A bench-to-bedside-and-back approach for microenvironment-based strategies will not only inform and enhance immunotherapies for solid tumors but also decrease irAEs.1,3 Therapeutic strategies must be designed to ensure that the TME is properly sensitized to ICBs without inducing alternative resistance mechanisms. This includes consideration of the dosing, timing, and sequencing of combination therapies and the host-organ and cancer-specific response of different microenvironments.4 For example, because vascular normalization can enhance drug delivery and distribution, clinicians may be able to lower the administered dose of ICBs because of higher drug concentrations throughout the tumor tissues, thus reducing the incidence and severity of irAEs.1 However, this will require judicious dosing of antiangiogenic agents to avoid aggressive ablation of the vasculature, which can compound hypoxia and immunosuppression. Low doses of antiangiogenics have been taken into account in the design of some ongoing trials with immunotherapies.1
Some tumors may benefit from vascular decompression therapies (e.g., stroma-reducing antihypertensives) to maximize vascular reprogramming and sensitize the TME to combined antiangiogenic and immunotherapies.3,9,11,13 Furthermore, for tumors that feature more normal or coopted vasculatures (e.g., recurrent glioblastoma3), combination approaches targeting the other TME facets described here—myeloid cells and Tregs—may provide benefit. Additional investigation should be made into emergent resistance mechanisms that may arise from the TME in the face of immunotherapy, beyond elevation of alternative immune checkpoint molecules (e.g., Tim-3, Lag-3, and TIGIT). For example, increased levels of circulating angiopoietin-2, a proangiogenic and immunosuppressive molecule, were found in patients with metastatic melanoma after ipilimumab therapy.47 Elevated angiopoietin-2 levels correlated with poorer survival and higher infiltration of protumor myeloid cells after ipilimumab. However, the combination of ipilimumab with bevacizumab was associated with reduced angiopoietin-2 levels and myeloid infiltration and better survival.47 Finally, careful consideration must be given to how TME-specific features (e.g., myeloid phenotypes, CTL infiltration patterns, and vascular morphology) are incorporated into emerging personalized strategies for combination approaches with immunotherapy to close the gap between responders and nonresponders. Likewise, TME factors should be included in biomarker development for predicting immunotherapeutic efficacy.1,19
Using these strategies, a combination of preclinical and clinical testing and validation will reveal how TME-driven resistance mechanisms can be overcome with effective combination therapies to enhance immunotherapy efficacy and durability for patients while reducing adverse effects.
PRACTICAL APPLICATIONS.
Abnormalities in the TME contribute to immunosuppression and dictate the outcome of various immunotherapeutic approaches.
Reprogramming specific facets of the immune compartment, such as immunosuppressive myeloid and lymphoid cell subsets, may overcome microenvironment-induced resistance mechanisms and enhance antitumor immunity.
Targeting nonimmune components of the TME, for example, by normalizing or decompressing the vasculature, represents a clinically translatable strategy to overcoming resistance to ICBs and other immunotherapies.
A bench-to-bedside-and-back approach for microenvironment-based strategies may not only enhance immunotherapies for solid tumors but also abrogate irAEs.
Improved survival in the phase III trial combining bevacizumab with atezolizumab and chemotherapy for metastatic nonsquamous non-small cell lung cancer represents a successful example of such an approach.
ACKNOWLEDGMENT
We thank Z. Amoozgar, PhD; D.G. Duda, DMD, PhD; D. Fukumura, MD, PhD; L.L. Munn, PhD; and T.P. Padera, PhD, for their helpful comments on our manuscript.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Disclosures provided by the authors and data availability (if applicable) are available with this article at DOI https://doi.org/10.1200/EDBK_237987.
REFERENCES
- 1.Fukumura D, Kloepper J,Amoozgar Z,et al. Enhancingcancerimmunotherapyusingantiangiogenics:opportunitiesandchallenges. NatRevClinOncol.2018; 15:325–340. [Google Scholar]
- 2.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019; 51:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164:1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Socinski MA, Jotte RM, Cappuzzo F, et al. ; IMpower150 Study Group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. [DOI] [PubMed] [Google Scholar]
- 7.Chauhan VP, Martin JD, Liu H,et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy bydecompressingtumour blood vessels. Nat Commun. 2013;4:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Incio J, Liu H, Suboj P, et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016;6:852–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Naxerova K, Pinter M, et al. Use of angiotensin system inhibitors is associated with immune activation and longer survival in nonmetastatic pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23:5959–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Cao J, Melamed A, et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Sci USA. 2019;116:2210–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin JD, Seano G, Jain RK. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu Rev Physiol. 2019;81:505–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Palma M, Jain RK. CD4+ T cell activation and vascular normalization: two sides of the same coin? Immunity. 2017;46:773–775. [DOI] [PubMed] [Google Scholar]
- 13.Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Sci Transl Med. 2017;9:eaan5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen IX, Chauhan VP, Posada J, et al. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci USA. 2019;201815515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro-versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. [DOI] [PubMed] [Google Scholar]
- 17.Saung MT, Muth S, Ding D, et al. Targeting myeloid-inflamed tumor with anti-CSF-1R antibody expands CD137+ effector T-cells in the murine model of pancreatic cancer. J Immunother Cancer. 2018;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ott PA, Hodi FS, Buchbinder EI. Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapyfor metastatic melanoma: an overview of rationale, preclinical evidence, and initial clinical data. Front Oncol. 2015;5:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shitara K, Nishikawa H. Regulatory T cells: a potential target in cancer immunotherapy. Ann N Y Acad Sci. 2018;1417:104–115. [DOI] [PubMed] [Google Scholar]
- 20.Peterson TE, Kirkpatrick ND, Huang Y, et al. Dual inhibitionofAng-2andVEGF receptorsnormalizestumorvasculatureand prolongssurvival in glioblastoma by altering macrophages. Proc Natl Acad Sci USA. 2016;113:4470–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Setoguchi R, Yagi H, et al. Naturallyarising Foxp3-expressingCD25+CD4+ regulatoryT cells in self-tolerance and autoimmune disease. CurrTop Microbiol Immunol. 2006;305:51–66. [DOI] [PubMed] [Google Scholar]
- 22.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–165. [DOI] [PubMed] [Google Scholar]
- 23.Gratz IK, Campbell DJ. Organ-specific and memory treg cells: specificity, development, function, and maintenance. Front Immunol. 2014;5:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. [DOI] [PubMed] [Google Scholar]
- 25.Yates K,Bi K, Haining WN, et al. Comparativetranscriptome analysis revealsdistinctgenetic modulesassociated with Heliosexpression in intratumoral regulatory T cells. Proc Natl Acad Sci USA. 2018;115:2162–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zemmour D, Zilionis R, Kiner E, et al. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped bythe TCR [published correction appears in Nat Immunol. 2018;19:645]. Nat Immunol. 2018;19:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa H, Sido JM, Reyes EE, et al. Instability of Helios-deficient Tregs is associated with conversion to a T-effector phenotype and enhanced antitumor immunity. Proc Natl Acad Sci USA. 2016;113:6248–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overacre-Delgoffe AE, Chikina M, Dadey RE, et al. Interferon-y drives Treg fragility to promote anti-tumor immunity. Cell. 2017;169:1130–1141.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Togashi Y, Shitara K, Nishikawa H. RegulatoryT cells in cancer immunosuppression - implicationsforanticancertherapy. Nat RevClin Oncol. Epub 2019January 31. [DOI] [PubMed] [Google Scholar]
- 30.Togashi Y, Kamada T, Sasaki A, et al. Clinicopathological, genomic and immunological features of hyperprogressive disease during PD-1 blockade in gastric cancer patients. J Clin Oncol. 2018;36;15s(suppl; abstr4106). [Google Scholar]
- 31.O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HJ, Barnitz RA, Kreslavsky T, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Vignali DA, Rudensky AY, et al. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. [DOI] [PubMed] [Google Scholar]
- 34.Sawant DV, Vignali DA. Once a Treg, always a Treg? Immunol Rev. 2014;259:173–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rech AJ, Mick R, Martin S, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4:134ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baban B, Chandler PR, Sharma MD,et al. IDO activates regulatoryT cellsand blockstheir conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akhmetzyanova I, Zelinskyy G, Littwitz-Salomon E, et al. CD137 agonist therapy can reprogram regulatory T cells into cytotoxic CD4+ T cells with antitumor activity. J Immunol. 2016;196:484–492. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, Goel S, Duda DG, et al. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tada Y, Togashi Y, Kotani D, et al. Targeting VEGFR2 with ramucirumab strongly impacts effector/ activated regulatory T cells and CD8+ T cells in the tumor microenvironment. J Immunother Cancer. 2018;6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein S, Pishvaian MJ, Lee MS, et al. Safety and clinical activity of 1Latezolizumab + bevacizumab in a phase Ibstudy in hepatocellular carcinoma (HCC). J Clin Oncol. 2018;36;15s(suppl; abstr 4074). [Google Scholar]
- 41.Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumabin patientswith advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018;19:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. Epub 2019. February 16. [DOI] [PubMed] [Google Scholar]
- 43.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. Epub 2019. February 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallin JJ, Bendell JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X, Giobbie-Hurder A, Liao X, et al. VEGF neutralization plus CTLA-4 blockade alters soluble and cellular factors associated with enhancing lymphocyte infiltration and humoral recognition in melanoma. Cancer Immunol Res. 2016;4:858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu X, Giobbie-Hurder A, Connolly EM, et al. Anti-CTLA-4 based therapy elicits humoral immunity to galectin-3 in patients with metastatic melanoma. OncoImmunology. 2018;7:e1440930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu X, Giobbie-Hurder A, Liao X, et al. Angiopoietin-2 as a biomarker and target for immune checkpoint therapy. Cancer Immunol Res. 2017;5:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


