Abstract
Background
People with HIV (PWH) have increased cardiovascular events, inflammation, and high-risk coronary atherosclerosis. Statin therapy has been shown to lower the risk of cardiovascular disease (CVD) in the general population, but whether this results from reductions in coronary atherosclerosis and is mediated by decreased inflammation remains unknown.
Methods
REPRIEVE is a randomized, placebo-controlled trial of pitavastatin calcium (4 mg/day) vs. placebo enrolling at least 7500 PWH between 40–75 years, on antiretroviral therapy (ART), with low to moderate traditional CVD risk. The Mechanistic Substudy of REPRIEVE (A5333s) is co-enrolling 800 participants from 31 US sites. These participants undergo serial contrast enhanced coronary computed tomography angiography (CCTA) and measurements of biomarkers of inflammation and immune activation at baseline and after 2 years of follow-up. The primary objectives are to determine the effects of pitavastatin on noncalcified coronary atherosclerotic plaque (NCP) volume, low attenuation plaque, and positive remodeling and on changes in immune activation and inflammation and to assess relationships between the two. Changes in CAD will be assessed in a standardized fashion by a core lab with expert readers blinded to time points and participant information; immune activation and inflammation assessment is also performed centrally.
Results
To date the Mechanistic Substudy has completed planned enrollment, with 805 participants.
Conclusion
This study represents the first large, randomized, CCTA-based assessment of the effects of a primary prevention strategy for CVD on high-risk CAD, immune activation and inflammation among PWH. The study will assess pitavastatin’s effects on coronary plaque, and the interrelationship of these changes with biomarkers of immune activation and inflammation in PWH to determine mechanisms of CVD prevention and improved outcomes in this population.
People with HIV (PWH) have an excess 1.5- to 2-fold risk of myocardial infarction (MI) as compared to HIV-uninfected individuals with a similar traditional cardio-vascular (CV) risk profile.1,2 This increased risk is thought to be due, in part, to immune dysfunction (e.g. reduced CD4+ T-cell counts) and to heightened immune activation and inflammation related to the virus, both of which may lead to acceleration of high-risk coronary artery disease (CAD), even in young PWH with relatively low traditional risk scores.3,4 Moreover, PWH have demonstrated increased noncalcified and high risk plaque5–7 suggesting increased vulnerable plaque as a mechanism of cardiovascular disease (CVD) HIV. Statins reduce major adverse cardiovascular events (MACE) in the HIV-uninfected population8 and have been shown to effectively reduce LDL cholesterol (LDL-C), key inflammatory markers, immune activation indices among HIV-infected patients.9,10 For example, 48 weeks of rosuvastatin treatment reduced significantly several markers of inflammation and lymphocyte and monocyte activation in ART-treated subjects.11 However, results have varied depending on endpoints and study design and a recent meta-analysis of studies in HIV positive patients could not identify a clear association between any of the immune markers and surrogate CVD outcomes.12 This maybe due to insensitivity of the primary surrogate markers used (CAC and arterial stiffness) or may be explained by heterogeneity across studies and lack of follow-up data. Overall though, it emphasizes the need for a well powered and conducted study to establish an association and moreover provide preliminary data on whether this association drives outcomes.
The REPRIEVE is a landmark trial to determine whether statin therapy (in this case pitavastatin) will prevent MACE in HIV-infected patients with low to moderate traditional CVD risk. An important aspect of REPRIEVE is the embedded Mechanistic Substudy of REPRIEVE (A5333s), hereafter referred to as the Mechanistic Substudy, that will simultaneously evaluate the mechanisms by which statins achieve an effect in PWH. This substudy will test whether statin therapy can reduce high-risk coronary plaque, modify inflammation and immune activation and the association of these effects with each other and with adverse cardiovascular outcomes.
Recent data in HIV demonstrate increased immune activation (monocyte activation, T cell activation), generalized inflammation, and arterial inflammation, as well as increased coagulation markers. Moreover, among PWH key indices of systemic immune activation and inflammation (including monocyte activation markers such as soluble CD14 (sCD14), soluble CD163 (sCD163) and T-cell activation markers) have been linked to subclinical carotid atherosclerosis, high-risk coronary atherosclerotic plaque, cardiovascular events, and mortality.6,9,13–21 Importantly, among PWH, ART lowers systemic immune activation, but may not be adequate to effectively reduce ongoing systemic and arterial inflammation and atherogenesis,22–24 highlighting the potential importance of adjunctive anti-inflammatory strategies for CVD prevention.12,25 Statin effects on arterial inflammation, oxidized LDL (oxLDL), sCD14, CRP, TNF-alpha, and immune activation in association with reductions in high-risk plaque (HRP), may provide a potential mechanism for statin effects on MACE.9,11,26–29
Coronary computed tomography angiography (CCTA) allows for non-invasive and accurate characterization of CAD, including atherosclerotic plaque and luminal stenosis.30 Across populations, both non-obstructive and obstructive (significant luminal narrowing) atherosclerosis have proven to be strong and independent predictors of MACE, with a three-fold and six-fold increased risk, respectively; as compared to individuals without these findings.30,31
Although most data on the prognostic value of CAD in asymptomatic populations are based on the measurement of coronary artery calcification (CAC),32–34 advanced CAD in PWH, especially in men is characterized by an increase in noncalcified plaque and HRP features such as plaque with low CT attenuation (correlated with necrotic lipid core) and positive remodeling (reflecting eccentric plaque extension) but not calcified plaque (Figures 1 and 2)6,13 independent of CAD risk factors.35 These data are consistent with the hypothesis that increased CV morbidity and mortality in the setting of HIV is driven by increased systemic immune activation and inflammation, a phenomenon that leads to an increased influx of inflammatory macrophages, which are more typically found in active non-calcified high risk morphology plaque.36,37 High risk CAD features are thought to identify individuals at greater risk of plaque rupture and have been associated with MACE in symptomatic HIV-uninfected populations.38–42 Serial CCTA is the preferred means to providing this assessment of high risk plaque features noninvasively and can detect and quantify changes in noncalcified coronary plaque volume in response to statin therapy43 (Table I). The potential utility of this technique was demonstrated in a smaller 12 month study among PWH, in which statin therapy reduced noncalcified plaque volume by 19% compared to a 20% increase with placebo, with accompanying decreases in high-risk plaque features and oxLDL.26,44
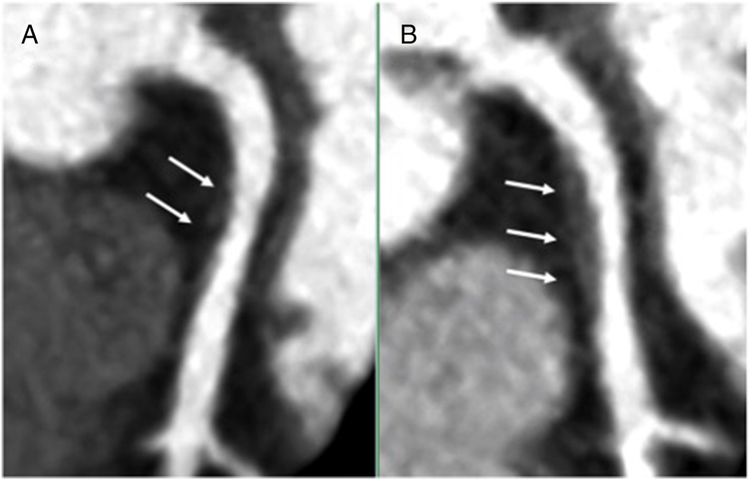
Figure 1.
Curved planar reformats of (A) baseline and (B) 1-year follow-up coronary CTAs in a 58-year-old woman living with HIV. A noncalcified proximal left anterior descending coronary artery plaque (arrows) increased in size while on placebo
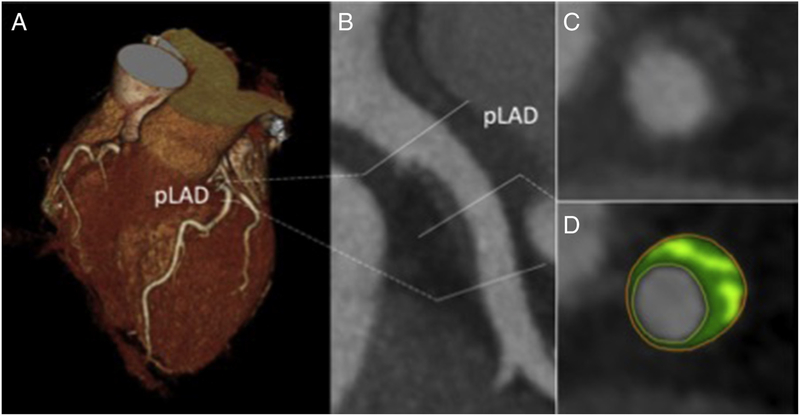
Figure 2.
Coronary computed tomography angiography (CCTA) (A) 3d volume rendering and (B) curved planar reformat through a proximal left anterior descending plaque. Plaque volume is quantified on the (C) short axis slice through the artery, with the (D) attenuation of plaque voxels categorized as noncalcified or calcified.
Table I.
Statin studies using change in coronary plaque volume on Serial CCTA as a measure of therapeutic efficacy
| Study | Population | Design | Therapy or Comparison | Main Finding |
|---|---|---|---|---|
| Inoue K et al. JACC Cardiovasc Imaging. 201048 | Non-HIV with CAD, n=32 | Prospective Interventional, not randomized | 12 months fluvastatin vs refused fluvastatin | Mean plaque volume: Fluvastatin −15.9 mm3 vs control 4 mm3 (p=0.01) |
| Lo J et al. Lancet HIV. 201521 | PWH, n=37 | Prospective Interventional Randomized Controlled | 12 months atorvastatin vs placebo in persons with HIV | Median Δ noncalcified plaque volume: atorvastatin −19% (−8.2 mm3) vs. placebo +20% (+6.7 mm3), p=0.009 |
| Auscher S et al. Atherosclerosis. 201549 | HIV-uninfected with acute MI, n=96 | Prospective Interventional Randomized Controlled | 12 months intensive rosuvastatin vs standard statin | Median Δ plaque volume intensive: +43.5 vs standard: +19.1 mm3, p<0.001 |
| Noguchi T et al. JACC 201550 | HIV-uninfected CAD | Prospective. Interventional, non-randomized | 12 months intensive pitavastatin vs propensity matched controls | Mean Δ low attenuation plaque volume: intensive −12.8 vs controls: +8.3 mm3, p=0.004 |
Abbreviations: CCTA, Coronary computed tomography angiography; CAD, coronary artery disease; PWH, people with HIV; Mi, myocardial infarction.
REPRIEVE is a landmark primary prevention trial assessing statin effects on MACE in PWH. Use of serial CCTA, in parallel with assessment of statin effects on key inflammatory and immune pathways in a large subset of participants enrolled in REPRIEVE, will provide a novel opportunity to assess the mechanisms of statin effects on CVD in PWH and provide important context to the findings in REPRIEVE.
Methods
Major objectives
PWH have evidence of high risk plaque and inflammation, often in the absence of increased LDL-C, that may contribute to increased CVD morbidity and mortality. The Mechanistic Substudy was designed to formally test whether statin therapy, results in reduction or non-progression of NCP volume, incident NCP, and the presence and extent of HRP among PWH. The addition of a wide panel of biomarkers of immune activation and inflammation will be key to identifying interactions between atherosclerosis and metabolic pathways including potential upstream mediators of statin effects on plaque and the impact of both mechanisms on outcomes.
The primary objective of the Mechanistic Substudy is to determine the effects of pitavastatin on the morphology and composition of NCP, including the progression of plaque volume and incident NCP and whether these effects are modulated by markers of inflammation and immune activation.
Key secondary objectives are to determine the effects of pitavastatin on the progression and incidence of HRP features, including low attenuation plaque and positive remodeling; to determine the effects of pitavastatin on immune markers, including immune function (CD4, HIV viral load), immune activation (%CD14+CD16+ monocytes, sCD163, sCD14, MCP-1 and T-cell markers), inflammation (Lp-PLA2, hsCRP, IL-6, oxLDL) and coagulation (D-Dimer and tissue factor), as well as traditional CVD risk indices including glucose homeostasis parameters (insulin, glucose and related indices of insulin resistance such as HOMA-IR, HgbA1c); to evaluate the relative contributions of baseline and pitavastatin-induced changes in HIV-specific immune activation and traditional CVD risk factors, including LDL-C, on the presence and progression of coronary plaque and high risk morphological features in HIV; and to assess the relationship of host genetics to study endpoints.
Primary mechanistic hypothesis
The primary hypothesis of the Mechanistic Substudy is that pitavastatin therapy will reduce progression of NCP volume over two years as measured by serial CCTA as compared with placebo in PWH on ART in whom traditional CVD risk is not significantly increased. The mechanisms underlying the effect of statins will include a) reduction in noncalcified coronary atherosclerotic plaque, b) reduction in vulnerability features of non-calcified coronary atherosclerotic plaque, and c) improvement in critical indices of immune activation and inflammation.
Secondary mechanistic hypotheses
The secondary hypothesis of the Mechanistic Substudy is that reduction in LDL-C levels associated with pitavastatin therapy will be predictive of improvement in noncalcified coronary atherosclerotic plaque burden and/or vulnerability features. Additionally, pitavastatin therapy will decrease indices of general inflammation, coagulation, monocyte activation, and arterial inflammation. More specifically, pitavastatin therapy will reduce levels of (a) pro-inflammatory monocyte populations and (b) T-cell activation and exhaustion. The observed changes in immune markers will be associated with changes in morphology and composition of NCP. Lastly, pitavastatin therapy will not have a clinically significant effect on glucose and insulin resistance.
Funding
Funding for this project comes from U01 HL123336 awarded to the Clinical Coordinating Center (CCC) and U01 HL123339 awarded to the Data Coordinating Center (DCC) from National Heart, Lung, and Blood Institute (NHLBI) and by the National Institute of Allergy and Infectious Diseases (NIAID) Division of AIDS (DAIDS), the Office AIDS Research (OAR), and the AIDS Clinical Trials Group (ACTG). Kowa Pharmaceuticals America, Inc. and Gilead Sciences, Inc. provided additional support. The authors are solely responsible for the design and conduct of this study including, all study analyses, the drafting and editing of this manuscript, and its final contents. The views expressed are those of the authors and do not necessarily represent the views of the NIH or Department of Health and Human Services.
Overall study design
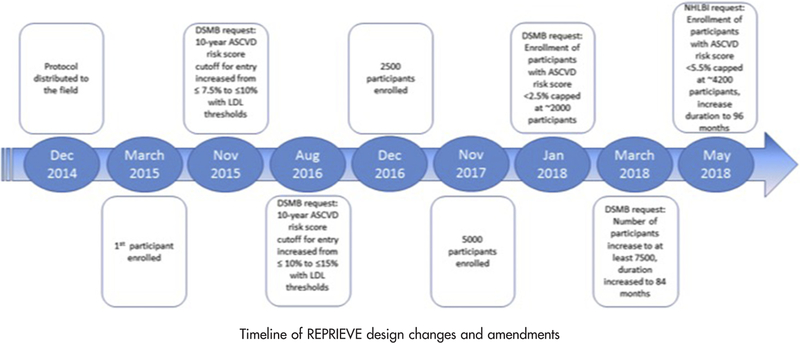
The Mechanistic Substudy is a prospective substudy of approximately 800 participants enrolled in REPRIEVE, a double-blind, randomized, placebo-controlled, multicenter, phase III efficacy trial for primary CVD prevention among HIV-infected patients on stable ART, randomized to 4mg daily pitavastatin vs. matching placebo (Figure 3). Participants co-enrolled in the Mechanistic Substudy will undergo CCTA and blood sampling at baseline and after two years in addition to the procedures for the main REPRIEVE trial, with continued follow up in the main REPRIEVE trial for approximately 48 to 96 months, depending on date of enrollment in the trial.
Figure 3.
Timeline of REPRIEVE design changes and amendments
Site selection and qualification
The Mechanistic Substudy is conducted at 31 US sites out of a total of 116 sites participating in the REPRIEVE trial. Substudy enrollment occurs at the same time as enrollment for the REPRIEVE trial using a separate additional informed consent. Prior to implementation of the Mechanistic Substudy, each site must have the substudy protocol and the informed consent form approved, as appropriate, by their local IRB/EC and any other applicable regulatory entity (RE). Site qualifications for the Mechanistic Substudy include: (a) participation in the REPRIEVE trial, (b) performance by the site radiology or cardiology department of >1000 CT/per year, (c) MD oversight/supervision of CT scans, (d) access to at least 2nd generation 64-slice CT scanner, (e) utilization of level III readers and (f) capability to perform prospective triggering/gating, using low kV and capacity to premedicate participants. Site certification and activation for participation in the Mechanistic Substudy includes mandatory protocol completion requirements such as IRB approval, contract execution, protocol training, and successful submission of two adequate test CCTAs for image quality and radiation safety assessment.
Study population
HIV-infected men and women ≥40 and ≤75 years of age who were eligible and enrolling in the main REPRIEVE trial co-enrolled in the Mechanistic Substudy. Requirements for enrollment in the main REPRIEVE trial include continuous use of ART for at least 6 months prior to study entry with CD4+ T-cell count >100 cells/mm3. Eligibility for REPRIEVE is further based on ASCVD score and LDL-C (see accompanying manuscript), with glomerular filtration rate (GFR) ≥60 mL/min/1.73m2 or creatinine clearance (CrCl) ≥60 mL/min.
Participant eligibility for the mechanistic Substudy of REPRIEVE
Enrollment into the Mechanistic Substudy occurred concurrently with enrollment and randomization into the main REPRIEVE trial. Eligibility for the Mechanistic Substudy was determined at a screening visit to determine participant’s willingness to complete study procedures and to rule out contraindications to CCTA (previous contrast reaction). CCTA was performed within 14 days after randomization in REPRIEVE. Depending on timing of the screening creatinine assessment in relationship to the entry CCTA, an additional serum creatinine was drawn to ensure an evaluation of GFR or CrCl within 14 days prior to CCTA. A GFR ≥60 mL/min/1.73m2 or creatinine clearance (CrCl) ≥60 mL/min, calculated using standard equations, is required for the subject to proceed with CCTA both at entry and at month 24. Additional information is collected as part of a participant’s enrollment in REPRIEVE on medical history, including CV risk history, physical exam, and medication history (Table II).
Table II.
Eligibility criteria in the Mechanistic Substudy of REPRIEVE (A5333s)
| Inclusion criteria |
| Enrollment in REPRIEVE (A5332) |
| Willingness to complete procedures required for the study |
| GFR ≥60 mL/min/1.73m2 or CrCl ≥60 mL/min, as per REPRIEVE (A5332) within 14 days prior to CCTA |
| Exclusion criteria |
| Known allergy to iodinated contrast agent |
| Currently symptomatic asthma |
| Allergy to beta blockers |
| Contraindication to beta blockers (i.e., taking daily asthma medications) Positive pregnancy test within 24 hours prior to study entry |
| Any condition that prohibits the individual from completing the CCTA BMI ≥40 kg/m2 |
| Cardiac arrhythmia at enrollment precluding CCTA; such as atrial fibrillation with heart rate >80 beats per minute or frequent ectopic beats |
Abbreviations: GFR, Glomerular filtration rate; CrCl, creatinine clearance; CCTA, coronary computed tomography angiography; BMI, body mass index.
Enrollment
Participants are enrolled in the Mechanistic Substudy via the enrollment system at the same time as they are randomized into REPRIEVE. As part of their participation in the main REPRIEVE trial, participants are randomly assigned in a ratio of 1:1 to either 4mg pitavastatin or identical placebo for pitavastatin, 1 pill daily. To ensure balanced treatment allocation in the Mechanistic Sub- study, REPRIEVE main study randomization is stratified by planned Mechanistic Substudy participation.
Intervention and subsequent study visits
After completion of the baseline CCTA, participants begin treatment with blinded study medication as per the REPRIEVE protocol. For the Mechanistic Substudy, blood is drawn at the 4 month follow up visit, and CCTA and blood are obtained at the 24 month follow up visit. The 24 month CCTA is conducted under the same protocol, including the same kVp setting as the baseline CCTA with identical blood collection as the baseline visit. Paired scans from baseline and month 24 visits are analyzed side by side with CT readers blinded to treatment assignment and CCTA time point (baseline vs follow-up).
Endpoints
Primary endpoint.
The primary outcome measure of the Mechanistic Substudy is change in NCP volume among participants with NCP evidence at entry or incident NCP among subjects without NCP evidence.
Secondary endpoints.
Secondary outcome measures include presence and number of HRP features (i.e. low attenuation and positive remodeling on CT); levels of immune, inflammatory and coagulation biomarkers at study entry, month 4, and month 24, and changes from study entry to month 4 and month 24 of immune, inflammatory and coagulation biomarkers; fasting lipid fractions (Total, HDL cholesterol [HDL-C], non-HDL-C, and LDL-C:HDL-C ratio) at study entry, month 4 and month 24; fasting insulin, HgbA1c, and HOMA-IR at study entry, month 4, and month 24 (excluding HgbA1c at month 4; HgbA1c will be available at entry and month 24 only); and time to the first major cardiovascular event. Flow cytometry is performed at Boston College from cryopreserved peripheral blood mononuclear cells (PBMCs) collected at baseline and 24 months, utilizing a standard ACTG preparation procedure to ensure cell viability. This analysis uses gating and analytical proce- dures to assess activated monocytes, see Table III for anticipated analyses. Immune activation and other inflammatory biomarker measurements are performed at Temple University. Biomarkers measured include: sCD163 (Trillium, now IQ Products), sCD14, monocyte Chemoattractant Protein-1 (MCP-1), Lipoprotein-associated phospholipase A2 (Lp-PLA2), high sensitivity Interleukin-6 (IL-6) (all R & D Systems) and the metabolic marker oxLDL (Mercodia). Samples are analyzed in batches every 6 months by ELISA assays from EDTA-coagulated plasma
Table III.
Anticipated flow cytometry panels to be performed in ana
| Panel | FITC | PE | TxRed-PE | Cy5-PE | Cy5.5-PerCP | Cy7-PE | APC | Alexa 700 | Cy7-APC | Pacific Blue | Aqua | QD655 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes | CCR7 | CD38 | CD45RA | CD16 | CD4 | CD56 | CD20 | CD3 | HLA-DR | PD-1 | Live/Dead | CD8 |
| Monocytes #1 | - | CCR2 | HLA-DR | - | CD163 | CD16 | CCR5 | CD3 | CD20 | CD14 | Live/Dead | CD4 |
| Monocytes #2 | CX3CR1 | CD169 | HLA-DR | - | - | CD16 | TF | CD3 | CD20 | CD14 | Live/Dead | - |
Statistical methods
Sample size and power calculations.
The target sample size for the Mechanistic Substudy was 800 participants approximately equally distributed between the study arms. This sample size was determined to have high power to detect clinically relevant differences between the two study groups with respect to progression of non-calcified plaque (NCP), defined as progression of baseline NCP among those with plaque at study entry and as rates of incident plaque (among those plaque-free at entry). Specifically, the total sample size of 800 participants will provide 90% power to detect a 6% difference between the study groups in the percent change in NCP volume over 2 years among those with plaque at entry and 90% power to detect 13 percentage point difference in the probability of plaque development over 2 years. These effect sizes translate to a combined estimated 14 percentage point difference in the probability of NCP progression over two years and are based on the following assumptions: a) 50% of study participants will have evidence of NCP at study entry5; b) a SD of 20% for the percent change over 2 years among participants with evidence of plaque at entry; c) an annual rate of incident plaque development of 12% among participants without plaque at entry; d) 15% of participants entering the substudy will not be evaluable for study entry or 2 year NCP volume. Together, the effects of statins acting in these two groups (those with and without evidence of NCP at study entry) will provide for a 7% lower prevalence of NCP after 2-years of statin treatment.
Primary statistical analysis.
Among participants with plaque at entry, descriptive statistics for the change and percentage change in NCP volume over 2 years will be provided by treatment group with group comparisons made with stratified t-test. Among those without NCP at entry, the prevalence of incident NCP over 2 years will be compared with stratified chi-squared test. To assess the mechanistic study population as a whole, participants will be classified as progressors (any progression/increase in NCP volume OR incident NCP) or non-progressors (no progression in NCP volume OR no incident NCP); the probability of progression over two years will be compared by treatment group using a stratified chi-squared test. All treatment group comparisons will be performed by intention to treat using a 5% type error. Unless otherwise noted, analyses will be performed by subgroups defined according to the presence of NCP at study entry.
Secondary and sensitivity analyses.
Statin effects on coronary plaque morphology.
The statin effect on HRP features, including low HU attenuation and positive remodeling, will be assessed by comparing differences in the 2-year prevalence of high-risk plaque morphology features between treatment groups using chi-squared (or Fisher’s exact) tests as appropriate; these analyses will be performed overall and by subgroups defined by the presence of NCP at study entry. Exploratory analysis will be performed for additional high-risk plaque features that have been described in CCTA and intravascular ultrasound (IVUS) studies including the napkin-ring sign, minimal luminal area, plaque burden, and segments with NCP. The analytic approach will be similar as described above.
Statin effects on blood biomarkers.
Statin effects on the distributions of blood biomarkers belonging to distinct pathways (i.e., monocyte activation, generalized inflammation, and coagulation) will be assessed via treatment group comparisons of these respective markers via t-tests; modification of statin effect on these markers by HIV-1 and traditional risk factors (including sex, age, screening CD4, duration of suppressive ART, and presence of NCP at study entry) will be assessed. Since the hypothesized mechanism is that sustained high levels of immune activation and inflammation precede and contribute to progression of NCP volume and HRP features, these analyses will relate short-term changes in these biomarkers (over 4 months) to longer term changes in NCP volume and morphology after two years.
LDL-C and blood biomarkers as mediators for plaque progression.
In the event that both statin effects on NCP progression and biomarker changes are apparent, the association between changes in LDL-C and these bio- markers and NCP progression will be examined using graphical techniques and normal errors and logistic regression (for the subpopulations with and without NCP at entry respectively. A mediating effect of these biological factors will be evaluated by examination of changes in the estimated statin effect on NCP after adjustment for these biological factors.
Plaque Progression and MACE.
These hypothesis generating analyses will assess the association of baseline CAD characteristics including NCP volume and HRP, 2-year changes in NCP volume and 6-month and 2-year changes blood biomarkers and the hazard of MACE to provide valuable insights to the mechanisms of CVD in HIV and the role of statins in reducing CVD burden in this population. Those biomarkers with the strongest mediating effect on plaque progression will then be measured in the entire REPRIEVE cohort to determine their association with MACE.
Organization and human Studies approval
The Mechanistic Substudy is conducted under the supervision of an Executive Committee (EC). The DCC coordinates all procedures associated with the Mechanistic Substudy, and performs all aspects of protocol monitoring; protocol development; and site selection, evaluation, and performance monitoring; guides image acquisition; and is responsible for receiving, tracking, and archiving CCTA images that are obtained during the study. Quality control (QC) checks for image quality and completeness of all CCTAs are also performed by the DCC. Radiation safety is supervised by the DCC. Frontier Science Foundation is the REPRIEVE data management center and provides and maintains infrastructure for data collection and supervises visit tracking for the substudy as well as the main REPRIEVE trial, AE reporting, and specimen handling and shipping.
The NHLBI appointed an independent Data and Safety Monitoring Board (DSMB) to monitor participant safety and provide recommendations regarding termination, continuation or modification the study protocol as necessary. Data from the Mechanistic Substudy is reviewed in parallel with data from the main REPRIEVE trial by REPRIEVE DSMB every 6 months. The Partners Health Care Institutional Review Board approved the protocol and provides oversight of central activities of the Coordinating Centers, including implementation of design changes and overall trial structure.
Results
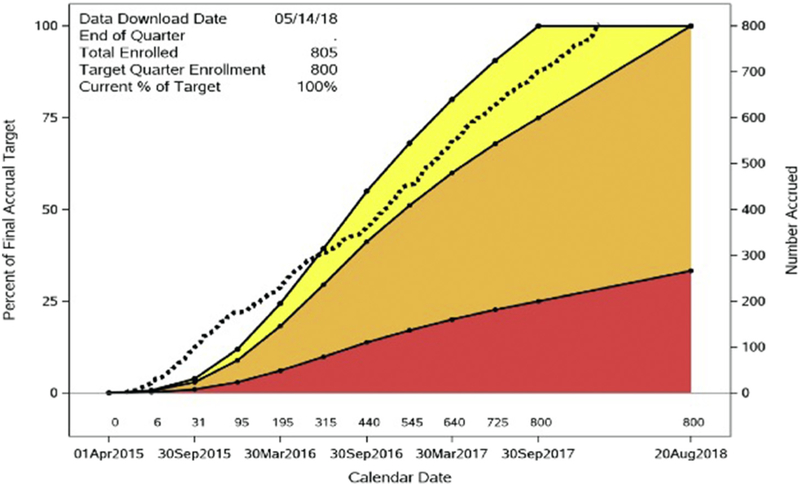
As of February 2018, the Mechanistic Substudy completed enrollment of 805 participants at 31 US-ACTG sites (Figure 4). Work has begun to analyze paired scans as well as inflammatory biomarkers from baseline and 2 years as participants complete the substudy.
Figure 4.
Mechanistic Substudy accrual figure. Dotted line shows actual accrual progress. Colored bands denote predefined NIH target enrollment zones: yellow (100%−75%), orange (75%−25%), red (<25%).
Discussion
Statin therapy lowers CVD events by 30% in classic primary and secondary prevention settings.45,46 However, the mechanisms of such effects remain unknown. This substudy of REPRIEVE was designed to assess potential mechanisms of statins on CVD in PWH, by determining serial plaque measurements, and key inflammatory and immune biomarkers in a large subset of patients enrolled in REPRIEVE. The Mechanistic Substudy will add significantly to the understanding of the results of REPRIEVE.
IVUS studies in non-HIV infected individuals have shown a variable association between statin therapy and change in atherosclerosis with changes ranging from minor regression (−2.3%) to minor progression (+1.5%)47 despite uniformly substantial lowering in LDL-C. The use of CCTA provides substantial benefits over traditional IVUS techniques for the assessment of CAD progression, including the ability to assess changes in a more generalizable manner across the entire coronary artery tree in a noninvasive fashion.
In addition, recent research has focused on the pleiotropic anti-inflammatory effects of statins as an additional explanation for their observed benefits. Indeed, JUPITER48 and CANTOS49 have tested the hypothesis that that reduction in inflammation may contribute to reducing CVD event rates. Unfortunately, none of these studies included imaging of CAD and hence a mechanistic explanation for the observed benefits, specifically whether a change in high-risk features of CAD such as NCP or HRP may have been causally responsible, could not be provided. In fact, to date, no such large trial combining coronary architecture with immune/inflammatory signals has been conducted either in people with or without HIV.
The Mechanistic Substudy of REPRIEVE will assess the mechanisms of statins within the context of a large randomized primary prevention trial of statin therapy in PWH with low to moderate traditional cardiovascular risk. Thus, this substudy offers a unique opportunity to study statin effects in a population whom traditional risks are not significantly increased, and in which inflammation and immune activation is thought to contribute to high risk plaque and potential plaque rupture. The Mechanistic Substudy of REPRIEVE will take advantage of the main study randomization, and rigorously determine statin benefits in this population utilizing serial CCTA and deep phenotyping of inflammatory and activation markers.
The study was launched in 2015 and has completed enrollment and baseline evaluations of 805 participants, utilizing 31 US sites, most of which are participating in the NIH-supported AIDS Clinical Trials Group (ACTG), with access to high quality CCTA and established biomarker preparation techniques, and is based on a highly successful, and novel collaboration among multiple stakeholders, including NHLBI, NIAID, OAR, and the ACTG.
An important goal of the REPRIEVE Mechanistic Substudy is to provide key scientific insights into how statin therapy affects CCTA-based plaque morphology and whether any plaque changes are predated by and related to statin-induced changes in immune activation and inflammation among PWH. In addition, we will determine whether the potential effects of statins on stabilization of atherosclerotic plaque—rendering plaques less likely to rupture and cause acute myocardial infarction—are mediated primarily through lipid-lowering effects or through immunomodulatory effects, and whether plaque stabilization is related to baseline traditional risk factors.
This trial was designed to demonstrate the effect of statin therapy on CAD among PWH in whom traditional risk factors are often not increased. This study has relevance to other disease populations with an increased inflammatory potential such as rheumatoid arthritis.50 Thus, PWH represent an important population in which to test whether CVD-protective effects of statins is mediated through pleotropic effects on immune activation and inflammation9,37,51 as well as lipid lowering.
REPRIEVE and its Mechanistic Substudy will help to provide information to guide primary CVD prevention for PWH, a population for whom appropriate algorithms for predicting CVD events have not been fully developed and among whom risk stratification is challenging.52–54 REPRIEVE relies on a recently developed algorithm employing assessment of 10-year ASCVD risk score via the 2013 ACC/AHA risk calculator, with paradigm modifications to permit enrollment of higher-risk participants with lower LDL-C levels. Data on the utility of the 10-year ASCVD risk score are retrospective, and no study has assessed this algorithm prospectively.53,54 We have shown that CAD, as detected by CCTA, effectively reclassifies ASCVD risk in individuals without HIV,31,55 and it will be important to assess how this new risk score relates to plaque morphology and ultimately MACE events in the HIV population.
Given that substudy enrolment was complete before changes in the ASCVD risk score requirements were made, proportionally more participants with lower ASCVD risk scores are expected in the substudy population than in a comparable US-based population in the main study. Given the timing of the enrollment restrictions relative to full enrolment of the parent study, this shift is expected to be modest.
Summary and significance
To our knowledge, the REPRIEVE Mechanistic Substudy represents the first large substudy of a primary CVD prevention trial to specifically assess statin mechanisms in any population. We will utilize state of the art CCTA assessment on high-risk plaque paired with detailed immunophenotyping to provide much needed insights into whether the benefits of statins on CVD prevention in PWH can be explained by modifications in coronary plaque as well as whether these changes are mediated by changes in key immune and inflammatory indices, and how both are related to adverse outcomes.
Acknowledgements
The study investigators would like to thank the study participants, site staff and study-associated personnel, REPRIEVE Community Advisory Board members and Clinical Trials Specialists (Barbara Bastow, Laura Moran and Jhoanna Roa) for their efforts to make this study possible.
Disclosures
Udo Hoffmann, M.D., M.P.H. has received grant support through his institution from Kowa Pharmaceuticals America, Inc. for the conduct of the study, from HeartFlow Inc., Medimmune Inc. unrelated to this project.
Michael T. Lu, M.D., M.P.H. has received grant support through his institution from Kowa Pharmaceuticals America, Inc. for the conduct of the study.
Devvora Olalere, M.S. has received grant support through her institution from Kowa Pharmaceuticals America, Inc. for the conduct of the study.
Elizabeth C. Adami, B.A. has received grant support through her institution from Kowa Pharmaceuticals America, Inc. for the conduct of the study.
Michael T. Osborne, M.D. has received grant support through his institution from Kowa Pharmaceuticals America, Inc. for the conduct of the study.
Alex Ivanov, B.S. has received grant support through his institution from Kowa Pharmaceuticals America, Inc. for the conduct of the study.
John Sukumar Aluru M.B.B.S., M.S. has received grant support through his institution from Kowa Pharmaceuticals America, Inc. for the conduct of the study.
Saeyun Lee, B.A. has received grant support through her institution from Kowa Pharmaceuticals America, Inc. for the conduct of the study.
Nadja Arifovic, B.S. has received grant support through her institution from Kowa Pharmaceuticals America, Inc. for the conduct of the study.
Edgar Turner Overton, M.D. has received grant support through his institution from Gilead unrelated to the study.
Carl Fichtenbaum, M.D., has received grant support through his institution from Gilead Sciences, ViiV Healthcare, Janssen, Pfizer, Merck, Amgen and Cytodyn unrelated to the study. He also receives consulting fees for advisory board from Janssen.
Judith A. Aberg, M.D. has received research grants from Bristol-Myers Squibb, Gilead Sciences and Viiv Healthcare, and received scientific advisory board personal fees from Gilead, Janssen, Merck, and ViiV Healthcare, all unrelated to the present study..
Beverly Alston-Smith, M.D. is an employee of the National Institutes of Health.
Karin L. Klingman, M.D. is an employee of the National Institutes of Health.
Myron Waclawiw, Ph.D. is an employee of the National Institutes of Health.
Tricia H. Burdo, Ph.D. has no disclosures to report.
Kenneth C. Williams, Ph.D. and has no disclosures to report.
Markella V. Zanni, M.D. has participated in a scientific advisory board meeting for Roche Diagnostics and has received grant support through her institution from Gilead for the conduct of the study.
Patrice Desvigne-Nickens, M.D. is an employee of the National Institutes of Health.
Katharine Cooper-Arnold, M.P.H. is an employee of the National Institutes of Health.
Kathleen V. Fitch M.S.N has no disclosures to report.
Heather Ribaudo, Ph.D. has no disclosures to report.
Pamela S. Douglas, M.D. has received support through her institution from HeartFlow, Inc unrelated to this project.
Steven K. Grinspoon, M.D. has received grant support through his institution from Kowa Pharmaceuticals America, Inc. and Gilead Sciences, Inc. for the conduct of the study; from Theratechnologies, and Navidea unrelated to this project and received consulting fees from Theratechnologies, Navidea, Gilead, Merck, and Bristol Myers Squibb, all unrelated to this Project.
Grants and Funding Support: U01 HL123336 (to SKG and PD); U01 HL123339 (to UH and HJR); UM1 AI068636 (to JSC); Investigator initiated grant, study drug and blinded matching placebo from Kowa Pharmaceuticals America, Inc. (to SKG); Investigator initiated grant from Gilead Sciences (to SKG and MVZ).
Footnotes
ClinicalTrials.gov Identifier: NCT02344290.
Publisher's Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institute of Allergy and Infectious Diseases; National Institutes of Health; or the United States Department of Health and Human Services.
References
- 1.Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92(7):2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg M, Chang C, Kuller L, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;173(8):614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA 2012;308(4):405–6. [DOI] [PubMed] [Google Scholar]
- 4.Zanni MV, Grinspoon SK. HIV-specific immune dysregulation and atherosclerosis. Curr HIV/AIDS Rep 2012;9(3):200–5. [DOI] [PubMed] [Google Scholar]
- 5.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 2010;24(2):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanni MV, Abbara S, Lo J, et al. Increased coronary atherosclerotic plaquevulnerabilityby coronarycomputed tomographyangiography in HIV-infected men. AIDS 2013;27(8):1263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller PE, Haberlen SA, Metkus T, et al. HIV and coronary arterial remodeling from the Multicenter AIDS Cohort Study (MACS). Atherosclerosis 2015;241(2):716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388(10059): 2532–61. [DOI] [PubMed] [Google Scholar]
- 9.Toribio M, Fitch KV, Sanchez L, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflamma- tion in HIV. AIDS 2017;31(6):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toribio M, Fitch KV, Stone L, et al. Assessing statin effects on cardiovascular pathways in HIV using a novel proteomics approach: Analysis of data from INTREPID, a randomized controlled trial. EBioMedicine 2018;35:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis 2014;58(4):588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vos AG, Hulzebosch A, Grobbee DE, et al. Association between immune markers and surrogate markers of cardiovascular disease in HIV positive patients: a systematic review. PLoS One 2017;12(1), e0169986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011;204(8): 1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 2011;203(4):452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelesidis T, Kendall MA, Yang OO, et al. Biomarkers of microbial translocation and macrophage activation: association with progres- sion of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012;206(10):1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merlini E, Luzi K, Suardi E, et al. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One 2012;7(9), e46073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortalityin HIV infection. J Infect Dis 2011;203 (6):780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012;308(4):379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giorgi JV, Hausner MA, Hultin LE. Detailed immunophenotype of CD8+ memory cytotoxic T-lymphocytes (CTL) against HIV-1 with respect to expression of CD45RA/RO, CD62L and CD28 antigens. Immunol Lett 1999;66(1–3):105–10. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Cumberland WG, Hultin LE, et al. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol 1997;16(2): 83–92. [DOI] [PubMed] [Google Scholar]
- 21.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS 2011;25(17): 2123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guihot A, Dentone C, Assoumou L, et al. Residual immune activation in combined antiretroviral therapy-treatedpatients with maximally suppressed viremia. AIDS 2016;30(2): 327–30. [DOI] [PubMed] [Google Scholar]
- 23.Wada K, Yoshikawa T, Lee JJ, et al. Sharp injuries in Japanese operating theaters of HIV/AIDS referral hospitals 2009–2011. Ind Health 2016;54(3):224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanni MV, Toribio M, Robbins GK, et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol 2016;1(4):474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nixon DE, Bosch RJ, Chan ES, et al. Effects of atorvastatin on biomarkers of immune activation, inflammation, and lipids in virologically suppressed, human immunodeficiency virus-1-infected individuals with low-density lipoprotein cholesterol <130 mg/dL (AIDS Clinical Trials Group Study A5275). J Clin Lipidol 2017;11(1): 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LoJ,Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015;2(2): e52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr 2015;68(4):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calza L, Vanino E, Salvadori C, et al. Tenofovir/emtricitabine/efavirenz plus rosuvastatin decrease serum levels of inflammatory markers more than antiretroviral drugs alone in antiretroviral therapy-naive HIV-infected patients. HIV Clin Trials 2014;15(1):1–13. [DOI] [PubMed] [Google Scholar]
- 29.De Wit S, Delforge M, Necsoi CV, et al. Downregulation of CD38 activation markers by atorvastatin in HIV patients with undetectable viral load. AIDS 2011;25(10):1332–3. [DOI] [PubMed] [Google Scholar]
- 30.Maurovich-Horvat P, Ferencik M, Voros S, et al. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 2014;11(7):390–402. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann U, Ferencik M, Udelson JE, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135(24): 2320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano Y, O’Donnell Q, Kuller L, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol 2017;2(9):986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann U, Massaro JM, D’Agostino RB Sr, et al. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc 2016;5(2), e003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358(13):1336–45. [DOI] [PubMed] [Google Scholar]
- 35.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014;160(7):458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kearns A, Gordon J, Burdo TH, et al. HIV-1-associated atheroscle- rosis: unraveling the missing link. J Am Coll Cardiol 2017;69(25): 3084–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanni MV, Schouten J, Grinspoon SK, et al. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol 2014;11(12): 728–41. [DOI] [PubMed] [Google Scholar]
- 38.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54(1): 49–57. [DOI] [PubMed] [Google Scholar]
- 39.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronarysyndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64(7): 684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol 2018;3(2):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nadjiri J, Hausleiter J, Jahnichen C, et al. Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. J Cardiovasc Comput Tomogr 2016;10 (2):97–104. [DOI] [PubMed] [Google Scholar]
- 42.Hell MM, Motwani M, Otaki Y, et al. Quantitative globalplaque characteristics from coronary computed tomography angiography for the prediction of future cardiac mortality during long-term follow-up. Eur Heart J Cardiovasc Imaging 2017;18(12): 1331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andelius L, Mortensen MB, Norgaard BL, et al. Impact of statin therapy on coronary plaque burden and composition assessed by coronary computed tomographic angiography: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2018;19(8): 850–8. [DOI] [PubMed] [Google Scholar]
- 44.Nou E, Lu MT, Looby SE, et al. Serum oxidized low-density lipoprotein decreases in response to statin therapy and relates independently to reductions in coronary plaque in patientswith HIV. AIDS 2016;30(4): 583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet 1994;344(8934):1383–9. [PubMed] [Google Scholar]
- 46.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350(15):1495–504. [DOI] [PubMed] [Google Scholar]
- 47.Tsujita K, Sugiyama S, Sumida H, et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol 2015;66(5):495–507. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359(21):2195–207. [DOI] [PubMed] [Google Scholar]
- 49.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapywith canakinumab for atherosclerotic disease. N Engl J Med 2017;377 (12):1119–31. [DOI] [PubMed] [Google Scholar]
- 50.Joshi AA, Lerman JB, Dey AK, et al. Association between aortic vascular inflammation and coronary artery plaque characteristics in psoriasis. JAMA Cardiol 2018;3(10):949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feinstein MJ, Achenbach CJ, Stone NJ, et al. A systematic review of the usefulness of statin therapy in HIV-infected patients. Am J Cardiol 2015;115(12):1760–6. [DOI] [PubMed] [Google Scholar]
- 52.Zanni MV, Fitch KV, Feldpausch M, et al. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS 2014;28 (14):2061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feinstein MJ, Nance RM, Drozd DR, et al. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus: a study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol 2017;2(2): 155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.TriantVA PerezJ, Regan S, et al. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation 2018;137(21):2203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA 2015;314(2):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]