Abstract
Background/Aims:
Although nonmedical use of prescription opioids (NMUPO) is a public health problem, few studies have examined the new-onset NMUPO in clinical populations. We estimated NMUPO incidence among veterans in medical care who had received prescription opioid medication and examined correlates of new-onset NMUPO.
Design:
Prospective cohort study.
Setting:
Veterans Health Administration primary care and infectious disease clinics in Atlanta, Baltimore, Bronx, Houston, Los Angeles, Manhattan, Pittsburgh, and Washington, DC.
Participants:
Patients enrolled in the Veterans Aging Cohort Study wave 3 (2005–2007) who received prescription opioids in the previous year and without lifetime NMUPO were followed at waves 4 and 5 (2008–2011).
Measurements:
Cox proportional hazards regression was used to examine the relationship between duration of prescription opioid receipt and incident NMUPO, adjusting for demographics, alcohol and tobacco use, substance use disorders, psychiatric and medical diagnoses, and medication-related characteristics.
Findings:
Among eligible participants (n = 815), the median age was 52 (IQR = 47–58) and 498 (59.8%) were Black; 122 (15.0%) reported new-onset NMUPO, for an incidence rate of 5.0 per 100 person-years. In a multivariable Cox model, compared to < 30 days, receipt of prescription opioids for 30–180 days (adjusted hazard ratio [AHR] = 1.65 95% CI: 1.06, 2.58) or > 180 days (AHR = 1.99, 95% CI: 1.21, 3.29) was associated with incident NMUPO.
Conclusions:
Duration of prescription opioid receipt is a risk factor for incident NMUPO among veterans receiving medical care. Providers who prescribe opioids should monitor for NMUPO, especially among those with a longer duration of opioid therapy.
Keywords: Opioids, Analgesics, Pain, HIV, Veterans
1. Introduction
Considerable debate surrounds the prescribing of opioid analgesics internationally for non-cancer chronic pain (Häuser et al., 2017; Katz, 2016; Novak et al., 2016; Okie, 2010). There is limited evidence supporting the efficacy of prescription opioids in managing chronic pain (Chou et al., 2015). Concerns have also emerged about the safety of long-term opioid therapy (i.e., greater than 90 days) because it may increase the risk of overdose death (Dunn et al., 2010), all-cause mortality (Ray et al., 2016), and nonmedical use of prescription opioids (NMUPO)(Becker et al., 2008). NMUPO is often defined as taking someone else’s opioid medication or taking the medication only for the experience it causes (SAMSHA, 2011), and is associated with the initiation and use of heroin (Banerjee et al., 2016; Compton et al., 2016) and psychiatric, medical, and non-opioid substance use problems (Becker et al., 2008; Campbell et al., 2018; Katz et al., 2013). NMUPO is also linked to pain complaints among untreated individuals with opioid use disorder (Barry et al., 2009, 2013). NMUPO comprises a challenge for clinicians in different settings, including office-based physicians and HIV providers (Barry et al., 2010; Keller et al., 2012; Lum et al., 2011; Starrels et al., 2016).
Veterans comprise a high-risk group for pain (Institute of Medicine, 2011). Among veterans in primary care, pain is associated with both receipt of opioid medication and NMUPO (Becker et al., 2009). Similar to other healthcare systems, rates of opioid prescribing escalated at the Veterans Health Administration (VHA) in the 1990s (Kuehn, 2007). The Department of Veterans Affairs/Department of Defense, Centers for Disease Control and Prevention (CDC), and some professional organizations have recently issued guidelines to promote appropriate prescribing practices for pain management (Chou et al., 2017; Department of Veterans Affairs Department of Defense, 2010; Dowell et al., 2016; Manchikanti et al., 2012).
To date, most research on NMUPO has focused on prevalence (using cross-sectional designs). Some longitudinal studies have examined NMUPO trajectories among middle and high school students (McCabe et al., 2016, 2014). One study of claims data examined incidence or new-onset of opioid use disorder among individuals prescribed opioid analgesics (Edlund et al., 2014). However, no studies to our knowledge have examined NMUPO incidence among individuals receiving prescription opioids. Estimating incident NMUPO in patients prescribed opioids require a relatively large sample that is systematically assessed for NMUPO over time, as well as access to detailed pharmacy information on prescription opioids. The Veterans Aging Cohort Study (VACS) meets these requirements (Justice et al., 2006). Previous studies involving the VACS have found that one-third of participants had been prescribed opioids and, of these individuals, more than one-third received opioids long-term (Edelman et al., 2013). Thirteen percent of all VACS wave-3 participants reported lifetime NMUPO (Barry et al., 2011). Substance use, medical status, and pain interference (but not HIV status) in this cross-sectional investigation were independent correlates of prevalent NMUPO (Barry et al., 2011).
The current study aimed to estimate the incidence of NMUPO among veterans with and without HIV who were prescribed opioids and to examine demographic, substance use and substance use disorder, psychiatric, medical, and medication predictors of new-onset NMUPO. Given that incident opioid use disorder risk among patients with chronic pain has been associated with longer durations of opioid use and with higher average daily opioid doses prescribed (Chou et al., 2015; Edlund et al., 2014), we hypothesized that incident NMUPO would be associated with these two opioid-medication characteristics. An enhanced understanding of the incidence of NMUPO and its associated risk factors among veterans with and without HIV may inform ongoing initiatives at the VHA and elsewhere to optimize opioid therapy benefits and minimize risks (Lin et al., 2017; Oliva et al., 2017).
2. Methods
2.1. Data sources
The Veterans Aging Cohort Study (VACS) (Justice et al., 2006) is a National Institute on Alcohol Abuse and Alcoholism (NIAAA)-funded prospective, longitudinal, multisite observational study of patients with and without HIV-infection receiving care in VHA primary care and infectious disease clinics. Uninfected participants were matched to HIV-infected ones by age, race, and site of care (Justice et al., 2006). Data for the current study were drawn from waves 3–5 of VACS follow-up surveys (hereafter referred to as waves 3–5). Since June 2002, VACS has enrolled over 7000 patients in Atlanta, Baltimore, Bronx, Houston, Los Angeles, Manhattan, Pittsburgh, and Washington, D.C. Wave 3–5 data were collected September 2005-January 2007, February 2008-August 2009, and September 2009-January 2011, and included 4133, 4182, and 3762 participants respectively. Survey data were linked to data on prescribed medications, medical and substance use diagnoses, and laboratory findings from VHA electronic medical records (EMR). Opioid prescription data were retrieved from the VHA Pharmacy Benefits Management records system. Other than NMUPO, all other data from self-report measures were collected at wave 3 (2005–2007). The VACS was approved by the institutional review boards at participating VHA facilities and affiliated academic institutions. More detailed information concerning the VACS, including the design and data collection procedures, are provided elsewhere (Justice et al., 2006).
2.2. Participant eligibility
The flowchart summarizing the selection of eligible participants for this study is illustrated in Fig. 1. Among the 4133 VACS participants at wave 3, a total of 1308 received at least one prescription opioid in the previous year (based on pharmacy data). Among those who received a prescription opioid, 486 (37.1%) reported lifetime NMUPO at wave 3 (see definition below) and were thus excluded. Among those who received a prescription opioid who reported no lifetime NMUPO (n = 822), seven (0.9%) participants provided invalid responses and were excluded, resulting in a final sample of 815 participants for analyses involving NMUPO incidence (at waves 4–5).
Fig. 1.
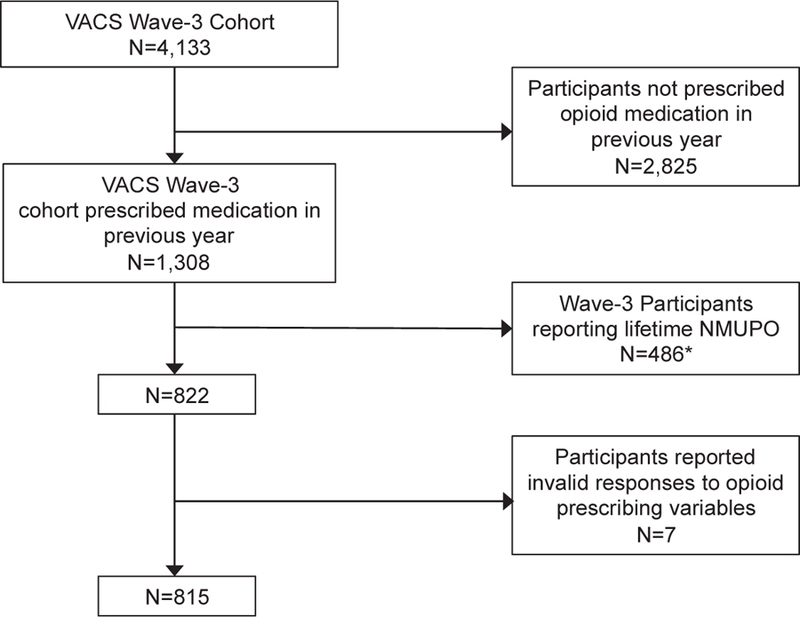
Flow Chart of eligible participants for incidence-related analyses.
Note: VACS: Veteran Aging Cohort Study, NMUPO: nonmedical use of prescribed opioids.
*262 participants reported past-year NMUPO.
2.3. Measures
2.3.1. Nonmedical use of prescription opioids
NMUPO was assessed by two self-report items at waves 3–5. The first question was derived verbatim from the National Survey on Drug Use and Health (NSDUH) (Substance Abuse and Mental Health Services Administration, 2007): “Have you ever, even once, used one of the medications listed below that was NOT prescribed for you or that you took only for the experience or feeling it caused?” For each medication listed, respondents checked whether or not they had “ever used” and “used in the past 12-months.” The list included the following prescription analgesics: buprenorphine, codeine, Darvocet, Darvon, Demerol, Dilaudid, Fioricet, Fiorinal, hydrocodone, methadone, morphine, Oxycontin, Percocet, Percodan, propoxyphene, Talwin, Tylenol with codeine, Tylox, Ultram, and Vicodin. As done previously, we excluded respondents whose only nonmedical use involved Fiorcet and/or Fiorinal because these medications are not opioids (Becker et al., 2008). The list of analgesics presented to participants at waves 4 and 5 also included Fentanyl. The second question that assessed NMUPO at waves 3–5 was: “Now think about the past 12-months. On average, how many days each week in the past 12-months did you use any prescription pain reliever that was not prescribed for you or that you took only for the experience or feeling it caused?” Participants who responded in the affirmative to either the first or second question were considered to exhibit NMUPO.
2.3.2. Demographics, substance use and substance use disorder, and psychiatric status
Participants provided information about their age, sex, and race/ ethnicity. Alcohol use was assessed using the 3-item Alcohol Use Disorder Identification Test (AUDIT-C) (Bush et al., 1998). The following questions assessed smoking status (never, current, former): “Have you smoked at least 100 cigarettes in your entire life?” (yes/no) and “Do you smoke cigarettes (as of 1-month ago)?” (yes/no). Diagnostic information related to alcohol use disorder, drug use disorder, and specific psychiatric disorders (major depression, bipolar disorder, schizophrenia, and post-traumatic stress disorder [PTSD]) were collected from the VHA EMR using the International Classification of Diseases, 9th Revision (ICD-9) codes (World Health Organization, 1975). Level of depression severity was assessed using the Patient Health Questionnaire (PHQ-9) (Spitzer et al., 1999).
2.3.3. Medical status and pain status
HIV data were collected from the VHA EMR system using ICD-9 codes (World Health Organization, 1975). Hepatitis C status was determined by the presence of an ICD-9 code or a positive HCV lab test. Pain intensity was assessed by the following 0–10 item: “On a scale of 0 to 10, where 0 means no pain, and 10 equals the worst possible pain, what is your current pain level?” Pain interference was measured by the following 0 (“no interference”)-6 (“extreme interference”) scored item: “In general, how much does your pain problem interfere with your day to day activities?”
2.3.4. Medication characteristics
Prescription opioid receipt was based on pharmacy data for all outpatient oral and transdermal opioids (Edelman et al., 2013). Medications used as part of opioid agonist treatment for opioid use disorder were not included. Days of opioid receipt were calculated from prescription information with the assumption that the prescription was taken as directed. Based on pharmacy data in the year preceding the wave 3 survey date, days of opioids supplied were analyzed both continuously and also categorized as fewer than 30, 30–180, and greater than 180. (This categorization was constructed in part based on the distribution of the data.) Morphine equivalent dose (MED) was calculated using a standard conversion chart, as described elsewhere (Von Korff et al., 2008). Total MED was calculated by multiplying the strength of the prescription (milligram of opioid per unit dispensed) by the prescription quantity. Average daily MED was calculated by dividing the total MED in the year by days supplied. Mean daily MED was categorized as follows: 0–49, 50–79, 80–119, ≥120 mg. Fifty milligrams were used as a threshold for high daily MED since the CDC opioid prescribing guideline recommends a reassessment of individual risks and benefits when this dosage is reached (Dowell et al., 2016). We also created a composite days supply/high-low dose variable, which combined data on both average daily MED (≤50 is low, > 50 is high) and days of opioids supplied (using the same classification as described above) to create mutually exclusive groups. Schedule II opioids were categorized as short-acting or long-acting per DEA classification (US Drug Enforcement Adminstration Controlled Substance Schedules, 2010).
Data about receipt and days supplied of benzodiazepines and non-benzodiazepine hypnotics were abstracted from the pharmacy data system. Benzodiazepines included alprazolam, chlordiazepoxide, clonazepam, estazolam, flurazepam, lorazepam, oxazepam, temazepam, and triazolam, whereas non-benzodiazepine hypnotics included eszopiclone, zaleplon, and zolpidem. Any benzodiazepine/non-benzodiazepine hypnotic receipt was defined as receipt of at least one prescription for an outpatient benzodiazepine/non-benzodiazepine hypnotic in the year preceding wave 3. Long-term receipt of benzodiazepines/non-benzodiazepine hypnotics was defined as at least 90 consecutive days of therapy, allowing for a 30-day refill window (Von Korff et al., 2008) in the year prior to the wave 3 survey date.
2.4. Data analysis
As a first step, we estimated the bivariable associations between each independent variable of interest (collected at wave 3) and incident NMUPO at waves 4 or 5 using chi-square tests, t-tests, and Wilcoxon rank sum tests for non-normally distributed data. We calculated the incidence of NMUPO over the study period using the standard Kaplan Meier method. For incident cases, we used the survey date during which the first instance of NMUPO was reported as the event time. All other individuals were right-censored at the date of their last survey completed or the end of the study period. Next, the independent variables of interest—days supplied (as a categorical variable) and high average daily dose—were entered into a Cox proportion hazards regression model, along with other potentially confounding covariates from the bivariate analyses whose associations were significant at p < 0.20. We adjusted for demographics, alcohol and tobacco use, substance use disorders, psychiatric and medical diagnoses, and medication-related characteristics. We assessed the assumption of proportional hazards by visual inspection of the Schoenfeld residual plots and by examining time-by-covariate interactions.
In a post hoc analysis, we used spline regression to examine further the relationship between days of opioid medication prescribed reported at wave 3 and risk of incident NMUPO at waves 4 or 5. Specifically, we used the %LGTPHCURV9 macro to fit a restricted cubic spline for proportional hazards regression models to examine the potentially non-linear relationship between days supplied and risk of NMUPO initiation (Li et al., 2011). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Participant characteristics
Demographic, substance use and substance use disorder, psychiatric, medical, and medication characteristics of wave 3 participants without a history of NMUPO who had received a prescription opioid in the prior year (n = 815) are summarized in Table 1. Wave-3 data are also presented separately for participants who did or did not exhibit incident NMUPO at waves 4 or 5. Participants were on average 52 years old and were predominantly male (93.3%) and African American (59.8%). The rates of the past-12 month and lifetime NMUPO reported among wave 3 respondents who were prescribed opioid medication in the previous year (n = 1308) were 20.0% and 37.2%, respectively (see Fig. 1).
Table 1.
Characteristics of participants prescribed opioids with and without incident NMUPO.
| Overall (N = 815) | Incident NMUPO (N = 122, 15.0%) | No incident NMUPO (N = 693, 85.0%) | p-valuea | |
|---|---|---|---|---|
| Demographics | ||||
| Age (median, IQR) | 52 (47−58) | 52 (47−57) | 52 (47−58) | 0.94 |
| Sex | 0.12 | |||
| Female | 54 (6.6) | 4 (3.3) | 50 (7.2) | |
| Male | 766 (93.3) | 118 (96.7) | 648 (92.7) | |
| Race/Ethnicity | 0.049 | |||
| White | 222 (27.2) | 28 (23.0) | 194 (28.0) | |
| Black | 498 (59.8) | 74 (60.7) | 413 (59.6) | |
| Hispanics | 68 (8.3) | 17 (13.9) | 51 (7.4) | |
| Other | 38 (4.7) | 3 (2.5) | 35 (5.0) | |
| Substance Use and SUD | ||||
| AUDIT-C (median, IQR) | 1 (0−4) | 2 (0−4) | 1 (0−4) | 0.23 |
| Risky alcohol useb | 212 (26.0) | 32 (26.2) | 180 (26.0) | 0.95 |
| Smoking Status | 0.51 | |||
| Never | 202 (25.0) | 27 (22.3) | 177 (25.5) | |
| Current | 381 (47.2) | 63 (52.1) | 318 (46.4) | |
| Former | 224 (27.8) | 31 (25.6) | 193 (28.1) | |
| Alcohol use disorder | 104 (12.8) | 15 (12.3) | 89 (12.8) | 0.87 |
| Drug use disorder | 121 (14.9) | 19 (15.6) | 102 (14.7) | 0.81 |
| Psychiatric Status | ||||
| Major depression | 86 (10.6) | 9 (7.4) | 77 (11.1) | 0.22 |
| Bipolar disorder | 43 (5.3) | 9 (7.4) | 34 (4.9) | 0.26 |
| Schizophrenia | 23 (2.7) | 3 (2.5) | 19 (2.7) | 1.00 |
| Posttraumatic stress disorder | 97 (11.9) | 12 (9.8) | 85 (12.3) | 0.46 |
| PHQ-9 (median, IQR) | 4 (0−9) | 3 (0−7) | 4 (0−9) | 0.26 |
| Medical Status | ||||
| HIV | 396 (48.6) | 62 (50.8) | 334 (48.2) | 0.59 |
| Hepatitis C | 307 (37.7) | 49 (40.2) | 258 (37.2) | 0.54 |
| Pain intensity (median, IQR) | 2 (0−6) | 3 (0 − 6) | 2 (0−5) | 0.51 |
| Pain interference (median, IQR) | 1 (0−4) | 1 (0−4) | 0.5 (0−3) | 0.26 |
| Medication-related Characteristics | ||||
| Opioid Medication | ||||
| Days supplied, continuous | 45 (15−176) | 56 (27−221) | 40 (15−162) | 0.03 |
| Days supplied, categorical | 0.045 | |||
| < 30 days | 276 (33.9) | 31 (25.4) | 245 (36.4) | |
| 30 to 180 days | 344 (42.2) | 53 (43.4) | 291 (42.0) | |
| > 180 days | 195 (23.9) | 38 (31.2) | 157 (22.7) | |
| Median daily MED (IQR) | 20 (13.5−3.0) | 19.3 (12.6−30.0) | 20 (13.5−33.4) | 0.51 |
| Mean daily MED (mg) | 0.54 | |||
| 0 – 49 | 692 (84.9) | 105 (86.1) | 587 (84.7) | |
| 50 –79 | 60 (7.4) | 11 (9.0) | 49 (7.1) | |
| 80 – 119 | 26 (3.2) | 3 (2.5) | 23 (3.3) | |
| ≥120 | 37 (4.5) | 3 (2.5) | 34 (4.9) | |
| High-dose receipt (> 50MED) | 123 (15.1) | 17 (13.9) | 106 (15.3) | 0.69 |
| Composite Days/Dosec | 0.09 | |||
| Low-dose, < 30 days | 260 (31.9) | 30 (24.6) | 230 (33.2) | |
| Low-dose, 30–180 days | 308 (37.8) | 51 (41.8) | 257 (37.1) | |
| Low-dose, > 180 days | 125 (15.2) | 24 (19.7) | 100 (14.4) | |
| High-dose, < 30 days | 16 (2.0) | 1 (0.8) | 15 (2.2) | |
| High-dose, 30–180 days | 36 (4.4) | 2 (1.6) | 34 (4.9) | |
| High-dose, > 180 days | 71 (8.1) | 14 (11.5) | 57 (8.2) | |
| Schedule II short-acting | 291 (35.7) | 47 (38.5) | 244 (35.2) | 0.48 |
| Schedule II long-acting | 116 (14.2) | 19 (15.6) | 97 (14.0) | 0.65 |
| Benzodiazepine Medicationd | ||||
| Any benzodiazepine receipt | 172 (21.0) | 24 (19.7) | 148 (21.2) | 0.71 |
| Long-term benzodiazepine receipt | 108 (13.3) | 14 (11.5) | 94 (13.6) | 0.53 |
Note: NMUPO = non-medical use of prescription opioids; IQR = interquartile range; SUD = substance use disorder; AUDIT-C = Alcohol Use Disorder Identification Test; PHQ-9=Patient Health Questionnaire; MED = morphine equivalent dose.
Bolded values were significant at p < 0.05.
Established AUDIT-C cutoffs (≥4 for men, ≥3 for women) were used to determine risky alcohol use.
Low-dose refers to ≤50MED; high-dose refers to > 50MED.
This medication category included both benzodiazepines and non-benzodiazepine hypnotics.
3.2. NMUPO incidence at waves 4–5
Of the 815 participants at wave 3 who received opioid medication in the previous 12 months and reported no past-year or lifetime NMUPO (see Fig. 1), 15.0% (n = 122) reported new-onset NMUPO at waves 4 or 5 (9.2% [n = 75] reported past 12-month NMUPO at wave 4, and 11.2% [n = 91] reported past 12-month NMUPO at wave 5). The incidence rate of NMUPO among the 815 eligible participants was 5.02 per 100 person-years (95% confidence interval [CI] 4.18–5.97 per 100-person-years).
3.3. Characteristics associated with NMUPO incidence
As summarized in Table 1, of the demographic, substance use and substance use disorder, psychiatric, medical, and medication characteristics examined in the bivariable model, only race/ethnicity and days of opioid medication supplied were significantly associated with NMUPO incidence (at p < 0.05). Respondents with incident NMUPO compared to those without were more likely to be Hispanic (13.9% vs. 7.4%), to have higher mean days of opioid medication prescribed (56 vs. 40 days), and to have been supplied opioid medication for 30 days or more (74.6% vs. 64.7%). Opioid dosing characteristics, including median or mean MED, high dose opioid receipt, composite days/dose, and presence of schedule II short-acting or long-acting medications, were not significantly associated with NMUPO. In the multivariable Cox regression analysis, days of opioids supplied remained positively associated with incident NMUPO (adjusted hazard ratio [AHR] = 1.65, 95% CI: 1.06–2.58 for 30–180 days; AHR = 1.99 95% CI: 1.21–3.29 for > 180, compared to < 30 days) (see Table 2). Fig. 2 summarizes the results of the spline regression model, which demonstrates a non-linear relationship between days of opioid medication supplied and risk of incident NMUPO. Specifically, the risk of new-onset NMUPO increases sharply until 75 days and then plateaus, followed by a slower increase in the risk after approximately 200 days of opioid medication supplied.
Table 2.
Cox proportional hazard regression model of factors associated with incident NMUPO among veterans participating in VACS, 2005–2011.
| Characteristic | Adjusted HR* (95% CI) |
P-value |
|---|---|---|
| Sex (ref: male) | 0.57 (0.21–1.55) | 0.27 |
| Race (ref: White) | ||
| Black | 1.13 (0.73–1.75) | 0.57 |
| Hispanic | 1.57 (0.86–2.89) | 0.14 |
| Other | 0.74 (0.22–2.44) | 0.62 |
| Days of opioid medication supplied (ref: < 30) | ||
| 30–180 | 1.65 (1.06–2.58) | 0.03 |
| > 180 | 1.99 (1.21–3.29) | 0.01 |
| High-dose opioid receipt (ref: none)† | 1.35 (0.78–2.33) | 0.29 |
Note: NMUPO = nonmedical use of prescription opioids; VACS = Veterans Aging Cohort Study.
HR = Hazard Ratio
refers to > 50 mg daily morphine equivalent dose.
Fig. 2.
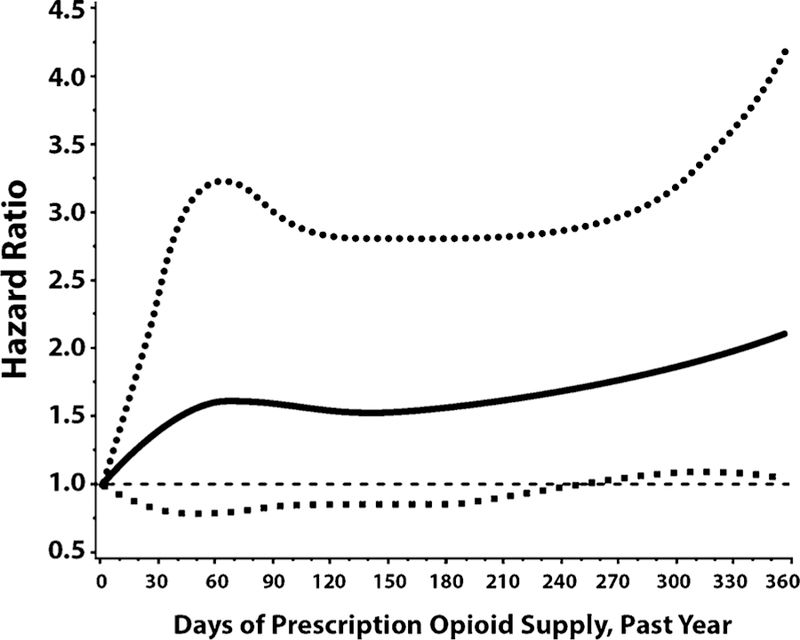
Spline regression showing non-linear relationship between days of prescription opioid supply and risk of NMUPO initiation at follow-up 4 or 5. Note: solid line indicates the hazard ratio estimate for risk of NMUPO initiation at a given number of days prescription opioid supply in the year prior to wave 3 survey date, compared to persons with no opioids prescribed during the same time period. The dashed lines indicate 95% confidence intervals around this estimate.
4. Discussion
The current study is among the first to assess the incidence of NMUPO among veterans with and without HIV who received prescription opioids, and to examine demographic, substance use and substance use disorder, psychiatric, medical, and medication predictors of new-onset NMUPO. Three main findings emerged. First, of wave-3 participants prescribed opioids in the previous year, 37% reported lifetime (and 20% past-year) NMUPO. Second, among wave-3 participants without prior NMUPO who were prescribed opioids in the previous year, 15% reported incident NMUPO at waves 4 or 5. Third, in multivariable analyses, NMUPO incidence was associated with duration of opioids prescribed.
In a prior study of wave-3 VACS participants (irrespective of opioid medication receipt), the estimate of lifetime NMUPO was 13% (Barry et al., 2011). By contrast, in the current study when the sample was restricted to those prescribed opioids in the previous year, the lifetime rate of NMUPO was markedly higher (37.2%). These findings support those from previous studies regarding the NMUPO risk associated with prescribed opioids. For example, in a study of veterans in primary care, pain was found to be associated with both receipt of opioids and NMUPO (Becker et al., 2009), while another study of patients with chronic pain found higher rates of opioid use disorder among patients prescribed (compared to those not prescribed) opioids (Edlund et al., 2014).
Prior studies of NMUPO in individuals prescribed opioids have generally used cross-sectional designs to estimate lifetime prevalence and associated risk factors (e.g., history of substance use disorder) (Barry et al., 2011; Morasco and Dobscha, 2008). Findings from the current longitudinal study highlight the importance of considering NMUPO risk at multiple time-points. Among wave-3 participants prescribed opioids in the previous year with no prior history of NMUPO, 15% reported incident NMUPO at waves 4 or 5. Consistent with the recent CDC opioid prescribing guidelines, our findings highlight the importance of clinicians’ continually assessing for NMUPO (and not just at opioid treatment initiation).
As predicted, in multivariable analyses, NMUPO was independently associated with duration of prescribed opioid receipt. Specifically, we found that the risk of new-onset NMUPO among veterans prescribed opioids increases until about 75 days and then plateaus, and is followed by a slower increase after approximately 200 days. While prior studies have demonstrated that the duration of initial opioid prescriptions is associated with greater odds of long-term opioid therapy among previously opioid-naïve patients (Deyo et al., 2017; Shah et al., 2017) and incident opioid use disorder among patients with chronic pain (Chou et al., 2015; Edlund et al., 2014), findings from the current study indicate that the duration of prescribed opioids among veterans in care also predicts new-onset NMUPO.
Notably, while substance use, medical status, and pain interference were previously found to be independent predictors of NMUPO prevalence among all wave-3 participants (Barry et al., 2011), none of these variables were significantly associated in the current study with incident NMUPO at the subsequent two waves. Contrary to the study hypothesis, in multivariable analyses, no significant association emerged between NMUPO incidence and average prescribed daily opioid dose. In prior studies of patients with chronic pain, higher average daily prescribed opioid doses have been found to be associated with longer duration of opioid use (Chou et al., 2015; Edlund et al., 2014). Similarly, in a recent study of an integrated healthcare system that used prescription opioid registry data, higher daily opioid doses were associated with increased risk of opioid misuse (Campbell et al., 2018). Differences in study design (e.g., cross-sectional vs. longitudinal, middle-aged vs. younger participants) and definition of opioid exposure (e.g., self-report vs. pharmacy records) may explain some of these discrepant findings. Our findings also raise the possibility that predictors for NMUPO prevalence and incidence are not identical.
Prior studies on the risk of NMUPO or opioid use disorder in clinical populations have generally focused on patients with chronic pain who were prescribed opioids for pain relief. The median pain intensity and pain interference ratings among veterans in care were relatively low and somewhat surprising. The reasons for these generally low scores (e.g., self-medication) and the extent to which the use of opioid medication (especially among the approximately 31% receiving long-term opioid therapy) was clinically indicated are unclear and merit further investigation.
Our study had several limitations. The main study variable, NMUPO, was measured by self-report, which may be subject to recall bias or under-reporting because of social desirability. The NSDUH has a purposeful community sampling strategy that targets adolescents and adults; in contrast, the current study sample comprised primarily middle-aged veterans in medical care, which restricts age-related comparisons. While the definition of NMUPO used in this study has been widely used by researchers (Barry et al., 2011), it does not address additional factors that are likely to be important to clinicians (e.g., frequency, motivation). The duration and patterns (e.g., continuous vs. non-continuous) of lifetime exposure to the medical use of prescription opioids were not assessed and consequently were not included in our analyses; thus, the potential impact of these factors on incident NMUPO is unclear. Data on opioid prescriptions filled were restricted to those drawn from the VHA Pharmacy Benefits Management records system; consequently, any opioid medications filled outside of the VHA were not captured (Gellad et al., 2018). Whereas we adjusted in the Cox regression analysis for clinician diagnoses of substance use disorders and self-reported alcohol and tobacco use, we did not adjust for illicit drug use. Neither were objective measures of substance use collected or used in the data analyses (e.g., urine toxicology). Although the VHA is one of the largest healthcare systems internationally, its patients are predominantly men who have been exposed to US military training and military conflict. Additionally, VHA policies on opioid prescribing for non-cancer chronic pain have shifted considerably in recent years. It is also important to note that the extent to which physicians incorporated opioid medications into the routine management of non-cancer chronic pain has varied considerably internationally. Consequently, both the characteristics of VHA patients and fluctuations in VHA opioid prescribing policies suggest caution in generalizing the findings of the current study to other countries, settings, and patient populations.
4.1. Conclusions
Despite these limitations, this study has several strengths. Unlike prior published studies on NMUPO, this study used a relatively large clinical sample of veterans in care with and without HIV infection, systematically assessed for NMUPO over time, and had access to data from participants’ pharmacy, medical, and psychiatric records. Consequently, unlike previous studies, we were able to assess NMUPO incidence and correlates among a clinical population with and without HIV who were prescribed opioid medications.
In summary, we found relatively high rates of both lifetime NMUPO among veterans in care who were prescribed opioids in the previous year (37%) and NMUPO incidence over the study period among those reporting no prior history of NMUPO (15%). In multivariable analyses, new-onset NMUPO was positively and independently associated with receipt of prescribed opioids for 30–180 days. Our findings suggest that prescribers should be aware of this risk when prescribing long-term opioids and should assess for NMUPO even among those without evidence of aberrant behavior.
Acknowledgments
This work was supported by grantsfrom the National Institute on Alcohol Abuse and Alcoholism (NIAAA: U10-AA013566, U01-AA020795, U01-AA020790, U24-AA020794, U10-AA013566, and P01-AA019072), the National Institute of Allergy and Infectious Diseases (P30-AI042853), and the US Department of Veterans Affairs (CIN 13-047). Dr. Julie Gaither is supported by the National Institute on Drug Abuse (F31-DA035567). E. Jennifer Edelman was supported by grants from the National Institute on Drug Abuse (K12-DA033312, R01-DA040471) during the conduct of this work. Dr. Brandon Marshall is supported by the National Institute on Drug Abuse (DP2-DA040236). Dr. Stephen Crystal is supported by AHRQ awards1U19HS021112 and R18-HS023258. The sponsors had no role in the study design; data collection, analysis or interpretation; the writing of the manuscript; or in the decision to submit the paper for publication. We would like to acknowledge the veterans who participate in the Veterans Aging Cohort Study (VACS) and the study coordinators and staff at each VACS site and at the West Haven Coordinating Center. We would also like to thank Melissa Skanderson for her assistance and support during data acquisition. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the funding agencies or affiliated institutions, including the Department of Veterans Affairs or the United States government.
Role of funding source
The sponsors had no role in the study design; the collection, analysis and interpretation of data; the writing of the report; and in the decision to submit the article for publication.
Footnotes
Conflict of Interest
No conflict declared.
References
- Banerjee G, Edelman EJ, Barry DT, Becker WC, Cerdá M, Crystal S, Gaither JR, Gordon AJ, Gordon KS, Kerns RD, 2016. Non‐medical use of prescription opioids is associated with heroin initiation among US veterans: a prospective cohort study. Addiction 111, 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Beitel M, Joshi D, Schottenfeld RS, 2009. Pain and substance-related pain-reduction behaviors among opioid dependent individuals seeking methadone maintenance treatment. Am. J. Addict 18, 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Irwin KS, Jones ES, Becker WC, Tetrault JM, Sullivan LE, Hansen H, O’Connor PG, Schottenfeld RS, Fiellin DA, 2010. Opioids, chronic pain, and addiction in primary care. J. Pain 11, 1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Goulet JL, Kerns RK, Becker WC, Gordon AJ, Justice AC, Fiellin DA, 2011. Nonmedical use of prescription opioids and pain in veterans with and without HIV. Pain 152, 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Savant JD, Beitel M, Cutter CJ, Moore BA, Schottenfeld RS, Fiellin DA, 2013. Pain and associated substance use among opioid dependent individuals seeking office-based treatment with buprenorphine-naloxone: a needs assessment study. Am. J. Addict 22, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA, 2008. Non-medical use, abuse and dependence on prescription opioids among US adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend 94, 38–47. [DOI] [PubMed] [Google Scholar]
- Becker WC, Fiellin DA, Gallagher RM, Barth KS, Ross JT, Oslin DW, 2009. The association between chronic pain and prescription drug abuse in veterans. Pain Med 10, 531–536. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, 1998. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch. Intern. Med 158, 1789–1795. [DOI] [PubMed] [Google Scholar]
- Campbell CI, Bahorik AL, VanVeldhuisen P, Weisner C, Rubinstein AL, Ray GT, 2018. Use of a prescription opioid registry to examine opioid misuse and overdose in an integrated health system. Prev. Med 110, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA, 2015. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med 162, 276–286. [DOI] [PubMed] [Google Scholar]
- Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Kraegel P, Griffin J, 2017. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline nonpharmacologic therapies for low back pain. Ann. Intern. Med 166 (7), 493–505. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT, 2016. Relationship between nonmedical prescription-opioid use and heroin use. N. Engl. J. Med 374, 154–163. [DOI] [PubMed] [Google Scholar]
- Department of Veterans Affairs Department of Defense, 2010. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain, v2.0 Washington, DC. [Google Scholar]
- Deyo RA, Hallvik SE, Hildebran C, Marino M, Dexter E, Irvine JM, O’Kane N, Van Otterloo J, Wright DA, Leichtling G, 2017. Association between initial pioid prescribing patterns and subsequent long-term use among opioid-naïve patients: a statewide retrospective cohort study. J. Gen. Intern. Med 32, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 315, 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, 2010. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann. Intern. Med 152, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Gordon K, Becker WC, Goulet JL, Skanderson M, Gaither JR, Brennan Braden J, Gordon AJ, Kerns RD, Justice AC, 2013. Receipt of opioid analgesics by HIV-infected and uninfected patients. J. Gen. Intern. Med 28, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund MJ, Martin BC, Russo JE, Devries A, Braden JB, Sullivan MD, 2014. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic non-cancer pain: the role of opioid prescription. Clin. J. Pain 30, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellad WF, Sileanu FE, Hale JA, Radomski TR, Gordon AJ, Good CB, Fine MJ, 2018. Impact of dual use of Department of Veterans Affairs and Medicare Part D drug benefits on potentially unsafe opioid use. Am. J. Public Health 108, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häuser W, Schug S, Furlan AD, 2017. The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain: a perspective from different continents. Pain Rep 2, e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine, 2011. Relieving Pain in America: a Blueprint for Transforming Prevention, Care, Education and Research The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, 2006. Veterans Aging Cohort Study (VACS): overview and description. Med. Care 44, S13–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MH, 2016. Opioid prescribing for chronic pain: not for the faint of heart. JAMA Intern. Med 176, 599–601. [DOI] [PubMed] [Google Scholar]
- Katz C, El-Gabalawy R, Keyes KM, Martins SS, Sareen J, 2013. Risk factors for incident nonmedical prescription opioid use and abuse and dependence: results from a longitudinal nationally representative sample. Drug Alcohol Depend 132, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CE, Ashrafioun L, Neumann AM, Van Klein J, Fox CH, Blondell RD, 2012. Practices, perceptions, and concerns of primary care physicians about opioid dependence associated with the treatment of chronic pain. Subst. Abus 33, 103–113. [DOI] [PubMed] [Google Scholar]
- Kuehn BM, 2007. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA 297, 249–251. [DOI] [PubMed] [Google Scholar]
- Li R, Hertzmark E, Louei M, Chen L, Spiegelman D, 2011. The SAS LGTPHCURV9 Macro Accessed 20 April 2017. https://www.hsph.harvard.edu/donna-spiegelman/software/lgtphcurv9/.
- Lin LA, Bohnert AS, Kerns RD, Clay MA, Ganoczy D, Ilgen MA, 2017. Impact of the Opioid Safety Initiative on opioid-related prescribing in veterans. Pain 158, 833–839. [DOI] [PubMed] [Google Scholar]
- Lum PJ, Little S, Botsko M, Hersh D, Thawley RE, Egan JE, Mitty J, Boverman J, Fiellin DA, 2011. Opioid-prescribing practices and provider confidence recognizing opioid analgesic abuse in HIV primary care settings. J. Acquir. Immune Defic. Syndr 56, S91–S97. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Abdi S, Atluri S, Balog CC, Benyamin RM, Boswell MV, Brown KR, Bruel BM, Bryce DA, Burks PA, 2012. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic noncancer pain: part I–evidence assessment. Pain Phys 15, S1–65. [PubMed] [Google Scholar]
- McCabe SE, Schulenberg JE, O’Malley PM, Patrick ME, Kloska DD, 2014. Non‐medical use of prescription opioids during the transition to adulthood: a multi‐cohort national longitudinal study. Addiction 109, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Kloska DD, Veliz P, Jager J, Schulenberg JE, 2016. Developmental course of non‐medical use of prescription drugs from adolescence to adulthood in the United States: national longitudinal data. Addiction 111, 2166–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasco BJ, Dobscha SK, 2008. Prescription medication misuse and substance use disorder in VA primary care patients with chronic pain. Gen. Hosp. Psychiatry 30, 93–99. [DOI] [PubMed] [Google Scholar]
- Novak SP, Håkansson A, Martinez-Raga J, Reimer J, Krotki K, Varughese S, 2016. Nonmedical use of prescription drugs in the European Union. BMC Psychiatry 16 (274). 10.1186/s12888-016-0909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okie S, 2010. A flood of opioids, a rising tide of deaths. N. Engl. J. Med 363, 1981–1985. [DOI] [PubMed] [Google Scholar]
- Oliva EM, Bowe T, Tavakoli S, Martins S, Lewis ET, Paik M, Wiechers I, Henderson P, Harvey M, Avoundjian T, 2017. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol. Serv 14, 34–49. [DOI] [PubMed] [Google Scholar]
- Ray WA, Chung CP, Murray KT, Hall K, Stein CM, 2016. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA 315, 2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMSHA, 2011. Results From the 2010 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-41, HHS Publication No. (SMA) 11–4658 Substance Abuse and Mental Health Services Administration, Rockville, MD; https://www.samhsa.gov/data/sites/default/files/SDUHNationalFindingsResults2010-web/2k10ResultsRev/NSDUHresultsRev2010.pdf [Google Scholar]
- Shah A, Hayes CJ, Martin BC, 2017. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb. Mortal. Wkly. Rep 66, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Group PHQPCS, 1999. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA 282, 1737–1744. [DOI] [PubMed] [Google Scholar]
- Starrels JL, Peyser D, Haughton L, Fox A, Merlin JS, Arnsten JH, Cunningham CO, 2016. When human immunodeficiency virus (HIV) treatment goals conflict with guideline-based opioid prescribing: a qualitative study of HIV treatment providers. Subst. Abus 37, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2007. Results From the 2006 National Survey on Drug Use and Health: National Findings Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07–4293. Accessed on 30 June 2010. http://www.oas.samhsa.gov/nsduh/2k6nsduh/2k6results.pdf. [Google Scholar]
- US Drug Enforcement Adminstration Controlled Substance Schedules Accessed 20 April 2017. https://www.deadiversion.usdoj.gov/schedules.
- Von Korff M, Saunders K, Ray GT, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter C, Silverberg M, Banta-Green C, 2008. Defacto long-term opioid therapy for non-cancer pain. Clin. J. Pain 24, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 1975. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, vol 1 ninth revision Geneva: http://www.who.int/iris/handle/10665/40492. [Google Scholar]


