Abstract
Background
Fibrinogen is produced in the liver and tends to be reduced in liver cirrhosis. Quantitative and qualitative tests exist to measure fibrinogen. We aimed to validate the functional fibrinogen thromboelastography assay (FF-TEG) and propose a new model to estimate fibrinogen levels via the Clauss method (Clauss) using data from a prothrombin time-derived fibrinogen assay (PT-Fg) in patients with liver cirrhosis.
Materials and methods
Clauss, PT-Fg, fibrinogen antigen (Fib-Ag) and FF-TEG were studied in 55 patients with liver cirrhosis (26 with Child-Turcotte-Pugh [CTP]-A disease, 14 with CTP-B and 15 CTP-C) and 20 healthy individuals.
Results
The results of all four assays correlated strongly with each other, but gave significantly different mean levels in all cohorts. PT-Fg gave the highest levels whereas the Clauss gave the lowest levels. The FF-TEG performed well with results which were in between the Clauss and the PT-Fg. Significant differences were only observed between CTP-A and CTP-C for the Clauss, PT-Fg and Fib-Ag but not functional fibrinogen level. We devised a simple linear regression model in order to estimate Clauss from the PT-Fg.
Discussion
The results of the FF-TEG correlate well with those of routine fibrinogen assays in patients with liver cirrhosis. However, the FF-TEG assay does not discriminate between early and late stages of disease, pointing to a preserved fibrin clot strength in cirrhosis. Through linear regression models, fibrinogen levels can be accurately estimated using the Clauss method based on fibrinogen levels obtained in the cheaper PT-Fg.
Keywords: fibrinogen, functional fibrinogen, cirrhosis, linear regression model
Introduction
Fibrinogen is a 340-kDa dimeric, soluble glycoprotein synthesised by hepatocytes1. It is found mainly in a soluble form circulating in blood2. Fibrinogen has numerous, important roles in the body, such as wound healing and inflammation, but one of the most important is in primary and secondary haemostasis3.
Due to its multiple roles in coagulation, disorders in fibrinogen can result in either bleeding or thrombotic tendencies4. Haemostasis and liver function are tightly linked since hepatocytes are responsible for the synthesis of the majority of clotting factors5 and also the natural anticoagulants. The liver is a very complex and vital organ, with metabolic, immunological and synthetic functions6. Patients with liver cirrhosis have coexisting haemostatic changes such as defects in primary haemostasis and abnormal clotting due to impaired synthesis of both pro-coagulant and anticoagulant factors7. Patients with a coagulopathy related to liver disease used to be considered at increased risk of bleeding. Nowadays, it is evident that such patients are also at increased risk of thrombosis which therefore makes their clinical management very difficult6.
Fibrinogen is an acute-phase protein and its plasma concentration increases during inflammatory conditions as well as with age, pregnancy, menopause, smoking, obesity and the use of the oral contraceptive pill. On the other hand, regular physical activity, alcohol consumption and fish intake tend to reduce fibrinogen levels1. Fibrinogen abnormalities in cirrhosis can be quantitative and/or qualitative. A decrease in hepatic synthetic function is associated with quantitative fibrinogen abnormalities. The more advanced the cirrhosis, the lower the production of coagulation factors8, hence the lower the fibrinogen levels and the more prolonged the results of screening tests such as the prothrombin time (PT) and activated partial thromboplastin time (APTT)5.
Plasma fibrinogen concentration can be within normal levels in patients with hepatocellular disease. However, this does not exclude the possibility of abnormal fibrinogen molecules (dysfibrinogens)5. Qualitative defects of fibrinogen in such patients are due to an increased sialic acid content in the molecule8 which causes abnormal fibrin monomer polymerisation thereby impairing aggregation9 but at the same time allowing normal release of fibrinopeptide A10. Sialic acid is an acetylated neuraminic acid derivative mainly terminating the carbohydrate chains of glycoproteins and glycolipids. Sialyation of carbohydrates changes and depends on liver function11. Acquired dysfibrinogens are mostly caused by excessive glycosylation2 and their incidence was found to be high in patients with liver cirrhosis12.
Coagulation in these patients is complex and differs between individuals, involving not only coagulant and fibrinolytic factors but also platelets, white blood cells and erythrocytes13. Screening tests such as the PT and APTT, as well as the thrombin time, are often prolonged in these patients8. However, these simple tests do not predict clinical outcome since complex abnormalities are present and more global tests are required to establish haemostatic status14. Fibrinogen levels are a reference point for clotting factor replacement in patients with cirrhosis who are either bleeding or who require invasive procedures. International guidelines recommend measurement of fibrinogen levels in similar scenarios in order to guide fibrinogen replacement15. However, there is no indication of which assay is best to use. The ones that were compared in this study have been selected since they are commonly employed in the investigation of fibrinogen deficiencies.
The Clauss method of determining the fibrinogen concentration is the most popular2 and standard technique used in clinical laboratories worldwide10. In some centres, the fibrinogen assays are performed along with the PT and APTT for haemostatic screening. Many laboratories determine the fibrinogen level derived directly from the PT, the so-called PT-Fg assay. While this does not involve extra expense and is very fast, it is not considered to be very accurate. Whole blood thromboelastography (TEG) is still considered more of an additional test for investigating liver disease and predicting prognosis rather than as an initial investigation16. Since it is a point-of-care assay, TEG has become widely used in operating theatres during liver transplantation to guide factor repletion and anti-fibrinolytic therapy17. The fibrinogen antigen (Fib-Ag) assay gives an overall estimate of the concentration of fibrinogen present in a sample but does not indicate qualitative defects. Each assay can give different information because of the different assay sensitivities. Where one assay is sensitive for detecting low fibrinogen levels, other assays might not show the same sensitivity. Some assays can be suitable for emergency situations while others might be laborious and time-consuming. In emergency situations, accuracy might not be mandatory: an estimate may be sufficient to identify whether the fibrinogen level is grossly increased or decreased2. One aim of this study was to check the performance of the Functional Fibrinogen Thromboelastography (FF-TEG) point-of-care method vs the more routine methods: Clauss fibrinogen, PT-Fg and the Fib-Ag. We also devised a mathematical model to predict Clauss fibrinogen results from PT-Fg measurements.
Materials and methods
Patients with liver cirrhosis were recruited from gastroenterology outpatients’ clinics at “Mater Dei” Hospital (Msida, Malta). The diagnosis of cirrhosis was made using standard clinical, radiological and histological criteria and the patients were graded according to severity using the Child-Turcotte-Pugh (CTP) classification system (A-C). The severity of liver disease was also assessed by the Model for End-stage Liver Disease (MELD) scoring system; however, for statistical purposes, the CTP system was used. Patients with liver cirrhosis were excluded if they were on anticoagulant or hormonal therapy. Healthy control individuals were also recruited.
This study was approved by the Faculty Research Ethics Committee and the University of Malta Research Ethics Committee (Study Approval n. 015/2016) and all individuals enrolled provided written informed consent to their inclusion in the study.
Blood was collected into vacutainers containing 3.2% (0.109 mol/L) buffered sodium citrate solution (Vacuette® Tube 2 mL 9NC Coagulation Sodium Citrate 3.2%, Greiner Bio-One, Kremsmünster, Austria). One tube was used for analysis on the TEG® 5000, whereas the rest were double spun at 2,500 rpm for 10 minutes and the platelet-poor plasma was frozen at −80 °C until analysis.
The Clauss fibrinogen, PT-Fg and Fib-Ag values were estimated using a Sysmex® CS-2100i analyser (Sysmex Corporation, Kobe, Japan). The Dade® Thrombin (Siemens Healthcare Diagnostics, Erlangen, Germany) reagent used for the Clauss fibrinogen assay consists of a lyophilised bovine thrombin preparation with stabilisers and buffers. The Liaphen® fibrinogen reagent (HYPHEN BioMed, Neuville-sur-Oise, France) for the Fib-Ag uses latex microparticles coated with polyclonal rabbit anti-human fibrinogen antibodies. The PT reagent used was Dade® Innovin® (Siemens Healthcare Diagnostics), a recombinant human tissue factor.
The FF-TEG was performed on a TEG® 5000 Thrombelastograph Hemostasis Analyser System (Haemoscope Corporation, Niles, IL, USA). Functional fibrinogen reagent vials containing lyophilised tissue factor with proprietary platelet inhibitor (TEG® Hemostasis System Function Fibrinogen Reagent, Haemoscope Corporation) were used to determine the functional fibrinogen level (FLEV).
While the other fibrinogen assays mentioned reflect the time it takes for plasma to clot, the TEG is a viscoelastic test that reflects overall clot integrity in whole blood. This method of investigating global haemostasis aims at reflecting in vivo coagulation and allows monitoring of changes that occur in blood18. It is a point-of-care test19 which measures a clot’s viscosity by using a cup and pin assembly. The pin is placed inside a heated (37 °C) cup filled with the whole blood sample and is connected to a detector system (torsion wire) which monitors motion20. As the fibrin strands form between the cup and the pin21, the viscosity of the blood increases thereby causing movement. The pin couples with the motion of the cup through which an electric signal is generated and a curve is plotted20. FF-TEG directly measures fibrinogen’s contribution to the overall clot strength22. It uses a platelet antagonist, which blocks the GPIIb/IIIa receptors on platelets23, thereby inhibiting the platelets and isolating fibrinogen’s contribution to overall clot strength19. The FLEV is calculated by the TEG® system’s software, from the transformation of the maximum amplitude24.
Statistical analysis
Data were analysed using IBM SPSS Statistics for Windows, version 20.0 (IBM Corporation, Armonk, NY, USA). All quantitative variables were first analysed for the presence of any outliers by inspecting their box plots. Outliers that were observed to be influential were excluded from the data set as they would have had an effect on the results. A 0.05 level of significance was used in all the statistical analyses. The Shapiro-Wilk test was performed to establish whether the assumption of normality was satisfied by the relevant variables and thereby determine whether parametric or non-parametric tests should be used.
Following normality testing, the paired samples t-test was used to look for pair-wise differences in the results of fibrinogen assays. A paired samples correlation table (Pearson’s correlation coefficients) is also generated during this test. As a result of the strong correlations obtained during the paired samples t-test, simple linear regression models were established for Clauss fibrinogen and FLEV, Clauss fibrinogen and Fib-Ag and Clauss fibrinogen and PT-Fg from the healthy control population. The last model was established twice, changing the dependent and independent variables of the model. The model that was of most interest in this research was that between Clauss fibrinogen (dependent) and PT-Fg (independent). Simple linear regression was used to establish a relationship in terms of y=b1x+b0 (y=mx+c). This technique estimates the regression coefficients b0 and b1 of the regression line (y=b0+b1x).
These regression models make it possible to predict the value of the dependent variable according to the values of the covariates. For this test to be carried out, the dependent variable must be normally distributed and there must be a correlation between the two covariates. Prior to establishing the simple linear regression models, scatter diagrams of the afore-mentioned pairs were plotted to ensure linearity. Analysis of variance (ANOVA) and coefficient tables were obtained while performing simple linear regression. The p-value for the ANOVA table was interpreted in order to accept or reject whether the model y=b0+b1x fitted the data set better than the constant model. The values of b0 and b1 are then given in the coefficient table, where the p-values also need to be interpreted in order to accept or reject whether their values are equal to 0 or not. The correct model equation and the fitted model for the relationships were then established.
In order to confirm that all the models were valid, the “studentised residual” values generated for every regression performed were tested for normality using the Shapiro-Wilk test. The “studentised residual” values generated for the first three regression tests performed were all normally distributed, indicating that the first three above-mentioned models were valid. However, normality for the model established between Clauss fibrinogen (dependent) and PT-Fg (independent) values was not established at first. Therefore, linear regression was performed multiple times for the latter model, until normality of the “studentised residual” values was established, thus validating the model. This was done by considering data points whose “studentised residual” values went beyond ±2, as outliers. After removing these data points from the data set and repeating the linear regression, the “studentised residual” obtained was again tested for normality. Normality was then established, confirming that the model Clauss fibrinogen =0.728 (PT-Fg) was valid. These data points were only removed for this linear regression model.
The fitted model was then tested on the population with liver disease so as to predict these patients’ Clauss fibrinogen levels. The mean square error of prediction (MSEP) equation was used to compare the original Clauss fibrinogen values with the predicted Clauss fibrinogen values obtained from the afore-mentioned model.
One-way ANOVA was used to determine whether there were any statistically significant differences between the means values from healthy controls and patients with CTP-A, CTP-B and CTP-C of the dependent variables (FLEV, Fib-Ag, Clauss fibrinogen and PT-Fg). Since statistically significant differences were found between the different categories (healthy controls, CTP-A, CTP-B and CTP-C patients) as a whole, the Bonferroni post-hoc test was performed to identify which population categories differed from each other.
Results
A total of 55 patients with liver cirrhosis (26 with CTP-A, 14 with CTP-B and 15 with CTP-C), aged between 39 and 80 years, were enrolled together with 20 healthy control individuals (10 males and 10 females; age range, 20–57 years).
The patients’ characteristics are detailed in Table I. In summary, the commonest causes of liver cirrhosis, accounting for 54.5% of cases, were alcoholic liver disease and non-alcoholic steatohepatitis (NASH). There was a preponderance of CTP-A patients with a mean MELD score of 10.5 and 72.7% of the whole group of patients were male.
Table I.
Patients’ demographics.
| Variable | Mean±SD | Normal ranges |
|---|---|---|
| Age (years) | 61.78±8.82 | - |
|
| ||
| Gender | ||
| Male | 40 (72.73%) | - |
| Female | 15 (27.27%) | - |
|
| ||
| Child-Turcotte-Pugh class | ||
| A | 26 (47.27%) | - |
| B | 14 (25.45%) | - |
| C | 15 (27.27%) | - |
|
| ||
| Aetiology of cirrhosis | ||
| Alcoholic liver disease | 16 (29.09%) | - |
| NASH | 14 (25.45%) | - |
| Autoimmune hepatitis | 2 (3.64%) | - |
| Hepatitis C virus | 5 (9.09%) | - |
| Indeterminate | 1 (1.82%) | - |
| Primary biliary cirrhosis | 3 (5.45%) | - |
| NAFLD | 1 (1.82%) | - |
| Multiple | 13 (23.64%) | - |
|
| ||
| Hepatocellular carcinoma | 11 (20.0%) | - |
|
| ||
| History of PVT | 5 (9.09%) | - |
|
| ||
| History of VTE | 1 (1.82%) | - |
|
| ||
| History of bleeding | 10 (18.18%) | - |
|
| ||
| INR | 1.14±0.23 | 0.8–1.2 |
|
| ||
| APTT | 36.10±12.01 | 23.2–28.9 |
|
| ||
| Platelet count (×109/L)1 | ||
| Males | 128.26±69.09 | 146–302 |
| Females | 154.67±66.29 | 132–349 |
|
| ||
| Bilirubin (μmol/L)2 | 55.32±115.07 | 0–21 |
|
| ||
| Alkaline phosphatase (U/L)2 | ||
| Males | 131.13±58.35 | 40–129 |
| Females | 167.71±99.79 | 40–104 |
|
| ||
| GGT (U/L)3 | ||
| Males | 130.44±103.68 | 8–61 |
| Females | 208.93±204.87 | 5–36 |
|
| ||
| ALT (U/L)4 | ||
| Males | 43.92±54.52 | 5–41 |
| Females | 27.36±13.56 | 5–33 |
|
| ||
| Creatinine (μmol/L)4 | ||
| Males | 77.61±24.58 | 59–104 |
| Females | 85.31±49.66 | 45–84 |
|
| ||
| Urea (mmol/L)2 | 8.32±11.56 | 1.7–8.3 |
|
| ||
| Sodium (mmol/L)5 | 136.92±5.16 | 135–145 |
|
| ||
| MELD4 | 10.47±4.94 | - |
Based on 54 patients;
based on 52 patients;
based on 50 patients;
based on 51 patients;
based on 53 patients.
NASH: non-alcoholic steatohepatitis; NAFLD: non-alcoholic fatty liver disease; PVT: portal vein thrombosis; VTE: venous thromboembolism; INR: international normalised ratio; APTT: activated partial thromboplastin time; GGT: gamma-glutamyl transferase; ALT: alanine aminotransferase; MELD: Model for End-stage Liver Disease. The normal ranges listed above were obtained from “Mater Dei” Hospital pathology laboratories at the time of the study.
We used citrated whole blood from one individual and analysed it five times during the space of 5 hours to generate the intra-assay coefficient of variation (CV) for FLEV, which was 3.6%. Since whole blood cannot be frozen without affecting its coagulation properties, we could not generate the inter-assay CV for this test. We felt that using plasma instead of fresh whole blood would not reflect the true inter-assay CV of the test. The intra-/inter-assay CV of the Clauss fibrinogen and PT in our laboratory are 2.1/9.7% and 0.6/0.7%, respectively.
Fibrinogen levels are higher in females than in males in the healthy control population
In the healthy control population, the values of FLEV (p=0.010, mean rank females 13.90, mean rank males 7.10) and PT-Fg (p=0.037, mean rank females 12.55, mean rank males 7.17) were affected by gender while Fib-Ag (p=0.070, mean rank females 12.90, mean rank males 8.10) and Clauss fibrinogen assay (p=0.088, mean rank females 12.75, mean rank males 8.25) values were not.
Pair-wise differences in the fibrinogen assays
In both healthy controls and patients with liver disease (Table II), all four fibrinogen assays gave statistically significant different results. PT-Fg gave the highest fibrinogen values of all four assays whereas the Clauss fibrinogen assay gave the lowest values when compared pair-wise. There was a strong, positive, linear relationship between the results of all the fibrinogen assays (Table III).
Table II.
Paired samples t-test to look for pair-wise differences in the four fibrinogen assays, in the healthy controls and patients with cirrhotic liver disease.
| Paired samples t-test table of the healthy control population | Paired samples t-test table of patients with liver disease | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Paired differences | p-value | Paired differences | p-value | ||||||||||
|
|
|
||||||||||||
| Mean | SD | SEM | 95% CI of the difference | Mean | SD | SEM | 95% CI of the difference | ||||||
|
|
|
||||||||||||
| Lower | Upper | Lower | Upper | ||||||||||
| Pair 1 | Clauss Fibrinogen - FLEV | −0.74745 | 0.53415 | 0.11944 | −0.99744 | −0.49746 | 0.000 | −0.71906 | 0.68011 | 0.09255 | −0.90469 | −0.53342 | 0.000 |
|
| |||||||||||||
| Pair 2 | Clauss Fibrinogen - Fibrinogen Antigen | −0.21425 | 0.14051 | 0.03142 | −0.28001 | −0.14849 | 0.000 | −0.46593 | 0.27040 | 0.03680 | −0.53973 | −0.39212 | 0.000 |
|
| |||||||||||||
| Pair 3 | Clauss Fibrinogen - PT-Fg | −1.10526 | 0.23446 | 0.05379 | −1.21827 | −0.99226 | 0.000 | −1.02778 | 0.50969 | 0.06936 | −1.16690 | −0.88866 | 0.000 |
|
| |||||||||||||
| Pair 4 | FLEV - Fibrinogen Antigen | 0.53320 | 0.49785 | 0.11132 | 0.30020 | 0.76620 | 0.000 | 0.25313 | 0.60680 | 0.08257 | 0.08751 | 0.41875 | 0.003 |
|
| |||||||||||||
| Pair 5 | FLEV - PT-Fg | −0.35805 | 0.55422 | 0.12715 | −0.62518 | −0.09093 | 0.011 | −0.30872 | 1.01330 | 0.13789 | −0.58530 | −0.03214 | 0.029 |
|
| |||||||||||||
| Pair 6 | PT-Fg - Fibrinogen Antigen | 0.88684 | 0.23030 | 0.05283 | 0.77584 | 0.99784 | 0.000 | 0.56185 | 0.60498 | 0.08233 | 0.39672 | 0.72698 | 0.000 |
SD: standard deviation; SEM: standard error of mean; 95% CI: 95% confidence interval; FLEV: functional fibrinogen level; PT-Fg: prothrombin-time derived fibrinogen assay.
Table III.
The Pearson correlation coefficient tables generated while performing the paired samples t-tests, to look for pair-wise correlations between the four fibrinogen assays.
| Paired samples correlation table for the healthy control population | Paired samples correlation table for patients with liver disease | ||||||
|---|---|---|---|---|---|---|---|
| N | Correlation | p-value | N | Correlation | p-value | ||
| Pair 1 | Clauss Fibrinogen - FLEV | 20 | 0.819 | 0.000 | 54 | 0.803 | 0.000 |
| Pair 2 | Clauss Fibrinogen - Fibrinogen Antigen | 20 | 0.979 | 0.000 | 54 | 0.972 | 0.000 |
| Pair 3 | Clauss Fibrinogen - PT-Fg | 19 | 0.982 | 0.000 | 54 | 0.961 | 0.000 |
| Pair 4 | FLEV - Fibrinogen Antigen | 20 | 0.840 | 0.000 | 54 | 0.853 | 0.000 |
| Pair 5 | FLEV - PT-Fg | 19 | 0.798 | 0.000 | 54 | 0.696 | 0.000 |
| Pair 6 | PT-Fg - Fibrinogen Antigen | 19 | 0.966 | 0.000 | 54 | 0.908 | 0.000 |
FLEV: functional fibrinogen level; PT-Fg: prothrombin-time derived fibrinogen assay.
Estimating Clauss fibrinogen from the prothrombin time-derived fibrinogen
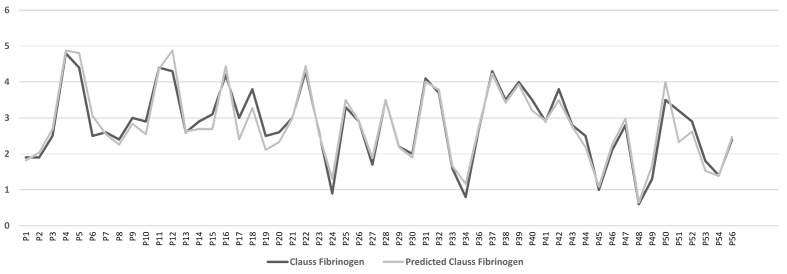
In view of the strong positive correlations, we proceeded to fit simple linear regression models from the results of the healthy control population. We then tested the predictive ability of the fitted model, Clauss fibrinogen =0.728 (PT-Fg), on the cohort with liver cirrhosis. The MSEP equation was used to mathematically compare the predicted Clauss fibrinogen values (obtained from the model) to the original Clauss fibrinogen values (Figure 1). The MSEP was equal to 0.079, which is very low, indicating that the model can be applied to the group with cirrhosis.
Figure 1.
Line chart of original Clauss fibrinogen values and predicted Clauss fibrinogen values of the liver patients categorised by patient (P) number.
Differences in fibrinogen levels depending on severity of cirrhosis
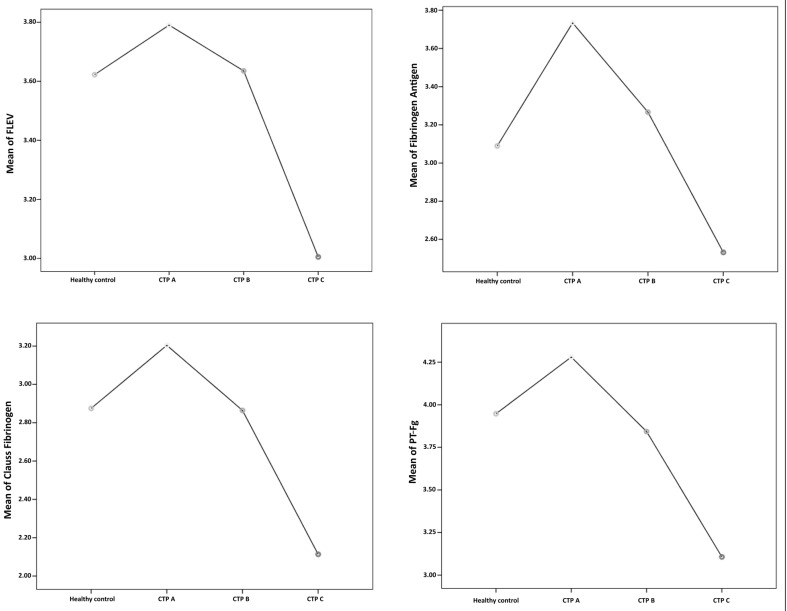
Upon applying one-way ANOVA, a significant difference was observed between the categories (healthy controls, patients with CTP-A, CTP-B and CTP-C cirrhosis) in the values of the Fib-Ag, Clauss fibrinogen and PT-Fg assays. There was no significant difference in FLEV between the four different categories. Following Bonferroni’s post-hoc test, PT-Fg, Fib-Ag and Clauss fibrinogen levels were found to be significantly higher in patients with CTP-A cirrhosis than in those with CTP-C cirrhosis. According to all four assays, the mean levels of fibrinogen in patients with CTP-A cirrhosis were higher than the mean levels of fibrinogen in healthy controls. A non-significant trend was observed in all assays showing higher fibrinogen levels in CTP-A, normal levels in CTP-B and decreased levels in CTP-C when compared to levels in the healthy controls (Figure 2).
Figure 2.
Mean plots of functional fibrinogen level (FLEV) (top left), fibrinogen antigen (top right), Clauss fibrinogen (bottom left) and prothrombin time-derived fibrinogen (PT-Fg) (bottom right) in healthy controls and patients grouped by Child-Turcotte-Pugh (CTP) class.
Discussion
In this study we confirmed that different fibrinogen assays give different results in patients with established cirrhosis, as in other pathological states2. Nevertheless, the results of all four assays showed strong correlation between each other. Importantly, the FF-TEG gave very similar results to those derived from the routine fibrinogen tests making it a suitable assay in patients with liver disease. We have shown that, according to FF-TEG findings, there is no difference in clot strength between normal individuals and subjects with liver cirrhosis of varying severity. We also derived an equation that could accurately predict the values of the more established Clauss fibrinogen from the cheaper PT-Fg. To our knowledge, this is the first study to compare these five different ways of assessing fibrinogen levels in patients with liver cirrhosis.
Our results show that FLEV and PT-Fg are affected by gender (p<0.05), whereas the Fib-Ag and Clauss fibrinogen assays are not. Even so, the mean ranks of all the aforementioned parameters were higher in females than in males indicating that females have higher fibrinogen levels than males. This is in agreement with the findings of other studies25,26. It has been suggested that the difference in fibrinogen levels between males and females can be explained by the fact that females have a lower haematocrit than males, which would result in a lower dilution of plasma when their blood samples are anticoagulated with sodium citrate27. However, this is debatable since even assays which are not affected by citrate tend to give similar results.
When the four fibrinogen assays were compared pair-wise, they all gave statistically significant different fibrinogen results with the PT-Fg giving the highest and the Clauss fibrinogen giving the lowest. This was true for both the healthy control population and the population with liver cirrhosis. This is important since several guidelines recommend specific fibrinogen thresholds for the administration of blood products, such as fibrinogen concentrates and cryoprecipitate, in patients with cirrhosis who are either bleeding or due to undergo invasive procedures15. These thresholds should ideally be qualified by a specified type of fibrinogen assay since these assays are not interchangeable.
Despite the statistical differences in the results between the four tests, the values determined by all the fibrinogen assays correlated strongly and positively with each other, in both healthy controls and the subjects with liver cirrhosis. The weakest correlation was observed between FLEV and PT-Fg in both the patients with liver disease and the healthy control population, while the strongest correlations were observed between the Clauss fibrinogen and Fib-Ag in the liver-diseased population and between the Clauss fibrinogen and PT-Fg in the healthy control population. In contrast to our study, Miesbach et al.28 did not find any correlation between Clauss fibrinogen and PT-Fg. It should be noted that they studied different groups of patients with dysfibrinogenaemia and used different reagents from different manufacturers. However, a correlation was found between PT-Fg and Fib-Ag levels measured by radial immunodiffusion and a heat fibrinogen method.
As a result of the strong correlations obtained, simple linear regression models were established. The model established between Clauss fibrinogen (dependent variable) and PT-Fg (independent variable) using data from the healthy control population was then tested on the group of patients with liver cirrhosis. In our study, the MSEP equation was then used to mathematically compare the predicted Clauss fibrinogen values (obtained from the model) to the original Clauss fibrinogen values obtained directly from the analysis of the patients with liver disease. The MSEP was very low, indicating that the model could be applied to the cirrhosis group since the differences between the original and predicted Clauss fibrinogen values were almost negligible. These findings suggest that this simple linear regression model could be used to estimate the value of Clauss fibrinogen from the PT-Fg in patients with cirrhotic liver disease. This could have significant clinical applications since the PT-Fg can be obtained without extra cost or extra reagents, in laboratories worldwide, given that it is derived from the PT. However, this assay varies greatly depending on the method of calibration, type of analyser and reagent used as well as the optical clarity of the calibrant and test plasmas and the nature of coagulopathy present. In fact, PT-Fg assays are not recommended for general use in laboratories. There might also be difficulty in the interpretation of fibrinogen results from patients’ records since PT-Fg results are not interchangeable between laboratories or hospitals due to differences in the reagents and analysers used2. Linear regression models might help to solve such problems by aiding the estimation of the Clauss fibrinogen levels from PT-Fg values. Similar linear regression models can also be established using other fibrinogen assays than the ones used in our study to predict unobserved fibrinogen levels in different populations from which the model was derived provided that all the required statistical assumptions are met. Such models can help to provide standardised management and treatment of patients by using the predicted Clauss fibrinogen results, which seem more reliable than PT-Fg.
We tried to determine whether there were significant differences in the means of each fibrinogen assay between healthy controls, and patients with CTP-A, CTP-B or CTP-C cirrhosis. The values obtained for the Fib-Ag, Clauss fibrinogen and PT-Fg assays indicate that there is a difference between the categories in these three fibrinogen assays. However, in the case of FLEV there was not a statistically significant difference between the four different clinical categories. Thus, the FLEV is incapable of differentiating between healthy controls, and patients with CTP-A, CTP-B or CTP-C cirrhosis. This contrasts with the Fib-Ag, Clauss fibrinogen and PT-Fg assays, which can differentiate between patients with early cirrhosis (CTP-A) and those with the most advanced disease (CTP-C). PT-Fg, Fib-Ag and Clauss fibrinogen levels are significantly higher in patients with CTP-A cirrhosis than in those with CTP-C cirrhosis. However, none of these assays can differentiate between all the other categories. Importantly, none of the four fibrinogen assays studied can differentiate a patient with CTP-B from one with CTP-A or CTP-C cirrhosis. This indicates that none of these fibrinogen assays is sensitive enough to distinguish between these categories of patients.
According to these assays, including the FF-TEG, the mean levels of fibrinogen in patients with CTP-A cirrhosis were higher than the mean levels of fibrinogen in healthy controls. The highest mean difference between CTP-A and CTP-C patients was observed in the Fib-Ag assay as was the highest mean difference between CTP-A and healthy controls. However, the latter mean difference was not found to be statistically significant in any of the assays. Although not statistically significant, the trends of all four fibrinogen assays shown in the mean plots (Figure 2) indicate that patients with CTP-A cirrhosis tend to have higher fibrinogen levels than healthy controls while patients with CTP-B cirrhosis have very similar fibrinogen levels to those of healthy controls. The FLEV and Clauss fibrinogen assay showed that patients with CTP-B cirrhosis and healthy control individuals have almost identical mean fibrinogen levels, while the Fib-Ag assay indicated that CTP-B patients have slightly higher mean fibrinogen levels and the PT-Fg indicated that they have slightly lower mean fibrinogen levels. As cirrhosis advances, the fibrinogen levels tend to decrease, with the lowest fibrinogen levels being observed in patients with CTP-C disease.
Our results are in concordance with those from Tytgat et al.29, who also showed that the average plasma fibrinogen concentration in 50 patients with cirrhosis were not different from those in 35 control subjects. In that study, the cirrhotic patients were not graded by disease severity. It was suggested that the fact that fibrinogen levels can be normal, despite evident accelerated catabolic rates, reflects the ability of the liver to increase the synthesis of fibrinogen.
It is unclear why patients with CTP-A cirrhosis were observed to have higher, albeit statistically non-significant, fibrinogen levels than healthy controls in our study. This could be due either to increased synthesis or a reduced rate of removal. Since fibrinogen is an acute-phase protein6, it is possible that patients with early disease might have more inflammation in the liver, resulting in increased fibrinogen release. A limitation of this study is that we did not test other inflammatory markers in order to correlate these with fibrinogen levels, since this was not the aim of the study.
Decreased fibrinogen levels, as observed in patients with CTP-C cirrhosis, may reduce the body’s ability to form a stable clot. However, our study suggests that this hypothesis is not robust. FLEV represents the measurement of the strength of a clot developed without platelet contribution and as such reflects the contribution of fibrinogen to overall clot strength22. In our study we found no statistical difference in the FLEV of the four cohorts of individuals tested. One possible explanation for this could be that although fibrinogen levels decrease in advancing liver cirrhosis (as observed from the mean plots), the clot strength remains stable. In fact, in a study by Hugenholtz et al.30, clot permeability, a surrogate marker of clot strength, was found to be decreased in patients with liver cirrhosis. This implies that decreased levels or functional defects of plasma fibrinogen in cirrhotic patients do not necessarily result in reduced clot resilience. On the contrary, decreased clot permeability may result in a potentially increased propensity towards thrombosis.
Another limitation of this study was the size of the populations recruited. A population of 30 individuals is usually considered statistically adequate. The trends observed between the different categories (i.e. healthy controls, patients with CTP-A, CTP-B or CTP-C cirrhosis) were similar in all four assays, indicating that a larger sample might have given statistically significant differences between all four categories. However, since liver cirrhosis is a relatively uncommon disease locally and a limited time was allocated for recruitment, we argue that the sample size was adequate. Another limitation is that several confounding factors that might affect fibrinogen levels (such as smoking, obesity, haematocrit) and other inflammatory markers were not all taken into consideration (or tested for) when recruiting the patients with liver disease. A reduction in the haematocrit has been shown to influence thromboelastometric parameters and Spiezia et al.31 found an inverse linear correlation between the haematocrit and maximum clot firmness in rotation thromboelastometry (ROTEM®) (an alternative to maximum amplitude in TEG®) in patients with sideropenic anaemia. Such patients, with low haematocrit values, were found to have increased maximum clot firmness, imitating a “hypercoagulable” profile. The authors suggested that this is likely to be due to the method itself rather than representing a marker of hypercoagulability in vivo. Even though anaemia might affect the maximum clot firmness in the unmodified ROTEM®, it is unknown whether it would have any effect on the FLEV.
Conclusions
Although all four fibrinogen assays gave statistically different results, they all showed a strong correlation between each other. The FF-TEG performed well giving results which were in between those of the Clauss fibrinogen and the PT-Fg. Clot strength, as judged by the FLEV determined using TEG, seems to be preserved in patients with various degrees of liver cirrhosis. We also derived a simple linear regression model in order to obtain the predicted Clauss fibrinogen values from the cheaper PT-Fg. This model should ideally be validated in larger cohorts of patients and using different reagents and analysers.
Footnotes
Funding
This project was supported by grants from the University of Malta Research Fund and the Faculty of Health Sciences, University of Malta.
Authorship contributions
AG and KR devised the study. KR collected the data. KR, KV, DZ, PG and CG analysed the samples. KR, MBI and AG performed the statistical analyses. JG, MV, PE, NV and JP were of critical importance in recruiting the patients. KR wrote the manuscript and all the authors critically revised it and approved the final version.
The Authors declare no conflicts of interests.
References
- 1.Iacoviello L, Vischetti M, Zito F, et al. Genes encoding fibrinogen and cardiovascular risk. Hypertension. 2001;38:1199–203. doi: 10.1161/hy1101.099478. [DOI] [PubMed] [Google Scholar]
- 2.Mackie I, Kitchen S, Machin S, et al. Guidelines on fibrinogen assays. Br J Haematol. 2003;121:396–404. doi: 10.1046/j.1365-2141.2003.04256.x. [DOI] [PubMed] [Google Scholar]
- 3.Moore G. Normal haemostasis. In: Moore G, Knight G, Blann A, editors. Haematology (Fundamentals of Biomedical Science) Oxford: Oxford University Press; 2010. pp. 435–476. [Google Scholar]
- 4.Acharya S, Dimichele D. Rare inherited disorders of fibrinogen. Haemophilia. 2008;14:1151–8. doi: 10.1111/j.1365-2516.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 5.Wada H, Usui M, Sakuragawa N. Hemostatic abnormalities and liver diseases. Semin Thromb Hemost. 2008;34:772–8. doi: 10.1055/s-0029-1145259. [DOI] [PubMed] [Google Scholar]
- 6.Joshi D, Keane G, Brind A. Hepatology at a Glance. 1st ed. Chichester: John Wiley & Sons Ltd; 2015. [Google Scholar]
- 7.Violi F, Basili S, Raparelli V, et al. Patients with liver cirrhosis suffer from primary haemostatic defects? Fact or fiction? J Hepatol. 2011;55:1415–27. doi: 10.1016/j.jhep.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Shao Z, Zhao Y, Feng L, et al. Association between plasma fibrinogen levels and mortality in acute-on-chronic hepatitis B liver failure. Dis Markers. 2015;2015:1–6. doi: 10.1155/2015/468596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez J, Palascak J, Kwasniak D. Abnormal sialic acid content of the dysfibrinogenemia associated with liver disease. J Clin Invest. 1978;61:535–8. doi: 10.1172/JCI108964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vucelic D, Jesic R, Jovicic S, et al. Comparison of standard fibrinogen measurement methods with fibrin clot firmness assessed by thromboelastometry in patients with cirrhosis. Thromb Res. 2015;135:1124–30. doi: 10.1016/j.thromres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Chrostek L, Supronowicz L, Panasiuk A, et al. Serum sialic acids levels according to the severity of liver cirrhosis. J Clin Lab Anal. 2014;28:465–8. doi: 10.1002/jcla.21711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis J, Armstrong D. Acquired dysfibrinogenaemia in liver disease. J Clin Pathol. 1982;35:667–72. doi: 10.1136/jcp.35.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatt A, Riddell A, Calvaruso V, et al. Enhanced thrombin generation in patients with cirrhosis-induced coagulopathy. J Thromb Haemost. 2010;8:1994–2000. doi: 10.1111/j.1538-7836.2010.03937.x. [DOI] [PubMed] [Google Scholar]
- 14.Lisman T, Porte R. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878–85. doi: 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 15.DeAngelis G, Khot R, Haskal Z, et al. Bleeding risk and management in interventional procedures in chronic liver disease. J Vasc Interv Radiol. 2016;27:1665–74. doi: 10.1016/j.jvir.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 16.Shin K, Kim I. Thromboelastographic evaluation of coagulation in patients with liver disease [abstract] Blood. 2015;126:1089. doi: 10.3343/alm.2017.37.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stravitz R. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol Hepatol (NY) 2012;8:513–20. [PMC free article] [PubMed] [Google Scholar]
- 18.Tripodi A, Primignani M, Chantarangkul V, et al. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb Res. 2009;124:132–36. doi: 10.1016/j.thromres.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal S, Johnson R, Shaw M. A comparison of fibrinogen measurement using TEG® functional fibrinogen and Clauss in cardiac surgery patients. Int J Lab Hematol. 2014;37:459–65. doi: 10.1111/ijlh.12311. [DOI] [PubMed] [Google Scholar]
- 20.Fluger I, Maderova K, Simek M, et al. Comparison of functional fibrinogen assessment using thromboelastography with the standard von Clauss method. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012;156:260–1. doi: 10.5507/bp.2011.035. [DOI] [PubMed] [Google Scholar]
- 21.Perry D, Todd T. TE and ROTEM [Internet] Practical-Haemostasis.com; 2012. [Accessed on: 23/03/2016]. Available at: http://www.practical-haemostasis.com/Miscellaneous/Miscellaneous%20Tests/teg.html. [Google Scholar]
- 22.Harr J, Moore E, Ghasabyan A, et al. Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 2013;39:45–9. doi: 10.1097/SHK.0b013e3182787122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlimp C, Solomon C, Ranucci M, et al. The effectiveness of different functional fibrinogen polymerization assays in eliminating platelet contribution to clot strength in thromboelastometry. Anesth Analg. 2014;118:269–76. doi: 10.1213/ANE.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 24.Haemonetics®. TEG® 5000 - TEG® Hemostasis System Functional Fibrinogen Reagent [leaflet] 2012. [Google Scholar]
- 25.Tabone C. Determinants of coagulation and myocardial infarction [master’s thesis] [Malta]: University of Malta; 2014. p. 184. [Google Scholar]
- 26.Krobot K, Hense H, Cremer P, et al. Determinants of plasma fibrinogen: relation to body weight, waist-to-hip ratio, smoking, alcohol, age, and sex. Results from the second MONICA Augsburg survey 1989–1990. Arterioscler Thromb Vasc Biol. 1992;12:780–8. doi: 10.1161/01.atv.12.7.780. [DOI] [PubMed] [Google Scholar]
- 27.Lowe G, Rumley A, Mackie I. Plasma fibrinogen. Ann Clin Biochem. 2004;41:430–40. doi: 10.1258/0004563042466884. [DOI] [PubMed] [Google Scholar]
- 28.Miesbach W, Schenk J, Alesci S, Lindhoff-Last E. Comparison of the fibrinogen Clauss assay and the fibrinogen PT derived method in patients with dysfibrinogenemia. Thromb Res. 2010;126:e428–e33. doi: 10.1016/j.thromres.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Tytgat G, Collen D, Verstraete M. Metabolism of fibrinogen in cirrhosis of the liver. J Clin Invest. 1971;50:1690–701. doi: 10.1172/JCI106658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hugenholtz G, Macrae F, Adelmeijer J, et al. Procoagulant changes in fibrin clot structure in patients with cirrhosis are associated with oxidative modifications of fibrinogen. J Thromb Haemost. 2016;14:1054–66. doi: 10.1111/jth.13278. [DOI] [PubMed] [Google Scholar]
- 31.Spiezia L, Radu C, Marchioro P, et al. Peculiar whole blood rotation thromboelastometry (Rotem) profile in 40 sideropenic anaemia patients [abstract] Thromb Haemost. 2008;100:1106–10. [PubMed] [Google Scholar]