Abstract
Background:
The actinomycetes strains isolated from unexplored ecosystems are a promising alternative for the biosynthesis of novel antimicrobial compounds. Depending on the interesting antifungal activity of the studied strain S19, the statistical method seems to be an effective tool for optimizing the production of anticandidal molecules.
Introduction:
This study was conducted in order to optimize the culture parameters (medium nutrients concentrations and initial pH value) affecting the production of antifungal metabolites from S. albidoflavus strain S19 (obtained from wastewater collected in Bejaia region, Algeria) using Response Surface Metho-dology (RSM). The best conditions for anti-Candida albicans compounds biosynthesis were determined.
Methods and Results:
The antimicrobial producer strain S. albidoflavus S19 was identified on the basis of morphological, chemicals characters and physiological characteristics along with 16S rRNA gene se-quencing analysis.
Response Surface Methodology by Central Composite Design (CCD) was employed to improve the anti-C. albicans agents production through the optimization of medium parameters. The highest antifungal ac-tivity was obtained by using a mixture of 2g l-1 starch, 4g l-1 yeast extract, 2g l-1 peptone at pH 11.
Conclusion:
The strain S19 isolated from wastewater showed a significant anti-C. albicans activity and this study revealed the effectiveness of RSM and CCD for increasing bioactive compounds production, rising the diameter of inhibition zones from 13 to 34 mm.
Keywords: Streptomyces albidoflavus, central composite design, RSM, optimization, antifungal production, antimicrobial activity, Candida albicans
1. Introduction
The increase of pathogens antibiotic resistance, the lack and the pharmacological restrictions of antibiotics availability are barriers that require the discovery of new antimicrobial substances. About 100 antibiotics have been marketed to treat human, animal and plant diseases [1].
Candida albicans is a common fungal pathogen responsible for several human fungal infections [2]. Immunocomp- romized patients, such as AIDS or neutropenic patients, are particularly high-risk for C. albicans infections that can become systemic [2, 3]. Among the diverse marketed drugs, antifungals represent a restricted class having an important role in controlling fungal infections. Recently, the need for new and effective antifungals is a major challenge for the pharmaceutical industry, particularly with the increase of opportunistic infections in immunocompromised patients [4]. It has been proven that natural sources are at the origin of the discoveries of new drugs [5]. Many compounds with highly biological activity have been extracted from microorganisms, plants and animals [1]. This implies the screening of microorganisms and plant extracts [6] but unfortunately, the search for new, more efficient, broad-spectrum antifungals progressed slowly [7]. Actinomycetes are Gram-positive bacteria including branching unicellular microorganisms [8]. They are extensively used in industries resulting from their aptitude to manufacture numerous antibiotics [9], anticandidal [10], or anti-inflammatory compounds [11], enzymes, vitamins, growth hormones and anti-cancer agents [12]. Members of Streptomyces genus can produce diverse active compounds with antimicrobial properties, developed for pharmaceutical uses [13]. Indeed, this genus accounts for the synthesis of about 60% of commercialized antibiotics [1]. In addition, valuable metabolites are also produced such as enzyme inhibitors, enzymes (lipases, cellulases, amylases and proteases) [14]. Therefore, Streptomyces are commonly investigated [15, 16], in particular from underexplored ecosystems, for new drugs discovery.
For the majority of antibiotics, including those recently marketed, fermentation is the most common procedure for bioactive compounds’ production as a consequence of the complexity and the high cost of chemical synthesis processes. Thereby, enhancing and improving microbial strains culture conditions remain the main tools for reducing volumes production and costs and guarantee quality and reproducibility of the drug production [6]. Secondary metabolites biosynthesis is significantly influenced by the strain and these cultural conditions [17]. Indeed, Parekh et al. [18] reported the impact of culture parameters (nature and concentration) on antibiotic production. So, carbon and nitrogen sources as well as minerals are highly required in the metabolism not only for the growth but also for the antibiotic production by Streptomyces strains [19, 20] and numerous studies showed the importance of the optimization for the metabolites production [21, 22]. Furthermore, Wang et al. [23] stated that the metabolic profile along with the kind and the amount of secondary metabolites production is highly affected by minor modifications in culture medium composition. So, in order to enhance the production of target metabolites, optimization of culture medium is required. One factor at a time (OFAT) approach was the main used procedure which is however time-consuming, claiming high number of experiments [24]. Consequently, statistical experimental design approach and response surface methodology (RSM) are widely applied to determine the most significant factors and their optimal levels affecting the production [23, 24]. RSM with Central Composite Design (CCD) is used for selected factors optimization [17, 23, 24].
Hence, the proposed study is an effort to screen the actinomycetes isolated from wastewater in Bejaia region (Algeria), as an unexplored diversity ecosystem. This diversity can be explored for isolation and characterization of native actinomycetes for antimicrobial metabolites, essentially displaying antifungal properties. In order to optimize the medium for the production of anticandidal compounds, the design of experimental methods was performed.
2. Materials and Methods
2.1. Actinobacteria Strain Isolation
The actinomycete strain designated S19 was isolated from a sample collected from wastewater treatment plant of Bejaia region in Algeria. Serially diluted suspensions up to 10-6 were prepared and spread (0.1 ml) onto Starch-Casein-Agar (SCA) medium [25] supplemented with K2Cr2O7 (50 μg ml-1) to inhibit the development of fungi. The plates were incubated for four weeks at 28°C. Based on the morphological characteristics such as aerial and substrate mycelia colour on the different tested media, sporulation according to the International Streptomyces project (ISP) [26] and its broad spectrum of antimicrobial activity (against both Gram positive and negative bacteria and C. albicans), the actinomycete isolate S19 was selected and purified on SCA medium, and then preserved at -20°C in 20% glycerol.
2.2. Characterization of Strain S19
The morphological and cultural characteristics such as the colour of aerial and substrate mycelia and also pigmentation, were noted from 7-14 days-old cultures grown at 28°C on the following ISP agar media: Yeast extract-tryptone (ISP 1), Yeast extract-malt extract (ISP 2), Oatmeal (ISP 3), Inorganic salts-starch (ISP 4), Glycerol-asparagine (ISP 5), Peptone-yeast extract-iron (ISP6), Tyrosine-asparagine (ISP 7) and YEGA [27]. For cell shape, arrangement for undisturbed hyphae, length of hyphae, branching pattern, reproductive structure and chains of spores, 7-14 days incubated cultures at 28°C on SCA medium were observed under the optic microscope with magnification up to x400.
The chemical examination of the diaminopimelic acid (DAP) isomers and whole-cell sugar were determined as described by Seong et al. [28] and Lechevalier and Lechevalier [29] respectively.
The biochemical characteristics of strain S19 were, in essence, based upon the assimilation of various carbohydrates and amino acids as unique carbon and nitrogen source respectively, according to Pridham and Gottlieb [30], decomposition of xanthine as described by Locci [31], degradation activity of several organic compounds as gelatin liquefaction, hydrolyzation of casein and tyrosine, utilization of acetate, citrate and oxalate as described by Gordon et al. [32], starch hydrolysis and reduction of nitrate according to Marchal et al. [33]. The lipasic activity was tested using Tween 80 according to Sierra [34]. All the results of the tests are noted after 7-14 days of incubation at 28° C.
For physiological growth characteristics, the strain S19 inoculated on SCA plates was incubated for 7-10 days at different temperatures (28, 30, 37 and 45°C), different pH values (3, 5, 7, 9 and 11 with an interval of 0.2 units) and different NaCl concentrations (1, 1.5, 2.0, 2.5, 3, 3.5, 4, 4.5 and 5 mol l-1). Moreover, sensitivities to phenol (at 0.05 and 0.1%), sodium azide (at 0.001 and 0.01%), potassium tellurite and five heavy metals (50 mg ml-1 of Cd3N2O6, CuSO4, FeN3O9, K2Cr2O7, and ZnSO4), as described by Daboor et al. [35], were evaluated on SCA medium after 7-10 days of incubation at 28°C.
2.3. The 16S rRNA Gene Amplification and Phylogenetic Analysis
A preculture of 50 ml of ISP medium was prepared with a single colony of the strain in a 250 ml flask, which was incubated under agitation at 28°C for 2 days. The genomic DNA used for PCR amplification was extracted with Wizard® Genomic DNA Extraction Kit from Promega Corp. and the concentration of the DNA used was determined using a NanoDrop™ Lite spectrophotometer (Thermo Scientific, Villebon sur Yvette, France). The 16S rDNA was amplified using the PCR method with Taq DNA polymerase (Master Mix x2) and universal primers S1 (5'- AGAGTTTGATC(A,C)TGGC-TCAG -3') and S2 (5'- GG(A,C)TACCTTGTTACGA (T,C)TTC-3'). Amplification was carried out in 50 µl reaction mixture containing 25 µl of Master Mix (Thermo Fisher Scientific) DreamTaq polymerase, 2.5 µl (20 µmol l-1) of each primer and 20 µl of genomic DNA. The determined sequences were compared with the GenBank databases using the basic local alignment search tools (BLAST) software provided online by the National Center for Biotechnology Information (Bethesda, MD, USA). Analyses were repeated at least three times. Sequence data were aligned with CLUSTAL_W [36] and the phylogenetic tree was constructed using MEGA 6.0 with the neighbor-joining method algorithm and 1000 bootstrap resampling interaction [37].
2.4. Selection of the Best Medium for Antibiotic Biosynthesis
The cylinder agar assay [38] was investigated to determine the antimicrobial activity of strain S19 against Gram-positive bacteria (Micrococcus luteus, Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 25923, MRSA ATCC 43300 and Listeria innocua CLIP 74915), Gram-negative bacteria (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and Salmonella typhi ATCC 14028) and a yeast (Candida albicans ATCC 10231). Strain S19 was grown on Starch-casein-agar (SCA), Mincer (M) [39], Mincer supplemented with 50% seawater (MSW), Gausse (G) [40], Gauss supplemented with 50% seawater (GSW), Czapeck Dox agar (Cz) [41], Czapeck supplemented with 50% seawater (CzSW), ISP 1 and ISP 2 media. The cultures were incubated at 30°C for 7 days and 6 mm diameter agar disks of the actinobacterial culture were prepared by using sterile Cork borers and aseptically transferred to Muller-Hinton (or Sabouraud dextrose for yeast) plates having 107 CFU ml-1 of the target microorganism. After at least 2 hours of diffusion of any antibiotic produced at 4°C, the dishes are incubated at 37° C and the diameters of the inhibition zones were determined after 18 to 24 h.
2.5. Optimization of Antifungal Production Using CCD and RSM
The number of experiments, time and material resources can be decreased using experimental design which is widely used for controlling the effects of parameters in many processes. Several experimental designs have been considered for studying such models.
In this study, Central Composite Design (CCD) was used to optimize four operating factors, namely starch concentration (X1), yeast extract concentration (X2), peptone concentration (X3) and pH of the medium (X4) on the biosynthesis of the anti-C. albicans compound after the study of their effect. The latter led to obtain a quadratic model with 24 factorial trails, eight star points to estimate quadratic effects and six central points to fit the second-order polynomial model. 30 experiments were required in this design where each factor was studied at five experimental levels: -α, -1, 0, +1, +α, with α = 2n/4, where n is the number of variables and 0 corresponded to the central point of each factor.
The levels of factors used for experimental matrix are given in Tables 1 and 2, respectively. The anti-C. albicans activity was tested for antibiotic production after 7 days of growth at 30°C. All the experiments were carried out in triplicate and the experimental results of CCD were fitted via the response surface regression procedure, using the following second order polynomial equation:
Table 1. Values and levels of operating parameters.
| Factors |
Levels
- α (-2) -1 0 +1 +α (+2) |
||||
|---|---|---|---|---|---|
| X1: Starch concentration (g l-1) | 2 | 6 | 10 | 14 | 18 |
| X2: Yeast extract concentration (g l-1) | 2 | 3 | 4 | 5 | 6 |
| X3: Peptone concentration (g l-1) | 0.5 | 1.25 | 2 | 2.75 | 3.5 |
| X4: pH | 3 | 5 | 7 | 9 | 11 |
Table 2. Central composite design (CCD) of factors in coded value for optimization of the antifungal activity yield.
| Run No. | Natural Values of Parameters | Coded Values of Parameters |
Observed Antifungal Activity
y (mm) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 (g l-1) | X2 (g l-1) | X3 (g l-) | X4 | x0 | x1 | x2 | x3 | x4 | ||
| 1 | 6 | 3 | 1.25 | 5 | 1 | -1 | -1 | -1 | -1 | 13.95 |
| 2 | 14 | 3 | 1.25 | 5 | 1 | 1 | -1 | -1 | -1 | 17.64 |
| 3 | 6 | 5 | 1.25 | 5 | 1 | -1 | 1 | -1 | -1 | 0 |
| 4 | 14 | 5 | 1.25 | 5 | 1 | 1 | 1 | -1 | -1 | 17.53 |
| 5 | 6 | 3 | 2.75 | 5 | 1 | -1 | -1 | 1 | -1 | 19.55 |
| 6 | 14 | 3 | 2.75 | 5 | 1 | 1 | -1 | 1 | -1 | 12.06 |
| 7 | 6 | 5 | 2.75 | 5 | 1 | -1 | 1 | 1 | -1 | 0 |
| 8 | 14 | 5 | 2.75 | 5 | 1 | 1 | 1 | 1 | -1 | 16.83 |
| 9 | 6 | 3 | 1.25 | 9 | 1 | -1 | -1 | -1 | 1 | 12.56 |
| 10 | 14 | 3 | 1.25 | 9 | 1 | 1 | -1 | -1 | 1 | 9.02 |
| 11 | 6 | 5 | 1.25 | 9 | 1 | -1 | 1 | -1 | 1 | 25.42 |
| 12 | 14 | 5 | 1.25 | 9 | 1 | 1 | 1 | -1 | 1 | 0 |
| 13 | 6 | 3 | 2.75 | 9 | 1 | -1 | -1 | 1 | 1 | 20.88 |
| 14 | 14 | 3 | 2.75 | 9 | 1 | 1 | -1 | 1 | 1 | 14.45 |
| 15 | 6 | 5 | 2.75 | 9 | 1 | -1 | 1 | 1 | 1 | 18.16 |
| 16 | 14 | 5 | 2.75 | 9 | 1 | 1 | 1 | 1 | 1 | 9.23 |
| 17 | 10 | 4 | 2 | 7 | 1 | 0 | 0 | 0 | 0 | 13.10 |
| 18 | 10 | 4 | 2 | 7 | 1 | 0 | 0 | 0 | 0 | 13.50 |
| 19 | 10 | 4 | 2 | 7 | 1 | 0 | 0 | 0 | 0 | 13.18 |
| 20 | 10 | 4 | 2 | 7 | 1 | 0 | 0 | 0 | 0 | 13.00 |
| 21 | 10 | 4 | 2 | 7 | 1 | 0 | 0 | 0 | 0 | 13.68 |
| 22 | 10 | 4 | 2 | 7 | 1 | 0 | 0 | 0 | 0 | 13.7 |
| 23 | 2 | 4 | 2 | 7 | 1 | -2 | 0 | 0 | 0 | 14.02 |
| 24 | 18 | 4 | 2 | 7 | 1 | 2 | 0 | 0 | 0 | 10.66 |
| 25 | 10 | 2 | 2 | 7 | 1 | 0 | -2 | 0 | 0 | 15.50 |
| 26 | 10 | 6 | 2 | 7 | 1 | 0 | 2 | 0 | 0 | 12.92 |
| 27 | 10 | 4 | 0.5 | 7 | 1 | 0 | 0 | -2 | 0 | 9.3 |
| 28 | 10 | 4 | 3.5 | 7 | 1 | 0 | 0 | 2 | 0 | 14.6 |
| 29 | 10 | 4 | 2 | 3 | 1 | 0 | 0 | 0 | -2 | 0 |
| 30 | 10 | 4 | 2 | 11 | 1 | 0 | 0 | 0 | 2 | 17.80 |
Y= β0+ ∑βiXi+∑βijXiXj+∑βiiXi2 i= 1, 2, 3…….k (1)
Where Y is the predicted response, β0 is the regression coefficient, βi is the linear coefficient, βii is the quadratic coefficient, βij is the interaction coefficient and Xi is the coded level of independent variables.
The antifungal production yield is the dependent output response variable which is expressed as the diameter of inhibition zone against C. albicans (mm). The independent variables are coded for statistical calculation according to the following equation:
| (2) |
where xi is the coded value of the ith variable, Xi is the original or real value, X̅is the value of Xi at the center point of the investigated area and ΔXi is the step size.
3. Results
3.1. Strain S19 Isolation
A total of 156 actinomycete isolates were obtained from a wastewater sample from Bejaia (Algeria). This ecosystem is unexplored for the isolation of actinomycetes. It was selected for its abundance of organic matters and organisms that are competitor for nutrients.
Among the strains isolated and tested for their ability to produce antimicrobial compounds, S19 showed the largest activity spectrum against the tested microorganisms (data not shown). So, this strain was selected for further study.
3.2. Characterization and Taxonomy of Strain S19
Significant growth of aerial mycelium of the actinomycetal strain S19 added to good sporulation was observed on six tested media, whereas no sporulation was observed on ISP 1 and ISP 5 media. Melanoid pigments were not produced on ISP 6 and ISP 7 media where grayish-green soluble pigment was produced on ISP 3 medium. Growth, aerial and substrate mycelia colour, sporulation and soluble pigment production of strain S19, after incubation at 28°C for 7-14 days are summarized in Table 3 and the light-microscopic observation of S19 (10x40 magnification) after 14 days of culture on ISP 4 medium is shown in Fig. (1).
Table 3. Morphological features of strain S19 on different media.
| Media | Mycelium Growth | Aerial Mycelium Colour | Substrat Mycelium Colour | Spores | Soluble Pigment |
|---|---|---|---|---|---|
| ISP 1 | ++ | White | White | No spore | Absent |
| ISP 2 | +++ | White-grayish | White | Grey | Absent |
| ISP 3 | +++ | Light greenish | Grayish green | Light grayish | Grayish green |
| ISP 4 | +++ | White | White | Yellow | Absent |
| ISP 5 | ++ | Grayish-white | White | Gray | Absent |
| ISP6 | +++ | Grayish-white | White | Gray | Absent |
| ISP7 | +++ | White | White | Grayish- white | Absent |
| YEGA | +++ | Grayish white | White | Gray | Absent |
ISP: International Streptomyces Project medium. +: poor; ++: moderate; +++: good.
Fig. (1).
Light microscopic observation of isolate S19 after 14 days of culture on ISP 4 medium (40x 10 magnifications).
The chemotaxonomic study of strain S19 showed the presence of LL-DAP in the peptidoglycan and glycine in the cell wall. The whole-cell hydrolysate did not contain characteristic sugars, typical of cell wall type IC [42].
Based on the morphological and chemotaxonomic features, the isolate S19 was found to be a member of the genus Streptomyces.
3.3. Phylogenetic Characteristics
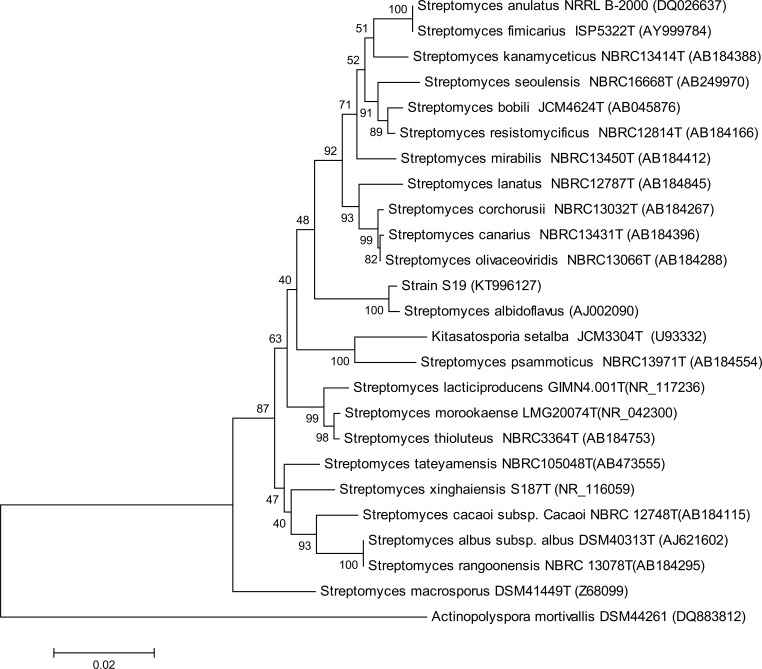
The 1,489 nt 16S rDNA sequence of the strain S19 has been deposited in the GenBank data library and has been assigned the accession number KT996127. This sequence was aligned with those of Streptomyces reference species available in the GenBank database indicating that S19 was related to the genus Streptomyces (similarity level between 96 and 99%). Its position in the 16S rDNA Streptomyces tree is shown in Fig. 2. This isolate has 99% similarity level with S. albidoflavus (AJ002090) [43], the most closely related species.
Fig. (2).
Neighbour-joining tree based on 16S rDNA sequence showing the relations between strain S19 and type species of the genus Streptomyces. The numbers at the nodes indicate the levels of bootstrap support based on Neighbour-joining analysis of 1,000 resampled datasets. Bar, 0.02 nt substitution per nt position. Actinopolyspora mortivalis DSM 44261 (DQ883812) has been used as outgroup.
The physiological and biochemical properties of strain S19 are summarized in Table 4. Isolate S19 growth occurs between pH 3 and pH 11 with an optimum at pH 9. The strain is also mesophilic with a growth optimum at 30°C; it assimilates 15 of the tested carbon sources, Sodium acetate and citrate but not inositol nor sodium oxalate. This isolate degrades starch, Tween 80, tyrosine, casein from milk, utilizes all tested nitrogen sources and liquefies gelatin. Moreover, it is not sensitive to the five tested heavy metals.
Table 4. Physiological and biochemical characteristics of strain S19.
| Characteristics | Results | Characteristics | Results |
|---|---|---|---|
| Range of temperature Optimum temperature Range of pH for growth Optimum pH Growth in the presence of NaCl Carbon source utilization Arabinose Adonitol Galactose Glucose Lactose Levulose Maltose Mannose Raffinose Ribose Saccharose Salicin Sorbitol Threalose Xylose Inositol Hydrolysis of: Starch Tween 80 Liquefaction of Gelatin Tyrosine Casein from milk |
25-45°C 30°C 3-11 9 1-2 mol l-1 + + + + + + + + + + + + + + + - + + + + + |
Nitrogen source utilization Alanine Arginine Asparagine Aspartic acid Cysteine Diphenylamine Glutamic acid Glycine Histidine Isoleucine Leucine Methionine Ornithine Phenylalanine Proline Serine Threonine Tryptophan Tyrosine Valine Xanthine Assimilation of sodium: Acetate Citrate Oxalate Resistance to: Phenol (0.01%) Potassium tellurite (0.01%) Cadmium Chromium Copper Iron Zinc |
+ + + + - - + + + + + + + + + + + + + + - + + - + + + + + + + |
+: positive test, -: negative test.
Based on the genotypic result, strain S19 belongs to the species S. albidoflavus. However, this strain could be distinguished from S. albidoflavus DSM 40455T by some phenotypic properties such as sporulation on some media, degradation of raffinose and colour of soluble pigment on ISP 3 medium. Strain S19 produces aerial spore mass at 40-45°C, while strain DSM 4055T is unable to produce this mass at 40°C.
3.4. Selection of Basic Medium for the Antimicrobial Activity of Strain S19
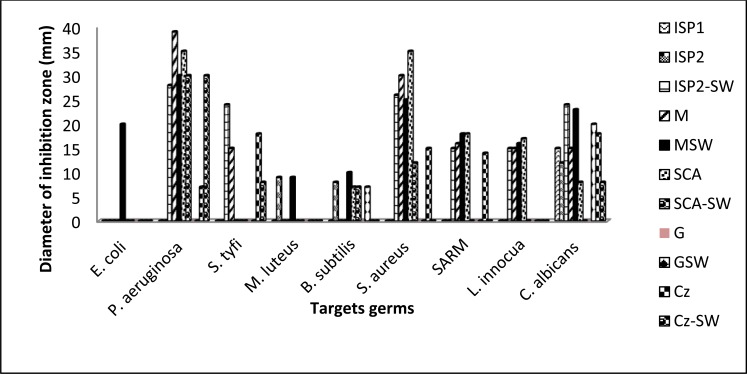
The nature of habitat of the microorganisms as well as the differences in the composition of the substrate affects significantly their antimicrobial activity. In addition, the strain and the target microorganisms can also have an influence. Eleven media: standards (ISP 1, ISP 2, M, SCA, G and Cz) or added with 50% of seawater (ISP 2-SW, M-SW, SCA-SW, G-SW and Cz-SW), reported in literature for production of various secondary metabolites, were investigated to determine the optimal nutrient medium for antibiotic biosynthesis by strain S19. The medium selected for further study is the one on which the isolate exhibited the largest antibiotic production spectrum against the tested microorganisms.
According to the results depicted in Fig. 3, all tested media exhibited an impact on the antimicrobial compounds production by strain S19. On M-SW, this strain was able to produce the largest spectrum of bioactive metabolites against all tested microorganisms. Indeed, by comparing antimicrobial activities of the isolate S19 against E. coli, P. aeruginosa, M. luteus, B. subtilis, S. aureus, MRSA, L. innocua and C. albicans on the tested media, culture on M-SW presented growth inhibition diameters of 20, 30, 9, 10, 25, 18, 16 and 23 mm, respectively. Based on these results, M-SW was selected to optimize the production of antifungal metabolites by strain S19 using CCD and RSM.
Fig. (3).
Comparison of the effect of culture media composition on bioactive metabolite production by strain S19.
3.5. Optimization of Antifungal Production Using CCD and RSM
In this study, 30 experiments were carried out to obtain optimal combinations of starch concentration (X1), Yeast-extract concentration (X2), Peptone concentration (X3), and initial pH (X4) for antifungal production. By applying multiple regression analysis to experimental data of central composite design (CCD) shown in Table 1, the effect of each factor and their second order interactions of S19 strain against C. albicans is explained by a second order polynomial model.
Table 2 summarizes the results of second-order CCD experiments. The model coefficients (Eq 1) are estimated by the standard least-squares regression method using the “EXCEL” software. Three statistical tests are necessary to evaluate the adequacy of the model, namely the Student's t-test for the significance of the factors, the R-squared test and Fisher's tests.
After the 30 experiments (Table 2) and the elimination of non-significant effects, the model equation obtained was as follows:
Y = 13.36- 0.85x1-1.58x2+1.07x3+1.99x4+0.86x12 - 4.68x14 -0.78x23+1.54x24 +1.03x34+0.4x22 - 0.17x23 - 0.93x24 (3)
Where is the predicted antifungal yield, X1 is starch, X2 is yeast extract, X3 is peptone concentration and X4 is initial pH.
The observed response variability values related to the experimental factors and their interaction is determined by the values of the coefficient of determination (R2) [44].
According to the obtained value of the determination coefficient (R2) of the model which was found to be 0.947, the variability of the response could be explained by the model at 94.7%.
According to Chen et al. [45] the R2 value greater than 0.9 is required for a very high correlation of the regression model. Indeed, the R2 obtained value indicates a good correlation between the observed and predicted responses and suggests that the model is reliable for antifungal production.
The obtained regression equation (Eq. 3) shows that concentrations of starch (x1), yeast extract (x2), peptone (x3) and pH (x4) all have an individual influence on the anti-C. albicans metabolite production. The pH (x4) has the strongest effect on the response since coefficient of x4 (b4 = +1.99) is larger than the coefficients of the other investigated factors. A positive sign of this coefficient indicates that the antifungal production is enhanced at high pH or alkaline media. The order for factors strength on antifungal production following pH was found as yeast extract concentration (x2) with a negative effect (b2= -1.58), peptone concentration (x3) with a positive influence (b3= +1.07) and finally starch concentration (x1) with a negative effect (b1= -0.85).
The significance interactions found by the design of experiments in order are essentially between the concentrations of starch and pH (x1x4) (b14 = - 4.68), concentrations of yeast extract and pH (x2x4), peptone concentration and pH (x3x4), starch and yeast extract (x1x2) and between concentrations of yeast extract and peptone (x2x3).
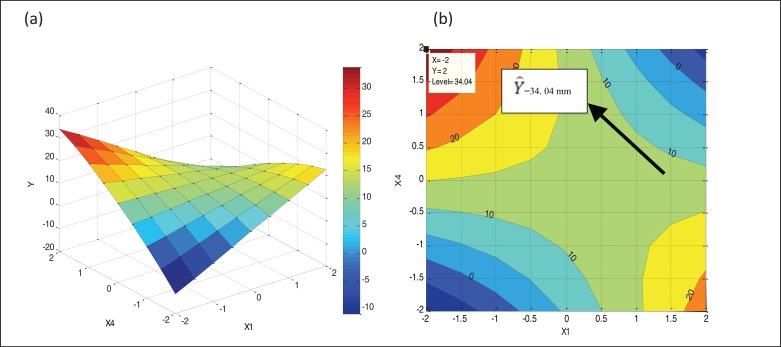
The corresponding 2D and 3D dimensional response surfaces of the quadratic model are shown in Figs. 4a and b. The figures are drawn in starch concentration(x1) – pH(x4) plan (the most important interaction affecting the response) for x2= 0 and x3= 0 using “MATLAB 7.0” software. The analysis of these figures showed that the maximum predicted diameter of inhibition zone against C. albicans (34 mm) is reached by decreasing the starch concentration and increasing the pH as indicated by the row in Fig. (4a). The corresponding coordinates of this optimum are as follows: starch concentration = 2 g l-1 (x1=-2), yeast extract = 4 g l-1 (x2= 0), peptone = 2 g l-1 (x3= 0) and pH = 11 (x4= 2).
Fig. (4).
2D and 3D Response surface of antifungal activity against C. albicans.
4. Discussion
In the way of actinomycetes isolation from underexplored sources, an antimicrobial producer, Streptomyces albidoflavus S19, isolated from wastewater of Bejaia region in Algeria, was selected. This strain exhibited important antimicrobial activities against five species of Gram-positive bacteria, especially against MRSA and S. aureus, three Gram-negative bacteria especially P. aeruginosa and S. typhi, and the yeast C. albicans.
Sarmiento-Vizcaino et al. have reported cultivable S. albidoflavus strains to be ubiquitous in most studied habitats (aerial, terrestrial, and marine) in a broad geographic region (Northern Spain, Northern Portugal and South of France, from 2008 until 2012) [46]. In the past few years, Actinobacteria isolated from several extreme environments and unexplored habitats around the world have been intensively studied. So, the exploration of particular ecosystems (temperature, pH, aeration or osmotic stress) promotes detection of actinomycetales bacteria which may optionally have a potential antibacterial and/or significant antifungal activity [47]. In addition, current research of novel molecules of antibiotics is directed towards the isolation of strains from specific ecosystems and hostile environments previously untapped [48].
Several genera affiliated with the Actinomycetales order are the source of various drugs used against common diseases and members of the genus Streptomyces are the main producers of bioactive molecules widely used in different biotechnology industries [49].
In fact, in current research programs; previously unexplored natural habitats are behind the isolation of various Streptomyces strains producing several antimicrobial agents [50, 51].
The results of the studies on morphological, chemotaxonomic, physiological characteristics and the phylogenetic analysis indicated that strain S19 had a high concordance with S. albidoflavus DSM 40455T strains described by Rong et al. [43], and a high similarity (99%) with its 16S rDNA sequence. However, some peculiar cultural properties are noted such as the colour of substrate mycelium on ISP 5 and ISP 6 media, the spore colour of strain S19 on ISP 4 medium, and the soluble pigment produced on ISP 3. For the physiological differences, raffinose is assimilated by strain S19 while this carbon source is not utilized by the type-strain S. albidoflavus described by [43]. The isolate S19 tolerates saline conditions up to 11% of NaCl and a wide range of pH (between pH 3 and pH 11) and temperatures (28-45°C), in agreement with previous reports [46, 52].
The influence on antibiotic production of S. albidoflavus S19 strain by culture medium was investigated and among eleven media reported for production of secondary metabolites, M-SW was selected because it allowed the largest spectrum of antibiotic production by this strain, thereby optimizing the production of anti-C. albicans compound. The composition of the growth medium has a relevant influence on metabolite production [53]. There is no universal medium applicable to all microorganisms; however, Starch Casein Agar medium (SCA) was reported to be the best one for antifungal production by S. albidoflavus PU 23 [54].
The combination of CCD design with RSM for optimizing the antifungal production by S. albidoflavus S19 against C. albicans is an effective and reliable tool to select the statistically significant factors and to find the optimal concentration and levels of those factors in the culture medium. Out of the four studied factors, pH and peptone concentration were found to have a positive influence on antifungal production, whereas the concentration of starch and yeast extract had a negative influence. Concerning the effect of pH, the results of this study seem opposite to those reported in the literature. Augustine et al. showed that the optimal pH for antifungal production by S. albidoflavus strain PU 23 was equal to 7 [54]; the same result was reported for S. albidoflavus strain ANU 2677 [55], and a value of this factor greater than 8 was unfavourable for antibiotic production. This value is probably strain-dependent.
The negative effect of starch concentration could be explained by the catabolite repression from its higher concentration [56]. The regulation of the rate of carbon consumption in Streptomyces seems to be unique; nonetheless, the catabolic mechanism in this genus has not been unraveled [57]. In particular, starch is the preferred carbon source by the majority of Streptomyces for the production of secondary metabolites [56, 58, 59]. Thus, it is not unexpected that starch is a significant factor, as shown by the results presented here. However, in this study, the anti-yeast production by the strain S19 decreases with the increasing of starch concentration. According to carbon sources, cell growth and secondary metabolite production in Streptomyces spp. are strongly related to species-specific variation [60]. In the complex nitrogen sources, yeast extract supports the greatest mycelium biosynthesis and favours a rapid growth [61]. Previous experiments have shown that it is the preferred nitrogen source of Streptomyces strains [62-64]. Moreover, Himabindu and Jetty found that yeast extract is favourable for growth but not for antibiotic production [65].
In this study, as shown by the second order equation developed, yeast extract is an important factor with a negative effect which means that it may decrease the anti-Candida molecule synthesis.
The interaction of starch with pH was the most important one. From CCD, the most optimal condition for anti-C. albicans production was found to be pH 11, 2 g l-1 of starch, 4 g l-1 of yeast extract and 2 g l-1 of peptone. In addition, validation of the model suggested, unequivocally, the reliability of RSM for optimization of media for antibiotic production.
Conclusion
The results of this study suggest that wastewater Actinobacteria are a particular group of microorganisms that can provide new metabolites.
The isolation of strains with antimicrobial activity suggests that wastewater may be an ecological niche, harbouring largely untapped microbial diversity and untapped potential for new secondary metabolites. The results obtained from this work are promising and deserve to be studied further as the kinetic production of the anticandidal compound(s), the purification, characterization and identification of the active secondary metabolites produced by S. albidoflavus strain S19.
ACKNOWLEDGEMENTS
Declared none.
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No Animals/Humans were used for studies that are the basis of this research.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Kalyani A.L.T., Ramya-Sravani K.M., Annapurna J. Isolation and characterization of antibiotic producing actinomycetes from marine soil samples. Int. J. Curr. Pharm. Res. 2012;4:109–112. [Google Scholar]
- 2.McCullough M.J., Ross B.C., Reade P.C. Candida albicans: A review of its history, taxonomy, epidemiology, virulence attributes, and methods of strain differentiation. Int. J. Oral Maxillofac. Surg. 1996;25:136–144. doi: 10.1016/s0901-5027(96)80060-9. [DOI] [PubMed] [Google Scholar]
- 3.Vazquez J.A. Options for the management of nosocomial candidiasis in patients with AIDS and HIV infection. Pharmacotherapy. 1999;19:76–87. doi: 10.1592/phco.19.1.76.30509. [DOI] [PubMed] [Google Scholar]
- 4.Bharti A., Kumar V., Gusain O., Bisht G.S. Antifungal activity of actinomycetes isolated from Garhwal region. J. Sci. Eng. Tech. Mgt. 2010;2:3–9. [Google Scholar]
- 5.Bevan P., Ryder H., Shaw I. Identifying small-molecule lead compounds: The screening approach to drug discovery. Trends Biotechnol. 1995;113:115–121. doi: 10.1016/S0167-7799(00)88916-7. [DOI] [PubMed] [Google Scholar]
- 6.Marinelli F., Genilloud O., Fedorenko V., Ron E.Z. Specialized bioactive microbial metabolites: From gene to product. BioMed Res. Int. 2015;••• doi: 10.1155/2015/276964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupte M., Kulkarni P., Ganguli B.N. Antifungal antibiotics. Appl. Microbiol. Biotechnol. 2002;58:46–57. doi: 10.1007/s002530100822. [DOI] [PubMed] [Google Scholar]
- 8.Waksman S.A. The Actinomycetes: Classification, identification and description of genera and species. Vol. 2 Baltimore: Williams and Wilkins Co.; 1961. [Google Scholar]
- 9.Raja A., Prabakarana P. Actinomycetes and drug-an overview. Am. J. Drug Discov. Dev. 2011;1:75–84. [Google Scholar]
- 10.Sanasam S., Ningthoujam D.S. Screening of local actinomycete isolated in Manipur for anticandidal activity. Asian J. Biotechnol. 2010;2:139–145. [Google Scholar]
- 11.Ponmurugan P., Nithya B. Plasmid DNA of antibiotic producing strains of Streptomyces sannanensis isolated from different states in Southern India. Biotechnology (Faisalabad) 2008;7:487–492. [Google Scholar]
- 12.Berdy J. Are actinomycetes exhausted as a source of secondary metabolites? Biotecnologica. 1995;7-8:13–34. [Google Scholar]
- 13.Wellington E.M., Stackebrandt E., Sanders D., Wolstrup J., Jorgensen N.O.G. Taxonomic status of Kitasatosporia and proposed unification with Streptomyces on the basis of phenotypic and 16S rRNA analysis and emendation of Streptomyces Waksman and Enrici 1943, 339AL. Int. J. Syst. Bacteriol. 1992;42:156–160. doi: 10.1099/00207713-42-1-156. [DOI] [PubMed] [Google Scholar]
- 14.Ravel J., Wellington E.M.H., Hill R.T. Interspecific transfer of Streptomyces linear plasmids in sterile amended soil microcosms. Appl. Environ. Microbiol. 2000;66:529–534. doi: 10.1128/aem.66.2.529-534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arasu M.V., Duraipandiyan V., Ignacimuthu S. Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp.AP-123 and its cytotoxic effect. Chemosphere. 2013;90:479–487. doi: 10.1016/j.chemosphere.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Jang K.H., Nam S-J., Locke J.B., Kauffman C.A., Beatty D.S., Paul L.A., Fenical W. Anthracimycin, a potent anthrax antibiotic from a marine derived actinomycete. Angew. Chem. Int. Ed. Engl. 2013;52:7822–7824. doi: 10.1002/anie.201302749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Huang L., Kang Z., Buchenauer H., Gao X. Optimization of the fermentation process of actinomycete strain Hhs.015T. J. Biomed. Biotechnol. 2010;••• doi: 10.1155/2010/141876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parekh S., Vinci V.A., Strobel R.J. Improvement of microbial strains and fermentation processes. Appl. Microbiol. Biotechnol. 2000;54:287–301. doi: 10.1007/s002530000403. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez S., Demain A.L. Metabolic regulation of fermentation processes. Enzyme Microb. Technol. 2002;31:895–906. [Google Scholar]
- 20.Yu J., Liu Q., Liu X., Sun Q., Yan J., Qi X., Fan S. Effect of liquid culture requirements on antifungal antibiotic production by Streptomyces rimosus MY02. Bioresour. Technol. 2008;99:2087–2091. doi: 10.1016/j.biortech.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Purama R.K., Goyal A. Screening and optimization of nutritional factors for higher dextransucrase production by Leuconostoc mesenteroides NRRL B-640 using statistical approach. Bioresour. Technol. 2008;99:7108–7114. doi: 10.1016/j.biortech.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Yuan L.L., Li Y.Q., Wang Y., Zhang X.H., Xu Y.Q. Optimization of critical medium components using response surface methodology for phenazine-1-carboxylic acid production by Pseudomonas sp. M-18Q. J. Biosci. Bioeng. 2008;105:232–237. doi: 10.1263/jbb.105.232. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Fang X., An F., Wang G., Zhang X. 2011.
- 24.Kanmani P., Karthik S., Aravind J., Kumaresan K. 2013. [DOI] [PMC free article] [PubMed]
- 25.Küster E., Williams S.T. Selection of media for isolation of Streptomycetes. Nature. 1964;202:928–929. doi: 10.1038/202928a0. [London]. [DOI] [PubMed] [Google Scholar]
- 26.Shirling E.B., Gottlieb D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966;16:313–340. [Google Scholar]
- 27.Athalye M., Lacey J., Goodfellow M. Selective isolation and enumeration of actinomycetes using rifampicin. J. Appl. Bacteriol. 1985;51:289–291. [Google Scholar]
- 28.Seong C.N., Kim Y.S., Baik K.S., Lee S.D., Hah Y.C., Kim S.B., Goodfellow M. Mycolic acid-containing actinomycetes associated with activated sludge foam. J. Microbiol. 1999;37:66–72. [Google Scholar]
- 29.Lechevalier M.P., Lechevalier H.A. Chemical composition as a criterion in the classification of aerobic actinomycetes. Int. J. Syst. Bacteriol. 1970;20:435–443. [Google Scholar]
- 30.Pridham T.G., Gottlieb G.D. The utilization of carbon compounds by some Actinomycetales as an aid for species determination. J. Bacteriol. 1948;56:107–114. doi: 10.1128/jb.56.1.107-114.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locci R. 1989. Streptomycetes and related genera. [Google Scholar]
- 32.Gordon R.E., Barnett D.A., Handarhan J.E., Hor-Nay-Pang C. Nocardia coeliaca, Nocardia autotrophica and the nocardin strains. Int. J. Syst. Bacteriol. 1974;24:54–63. [Google Scholar]
- 33.Marchal N., Bourdon J.L., Richard C.L. Les milieux de culture pour l’isolement et l’identification biochimique des bactéries. Paris: Doin Press; 1987. [Google Scholar]
- 34.Sierra G. A simple method for the detection of lipolytic activity of microorganisms and some observations on the influence of the contact between cells and fatty substrates. J. Microbiol. Serol. 1957;23:15–22. doi: 10.1007/BF02545855. [DOI] [PubMed] [Google Scholar]
- 35.Daboor S.M., Amany M.H. Neven AbdElfatah E.; Hanouna S.I. Heavy metal adsorption of Streptomyces chromofuscus. J. Coast. Life Med. 2014;2:431–437. [Google Scholar]
- 36.Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic tree. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 38.Bastide A., De Méo M., Andriantsoa M., Laget M., Duménil G. Isolement et sélection de souches d’actinomycète productrices de substances antifongiques de structure non-polyéniques. Mircen J. Appl. Microbiol. 1986;2:453–466. [Google Scholar]
- 39.Mincer T.J., Jensen P.R., Kauffman C.A., Fenical W. Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl. Environ. Microbiol. 2002;68:5005–5011. doi: 10.1128/AEM.68.10.5005-5011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivantiskaya L.P., Singal S.M., Bibikova M.V., Vostrov S.N. Direct isolation of Micromonospora on selective media with gentamicin. Antibiotiki. 1978;23:690–692. [PubMed] [Google Scholar]
- 41.Lawrence C.H. A method for isolating actinomycetes from scabby potato tissue and soil with minimal contamination. Can. J. Bot. 1956;34:44–47. [Google Scholar]
- 42.Holt J.G., Krieg N.R., Sneath P.H.A., Staley J.T., Williams S.T. Bergey’s Manual of Determinative Bacteriology. 9th ed. Baltimore: Williams and Wilkins; 1994. [Google Scholar]
- 43.Rong X., Guo Y., Huang Y. Proposal to reclassify the Streptomyces albidoflavus clade on the basis of multilocus sequence analysis and DNA-DNA hybridization, and taxonomic elucidation of Streptomyces griseus subsp. solvifaciens. Syst. Appl. Microbiol. 2009;32:314–322. doi: 10.1016/j.syapm.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Kaushik R., Saran S., Isar J., Saxena R.K. Statistical optimization of medium components and growth conditions by response surface methodology to enhance lipase production by Aspergillus carneus. J. Mol. Catal. B-Enz. 2006;40:121–126. [Google Scholar]
- 45.Chen X.C., Bai J.X., Cao J.M., Li Z.J., Xiong J., Zhang L., Hong Y., Ying H.J. Medium optimization for the production of cyclic adenosine 30, 50-monophosphate by Microbacterium sp. no. 205 using response surface methodology. Bioresour. Technol. 2009;100:919–924. doi: 10.1016/j.biortech.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 46.Sarmiento-Vizcaíno A., Braña A.F., González V., Nava H., Molina A., Llera E., Fiedler H-P., Rico J.M., García-Flórez L., Acuña J.L., García L.A., Blanco G. Atmospheric dispersal of bioactive Streptomyces albidoflavus strains among terrestrial and marine environments. Microb. Ecol. 2015;71:375–386. doi: 10.1007/s00248-015-0654-z. [DOI] [PubMed] [Google Scholar]
- 47.Willams S.T., Cross T. 1971. [Google Scholar]
- 48.Lam K.S. Discovery of novel metabolites from marine actinomycetes. Curr. Opin. Microbiol. 2006;9:245–251. doi: 10.1016/j.mib.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Medema M.H., Breitling R., Takano E. Synthetic biology in Streptomyces bacteria. Methods Enzymol. 2011;497:485–502. doi: 10.1016/B978-0-12-385075-1.00021-4. [DOI] [PubMed] [Google Scholar]
- 50.Hamedi J., Mohammadipanah F., Ventosa A. Systematic and biotechnological aspects of halophilic and halotolerant actinomycetes. Extremophiles. 2012;17:1–13. doi: 10.1007/s00792-012-0493-5. [DOI] [PubMed] [Google Scholar]
- 51.Jose P.A., Jebakumar S.R.D. Phylogenetic appraisal of antagonistic, slow growing actinomycetes isolated from hypersaline inland solar salterns at Sambhar salt Lake, India. Front. Microbiol. 2013;4:190. doi: 10.3389/fmicb.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amato P., Parazols M., Sancelme M., Laj P., Mailhot G., Delort A.M. Microorganisms isolated from the water phase of tropospheric clouds at the Puy de Dôme: major groups and growth abilities at low temperatures. FEMS Microbiol. Ecol. 2007;59:242–254. doi: 10.1111/j.1574-6941.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 53.Porter J.N. 1975. Cultural conditions for antibiotic producing microorganisms. [DOI] [PubMed] [Google Scholar]
- 54.Augustine S.K., Bhavsar S.P., Baserisalehi M., Kapadnis B.P. Isolation, characterization and optimization of antifungal activity of an actinomycete of soil origin. Indian J. Exp. Biol. 2004;42:928–932. [PubMed] [Google Scholar]
- 55.Narayana K.J.P., Vijayalakshmi M. Optimization of antimicrobial metabolites production by Streptomyces albidoflavus. Res. J. Pharmacol. 2008;2:4–7. [Google Scholar]
- 56.Jia B., Jin Z.H., Mei L.H. Medium optimization based on statistical methodologies for pristinamycins production by Streptomyces pristinaespiralis. Appl. Biochem. Biotechnol. 2008;144:133–143. doi: 10.1007/s12010-007-8012-3. [DOI] [PubMed] [Google Scholar]
- 57.Paulsen I.T. Carbon metabolism and its regulation in Streptomyces and other high GC gram-positive bacteria. Res. Microbiol. 1996;147:535–541. doi: 10.1016/0923-2508(96)84009-5. [DOI] [PubMed] [Google Scholar]
- 58.Gupte T.E., Naik S.R. Optimisation of nutritional requirements and process control parameters for the production of HA-2-91, a new tetraene polyene antibiotic. 1998. [PubMed]
- 59.Syed D.G., Lee J.C., Li W.J., Kim C.J., Agasar D. Production, characterization and application of keratinase from Streptomyces gulbargensis. Bioresour. Technol. 2009;100:1868–1871. doi: 10.1016/j.biortech.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 60.Jonsbu E., McIntyre M., Nielsen J. The influence of carbon source and morphology on nystatin production by Streptomyces noursei. J. Biotechnol. 2002;95:133–144. doi: 10.1016/s0168-1656(02)00003-2. [DOI] [PubMed] [Google Scholar]
- 61.Suutari M., Lignell U., Hyvarinen A., Nevalainen A. Media for cultivation of indoor Streptomycetes. J. Microbiol. Methods. 2002;51:411–416. doi: 10.1016/s0167-7012(02)00100-8. [DOI] [PubMed] [Google Scholar]
- 62.Gill P.K., Sharma A.D., Harchand R.K., Singh P. Effect of media supplements and culture conditions on inulinase production by an actinomycete strain. Bioresour. Technol. 2003;87:359–362. doi: 10.1016/s0960-8524(02)00262-6. [DOI] [PubMed] [Google Scholar]
- 63.Tuncer M., Kuru A., Isikli M., Sahin N., Celenk F.G. Optimization of extracellular endoxylanase, endoglucanase and peroxidase production by Streptomyces sp. F2621 isolated in Turkey. J. Appl. Microbiol. 2004;97:783–791. doi: 10.1111/j.1365-2672.2004.02361.x. [DOI] [PubMed] [Google Scholar]
- 64.Lazim H., Mankai H., Slama N., Barkallah I., Limam F. Production and optimization of thermophilic alkaline protease in solid-state fermentation by Streptomyces sp. CN902. J. Ind. Microbiol. Biotechnol. 2009;36:531–537. doi: 10.1007/s10295-008-0523-6. [DOI] [PubMed] [Google Scholar]
- 65.Himabindu M., Jetty A. Optimization of nutritional requirements for gentamicin production by Micromonospora echinospora. Indian J. Exp. Biol. 2006;44:842–848. [PubMed] [Google Scholar]