Abstract
Background:
Antibiotics play an important role in the treatment of infections to the hu-mans and at the same time, irrational, frequent prescription of higher antibiotics, change in gene com-position of microorganisms are all the reasons behind the development and introduction of new anti-biotics against different microorganisms.
Objective:
In this project, an attempt has been made to synthesize some derivatives of diazenyl con-taining phenyl styryl ketones and also their in vitro screening was conducted against Mycobacterium tuberculosis, Escherichia coli, Klebsiella pneumonia, Bacillus subtilis, Staphylococcus aureus, As-pergillus niger and Candida albicans.
Methods:
Ten molecules were synthesized which are diazenyl containing chalcones. 4-aminoacetophenone was diazotised and piperidine was coupled with the formed diazonium chloride. Further, the acetoxy group underwent Claisen-Schmidt condensation with differently substituted al-dehydes to form the final compounds- the chalcones. The proposed chemical structures were con-firmed by different spectroscopic techniques like FTIR, 1H NMR and Mass spectroscopy. TLC was used to know that the reactants were exhausted and the formation of the product occurred. Sharp melting point of the compounds concludes the purity.
Results:
The MIC of the compounds 3CP, 3DP, 3EP and 3GP is 20 times the MIC of the standard fluconazole drug against Aspergillus niger. The compound 3GP is as equipotent as the standard drug Pyrazinamide with MIC of 3.12 µg/ml against Mycobacterium tuberculosis.
Conclusion:
The results are quite promising which on further studies may lead to drug molecules against different microorganisms. Especially, 3EP can be considered as a broad spectrum agent due to its potent activity against different microorganisms like Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia and Candida albicans
Keywords: Diazenyl styryl chalcones, antimicrobial, antifungal, piperidine, claisen-schmidt, pyrazinamide
1. INTRODUCTION
Antimicrobial drugs which comprise of antibiotics, antifungal, antiprotozoals, and antivirals have a major threat of drug resistance, which is the reason for the need for new drugs in this class. Multidrug Resistance (MDR) strains are mostly reported in Mycobacterium tuberculosis worldwide. MDR is also reported and needs attention in the treatment of fungal infections. Over 300 million people suffer from serious fungal-related diseases, which kill over 1.6 million people annually [1]. Oropharyngeal candidiasis is the most frequent fungal infection in patients with HIV/AIDS [2, 3]. Fluconazole is the first agent to treat the candidial fungal infections [4]. The use of antineoplastic, immunosuppressive agents, broad-spectrum antibiotics and more aggressive surgery are the few important remedies for fungal infections [5]. α,β unsaturated ketones containing acyclic or aromatic rings are known as chalcones (phenyl styryl ketones), which have varied biological activities and are easy to synthesize. Top selling drugs with piperidine heterocyclic compounds are Tiagabine, Solifenacin and Carmegliptine [6]. Diazenyl Schiff's bases seem to have antimicrobial, cytotoxic activity on human colorectal carcinoma cell line (HCT-116) [7]. The azo oxychinolin compounds attached with isoxazolyl, pyrazolone and 2-carboxy phenyl chemical entities showed significant biological activities like antimicrobial, analgesic, wound healing and antioxidant [8].
The azo linkage (N=N) is successfully used to link two bioactive moieties to enhance the therapeutic effects. The hybrid derivatives linked by azo linkage have shown their activity by acting on target proteins in microorganisms, cell wall inhibitors, DHPS inhibitors, RNA Editing Ligase 1 inhibitors, the general protein secretory (Sec) pathway inhibitors, neuraminidase inhibitors etc. [9]. Various amides of 2-amino-5-(4-methylphenyl)-diazenyl-4-phenyl-1, 3-thiazole were synthesized and were active at a concentration of 1 μg/ mL against bacterial and fungal microorganism [10]. Novel (phenyl-diazenyl)phenols showed the highest activity against S. aureus and C. albicans, with remarkable MIC values of 10 µg/mL and 3µg/mL, respectively. The effect of different substitutions on the phenyl ring of the azobenzene on antimicrobial activity was noted [11]. Antipyrin analogues of azo molecules may be suggested for new establishment chemical class of antimicrobial agents and to create an opportunity in new drug discovery and medicinal research [12]. Benzostyrene incorporated Phenyl Styryl Ketone derivatives can act as COX-2 inhibitors with Anti-Inflammatory activity [13]. 5.9 Some novel (phenyl-diazenyl)phenols were selectively active against gram-positive bacteria and the highest activity against S. aureus and Listeria monocytogenes was noticed which reached remarkable MIC values of 4 µg/mL and 8 µg/mL [14]. Antimicrobial activity was noticed in synthetic quinazoline derivative with substituted azo salicylaldehyde [15]. A number of azo compounds were synthesized via diazotization of primary aromatic amine and subsequent coupling with naphthols or other coupling partners and antimicrobial activity was reported [16]. A series of novel tetrazole substituted piperidine derivatives were synthesized and evaluated for their antimicrobial activity using serial dilution method [17]. Inhibition of H+-ATPase-mediated proton pumping was observed in Cryptococcus by a novel conjugated styryl ketone [18]. Azosulfonamide reported to possess antimicrobial activities [19]. Vasodilation properties of Indanone-based chalcones were reported [20]. Structurally simple synthetic 1, 4- disubstituted piperidines with high selectivity for resistant Plasmodium falciparum were notified [21]. Structure modification of an active Azo-Compound as a route to new antimicrobial compounds was done [22]. Synthesis of new piperidine and cyclohexylamino-spiro derivatives acted as potential anticalcium agents [23]. A new series of 3-[phenyldiazenyl] benzaldehyde N-phenylthiosemicarbazones exhibited considerable inhibition against the bacteria and fungi tested [24]. A few conventional methods for the synthesis of chalcones are Claisen-Schmidt condensation, Suzuki reaction and Heck reaction. In Microwave irradiated synthesis of chalcones, the heterogeneous catalysts that have been used for the synthesis of chalcones and their analogous under microwave irradiation are Potassium carbonate, Barium hydroxide, p-Toluenesulphonic acid, KF-Al2O3, Zirconium tetrachloride, Piperidine and Aqueous alkali. Heterogeneous catalysts have been successfully used for the synthesis of chalcones and their analogues under ultrasound irradiation and the heterogeneous catalysts include Potassium carbonate, Basic Al2O3, Amino grafted Zeolite, Ba(OH)2, Pulverized KOH, KF-Al2O3 [25]. From the above literature, we decided to synthesize molecules containing diazenyl styryl moiety along with piperidine heterocyclic structure, conforming the structures of the synthesized compounds by various spectral analysis and screen these molecules for in vitro antimicrobial and antitubercular activities. The different microbes used in the study are Mycobacterium tuberculosis, Escherichia coli, Klebsiella pneumonia, Bacillus subtilis, Staphylococcus aureus, Aspergillus niger and Candida albicans.
2. MATERIALs AND METHODS
2.1. Synthesis of Diazenyl Styryl Ketone Derivatives
In the present work, chalcones with two aromatic rings were synthesized, out of which one of the aromatic rings was linked to a diazenyl moiety and other contained different substituents such as chloro, nitro, etc. at ortho or meta or para positions. The synthesized compounds were screened for anti-tubercular activity, anti-bacterial activity and anti-fungal activity by in vitro methods. Characterization of the synthesized molecules was done by using a physical technique like melting point determination by the open capillary method, TLC and spectral methods including FTIR, NMR and Mass.
The different instruments used in this project include hot air oven (Vision lab equipments), Magnetic stirrers (Vision lab equipments), Weighing Balance (Contech, 0.1mg presion), FTIR Spectrophotometer (BRUKER Alpha Transmission mode), UV Cabinet (Vision lab equipments), NMR (BRUKER) and Mass spectroscopy (Shimadzu lab solutions).
2.1.1. Scheme for the Proposed Synthesis of Compounds
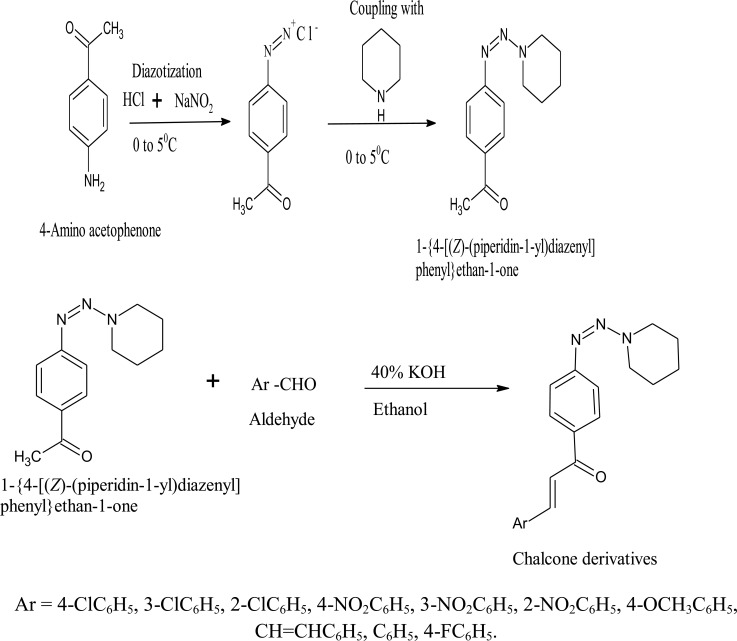
The scheme for the synthesis of compounds is outlined in the Fig. (1).
Fig. (1).
Scheme for the proposed synthesis of compounds.
2.1.2. Procedure
2.1.2.1. Diazotization and Coupling of Amine Group in 4-amino Acetophenone with Piperidine
0.02 mol of 4-amino acetophenone (2.7 g) was dissolved in a mixture of 6 ml of conc HCl and 12 ml of water and 0.02 mol of NaNO2 (2.7 g) was dissolved in 6 ml of water in another beaker. Then both the solutions were made to cool at 0-5°C in an ice bath. Now the solution of NaNO2 was added dropwise to 4-amino acetophenone solution with continuous stirring by adding a few pieces of ice to avoid the rise in temperature during the reaction. After the addition of NaNO2 was completed, piperidine was added to the reaction mixture with continuous stirring and maintaining the same temperature i.e., 0-5 °C till a yellow coloured precipitate was obtained. The obtained precipitate was filtered, washed with ice-cold water and dried [26-28].
2.1.2.2. Preparation of Chalcones
In a round-bottomed flask containing 50 ml of ethanol, 0.005 mol of diazotized 4-aminoacetophenone (1.15g) was added, coupled with piperidine and an equimolar amount of substituted benzaldehydes (0.70g). Then 1 ml of 40% ethanolic KOH was added at room temperature with continuous stirring on a magnetic stirrer for 4 hrs. In between, the reaction was monitored by TLC. After confirming the completion of the reaction, the reaction mixture was poured into a beaker containing finely crushed ice with a little amount of dilute HCl in order to neutralize KOH. Then the solid was filtered and washed with cold water, dried and recrystallized from hot ethanol [29-32].
3. IN VITRO ANTIMICROBIAL ACTIVITIES
3.1. Anti-Tubercular Activity (Alamar Blue Dye)
The Microplate Alamar blue assay (MABA) method was used to evaluate the anti-tubercular activity of synthesized compounds against mycobacterial strain Mycobacterium tuberculosis H37Rv ATCC NO- 27294. The medium used for this evaluation was 7H9 media. The antimycobacterial activity of compounds was assessed against Mycobacterium tuberculosis using Microplate Alamar Blue Assay (MABA). This methodology is non-toxic, uses a thermally stable reagent and shows good correlation with proportional and BACTEC radiometric method. Briefly, 200µ1 of sterile deionized water was added to all outer perimeter wells of sterile 96 wells plate to minimize evaporation of medium in the test wells during incubation. The 96 wells plate received 100µ1 of the Middlebrook 7H9 broth and serial dilution of compounds was made directly on the plate. The final drug concentration tested were 100 to 0.2µg/ml. Plates were covered and sealed with parafilm and incubated at 37°C for five days. After the time, 25µl of freshly prepared 1:1 mixture of Alamar Blue reagent and 10% tween-80 was added to the plate and incubated for 24hrs. A blue colour in the well was interpreted as no bacterial growth, and pink colour was scored as growth. MIC was defined as the lowest drug concentration which prevented the colour change from pink to blue [33-36].
3.2. Anti-Microbial Activity
3.2.1. Evaluation of Anti Microbial Activity
Serial dilution method was used to find the MIC of the compounds. Microorganisms used are Escherichia coli ATCC No: 25922, Klebsiella pneumonia ATCC No: 29665, Bacillus subtilis ATCC No: 6051, Staphylococcus aureus ATCC No: 12598, Aspergillus niger ATCC No: 9029, Candida albicans ATCC No. 2091.
3.2.2. MIC Procedure
Nine dilutions of each drug have to be done with BHI for MIC. In the initial tube, 20µl of drug was added into the 380 µl of BHI broth. For dilutions, 200 µl of BHI broth was added into the next 9 tubes separately. Then from the initial tube, 200 µl was transferred to the first tube containing 200 µl of BHI broth. This was considered as 10-1 dilution. From 10-1 diluted tube, 200 µl was transferred to the second tube to make 10-2 dilution. The serial dilution was repeated up to 10-9 dilution for each drug. From the maintained stock cultures of required organisms, 5 µl was taken and added into 2ml of BHI (brain heart infusion) broth. In each serially diluted tube, 200 µl of above culture suspension was added. The tubes were incubated for 24 hours and observed for turbidity. For facultative anaerobes, tubes were incubated at 37°C for 48-72 hours in CO2 Jar. For strict anaerobes, tubes were incubated in anaerobic Jars for 48-72 hours [37, 38].
4. RESULTS
4.1. Physical-Chemical Characterization of the Compounds
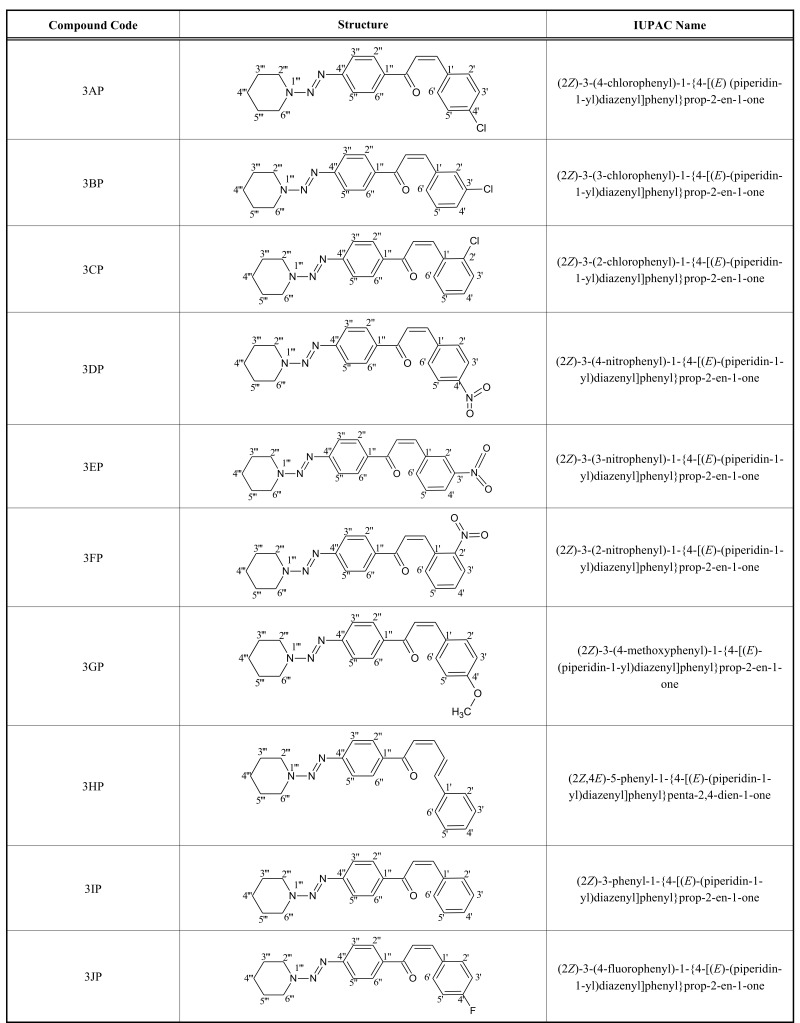
Structures along with their IUPAC names and Physical chemical characterization of compounds are listed in the Table 1 and 2 respectively.
Table 1.
Structure of compounds along with their IUPAC names.
4.2. Spectral Data of the Synthesized Compounds
COMPOUND 3AP: FTIR- C-H stretching (Aromatic) - 2945.52 cm-1, C-H stretching (Olefins) - 2862.54 cm-1, C=O
stretching -1664.94 cm-1, C=C stretching (Olefins) - 1603.91 cm-1, -C=C stretching (Aromatic) - 1429.92 cm-1, -C-H bending (Aromatic) -1107.37 cm-1, N=N stretching - 1489.81 cm-1,C-Cl stretching - 819.94 cm-1. 1H NMR: δ 7.526 (m) 1H COCH J=16.8 ; δ 7.722 (d) 1H CH=CH J=15.6 ; δ 8.039 (d) 2ArH “2 & “6 ; δ 7.590 (d) 2ArH '2 & '6 ; δ 7.515 (m) 2ArH “3 & “5 ; δ 7.40 (d) 2ArH '3 & '5 ; δ 3.868 (s) 4H of piperidine “'2 & “'6 (ortho position of nitrogen atom) ; δ 1.741 (s) 4H of piperidine “'3 & “'5 (meta position of nitrogen atom) ; δ 1.560 (s) 2H of piperidine “'4 (para position of nitrogen atom). Mass Spectrum (EI, m/z): Actual molecular mass = 353.84, Predicted molecular mass = 376.35 (M + Na+).
COMPOUND 3BP: FTIR- C-H stretching (Aromatic) - 2923.16 cm-1, C-H stretching (Olefins) - 2548.78 cm-1, C=O stretching -1698.41 cm-1, C=C stretching (Aromatic) -1418.00 cm-1, -C=C stretching (Olefins) - 1574.64 cm-1, -C-H bending (Aromatic) - 898.00 cm-1, N=N stretching -1574.64 cm-1, C-Cl stretching -750.11 cm-1. 1H NMR: Sample was not sufficient. Mass Spectrum (EI, m/z): Actual molecular mass = 353.84, Predicted molecular mass = 353.
COMPOUND 3CP: FTIR- C-H stretching (Aromatic) - 2934.23 cm-1, C-H stretching (Olefins) - 2855.74 cm-1, C=O stretching - 1655.17 cm-1, C=C stretching (Aromatic) - 1603.82 cm-1, -C=C stretching (Olefins) - 1328.87 cm-1, -C-H bending (Aromatic) - 837.49 cm-1, N=N stretching - 1430.60 cm-1, C-Cl stretching - 760.67 cm-1.1H NMR: δ 8.191 (d) 1H COCH J= 15.6, δ 7.526 (m) 1H CH=CH J=16.8, δ 8.045 (s) 2ArH “2 & “6 ; δ 7.546 (m) 2ArH “3 & “5 ; δ 7.517 (m) 1ArH '3 ; δ 7.462 (m) 1ArH '6 ; δ 7.348 (m) 1ArH '5 ; δ 7.324 (m) 1ArH '4 ; δ 3.867 (s) 4H of piperidine “'2 & “'6 (ortho position of nitrogen atom) ; δ 1.739 (s) 4H of piperidine “'3 & “'5 (meta position of nitrogen atom) ; δ 1.570 (s) 2H of piperidine “'4 (para position of nitrogen atom). Mass Spectrum (EI, m/z): Actual molecular mass = 353.84 Predicted molecular mass = 353.
COMPOUND 3DP: FTIR-C-H stretching (Aromatic) - 2924.84cm-1, C-H stretching (Olefins) - 2855.28 cm-1, C=O stretching -1652.90 cm-1, C=C stretching (Aromatic) - 1596.26 cm-1, -C=Cstretching (Olefins) - 1427.35 cm-1, -C-H bending (Aromatic) - 851.23 cm-1, N=Nstretching - 1344.50 cm-1, N=O (Nitro) - 1516.73 cm-1. 1H NMR: δ 7.703 (d) 1H COCH J=15.6 ; δ 7.848 (m) 1H CH=CH J=16.0 ; δ 8.295 (d) 2ArH “2 & “6 ; δ 8.077 (d) 2ArH '2 & '6 ; δ 7.578 (d) 2ArH “3 & “5 ; 2ArH of '3 & '5 were not detected in NMR spectra ; δ 3.902 (s) 4H of piperidine “'2 & “'6 (ortho position of nitrogen atom) ; remaining 6H of piperidine (“'3, “'4 and “'5) were not detected in NMR spectra. Mass Spectrum (EI, m/z): Actual molecular mass = 364.32, Predicted molecular mass = 383.
COMPOUND 3EP: FTIR- C-H stretching (Aromatic) - 2924.84 cm-1, C-H stretching (Olefins) - 2855.28 cm-1, C=O stretching - 1652.90 cm-1, C=C stretching (Aromatic) - 1596.26 cm-1, -C=C stretching (Olefins) - 1427.35 cm-1, -C-H bending (Aromatic) - 851.23 cm-1, N=N stretching - 1344.50 cm-1, N=O (Nitro) - 1516.73 cm-1. 1H NMR : δ 7.722 (d) 1H COCH J=15.6 ; δ 7.854 (d) 1H CH=CH J=16.0 ; δ 8.528 (s) 2ArH “2 & “6 ; δ 7.562 (d) 2ArH “3 & “5 ; δ 8.258 (d) 1H '2 ; δ 8.076 (d) 1ArH '4 ; δ 8.072 (d) 1H '6 ; δ 7.634 (d) 1H '5 ; δ 3.884 (s) 4H of piperidine “'2 & “'6 (ortho position of nitrogen atom) ; δ 1.560 (s) 4H of piperidine “'3 & “'5 (meta position of nitrogen atom) ; δ 1.744 (s) 2H of piperidine “'4 (para position of nitrogen atom). Mass Spectrum (EI, m/z): Actual molecular mass = 364.32, Predicted molecular mass = 365 (M + H+).
COMPOUND 3FP: FTIR-C-H stretching (Aromatic) - 2936.06 cm-1, C-H stretching (Olefins - 2855.95 cm-1, C=O stretching - 1656.44 cm-1, C=C stretching (Aromatic) - 1603.89 cm-1, -C=C stretching (Olefins) - 1427.38 cm-1, -C-H bending (Aromatic) - 838.88 cm-1, N=N stretching - 1521.77 cm-1, N=O (Nitro) - 1344.69 cm-1. 1H NMR: δ 7.403 (d) 1H COCH J=15.6 ; δ 8.161 (d) 1H CH=CH J=15.6 ; δ 8.050 (m) 2ArH “2 & “6 ; δ 7.530 (m) 2ArH “3 & “5 ; δ 8.050 (m) 1H '4 ; δ 7.771 (m) 1ArH '3 ; δ 7.701 (m) 1H '6 ; δ 7.558 (m) 1H '5 ; δ 3.867 (s) 4H of piperidine “'2 & “'6 (ortho position of nitrogen atom) ; δ 1.560 (s) 4H of piperidine “'3 & “'5 (meta position of nitrogen atom) ; δ 1.744 (s) 2H of piperidine “'4 (para position of nitrogen atom). Mass Spectrum (EI, m/z):
COMPOUND 3GP: FTIR-C-H stretching (Aromatic) - 2943.42 cm-1, C-H stretching (Olefins) - 2855.70 cm-1, C=O stretching - 1678.04 cm-1, C=C stretching (Aromatic) - 1596.77 cm-1, -C=C stretching (Olefins) - 1432.48 cm-1, -C-H bending (Aromatic) - 850.06 cm-1, N=N stretching - 1512.85 cm-1, C-O-C stretching - 1105.73 cm-1, CH3 bending – 1355.29 cm-1. 1H NMR: δ 7.503 (m) 1H COCH J=14.0 ; δ 7.808 (d) 1H CH=CH J=15.6 ; δ 7.949 (d) 2ArH “2 & “6 ; δ 7.622 (d) 2ArH '2 & '6 ; δ 7.509 (m) 2ArH “3 & “5 ; δ 6.949 (d) 2ArH '3 & '5 ; δ 2.58 (s) 3H '4OCH3 ; δ 3.858 (s) 4H of piperidine “'2 & “'6 (ortho position of nitrogen atom) ; δ 1.731 (d) 4H of piperidine “'3 & “'5 (meta position of nitrogen atom) ; δ 1.610 (d) 2H of piperidine “'4 (para position of nitrogen atom). Mass Spectrum (EI, m/z): Actual molecular mass = 349. Predicted molecular mass = 350 (M + H+).
COMPOUND 3HP: FTIR- C-H stretching (Aromatic) - 2933.48 cm-1, C-H stretching (Olefins) - 2854.53 cm-1, C=O stretching - 1647.08 cm-1, C=C stretching (Alkenes) 1625 cm-1, C=C stretching (Aromatic) - 1429.42 cm-1, -C=C stretching (Olefins) - 1350.97 cm-1, -C-C stretching (Alkane) - 826.71 cm-1, N=N stretching - 1576.00 cm-1. 1H NMR: δ 7.356 (m) 1H COCH J=13.6 ; δ 7.166 (d) 1H COCH=CH J=14.8 ; δ 6.980 (m) 1H COCH=CH-CH=CH ; δ 7.03 (m) 1H COCH=CH-CH=CH ; δ 7.985 (d) 2ArH “2 & “6, δ 7.522 (d) 2ArH '2 & '6 ; δ 7.512 (d) 2ArH “3 & “5 ; δ 7.375 (m) 2ArH '3 & '5 ; δ 7.078 (d) 1ArH '6 ; δ 3.861 (s) 4H of piperidine “'2 & “'6 (ortho position of nitrogen atom) ; δ 1.571 (s) 4H of piperidine “'3 & “'5 (meta position of nitrogen atom) ; δ 1.736 (s) 2H of piperidine “'4(para position of nitrogen atom). Mass Spectrum (EI, m/z): Actual molecular mass = 345 Predicted molecular mass = 346 (M+H+).
COMPOUND 3IP: FTIR- C-H stretching (Aromatic) - 2942.00 cm-1, C-H stretching (Olefins) - 2857.15 cm-1, C=O stretching - 1651.08 cm-1, C=C stretching (Aromatic) - 1600.94 cm-1, -C=C stretching (Olefins) - 1338.19 cm-1, -C-H bending (Aromatic) - 841.30 cm-1, N=N stretching - 1404.55 cm-1. 1H NMR: δ 7.602 (s) 1H COCH J=18.0 ; δ 7.834 (d) 1H CH=CH J=15.60 ; δ 8.054 (d) 2ArH “2 & “6 ; δ 7.656 (d) 2ArH '2 & '6, δ 7.539 (d) 2ArH “3 & “5 ; δ 7.419 (d) 2ArH '3 & '5 ; δ 7.411 (d) 1ArH '6 δ 3.865 (s) 4H of piperidine “'2 & “'6 (ortho position of nitrogen atom) ; δ 1.596 (s) 4H of piperidine “'3 & “'5 (meta position of nitrogen atom) ; δ 1.737 (s) 2H of piperidine “'4 (para position of nitrogen atom). Mass Spectrum (EI, m/z): Actual molecular mass = 319. Predicted molecular mass = 320 (M+ H+).
COMPOUND 3JP: FTIR- C-H stretching (Aromatic) - 2925.54 cm-1, C-H stretching (Olefins) - 2856.60 cm-1, C=O stretching - 1655.98 cm-1, C=C stretching (Olefins) - 1595.56 cm-1, -C=C stretching (Aromatic) - 1429.04 cm-1, -C-H bending (Aromatic) - 825.02 cm-1, N=N stretching - 1508.53 cm-1, C-F stretching - 1331.59 cm-1. 1H NMR: δ 7.132 (m) 1H COCH J=17.2 ; δ 7.795 (d) 1H CH=CH J=15.6 ; δ 8.044 (d) 2ArH “2 & “6 ; δ 7.661 (m) 2ArH '2 & '6 ; δ 7.538 (m) 2ArH “3 & “5 ; δ 7.111 (m) 2ArH '3 & '5 ; δ 3.869 (s) 4H of piperidine “'2 & “'6 (ortho position of nitrogen atom) ; δ 1.742 (s) 4H of piperidine “'3 & “'5 (meta position of nitrogen atom) ; δ 1.558 (s) 2H of piperidine “'4 (para position of nitrogen atom). Mass Spectrum (EI, m/z): Actual molecular mass = 337, Predicted molecular mass = 338 (M+H+).
4.3. In Vitro Biological Activity Studies
4.3.1. In Vitro Anti-tubercular Activity Studies
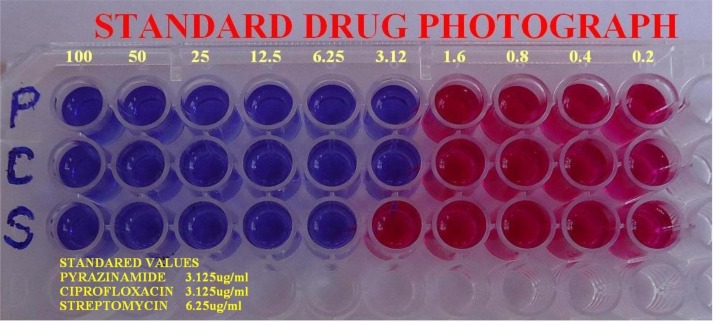
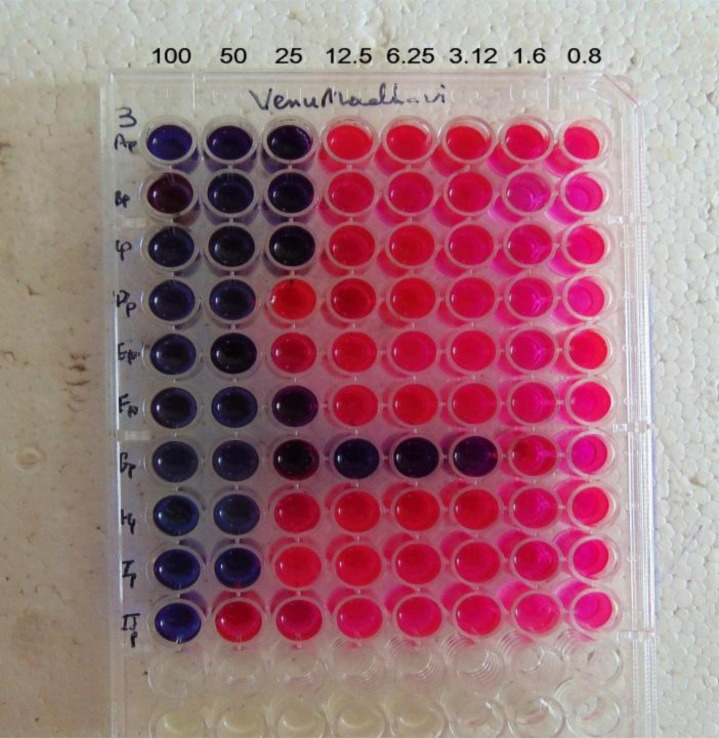
The antitubercular activity of the synthesized compounds and standard drugs in terms of their MIC are tabulated in Table 3. Fig. (2) depicts the image of Anti-Tubercular MIC results for Standard drugs- Pyrazinamide, Ciprofloxacin, Streptomycin. Fig. (3) depicts the image of Anti-Tubercular MIC results for the synthesized compounds.
Table 3.
MIC against Mycobacterium tuberculosis for the synthesized chalcones (3AP-3JP).
| S.No. | Compound Code | 100 µg/ml | 50 µg/ml | 25 µg/ml | 12.5 µg/ml | 6.25 µg/ml | 3.12 µg/ml | 1.6 µg/ml | 0.8 µg/ml |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3AP | S | S | S | R | R | R | R | R |
| 2 | 3BP | S | S | S | R | R | R | R | R |
| 3 | 3CP | S | S | S | R | R | R | R | R |
| 4 | 3DP | S | S | R | R | R | R | R | R |
| 5 | 3EP | S | S | R | R | R | R | R | R |
| 6 | 3FP | S | S | S | R | R | R | R | R |
| 7 | 3GP | S | S | S | S | S | S | R | R |
| 8 | 3HP | S | S | R | R | R | R | R | R |
| 9 | 3IP | S | S | R | R | R | R | R | R |
| 10 | 3JP | S | R | R | R | R | R | R | R |
| 11 | Pyrazinamide | S | S | S | S | S | R | R | R |
| 12 | Streptomycin | S | S | S | S | R | R | R | R |
| 13 | Ciprofloxacin | S | S | S | S | S | R | R | R |
S = Sensitive; R = Resistant; Strain used: M. tuberculosis (H37 RV strain): ATCC No: 27294.
Fig. (2).
Anti-TB microplate alamar blue assay for standard. Image showing anti-tubercular results for standard drugs- pyrazinamide, ciprofloxacin, streptomycin.
Fig. (3).
Anti-TB Microplate Alamar Blue assay for piperidine linked substituted benzaldehyde chalcone derivatives (3AP-3JP).
All the compounds are active against Mycobacterium tuberculosis at a concentration of 100 µg/ml. Out of ten compounds, half of them are active at 25 µg/ml concentration, The compound 3GP is the most potent one and its MIC value is equal to the standard drug Pyrazinamide and Ciprofloxacin (MIC-3.12 µg/ml). The compound 3GP is also twice as potent as the standard drug-Streptomycin (MIC-6.25 µg/ml). The difference in the potency of compound 3GP may be attributed to the structural differences when compared with other less active compounds.
4.3.2. Anti-microbial Studies
The synthesized chalcone derivatives (3AP-3JP) were tested for their anti-microbial activity. The MIC results of standard drugs and synthesized compounds are shown in Table 4.
Table 4.
Results of Anti-microbial activity of synthesized compounds.
| E. coli | 100 µg/ml | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.6 | 0.8 | 0.4 | 0.2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 3AP | S | S | R | R | R | R | R | R | R | R |
| 3BP | S | S | S | R | R | R | R | R | R | R |
| 3CP | S | S | S | R | R | R | R | R | R | R |
| 3DP | S | S | S | R | R | R | R | R | R | R |
| 3EP | S | S | S | S | S | R | R | R | R | R |
| 3FP | S | S | R | R | R | R | R | R | R | R |
| 3GP | S | S | S | R | R | R | R | R | R | R |
| 3HP | S | S | S | R | R | R | R | R | R | R |
| 3IP | S | S | R | R | R | R | R | R | R | R |
| 3JP | S | S | R | R | R | R | R | R | R | R |
| E. coli | 100 µg/ml | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.6 | 0.8 | 0.4 | 0.2 |
| Klebsiella | - | - | - | - | - | - | - | - | - | - |
| 3AP | S | R | R | R | R | R | R | R | R | R |
| 3BP | S | R | R | R | R | R | R | R | R | R |
| 3CP | S | S | R | R | R | R | R | R | R | R |
| 3DP | S | R | R | R | R | R | R | R | R | R |
| 3EP | S | S | S | S | R | R | R | R | R | R |
| 3FP | S | S | S | R | R | R | R | R | R | R |
| 3GP | S | S | R | R | R | R | R | R | R | R |
| 3HP | S | S | S | R | R | R | R | R | R | R |
| 3IP | S | S | R | R | R | R | R | R | R | R |
| 3JP | S | S | R | R | R | R | R | R | R | R |
| Staph | - | - | - | - | - | - | - | - | - | - |
| 3AP | S | S | S | R | R | R | R | R | R | R |
| 3BP | S | S | R | R | R | R | R | R | R | R |
| 3CP | S | S | R | R | R | R | R | R | R | R |
| 3DP | S | S | S | R | R | R | R | R | R | R |
| 3EP | S | S | S | S | R | R | R | R | R | R |
| 3FP | S | R | R | R | R | R | R | R | R | R |
| 3GP | S | R | R | R | R | R | R | R | R | R |
| 3HP | S | S | R | R | R | R | R | R | R | R |
| 3IP | S | S | R | R | R | R | R | R | R | R |
| 3JP | S | S | R | R | R | R | R | R | R | R |
| Bacillus | - | - | - | - | - | - | - | - | - | - |
| 3AP | S | S | R | R | R | R | R | R | R | R |
| 3BP | S | S | S | R | R | R | R | R | R | R |
| 3CP | S | S | R | R | R | R | R | R | R | R |
| 3DP | S | S | R | R | R | R | R | R | R | R |
| 3EP | S | S | S | R | R | R | R | R | R | R |
| 3FP | S | S | S | S | S | R | R | R | R | R |
| 3GP | S | S | R | R | R | R | R | R | R | R |
| 3HP | S | S | S | R | R | R | R | R | R | R |
| 3IP | S | S | R | R | R | R | R | R | R | R |
| 3JP | S | S | R | R | R | R | R | R | R | R |
| A.niger | - | - | - | - | - | - | - | - | - | - |
| 3AP | S | S | R | R | R | R | R | R | R | R |
| E. coli | 100 µg/ml | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.6 | 0.8 | 0.4 | 0.2 |
| 3BP | S | S | S | S | S | S | S | S | R | R |
| 3CP | S | S | S | S | S | S | S | S | S | R |
| 3DP | S | S | S | S | S | S | S | S | S | R |
| 3EP | S | S | S | S | S | S | S | S | S | R |
| 3FP | S | S | S | S | S | S | S | S | R | R |
| 3GP | S | S | S | S | S | S | S | S | S | R |
| 3HP | S | S | S | S | S | S | S | S | R | R |
| 3IP | S | S | S | S | S | S | S | S | R | R |
| 3JP | S | S | S | S | S | S | S | S | R | R |
| Candida | - | - | - | - | - | - | - | - | - | - |
| 3AP | S | S | R | R | R | R | R | R | R | R |
| 3BP | S | S | S | S | S | S | R | R | R | R |
| 3CP | S | S | S | R | R | R | R | R | R | R |
| 3DP | S | S | S | R | R | R | R | R | R | R |
| 3EP | S | S | R | R | R | R | R | R | R | R |
| 3FP | S | S | R | R | R | R | R | R | R | R |
| 3GP | S | S | R | R | R | R | R | R | R | R |
| 3HP | S | S | S | R | R | R | R | R | R | R |
| 3IP | S | S | R | R | R | R | R | R | R | R |
| 3JP | S | S | R | R | R | R | R | R | R | R |
S-Sensitive; R-Resistant; Fluconazole standard against C.albicans- (ATCC No: 2091) MIC 16µg/ml, A.niger - (ATCC No: 9029) MIC 8µg/ml.
The synthesized compounds were screened against two gram-positive (Staphylococcus aureus and Bacillus subtilis and two gram-negative microorganism (E.coli and Klebsiella). All the synthesized compounds were active against E.coli at 50 µg/ml and a few of them were active at MIC value of 25 µg/ml. The compound 3EP was the most potent of all the synthesized compounds (MIC of 6.25 µg/ml) against E.coli and one-third potency of standard drug-Ciprofloxacin (MIC 2 µg/ml). The same compound was also active against Klebsiella but with half the potency seen against E.coli and MIC value of 12.5µg/ml. At MIC of 100 µg/ml, all the compounds were active against Klebsiella. Compound 3EP was also active against Staphylococcus aureus with MIC value of 12.5 µg/ml which is six times less potent than Ciprofloxacin standard (MIC 2 µg/ml) and all the compounds are active at 100 µg/ml of MIC. Least potency is noticed against Bacillus subtilis with MIC of 25 µg/ml. On the whole, the compounds are more active against gram-negative microorganisms than the antimicrobial activity against gram-positive microorganisms.
Ciprofloxacin standard against S.aureus- (ATCC No: 12598) MIC 2µg/ml, Bacillus sps - (ATCC No: 6051) MIC 2 µg/ml), E.coli -(ATCC No: 25922) MIC 2µg/ml), K.Pneumoniae -(ATCC No: 29665) MIC 1µg/ml.
Two fungal microorganisms, namely A. niger and C. albicans were chosen to check the antifungal activity of the synthesized compounds. Four compounds of MIC 0.4 µg/ml (3CP, 3DP, 3EP and 3GP), were twenty times more active than the standard antifungal drug-Flucanazole (MIC 8 µg/ml) against A. niger. Except for 3AP, remaining nine compounds were also ten times more potent than the standard drug. The MIC of the least potent drug against A. niger is 50 µg/ml. The compounds were less active against C. albicans in comparison to A. niger. The most active compound against C. albicans was 3BP with MIC of 6.25 µg/ml and was 2.5 times more active than the standard drug Flucanazole (MIC 16 µg/ml). All the compounds were active at MIC of 50 µg/ml.
5. DISCUSSION
All the synthesized compounds are in accordance with the proposed scheme which has been confirmed from the spectral data. The trans type of isomers confirmed by the coupling constant(J) value was greater than 12Hz. The mass spectral and the proton NMR spectral data have lent sufficient information to conclude the exact structures.
The synthesized compounds have some structural similarity with that of the fluoroquinolone class of antimicrobial agent. Structural activity relationship (SAR) of fluoroquinolones reveals that modification at N-1, C-6, C-7 and C-8 lead to more successful antimicrobial agents [39].
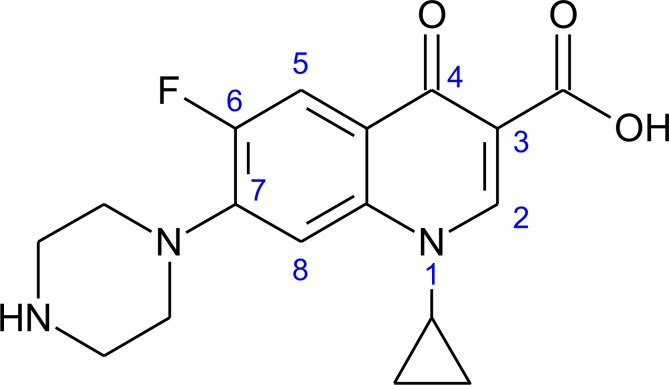
The structure of Ciprofloxacin is shown in Fig. (4).
Fig. (4).
Structure of ciprofloxacin.
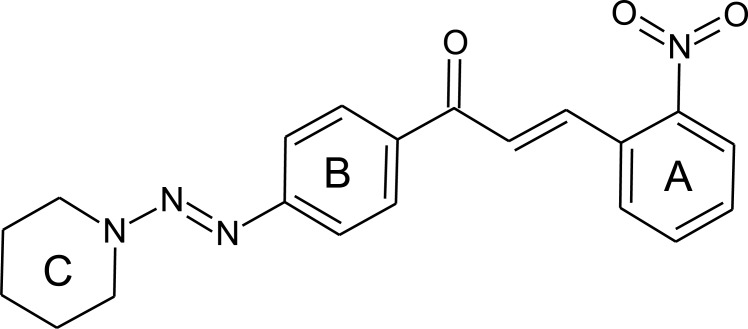
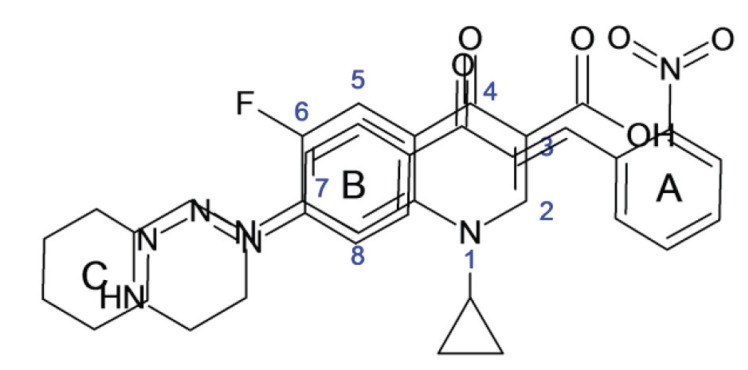
The Fig. (5) represents the chemical structure of compound 3FP and the Fig. (6) is the overlap of structures of ciprofloxacin and compound 3FP.
Fig. (5).
Structure of compound 3FP.
Fig. (6).
Overlapped structures Ciprofloxacin and Compound 3FP.
The structure of compound 3FP, when stacked over the structure of Ciprofloxacin, seems to be an overlap of the pharmacophore of ciprofloxacin which binds to the DNA gyrase enzyme of the microorganism. The carbonyl and carboxylate groups of ciprofloxacin bind with the DNA by hydrogen bonding and similar binding can occur with the carbonyl and nitro group of ring A substituent in 3FP compound with the DNA. The fluoro substituent, the substituent at C-7 and the carboxylate ion of Ciprofloxacin are involved in binding interactions with the enzyme whereas, the piperidine (ring C) substituent and the diazenyl nitrogen of 3FP compound may be involved in enzyme binding [40]. Thus, we can conclude that the reported compounds with nitro group have better activity (mainly 2-nitro and 3-nitro and to some extent 4-nitro substituent on A ring) as they can bind better with the DNA, while other compounds have shown less activity because they lack group to form hydrogen bonding with the DNA unlike the nitro group of 3FP compound which form the hydrogen bonding.
CONCLUSION
The synthesized compounds with the nitro substituent are having better activity when compared with the other substituted compounds. The compound 3EP was found to have broad-spectrum activity i.e. active against Escherichia coli, Klebsiella pneumonia, Bacillus subtilis, Staphylococcus aureus, and Aspergillus niger. The compound 3GP is most active against Mycobacterium tuberculosis. The synthesized compounds have structural similarity with that of quinolone pharmacophore and hence DNA gyrase may be the target by which the compounds are eliciting antimicrobial activity.
Table 2.
Physico-chemical data of synthesized Chalcone derivatives (3AP-3JP).
| Compound Code | Mol. Formula | Mol.wt | Melting Point (°C) | Color | Rf | Solubility | Yield (% w/w) |
|---|---|---|---|---|---|---|---|
| 3AP | C20H20ClN3O | 353.84 | 137-140 °C | Pale orange, amorphous | 0.24* | Soluble in petroleum ether, Chloroform, DMSO, DMF | 45.58% |
| 3BP | C20H20ClN3O | 353.84 | 115-120 °C | Light brown, amorphous | 0.51* | 55.65% | |
| 3CP | C20H20ClN3O | 353.84 | 120-125 °C | Light brown, crystals | 0.41* | 42.85% | |
| 3DP | C20H20N4O3 | 364.32 | 142-145 °C | Lemon yellow, amorphous | 0.30* | 69.44% | |
| 3EP | C20H20N4O3 | 364.32 | 130-135 °C | Pale yellow, amorphous | 0.31* | 66.66% | |
| 3FP | C20H20N4O3 | 364.32 | 126-132 °C | Red, amorphous | 0.33* | 22.39% | |
| 3GP | C21H23N3O2 | 349.00 | 130-134 °C | Yellow, amorphous | 0.26** | 56.65% | |
| 3HP | C22H23N3O | 345.00 | 112-116 °C | Lemon yellowish, crystals | 0.35* | 48.53% | |
| 3IP | C20H21N3O | 319.00 | 83-88 °C | Light brown, crystals | 0.39** | 48.42% | |
| 3JP | C20H20FN3O | 337.00 | 123-126 °C | Lemon yellow, crystals | 0.21** | 63.36% |
Solvent front used for TLC are * = n-Hexane: Ethyl acetate (6:4) * * = n-Hexane: Ethyl acetate (8:2).
Acknowledgements
This researcher did not receive any specific grant or funding from the public, commercial, or not for profit sectors.
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No Animals/Humans were used for studies that are the basis of this research.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.2017 http://go.nature.com/2sMKpuN
- 2.Barr C.E. Oral diseases in HIV-1 infection. Dysphagia. 1992;7(3):126–137. doi: 10.1007/BF02493444. [DOI] [PubMed] [Google Scholar]
- 3.Rasika S.B., Mona U.S., Yogesh S.D., Vidhi A.S., Supriya P.K. Antibacterial activity of curcumin (turtmeric) against periopathogens-An in vitro evaluation. J. Adv. Clin. Res. Insig. 2017;4:175–180. [Google Scholar]
- 4.Kaplan J.E., Benson C., Holmes K.K., Brooks J.T., Pau A., Masur H. Centres for disease control and prevention. guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. recommendations from cdc, the national institutes of health and the HIV medicine association of the infectious diseases society of america. MMWR Morb. Mortal. Wkly. Rep. 2009;58:45–48. [PubMed] [Google Scholar]
- 5.Eggimann P., Garbino J., Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 2003;3(11):685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 6.Marcus B., Ian B.R. An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem. 2013;9:2265–2319. doi: 10.3762/bjoc.9.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harmeet K., Siong M.L., Kalavathy R., Mani V., Syed A.A.S., Narasimhan B., Kaur H. Diazenyl schiff bases: Synthesis, spectral analysis, antimicrobial studies and cytotoxic activity on human colorectal carcinoma cell line (HCT-116). Arab. J. Chem. doi: 10.1016/j.arabjc.2017.05.004. In Press. [DOI] [Google Scholar]
- 8.Jyotirmaya S., Sudhir Kumar P. Study of antimicrobial, analgesic wound healing and antioxidant activities of some newly synthesized oxychinolin derivatives and their characterization. Beni-Suef Univ. J. Basic App. Sci. 2015;4:232–245. [Google Scholar]
- 9.Harmeet K., Balasubramanian N. Antimicrobial activity of diazenyl derivatives: An update. Curr. Top. Med. Chem. 2018;18:3–21. doi: 10.2174/1568026618666180206093107. [DOI] [PubMed] [Google Scholar]
- 10.Ashishkumar K.P., Vishal Pankajkumar M. Synthesis and biological activity of n-{5-(4-methylphenyl) diazenyl-4-phenyl- 1, 3-thiazol-2-yl}benzamide derivatives. Quim. Nova. 2011;34(5):771–774. [Google Scholar]
- 11.Simona C., Lucia S., Anna M.P., Amalia P., Rosita D. Pio Iannelli.; Stefano, P. Structure modification of an active azo-compound as a route to new antimicrobial compounds. Molecules. 2017;22:E875. doi: 10.3390/molecules22060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jyotirmaya S., Sudhir Kumar P. A Study on antimicrobial evaluation of newly synthesized antipyrin analogues. Indian J. Pharm. Ed. Res. 2017;4(51):740–747. [Google Scholar]
- 13.Heena S., Mehul Z., Gourav J., Neha K., Amita P. Design, synthesis, and biological evaluation of some new benzostyrene incorporated phenyl styryl ketone derivatives as COX-2 inhibitors with anti-inflammatory activity. Int. J. Pharm. Pharmaceutical Res. 2017;2(10):424–442. [Google Scholar]
- 14.Stefano P., Simona C., Lucia S., Rosita D., Gabriel T., Carlos J. Ugo.; Cand Pio, I. Synthesis and antimicrobial studies of new antibacterial azo-compounds active against staphylococcus aureus and listeria monocytogenes. Molecules. 2017;22:E1372. doi: 10.3390/molecules22081372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shweta T., Vikas M. Vasudha Sharma.; Pushplata, S.; Manjul, S. Synthesis and evaluation of schiff’s base of 4-quinazolinone analogues as antimicrobial agents. Asian J. Pharmaceutical Clin. Res. 2012;5(1):98–100. [Google Scholar]
- 16.William K., Cedric D., Reimmel K.A. Synthesis and evaluation of antimicrobial properties of azo dyes. Int. J. Pharm. Pharm. Sci. 2014;7(4):398–401. [Google Scholar]
- 17.Thangasamy E., Durairaj P.B., Gopalakrishnan M. Synthesis, Spectral Analysis, in vitro microbiological evaluation, and molecular docking studies of some novel 1-(1-Aryl-1H-tetrazol-5-yl)-2-(piperidin-1-yl)ethanone derivatives. ISRN Org. Chem. 2014;2014:1–9. doi: 10.1155/2014/120173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias K.M., Jonathan R.D., Sarvesh C.V., Chandrasekar P.H. Novel styryl ketones acted as novel fungicidal agent by inhibiting H+-ATPase-mediated proton pumping in Cryptococcus. J. Antimicrob. Chemother. 2001;47:491–494. doi: 10.1093/jac/47.4.491. [DOI] [PubMed] [Google Scholar]
- 19.Vora P.J., Mehta A.G. Synthesis, characterization and antimicrobial efficacy of quinoline based compounds. IOSR J. App. Chem. 2012;4:34–39. [Google Scholar]
- 20.Mohamad Syahir M.S., Oo Chuan W., Yam Mun F. Synthesis, characterisation and vasolidation properties of indanone-based chalcones. J. Phy. Sci. 2018;29(Suppl. 1):99–106. [Google Scholar]
- 21.Moses N.N., Grace N.A., Hermia N.I., Denis Z., Fidele N.K., Simon M.N.E. Structurally simple synthetic 1, 4-disubstituted piperidines with high selectivity for resistant Plasmodium falciparum. BMC Pharmacol. Toxicol. 2018;19:1–7. doi: 10.1186/s40360-018-0233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simona C., Lucia S., Anna M.P., Amalia P., Rosita D., Pio I., Stefano P. Structure modification of an active azo-compound as a route to new antimicrobial compounds. Molecules. 2017;22:875. doi: 10.3390/molecules22060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez I., Pujol Maria D., Guillaumet G. Massingham Roy.; Andre M. Synthesis of new piperidine and cyclohexylamino-spiro derivatives as potential anticalcium agents. Sci. Pharm. 2002;70:177–187. [Google Scholar]
- 24.Anand K.H., Bhashkar B., Vasudha S., Raman B., Amit K., Ajay S., Kiran T. Synthesis and in vitro antimicrobial studies of some new 3-[phenyldiazenyl] benzaldehyde N-phenyl thiosemicarbazones. J. Enzyme Inhib. Med. Chem. 2008;23:77–81. doi: 10.1080/14756360701408614. [DOI] [PubMed] [Google Scholar]
- 25.Aastha P., Priyanka R., Navneet K., Pratima S., Kishore D. An efficient synthesis and applications of chalcones in organic synthesis. Int. J. Chem. Pharm. Sci. 2013;4:19–23. [Google Scholar]
- 26.Siva S.R.L., Bhagavanraju M., Sridhar C. Synthesis and evaluation of novel morpholine linked substituted chalcone derivatives. Med. Chem. 2015;2015:133–141. [Google Scholar]
- 27.Siva S.R.L., Bhagavanraju M., Sridhar C. Synthesis and evaluation of novel pyrrolidine chalcone derivatives with anticancer, anti-inflammatory and antibacterial activities. J. Chem. Pharm. Res. 2015;7:211–219. [Google Scholar]
- 28.Boon K.H., Zainab N., Paul M.N., Siaw S.H., Reagan E.L., Ee L.K., Boon K.L. Synthesis and anticancer activities of 4-[(halophenyl)diazenyl]phenol and 4-[(halophenyl)diazenyl]phenyl aspirinate derivatives against nasopharyngeal cancer cell lines. J. Chem. 2017;2017:1–7. [Google Scholar]
- 29.Mohammed R.A., Girija S.V., Nasreen B., Syed A. Synthesis of novel chalcone derivatives by conventional and microwave irradiation methods and their pharmacological activities. Arab. J. Chem. 2016;9:931–935. [Google Scholar]
- 30.Shailendra M., Hemendra P., Dutta G.S., Hari Narayana Moorthy N.S. Synthesis and characterization of some chalcone derivatives. Trends Appl. Sci. Res. 2007;2:52–56. [Google Scholar]
- 31.Prasada R.M.M., Dhachinamoorthi D., Mounika M. Pavan,i M. Shireennaaz, S.; Sravani Reddy, C.; Sri Annapurna, M. Synthesis, characterization and anti microbial activity of novel chalcones and its di hydro pyrimidinenones. Int. J. Life Sci. Pharma Res. 2015;5:13–16. [Google Scholar]
- 32.Rajendra P.Y., Praveen Kumar P., Ravi Kumar P., Srinivasa Rao A. Synthesis and antimicrobial activity of some new chalcones of 2-Acetyl Pyridine. E-J. Chem. 2008;5:144–148. [Google Scholar]
- 33.Maria C.S.L., Marcus de Souza V.N., Alessandra P.C., Marcelle de Ferreira L., Raoni G.S.B., Thais C.N.M., Monica P.A. Evaluation of anti tubercular activity of nicotinic and isoniazid analogues. ARKIVOC. 2007;15:181–191. [Google Scholar]
- 34.Lisa A.C., Scott G.F. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanithaa J.D., Paramasivan C.N. Evaluation of microplate Alamar blue assay for drug susceptibility testing of Mycobacterium avium complex isolates. Diagn. Microbiol. Infect. Dis. 2004;49:179–182. doi: 10.1016/j.diagmicrobio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Kimberly K., Repp S.A., Menor Robin K.P. Microplate Alamar blue assay for susceptibility testing of Candida albicans biofilms. Med. Mycol. 2007;45:603–607. doi: 10.1080/13693780701581458. [DOI] [PubMed] [Google Scholar]
- 37.Schwalbe R., Lyn Steele M., Avery Goodwin C. Antimicrobial susceptibility testing protocols. Crc Press. 2007.
- 38.Shrinath S.M., Basavaraj R.P., Aishakhanam H.P., Ganesh N.K., Shashikala G.L., Kalagouda B.G. Synthesis, antimicrobial and antimycobacterial evaluation of star shaped hydrazones derived from 1,3,5-triazine. Pharma Chem. 2012;4(2):600–607. [Google Scholar]
- 39.Daniel Chu T.W., Prabhavathi Fernandesh B. Mini review- structural activity relationships of fluoroquinolones. Antimicrob. Agents Chemother. 1989;33(2):131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prabodh C.S., Ankit J., Sandeed J. Fluoroquinolone antibacterials- A review on chemistry, microbiology and therapeutic prospects. Acta Pol. Pharm. 2009;66(6):587–604. [PubMed] [Google Scholar]