Abstract
Background
Human skeletal muscle is composed of a functional and metabolic continuum of slow (Type I) and fast fibers (IIa and IIx). Hybrid fibers co‐expressing different myosin heavy chains are also present and seem to be more prominent in aging muscle. Their role is debated; hybrid fibers were reported either in a transitional state, between slow and fast fibers, or as fixed individual entities. This study examined the fate of hybrid fibers with an endurance exercise intervention in an elderly sedentary population.
Methods
Twenty‐two sedentary healthy elderly men and women underwent a 16‐week supervised endurance exercise intervention. Eighteen endurance‐trained age‐ and gender‐matched volunteers served as controls. Fiber type distribution was determined by immunohistochemistry on vastus lateralis muscle biopsies pre‐intervention and post‐intervention.
Results
A total of 13840 fibers were analyzed. At baseline, a Type II dominant fiber profile was observed compared with the control group, with more Type IIa (P = 0.0301) and Type IIx fibers (P = 0.0328). Hybrid fibers represented almost 5% of total muscle fibers in both groups. There was no significant difference between groups (I–IIa, P = 0.6719 and IIa–IIx, P = 0.0998). Intervention triggered qualitative dynamics towards an increase in Type I, and decrease in Type II fibers, paralleled by an increase in I–IIa hybrids (P = 0.0301).
Conclusions
The present study is, to our knowledge, the first to examine hybrid muscle fiber type adaptations to an endurance exercise intervention in the elderly. Hybrid fiber proportions did not differ between chronic sedentary state and chronic endurance‐trained state. Exercise intervention increased Type I–IIa hybrid fibers along with shift dynamics in other fiber types suggesting the contribution of hybrid fiber to a fast‐to‐slow fiber type transition, eventually serving as intermediate reservoir from one monomorphic myosin heavy chain expressing fiber type to another. This finding favours the transitional theory regarding hybrid muscle fibers and exercise, crucial to understanding reversible mechanisms of sarcopenia and development of prevention measures.
Keywords: Skeletal muscle fibre type, Myosin heavy chain, Aging, Endurance training
Introduction
Human muscles are composed of three main muscle fibers, which are defined by their functional and biochemical characteristics such as their content in myosin heavy chain (MHC) isoforms.1 The first is Type I fibers (MHC‐I), which are typically slow‐twitch fibers with a high myoglobin content and high oxidative metabolism. The two others are Type IIa (MHC‐IIa) and Type IIx fibers (MHC‐IIx), which are fast‐twitch fibers with lower myoglobin content and a more anaerobic glycolytic metabolism, sometimes referred together collectively as Type II fibers. In this functional and metabolic continuum, Type IIa fibers have an intermediate phenotype between Type I and Type IIx.1
In addition to these monomorphic MHC expressing fiber types, skeletal muscle contains hybrid fibers, which co‐express various MHC isoforms in different combinations and proportions.1, 2 Their origin and role are debated. Previous research suggested that these fibers might either represent fibers in a transitional state between different monomorphic MHC expressing fiber types, undergoing progressive replacement of one myosin isoform with another,3, 4, 5, 6 or fixed individual entities conferring mixed mechanical properties to the muscle in order to fine tune its kinetics.7, 8, 9
Hybrid fiber adaptation to physical training is yet to be understood. Previous intervention studies reported variations depending on the type and volume of exercise. The fact that exercise protocols were different partly explains why it is difficult to identify an adaptation pattern. For example, in the vastus lateralis muscle of healthy young sedentary men, 8 weeks of sprint training did not induce changes in hybrid fibers,9 whereas an increase in Type I–IIa hybrid fibers was observed in resistance training,3, 6 with or without a concomitant decrease in IIa–IIx hybrids.3, 4 Endurance training for 13 weeks showed a decrease in I–IIa hybrid and total hybrid fibers with no change in IIa–IIx fibers in the gastrocnemius.10 Cross‐sectional studies report that endurance‐trained runners have higher proportions of I–IIa hybrids,11 sometimes over 30%,12 and lower proportions of IIa–IIx hybrid fibers as compared with non‐runners.11, 13
In contrast to exercise, physically inactive muscle has shown a high proportion of all hybrid fibers.6, 8 Studies in young sedentary volunteers have reported 27% of fibers being hybrid in the gastrocnemius 8 and about 19% of IIa–IIx fibers in the vastus lateralis.12 The same is true regarding aging muscle, in which hybrid fiber proportions have been reported higher than in the young,14, 15 with over 50% of the muscle fibers co‐expressing multiple MHC isoforms in the vastus lateralis.14, 16 It is well known that reduction in the number and size of both slow and fast monomorphic MHC expressing fibers contributes to the loss of muscle mass due to aging.17, 18 Exercise in this context induces muscle fiber hypertrophy and a decrease in the proportion of Type IIx fibers, with or without an increase in other monomorphic fibers.19, 20 A decrease in all hybrid fiber isoforms was shown in the vastus lateralis of seven healthy septuagenarians with resistance training.21 To our knowledge, no investigations have examined the fate of hybrid fibers with an endurance exercise intervention in an elderly sedentary population.
Given the importance of muscle health in aging, particularly in the quest to prevent sarcopenia, the purpose of this study was to investigate the proportion of hybrid muscle fibers in a sedentary healthy elderly population as well as its adaptation to an endurance exercise intervention. We hypothesized that hybrid fibers are related to muscle undergoing functional transformation, particularly that these fibers take part in the transition to a more aerobic profile with endurance exercise in muscle of 60‐ to 80‐year‐old healthy volunteers.
Materials and methods
Study design and population
A pre–post study design was used to examine the adaptation of hybrid muscle fibers to endurance exercise intervention in a sedentary population (S, n = 22) between 60 and 80 years old. Endurance‐trained volunteers matched by gender and age served as controls (C, n = 18). Recruitment was done through local newspapers and information flyers. To be included, volunteers had to be healthy, non‐smoker, and stable weight. Sedentary was defined as engaging in <20 min of a structured exercise session per week. Endurance‐trained was defined as engaging in three or more >20‐min periods of structured aerobic exercise sessions per week for >1 year preceding study enrollment. Volunteers with chronic co‐morbidities or medications known to affect muscle metabolism, such as diabetes, were excluded. The Ethics Committee of the Canton of Vaud accepted the research protocol, and all volunteers gave written informed consent.
Clinical outcome measures
Height was measured with a wall‐mounted stadiometer. Weight was measured using a calibrated digital scale (Seca, Semur‐en‐Auxois, France) in hospital gown and fasted state. Lean body mass (LBM) was determined by dual energy X‐ray absorptiometry (DiscoveryA, Hologic Inc., Marlborough, MA). Peak oxygen consumption (V̇O2peak) and heart rate (HR) were determined with a graded exercise test on an electronically braked cycle ergometer (Lode B.V., Groningen, The Netherlands). Oxygen consumption was computed with indirect calorimetry (Metalyzer3B, Cortex GmbH, Leipzig, Germany). All subjects performed the test until volitional exhaustion or when one of the American College of Sports Medicine established criteria for maximal testing was reached.22 These measures were performed in tightly controlled conditions as described previously.23 The exact same measurements in the same conditions were performed after intervention.
Exercise training intervention
Sedentary subjects followed an exercise training intervention as previously reported.23 It consisted of a moderate‐intensity aerobic protocol of tri‐weekly supervised exercise sessions over 16 weeks. Each session was progressively increased from 30 to 60 min. Half of the training was performed on a stationary bike and the other half on a treadmill (mostly walking or some light jogging). Exercise intensity was progressive and adapted to each subject based on his or her peak HR to achieve 60–75% of the maximal HR deduced from the baseline V̇O2peak test. Exercise prescription was adapted at the midpoint of the intervention with a submaximal ergometer test as described in details by Dubé et al.24 HR monitors (Polar Electro Oy, Kempele, Finland) and exercise logs were utilized to monitor intensity.
Muscle biopsies
Percutaneous muscle biopsies were obtained from the vastus lateralis under local anaesthesia as previously described.25, 26 Prerequisites to biopsies included no exercise for 72 h and an overnight stay with a controlled dinner in the evening prior to the biopsy followed by fasting until the biopsy was taken at 8 a.m. After trimming of visible adipose tissue with a dissecting microscope (MZ6, Leica Microsystems, Wetzlar, Germany), 3–4 portions (~30 mg each, wet weight) were mounted on a cork with embedding resin (Shandon Cryomatrix OCT, Thermo Fisher Scientific, Waltham, MA) oriented so that the muscle fibers were completely embedded and perpendicular to the cork. The samples were then immediately flash frozen in liquid nitrogen‐cooled isopentane and stored at −80°C prior to cryosectioning.27, 28
Immunohistochemistry
Pre‐intervention and post‐intervention samples for the same subject were simultaneously cryosectioned (10‐μm sections at −30°C; microtome Leica CM3050 S, Leica Biosystems), placed on the same slide and air dried for 15 min before being fixed in phosphate‐buffered saline (PBS) containing 3.7% of formaldehyde solution (Sigma‐Aldrich, St Louis, MO) for 1 h at 4°C. Sections were incubated overnight at 4°C in a solution of primary antibodies (Ab) against MHC Types I (MHC7 sc‐53089, Santa Cruz, Iowa City, IA; dilution 1:200) and IIa (MHC2 sc‐53095, Santa Cruz; dilution 1:100) diluted in PBS containing 2% of bovine serum albumin (BSA, Sigma‐Aldrich). Sections were then rinsed 3 × 5 min in PBS and incubated for 1 h at room temperature in a solution of fluorescent‐conjugated secondary Ab against various immunoglobulin subtypes (Ab anti‐IgM‐R AF 594, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA; dilution 1:800; Ab anti‐IgG‐fluorescein isothiocyanate (FITC) sc‐2010, Santa Cruz; dilution 1:250) diluted in PBS containing 2% of BSA. After a final rinse of 3 × 5 min in PBS containing 0.5% of BSA, sections were dried and mounted using mounting medium (UltraCruz, Santa Cruz).
Image acquisition
All images were captured with a black and white digital camera on an upright fluorescent microscope (Eclipse 90i, Nikon Instruments Europe BV, Amsterdam, The Netherlands) at ×20 magnification using Image‐Pro Plus 7.0 software (Media Cybernetics, Rockville, MD). For every field pictured, photos were taken with fluorescent Cy3 and FITC settings. A merge of these pictures with color attribution was generated. Images were saved in a de‐identified manner to allow blinded analysis.
Image quantification
Image‐Pro Plus 7.0 software (Media Cybernetics) was used to identify and map fiber types on the color attributed merged histology images based on the corresponding fluorescent channels: Type I = positive in Cy3 channel and negative in FITC channel; Type IIa = negative in Cy3 channel and positive in FITC channel; Type IIx = negative in Cy3 and FITC channels; hybrid I–IIa = intermediate in Cy3 channel and in FITC channel; and hybrid IIa–IIx = negative in Cy3 channel and intermediate in FITC channel. This mapping enabled classifying and quantifying precisely each type of fiber. A representative image with color attribution is shown in Figure 1 . To verify this classification in regard to the fact that hybrid fibers have dual or intermediate staining, a subset of samples was used to perform single MHC staining (including Type IIx) on consecutive serial sections. This allowed assessing that the subtraction technique was specific and did not lead to Ab‐related overlapping staining, confirming the identification of hybrid fibers expressing multiple MHC isoforms.
Figure 1.
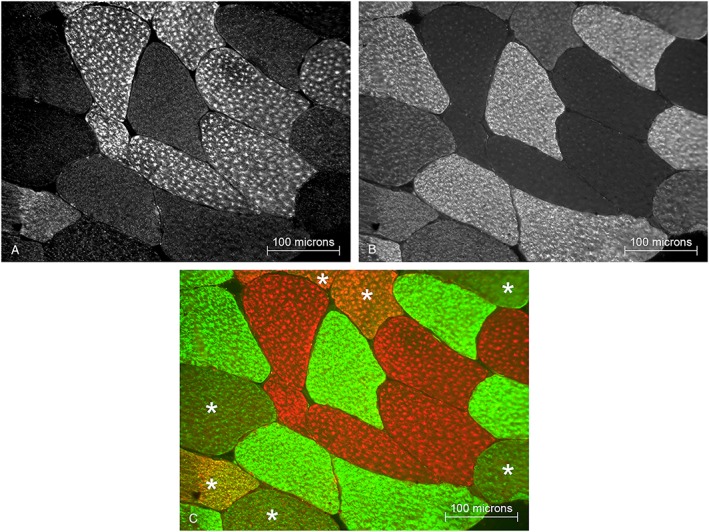
Representative muscle biopsy section with immunohistochemical staining. (A) Myosin heavy chain (MHC)‐I expressing fibers are revealed with Cy3 fluorescence. (B) MHC‐IIa expressing fibers are revealed with fluorescein isothiocyanate (FITC) fluorescence. (C) Merged image with color attribution: red for MHC‐I (Cy3), green for MHC‐IIa (FITC), no color (black) for MHC‐IIx (not present in this image), and mix of colors for hybrid fibers co‐expressing multiple MHC isoforms (*).
Computations
Muscle fiber types are expressed as relative proportions, total being 100%. An ‘aerobic score’ (AS) was generated according to fiber‐specific oxidative metabolic properties. This was in order to evaluate exercise adaptations in the muscle's global aerobic profile resulting from cumulative changes in fiber types. To obtain the AS, relative fiber proportions were multiplied according to aerobic capacity by 1 (Type I), 0.75 (Type I–IIa), 0.5 (Type IIa), 0.25 (Type IIa–IIx), or 0 (Type IIx).
Statistical procedures
Analyses were achieved for each individual samples in each group. Baseline group comparisons were performed using an independent t‐test. Welch's correction was used if the variances were not equal (tested with the following: O'Brien, Brown‐Forsythe, Levene, and Bartlett). Pre‐intervention vs. post‐intervention differences were tested using a paired t‐test, thus taking into account each individual baseline sample as its own control. Clinical outcome measures are represented as mean ± SD. Fiber proportions and AS are represented as mean ± SEM. All analyses were performed with JMP 11 software (SAS Institute Inc., Cary, NC). Significance was set at P < 0.05.
Results
Study population and clinical outcome measures
Twenty‐two S and 18 C were included in this study. Both groups were comparable in terms of gender, age, and LBM at baseline (Table 1). S had a higher weight (+11.9%, P = 0.0163) and body mass index (BMI, +6.5%, P = 0.0216), and lower V̇O2peak corrected for LBM (V̇O2peak/LBM) than C (−11.3%, P = 0.0274). The exercise intervention induced a 1.74 ± 0.75% (SEM) increase in LBM (P = 0.0263) and a 9.38 ± 2.24% increase in V̇O2peak/LBM (P = 0.0007), while there was no change in weight (−1.18 ± 0.75%, P = 0.1305) or BMI (−1.16 ± 0.75%, P = 0.1545) (Table 1). Two volunteers were not included in the interventional data analyses due to study drop out for time constraint and religious fasting (one woman and one man).
Table 1.
Study population characteristics
| Endurance exercise | Trained controls | ||
|---|---|---|---|
| Before | After | ||
| n subjects | 22 | 20 | 18 |
| Men/women | 11/11 | 10/10 | 7/11 |
| Age (years) | 65.50 ± 3.91 | 67.78 ± 5.86 | |
| Weight (kg) | 68.59 ± 10.95* | 68.72 ± 11.22 | 61.27 ± 7.33 |
| LBM (kg) | 48.17 ± 9.74 | 49.30 ± 10.12§ | 46.30 ± 7.64 |
| BMI (kg/m2) | 23.41 ± 2.14* | 23.37 ± 2.33 | 21.98 ± 1.63 |
| V̇O2peak (mL/min/LBM kg) | 40.69 ± 6.02* | 44.18 ± 5.89§ | 45.88 ± 7.79 |
BMI, body mass index; LBM, body mass; V̇O2peak, peak oxygen consumption.
Data are means ± SD.
P < 0.05 vs. ‘trained controls’ by independent t‐test.
P < 0.05 vs. ‘before endurance exercise’ by paired t‐test.
Muscle biopsies
Muscle biopsies were equally distributed in each group (S = 21 vs. C = 18), including S pre‐exercise and post‐exercise intervention biopsies (21 vs. 19). In addition to the two S dropouts that did not have a post‐intervention biopsy, 1 pre‐intervention biopsy and 1 post‐intervention biopsy were poorly interpretable for technical reasons (both women). Eighteen pre‐intervention and post‐intervention matched biopsy pairs were therefore included in the interventional data analysis (8 women and 10 men).
The total number of fibers analyzed was 13840 (4668 pre‐intervention, 4393 post‐intervention in S, and 4419 in C). On average, 222 ± 6 fibers per individual sample were analyzed at baseline (range 136–409), and 231 ± 7 fibers per individual sample were analyzed at post‐intervention (range 160–284).
Muscle fiber type and aerobic profile analysis
At baseline, S showed a Type II dominant fiber profile, with more Type IIa (P = 0.0301) and Type IIx fibers (P = 0.0328) than C (Table 2). In contrast, C showed a Type I dominant fiber profile, with more Type I fibers than S (P = 0.0021). This was consistent with the AS, which was lower in S than C (P = 0.0017). No significant difference between the two groups was noted in hybrid fibers I–IIa (P = 0.6719) and IIa–IIx (P = 0.0998). Across all groups, IIa–IIx hybrid fibers were significantly higher than I–IIa hybrid fibers (P < 0.0001).
Table 2.
Fiber type distribution determined from immunohistochemistry and relative aerobic score
| Endurance exercise | Trained controls | ||
|---|---|---|---|
| Before | After | ||
| Fiber Type I (%) | 43.95 ± 2.76* | 46.29 ± 2.53 | 59.80 ± 3.85 |
| Fiber Type I–IIa (%) | 0.15 ± 0.09 | 0.78 ± 0.30§ | 0.20 ± 0.07 |
| Fiber Type IIa (%) | 45.29 ± 2.08* | 44.46 ± 1.73 | 36.40 ± 3.30 |
| Fiber Type IIa–IIx (%) | 3.57 ± 0.92 | 4.14 ± 1.03 | 1.62 ± 0.69 |
| Fiber Type IIx (%) | 7.04 ± 1.86* | 4.34 ± 1.66 | 1.97 ± 1.31 |
| Aerobic score (arbitrary unit) | 60.02 ± 2.32* | 62.54 ± 2.14 | 72.44 ± 2.82 |
Data are means ± SEM.
P < 0.05 vs. ‘trained controls’ by independent t‐test.
P < 0.05 vs. ‘before endurance exercise’ by paired t‐test.
Looking at the overall picture (Figure 2), endurance exercise intervention triggered qualitative dynamics towards an increase in Type I, and decrease in Type II fibers, paralleled by an increase in hybrid fibers. The proportion of I–IIa hybrid fibers increased by 402% (P = 0.0301). Changes in the individual proportion of each other fiber type were not statistically significant: Types I (+5.33%, P = 0.7937), IIa (−1.84%, P = 0.7902), IIx (−38.4%, P = 0.2912), and IIa–IIx (+16%, P = 0.6729). The increase of AS observed was not statistically significant (+4.17 ± 4.33%, P = 0.5529).
Figure 2.
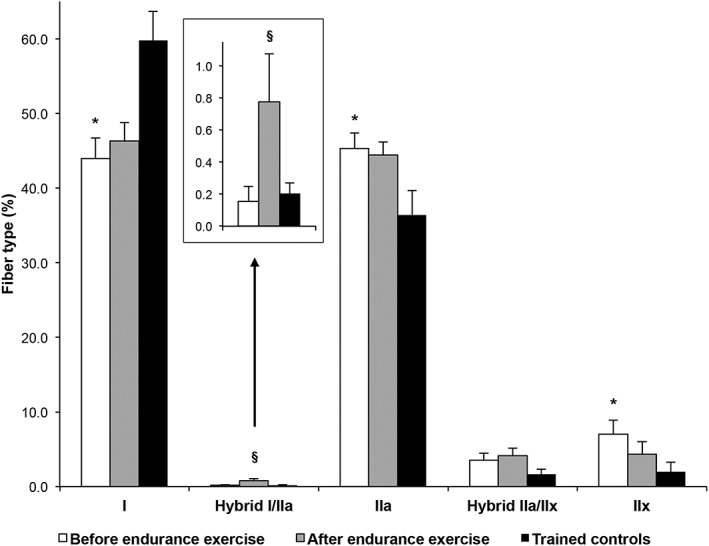
Fiber type distributions and exercise‐induced shift from fast glycolytic towards slow oxidative fibers. Bars are means ± SEM. *P < 0.05 vs. ‘trained controls’ by independent t‐test. § P < 0.05 vs. ‘before endurance exercise’ intervention by paired t‐test.
Discussion
The present study investigated hybrid muscle fibers in sedentary elderly volunteers and the effect of 16 weeks of moderate‐intensity endurance training on muscle fiber composition. Exercise intervention increased Type I–IIa hybrid fibers along with qualitative shifts in other fiber types towards slow‐twitch fibers. These findings suggest the contribution of hybrid fibers to a fast‐to‐slow fiber type transition along the metabolic and functional fiber type continuum. Exercise intervention significantly increased V̇O2peak/LBM, illustrating the increase in aerobic capacity. LBM was also significantly increased with an average gain slightly above 1 kg, which is clinically significant and comparable with interventions geared at treating sarcopenia.29, 30
Baseline muscle fiber profile was less aerobic in S than C. This was confirmed by the AS and is consistent with what was observed between inactive and endurance‐trained younger individuals.11 The global picture of monomorphic MHC muscle fiber distribution in S was thus the opposite image of the fiber distribution in C, as the profile of S was fast Type II fiber dominant, whereas the profile of C was slow Type I fiber dominant. After intervention, our 60‐ to 79‐year‐old previously sedentary volunteers did not show significant changes in monomorphic MHC fiber types but revealed a statistically significant exercise‐induced increase in hybrid I–IIa fibers, which was accompanied by a general qualitative shift from fast glycolytic towards slow oxidative fibers (Figure 2 ). This histological snapshot after 16 weeks of training was compatible with a training‐dependent response of fast‐to‐slow fiber types over time, which is the premise of more consistent functional and morphological changes. This was indeed shown in our previous work using a more stringent exercise schedule (up to five weekly training sessions vs. three weekly sessions in the present work),27 which disclosed an exercise‐induced 11% increase in Type I fibers in overweight and obese elderly individuals. Similarly, Pruchnic et al.28 observed a 4.1% increase in Type I fibers with a 12‐week endurance exercise protocol in an overweight population. Other endurance exercise interventional studies in healthy 60‐ to 75‐year‐old sedentary subjects observed that exercise induced a decrease in Type IIx fibers, with or without increase in Type IIa, but with no significant changes in the proportions of Type I fibers in gastrocnemius and vastus lateralis.19, 20 While these studies disclose decreases in IIx fibers, they did not provide detailed analyses of hybrid fibers, which might have acted as intermediate between a fast‐to‐slow fiber shift.
Hybrid fibers did not significantly differ in S vs. C, which is in agreement with several previous cross‐sectional studies in a younger healthy population reporting a similar proportion of hybrid fibers in both the untrained and the trained.8, 12 This was expected, as the two groups in our study were either chronically sedentary or chronically active in the cross‐sectional comparison (in pre‐intervention). Thus, their muscles could be considered respectively in a steady state rather than in functional transformation. Similarly, St‐Jean‐Pelletier et al.15 have shown recently that proportions of I–IIa and IIa–IIx fibers were not statistically different between active, sedentary, or frail 65+‐year‐old seniors. This was not the case of a bed‐rest intervention that has shown an increase in both I–IIa and IIa–IIx fibers in the vastus lateralis 6 or with our exercise intervention that resulted in an increase in hybrid I–IIa fibers. These results are suggestive of an intermediary transition reservoir consistent with the dynamics of shift from fast glycolytic towards slow oxidative monomorphic MHC fibers (Figure 2 ).
Volume, intensity, frequency, mode, and duration are all endurance exercise training attributes influencing skeletal muscle responses to intervention. For example, Kohn et al.11 reported that 20‐ to 25‐year‐old runners had higher proportions of Type I fibers and lower proportions of Type IIx and hybrid IIa–IIx fibers than non‐runners. They also showed that exercise volume and running distance were positively related to the proportion of hybrid Type I–IIa fibers and negatively related to the proportion of IIa–IIx fibers. Likewise, Trappe et al.10 have shown that there was a decrease in relative quantity of hybrid Type I–IIa fibers to the benefit of an increase in relative quantity of Type I fibers after 13 weeks of training for a marathon in 22‐year‐olds. While several evidence, including the present study, point towards a fast‐to‐slow fiber type shift induced by endurance exercise, we did not measure statistically significant increases in Type I fibers in our subjects. It is possible that our moderate‐intensity exercise routine proposed for general health in 60‐ to 80‐year‐old volunteers was not long or intense enough to create significant increases in Type I fibers. On the other hand, this allowed us to capture the likely transient character of hybrid fibers. Taken together, these observations represent complementary snapshots of a similar phenomenon in which hybrid fibers, in response to endurance exercise, ensure the progressive passage along the continuum between fast‐to‐slow monomorphic MHC expressing fibers.
The purpose of hybrid fibers in human muscle is debated. The MHC composition of hybrid fibers has been theorized to be a specialized group providing muscle with a wide spectrum of contractile properties, thus enabling to fine tune muscle power efficiency and resistance to fatigue.7, 8, 9 This theory was supported by Neunhauserer et al.7 who identified three groups with different kinetics (fast, slow, and transition zone) when studying fiber Types I, IIa, and their related I–IIa hybrid types in humans. The group with intermediate properties was composed of specific Type I fibers and MHC‐I dominant hybrid fibers (7:3 ratio) rather than a transitional spectrum of hybrid fiber type. It has also been theorized that hybrid fibers play a transitional role between two monomorphic MHC expressing fiber types.15 This theory was supported by several studies previously mentioned in which either a slow‐to‐fast fiber transition was shown with bed‐rest and resistance exercise intervention, or a fast‐to‐slow fiber transition with endurance exercise intervention.3, 4, 6, 10 In the present study, the general adaptation of fiber types to exercise suggests a transition from fast‐to‐slow fibers with an increase in hybrid Type I–IIa fibers favoring the transitional theory. Only a careful time‐course study with multiple biopsies would be able to ascertain the exact kinetics of this shift, which was beyond the scope of this work. Our observations also raise the question of the origin of hybrid fibers in the transforming muscle. Whether they are pre‐existing MHC monomorphic fibers undergoing MHC protein turnover and modifications of gene expression to adapt to new conditions as suggested by previous data,5 or de novo cells in the process of adjusting to exercise‐related mechanic and metabolic demand of the muscle, is poorly understood.
Determining muscle fiber profiles may be subject to technical pitfalls that may account for different results. In the present immunohistochemistry study, hybrid fibers represented almost 5% of total muscle fibers at baseline, which was higher than the <0.5% reported in the gastrocnemius of healthy 60‐ to 70‐year‐olds determined by myosin adenosine triphosphatase histochemistry.19 Staron et al.31 suggested that immunohistochemistry may generate a misclassification of hybrid fibers, especially those that express only a small amount of one of the MHC isoforms, leading to an underestimation of hybrid fibers. An important consideration is that we used two Ab as reported by multiple authors25, 27, 28, 32, 33, 34, 35, 36 and confirmed the specificity of our subtraction technique to minimize interpretation biases. Three Ab have been used in a recent cross‐sectional study by St‐Jean‐Pelletier et al.15 Similarly to our results, the authors report no difference in the proportion of I–IIa and IIa–IIx hybrid fibers between groups of sedentary or active 65 years and older individuals. The comparison of possible biases between these two methods is yet to be performed.
In 68 years and older elderly, greater proportions of hybrid fibers (up to 50%) were reported when determined by electrophoretic analysis of single cells.14, 16, 20 Andersen et al. 37 measured a high proportion of hybrid fibers (close to one‐third) in human subjects ranging from 85 to 97 years old by MHC electrophoresis, which was not found with histochemistry analysis. They thus suggest fiber type shifting along the length of the fiber due to different MHC isoform expression within myonuclear domains. The greater proportion of hybrid fibers found by electrophoretic techniques is most probably due to the fact that it examines the MCH expressed in a total muscle fiber, whereas immunohistochemistry examines the MHC expressed in a fiber cross‐section, thus in a single myonuclear domain.37 Nevertheless, MHC electrophoresis pitfalls are not insignificant either as results depend on several variables like run times, voltage settings, buffer ingredients, staining and gel settings. For example, Bamman et al.38 have shown that a 1% difference in polyacrylamide separating gel could hinder myosin band analysis.
In the present study, the large number of fiber cross‐sections examined, close to 1400, may offset, at least in part, the limitations alluded to above. Moreover, one main advantage of muscle fiber histochemical identification is that it enables investigations without denaturing cells, as for phenotypic and metabolic properties of fibers. In addition, while different muscles exhibit different fiber type profiles, this study focused on vastus lateralis, which is a primordial muscle for activities of daily living and thus has major implication in the context of aging muscle and sarcopenia. Therefore, without an ultimate methodology to integrate all muscle cell characteristics in realistic in vivo three‐dimensional imaging, the present results are likely to present the picture of the dynamics of exercise‐induced muscle fiber type transition. Future work examining the effect of an exercise protocol on fiber type composition, specifically hybrid fibers, should include multiple serial time‐course biopsies with multiple fiber type identification methods to confirm the results.
In conclusion, the present study is the first to examine hybrid muscle fiber type adaptations to endurance exercise in 60‐ to 79‐year‐old elderly. Hybrid fiber proportions were not different in the chronically sedentary than the chronically active group, although their respective dominant monomorphic MHC expressing fiber type profile was a mirror image of one another (fast vs. slow dominant). Exercise intervention increased Type I–IIa hybrid fibers along with a comprehensive dynamics in other fiber types suggesting hybrid contribution to a fast‐to‐slow fiber type transition in a communicant‐vessel‐like manner, the hybrid fibers eventually serving as intermediate transition reservoirs from one monomorphic MHC expressing cell type to another. This results in one more step towards favouring the transitional theory regarding hybrid muscle fibers and exercise, a key to understanding reversible mechanisms for sarcopenia and development of therapeutic and preventive measures. More research is needed to fully understand the underlying mechanisms and implications of hybrid fibers in transforming muscle. Future work on the multiple interlinked actors likely to be involved in this process—including motor unit plasticity efficiency and patterns as well as molecular and gene expression pathways (e.g. MCH protein half‐life and replacement)23, 39, 40, 41, 42—will be critical to develop novel strategies to improve functional capacity at all ages.
Funding
This work was supported by the Swiss National Science Foundation (SNSF) grant nos PZ00P3_126339 (to FA), PZ00P3_149398 (to FA) and 320030_170062.
Author contributions
F.A. designed and supervised all aspects of this research; M.M., N.B., C.G., C.B., and F.A. recruited and performed clinical experiments; N.B. conducted the exercise intervention; M.M., C.G. and F.A. performed bench experiments; M.M. blindly analyzed images; M.M. and F.A. interpreted results; V.R. supervised statistical analyses; M.M. prepared all figures; M.M. and F.A. wrote the manuscript; all authors edited and approved the final version of the manuscript.
Conflict of interest
M.M., S.C.A., N.T.B., C.G., C.B., V.R., and F.A. declare that they have no conflict of interest.
Acknowledgements
We want to thank all of our volunteers for their commitment to the study. We also want to acknowledge the contributions of Dr. Leo Schluetter for his expert cardiology supervision of the stress tests, Dr. Didier Hans and the technicians for the DXAs, and Jean‐Pierre Sacco for the use of the Let's Go fitness centre.
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.43
Moreillon M., Conde Alonso S., Broskey N. T., Greggio C., Besson C., Rousson V., and Amati F. (2019) Hybrid fiber alterations in exercising seniors suggest contribution to fast‐to‐slow muscle fiber shift, Journal of Cachexia, Sarcopenia and Muscle, 10: 687–695. 10.1002/jcsm.12410.
References
- 1. Billeter R, Weber H, Lutz H, Howald H, Eppenberger HM, Jenny E. Myosin types in human skeletal muscle fibers. Histochemistry 1980;65:249–259. [DOI] [PubMed] [Google Scholar]
- 2. Staron RS, Hikida RS. Histochemical, biochemical, and ultrastructural analyses of single human muscle fibers, with special reference to the C‐fiber population. J Histochem Cytochem: Off J Histochem Soc 1992;40:563–568. [DOI] [PubMed] [Google Scholar]
- 3. Putman CT, Xu X, Gillies E, MacLean IM, Bell GJ. Effects of strength, endurance and combined training on myosin heavy chain content and fibre‐type distribution in humans. Eur J Appl Physiol 2004;92:376–384. [DOI] [PubMed] [Google Scholar]
- 4. Williamson DL, Gallagher PM, Carroll CC, Raue U, Trappe SW. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol 2001;91:1955–1961. [DOI] [PubMed] [Google Scholar]
- 5. Andersen JL, Gruschy‐Knudsen T, Sandri C, Larsson L, Schiaffino S. Bed rest increases the amount of mismatched fibers in human skeletal muscle. J Appl Physiol 1999;86:455–460. [DOI] [PubMed] [Google Scholar]
- 6. Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed‐rest and resistance exercise. J Physiol 2004;557:501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neunhauserer D, Zebedin M, Obermoser M, Moser G, Tauber M, Niebauer J, et al. Human skeletal muscle: transition between fast and slow fibre types. Pflugers Archiv: Eur J Physiol 2011;461:537–543. [DOI] [PubMed] [Google Scholar]
- 8. Parcell AC, Sawyer RD, Craig Poole R. Single muscle fiber myosin heavy chain distribution in elite female track athletes. Med Sci Sports Exerc 2003;35:434–438. [DOI] [PubMed] [Google Scholar]
- 9. Parcell AC, Sawyer RD, Drummond MJ, O'Neil B, Miller N, Woolstenhulme MT. Single‐fiber MHC polymorphic expression is unaffected by sprint cycle training. Med Sci Sports Exerc 2005;37:1133–1137. [DOI] [PubMed] [Google Scholar]
- 10. Trappe S, Harber M, Creer A, Gallagher P, Slivka D, Minchev K, et al. Single muscle fiber adaptations with marathon training. J Appl Physiol 2006;101:721–727. [DOI] [PubMed] [Google Scholar]
- 11. Kohn TA, Essen‐Gustavsson B, Myburgh KH. Exercise pattern influences skeletal muscle hybrid fibers of runners and nonrunners. Med Sci Sports Exerc 2007;39:1977–1984. [DOI] [PubMed] [Google Scholar]
- 12. Klitgaard H, Bergman O, Betto R, Salviati G, Schiaffino S, Clausen T, et al. Co‐existence of myosin heavy chain I and IIa isoforms in human skeletal muscle fibres with endurance training. Pflugers Archiv: Eur J Physiol 1990;416:470–472. [DOI] [PubMed] [Google Scholar]
- 13. Harber MP, Gallagher PM, Trautmann J, Trappe SW. Myosin heavy chain composition of single muscle fibers in male distance runners. Int J Sports Med 2002;23:484–488. [DOI] [PubMed] [Google Scholar]
- 14. Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand 1990;140:55–62. [DOI] [PubMed] [Google Scholar]
- 15. St‐Jean‐Pelletier F, Pion CH, Leduc‐Gaudet JP, Sgarioto N, Zovile I, Barbat‐Artigas S, et al. The impact of ageing, physical activity, and pre‐frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J Cachexia Sarcopenia Muscle 2017;8:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve 1999;22:449–454. [DOI] [PubMed] [Google Scholar]
- 17. Lexell J. Human aging, muscle mass, and fiber type composition. The journals of gerontology Series A, Biological sciences and medical sciences. 1995;50 Spec No:11–6. [DOI] [PubMed]
- 18. Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15‐ to 83‐year‐old men. J Neurol Sci 1988;84:275–294. [DOI] [PubMed] [Google Scholar]
- 19. Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, et al. Skeletal muscle adaptations to endurance training in 60‐ to 70‐yr‐old men and women. J Appl Physiol 1992;72:1780–1786. [DOI] [PubMed] [Google Scholar]
- 20. Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, et al. Aerobic exercise training induces skeletal muscle hypertrophy and age‐dependent adaptations in myofiber function in young and older men. J Appl Physiol 2012;113:1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol 2000;88:627–633. [DOI] [PubMed] [Google Scholar]
- 22. American College of Sports Medicine , Riebe D, Ehrman JK, Liguori G, Magal M. ACSM's guidelines for exercise testing and prescription, 10th ed. Philadelphia: Wolters Kluwer; 2018. [Google Scholar]
- 23. Broskey NT, Greggio C, Boss A, Boutant M, Dwyer A, Schlueter L, et al. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab 2014;99:1852–1861. [DOI] [PubMed] [Google Scholar]
- 24. Dube JJ, Allison KF, Rousson V, Goodpaster BH, Amati F. Exercise dose and insulin sensitivity: relevance for diabetes prevention. Med Sci Sports Exerc 2012;44:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amati F, Dube JJ, Alvarez‐Carnero E, Edreira MM, Chomentowski P, Coen PM, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance‐trained athletes? Diabetes 2011;60:2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Broskey NT, Boss A, Fares EJ, Greggio C, Gremion G, Schluter L, et al. Exercise efficiency relates with mitochondrial content and function in older adults. Physiol Rep 2015;3:e12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dube JJ, Amati F, Stefanovic‐Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise‐induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 2008;294:E882–E888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab 2004;287:E857–E862. [DOI] [PubMed] [Google Scholar]
- 29. Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med 2009;169:122–131. [DOI] [PubMed] [Google Scholar]
- 30. Kalyani RR, Corriere M, Ferrucci L. Age‐related and disease‐related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014;2:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Staron RS, Herman JR, Schuenke MD. Misclassification of hybrid fast fibers in resistance‐trained human skeletal muscle using histochemical and immunohistochemical methods. J Strength Cond Res 2012;26:2616–2622. [DOI] [PubMed] [Google Scholar]
- 32. Gueugneau M, Coudy‐Gandilhon C, Theron L, Meunier B, Barboiron C, Combaret L, et al. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J Gerontol A Biol Sci Med Sci 2015;70:566–576. [DOI] [PubMed] [Google Scholar]
- 33. He J, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on muscle lipid content and droplet size. Obes Res 2004;12:761–769. [DOI] [PubMed] [Google Scholar]
- 34. Dube JJ, Amati F, Toledo FG, Stefanovic‐Racic M, Rossi A, Coen P, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 2011;54:1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chow LS, Mashek DG, Wang Q, Shepherd SO, Goodpaster BH, Dube JJ. Effect of acute physiological free fatty acid elevation in the context of hyperinsulinemia on fiber type‐specific IMCL accumulation. J Appl Physiol 2017;123:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dube JJ, Broskey NT, Despines AA, Stefanovic‐Racic M, Toledo FG, Goodpaster BH, et al. Muscle characteristics and substrate energetics in lifelong endurance athletes. Med Sci Sports Exerc 2016;48:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 2003;13:40–47. [DOI] [PubMed] [Google Scholar]
- 38. Bamman MM, Clarke MS, Talmadge RJ, Feeback DL. Enhanced protein electrophoresis technique for separating human skeletal muscle myosin heavy chain isoforms. Electrophoresis 1999;20:466–468. [DOI] [PubMed] [Google Scholar]
- 39. Barbieri E, Agostini D, Polidori E, Potenza L, Guescini M, Lucertini F, et al. The pleiotropic effect of physical exercise on mitochondrial dynamics in aging skeletal muscle. Oxid Med Cell Longev 2015;2015:917085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown WM. Exercise‐associated DNA methylation change in skeletal muscle and the importance of imprinted genes: a bioinformatics meta‐analysis. Br J Sports Med 2015;49:1567–1578. [DOI] [PubMed] [Google Scholar]
- 41. Tarnopolsky MA. Mitochondrial DNA shifting in older adults following resistance exercise training. Appl Physiol Nutr Metab 2009;34:348–354. [DOI] [PubMed] [Google Scholar]
- 42. Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol 1960;150:417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8: 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


