Abstract
Background
Living tissues maintain a fine balance between protein synthesis and protein breakdown rates. Animal studies indicate that protein synthesis rates are higher in organs when compared with skeletal muscle tissue. As such, organ and tumour protein synthesis could have major effects on whole‐body protein metabolism in wasting disorders such as cancer cachexia. We aimed to assess protein synthesis rates in pancreatic tumour tissue and healthy pancreas, liver, and skeletal muscle tissue in vivo in humans.
Methods
In eight patients with pancreatic cancer undergoing pancreaticoduodenectomy, primed continuous infusions with L‐[ring‐13C6]phenylalanine and L‐[3,5‐2H2]tyrosine were started prior to surgery and continued throughout the surgical procedures. During surgery, plasma samples and biopsies from the pancreas, pancreatic tumour, liver, and vastus lateralis muscle were taken. Post‐absorptive fractional protein synthesis rates were determined by measuring incorporation of labelled L‐[ring‐13C6]phenylalanine in tissue protein using the weighed plasma L‐[ring‐13C6]phenylalanine enrichments as the precursor pool.
Results
Five male patients and three female patients with a mean age of 67 ± 2 years were included into this study. Plasma L‐[ring‐13C6]phenylalanine enrichments (6–9 mole per cent excess) did not change during surgery (P = 0.60). Pancreatic tumour protein synthesis rates were 2.6‐fold lower than surrounding pancreatic tissue protein synthesis rates (0.268 ± 0.053 vs. 0.694 ± 0.228%/h, respectively; P = 0.028) and 1.7‐fold lower than liver protein synthesis rates (0.268 ± 0.053 vs. 0.448 ± 0.043%/h, respectively; P = 0.046). Among healthy organ samples, protein synthesis rates were 20‐fold and 13‐fold higher in pancreas and liver, respectively, compared with skeletal muscle tissue (0.694 ± 0.228 and 0.448 ± 0.043 vs. 0.035 ± 0.005%/h, respectively; P < 0.05).
Conclusions
Liver and pancreas tissue protein synthesis rates are higher when compared with pancreatic tumour and skeletal muscle tissue protein synthesis rates and can, therefore, strongly impact whole‐body protein metabolism in vivo in humans.
Keywords: Protein metabolism, Pancreatic cancer, Pancreas, Liver
Background
All tissues are in a constant state of turnover, with a tightly controlled regulation of tissue protein synthesis and protein breakdown rates.1 Because of the relative ease of sampling skeletal muscle tissue,2 ample information is available on the synthesis rates (average 1–2% per day3) and factors influencing skeletal muscle protein synthesis in humans. Prior work in animals suggests that protein synthesis rates in organ tissue are much higher compared with skeletal muscle tissue protein synthesis rates.1, 4 This likely applies to humans as well, and because ~40% of total human protein content is stored in non‐muscle tissue,5 it suggests that protein synthesis in organs could play a significant role in whole‐body protein metabolism. In addition, protein synthesis rates in tumours contribute to whole‐body protein metabolism as indicated by data from rats bearing hepatomas and sarcomas.6 These observations suggest that tumour protein synthesis might be a potential driver of wasting diseases such as cancer cachexia and calls for human studies of protein synthesis rates in tumour tissue relative to surrounding healthy organs and skeletal muscle.
In the present study, we applied contemporary stable isotope methodology using primed continuous intravenous L‐[ring‐13C6]phenylalanine infusions prior to and during pancreaticoduodenectomy in eight patients with pancreatic cancer. During the surgical procedures (5–7 h), biopsies were taken from the pancreatic tumour as well as healthy pancreas, vastus lateralis muscle, and liver tissue, allowing us to assess tissue protein synthesis rates in vivo in these four different tissues.
Methods
Subjects
Eight consenting patients with cancer of the pancreatic head undergoing surgery were enrolled in this study (Table 1). Inclusion criteria were (i) age ≥18 years, (ii) pancreaticoduodenectomy for suspected adenocarcinoma of the pancreas head, and (iii) presence of cachexia [weight loss >5% in the last 6 months; weight loss >2% and body mass index <20; or weight loss >2% and sarcopenia (L3‐skeletal muscle index of <55 cm2/m2 for men and <39 cm2/m2 for women)].7 Patients were excluded from participation if they met any of the following criteria: active acute pancreatitis, chronic pancreatitis, previous pancreatic surgery, inflammatory bowel disease, use of systemic steroids in the past 4 weeks, abdominal surgery in the past 4 weeks, phenylketonuria, insulin‐dependent diabetes mellitus, global initiative for chronic obstructive lung disease III or IV, heart failure, receiving total parenteral nutrition at day of surgery, pregnancy, neoadjuvant chemotherapy, or radiotherapy. Patients on systemic steroid use were excluded as it can induce muscle atrophy through the ubiquitin proteasome pathway and inhibit insulin and insulin‐like growth factor 1 mediated protein synthesis.8 In rats, the inhibiting effect on the skeletal muscle fractional synthetic rate (FSR) is restored to normal 3 days after steroid administration.9 Therefore, we found a period of 4 weeks without systemic steroid use before surgery to be appropriate. Patients were included consecutively at the Department of Surgery of the Maastricht University Medical Centre. Nutritional status was assessed by a trained physician in the outpatient department and included measurements of weight and height, patient‐reported weight loss in the last 6 months, triceps skinfold thickness, handgrip strength, patient‐generated subjective global assessment, and malnutrition universal screening tool. Systemic inflammation was assessed by measuring plasma C‐reactive protein (CRP) and albumin levels preoperatively (routine in‐hospital laboratory test) and by measuring preoperative levels of interleukin (IL)‐1β, IL‐6, and tumour necrosis factor‐α (TNF‐α) by enzyme‐linked immunosorbent assay. Preoperative muscle mass was measured using computed tomography imaging on the third lumbar vertebra and sliceOmatic (Tomovision, Magog, Canada), which is a reliable method for assessing total body skeletal muscle mass.10
Table 1.
Patient characteristics
| Patients (n = 8) | |
|---|---|
| Age (years) | 67 ± 2 |
| Female (n, %) | 3, 37.5% |
| Male (n, %) | 5, 62.5% |
| BMI (kg/m2) | 24.3 ± 1.4 |
| Weight loss over the last 6 months (%) | 13.0 ± 2.2 |
| L3‐skeletal muscle index (cm2/m2) | 45.1 ± 2.5 |
| L3‐visceral adipose tissue index (cm2/m2) | 58.1 ± 16.7 |
| L3‐subcutaneous adipose tissue index (cm2/m2) | 57.3 ± 11.5 |
| Type of surgery (n, %) | |
| Pylorus‐preserving pancreaticoduodenectomy | 6, 75% |
| Double bypass | 2, 25% |
| Histopathological diagnosis (n, %) | |
| Pancreatic adenocarcinoma | 5, 62.5% |
| Ampullary carcinoma | 1, 12.5% |
| Intrapancreatic cholangiocarcinoma | 2, 25% |
| PG‐SGA score | 15.4 ± 2.0 |
| MUST score | 1.8 ± 0.2 |
| Triceps skinfold (mm) | 12.8 ± 1.7 |
| Handgrip strength (kg) | 30.4 ± 2.4 |
| C‐reactive protein (mg/L) | 5.9 ± 1.3 |
| Albumin (g/L) | 30.7 ± 1.3 |
| Interleukin‐1β (pg/mL) | 5.1 ± 0.8 |
| Interleukin‐6 (pg/mL) | 13.1 ± 9.1 |
| TNF‐α (pg/mL) | Not detecteda |
Data are presented as mean ± standard error of the mean. BMI, body mass index; MUST, malnutrition universal screening tool; PG‐SGA, patient generated selective global assessment; TNF‐α, tumour necrosis factor‐α.
Plasma concentrations <2 pg/mL for all patients.
Study protocol
Patients were admitted to the hospital 1 day prior to surgery. Two hours before surgery, a venous blood sample was taken (t = 0). Thereafter, a primed continuous intravenous infusion of L‐[ring‐13C6]phenylalanine and L‐[3,5‐2H2]tyrosine was started and continued throughout surgery (5–7 h). Before surgery, a catheter was placed in the radial artery from which arterial blood samples were drawn every 15–30 min. Following the start of surgery, the first biopsies of the liver and vastus lateralis muscle were taken (t = 1). During surgery, the surgeon judged the feasibility to perform a resection of the tumour [pylorus‐preserving pancreaticoduodenectomy (PPPD)] or alternatively palliative surgery in which the pancreas remains untouched (double bypass). At t = 2 (just before the blood supply to the tumour containing pancreas head was interrupted), repeated biopsies of the liver and vastus lateralis muscle, and from the non‐tumorous pancreas, were collected. Muscle biopsy samples from the vastus lateralis were taken from the same incision; the first sample was taken from different fibres (3 cm distal of the incision with the biopsy needle pointing inwards) from the second (3 cm proximal of the incision with the biopsy needle pointing outwards). A schematic overview of the study protocol is shown in Figure 1. The study protocol was reviewed and approved by the medical ethics committee of the Maastricht University Medical Centre (No: NL45969.068.13).
Figure 1.
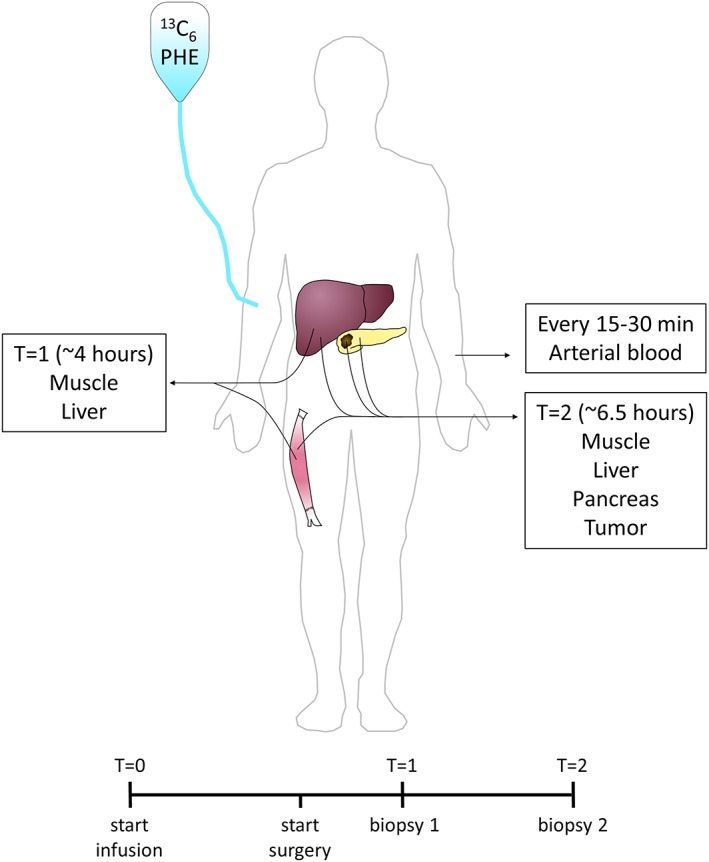
Schematic overview of the study protocol. Patients received a primed continuous infusion of L‐[ring‐13C6]phenylalanine and L‐[3,5‐2H2]tyrosine before and during surgery. Arterial plasma samples were drawn every 15–30 min. At t = 1 (~4 h after start of infusion), muscle and liver biopsies were obtained. At t = 2 (~6.5 h after start of infusion), muscle, liver, pancreas, and pancreatic tumour biopsies were obtained.
Biopsy and plasma analyses
The PPPD specimen was transferred to the pathology laboratory immediately after resection, where the pathologist identified the tumour macroscopically and provided a fresh approximately 5 mm tumour containing tissue slice. Samples were taken within 15 min after removal from the body. Frozen sections were cut and thereafter stained with haematoxylin and eosin for histologic examination. A pathologist specialized in pancreas pathology examined all tumour biopsies using light microscopy to confirm the presence of malignant cells in the biopsies, which is considered to be the gold standard for diagnosing malignancies of the pancreas.11 None of the pancreatic tumours showed signs of necrosis or ischaemia on histologic examination.
Biopsies from healthy pancreas, liver, and skeletal muscle were cleaned of any visible blood and immediately frozen in liquid nitrogen in the operating theatre and stored at −80°C until analysis. Fifty milligrams of tissue were freeze‐dried and homogenized in ice‐cold 2% perchloric acid with a sonicator and subsequently centrifuged at 1000 g at 4°C. The supernatant was separated from the protein pellet for assessment of tissue‐free phenylalanine enrichments.
Blood was collected in EDTA tubes pre‐chilled on ice and centrifuged at 1000 g at 4°C for 5 min. Plasma aliquots were frozen in liquid nitrogen and stored at −80°C until analysis. Plasma IL‐1β, IL‐6, and TNF‐α concentrations were measured by enzyme‐linked immunosorbent assays according to the manufacturer's instructions (U‐CyTech Biosciences, Utrecht, the Netherlands; assays CT576‐10, CT744‐10, and CT747‐10, respectively). Plasma proteins were extracted by adding ice‐cold 2% perchloric acid up to a concentration of 2% and subsequently centrifuged at 1000 g at 4°C.
For plasma and tissue supernatants, phenylalanine was derivatized to its t‐butyldimethyl‐silyl derivative, and its 13C enrichment was determined in duplicates by electron impact ionization gas chromatography mass spectrometry (model 6890N GC/5973N MSD; Agilent, Little Falls, DE) by using selected ion monitoring of masses of 336 and 342 for unlabelled and labelled phenylalanine, respectively. Enrichment was corrected for the natural presence of the 13C isotope. The tissue protein pellets were washed three times with 2% perchloric acid, vacuum‐dried overnight, and hydrolysed in 6 M HCL at 120°C for 15–18 h. The hydrolysed protein fraction was dried under a nitrogen stream while heated to 120°C before adding 50% acetic acid solution and passing the hydrolysed protein over a Dowex exchange resin (AG 50W‐X8, 100–200 mesh hydrogen form; Biorad, Hercules, CA) by using 2 M NH4OH. The eluate was collected for the measurement of L‐[ring‐13C6]phenylalanine enrichment in the samples. L‐[ring‐13C6]phenylalanine was derivatized to its N(O,S)‐ethoxycarbonyl ethyl esters. Thereafter, the ratios of labelled to unlabelled derivatives were determined in quadruplicates by gas chromatography combustion isotope ratio mass spectrometry (MAT 252; Finnigan, Bemen, Germany). Standard regression curves were applied to assess the linearity of the mass spectrometer and to control for loss of tracer.
Calculations
Protein synthesis rates were calculated as FSR, expressed as %/h, using tissue and plasma L‐[ring‐13C6]phenylalanine enrichments. The FSR was calculated using the standard precursor‐product equation:
E p2 and E p1 are the protein‐bound enrichments measured in the second biopsy (or only biopsy in the case of tumour tissue12) (t = 2) and basal mixed plasma proteins (t = 0), respectively (single biopsy approach). E precursor indicates the average plasma free L‐[ring‐13C6]phenylalanine enrichment, and t indicates the tracer incorporation time. For muscle and liver tissue, FSRs were also calculated using the two successive biopsy samples (successive biopsy approach). In this case, E p1 was the protein‐bound enrichment measured in the first biopsy (t = 1).
Although theoretically the ideal precursor pool for FSR calculations would be the intracellular free‐phenylalanine enrichment, the average plasma phenylalanine enrichment was chosen as preferred precursor pool because it is calculated over the entire tracer incorporation period, whereas the intracellular free‐phenylalanine enrichment can only be assessed at the time of biopsy. For comparing both methods, FSRs were also calculated by using the tissue‐free phenylalanine enrichments as E precursor measured in the supernatant of each processed biopsy. Comparing the calculation methods using plasma and tissue free‐tracer enrichments as the precursor pool, we found an almost linear relationship between the methods for both non‐tumour pancreas (R 2 = 0.99) and tumorous pancreas (R 2 = 0.87; data not shown).
Statistical analyses
Data were analysed using IBM SPSS 23 for Microsoft Windows®. All data are presented as mean and standard error of mean. Phenylalanine enrichments are expressed in mole per cent excess. Differences among tissues were analysed using the Wilcoxon signed‐rank test. Differences in plasma enrichments over time were analysed using Friedman ANOVA. A P‐value of <0.05 was considered significant.
Results
From the eight enrolled patients with cancer of the pancreatic head (Table 1), six patients underwent PPPD and two patients had palliative double bypass surgery without pancreatic resection. None of the patients had a history of systemic steroid or anti‐diabetic drug use within a year prior to surgery. Skeletal muscle and liver biopsies were taken from all eight patients without any complications. In the six patients receiving PPPD, healthy and tumour tissue from the pancreas were also obtained. In all patients, a plasma L‐[ring‐13C6]phenylalanine isotopic steady state was reached within 2 h after starting the tracer infusion, with plasma L‐[ring‐13C6]phenylalanine concentrations remaining stable throughout surgery (Figure 2).
Figure 2.
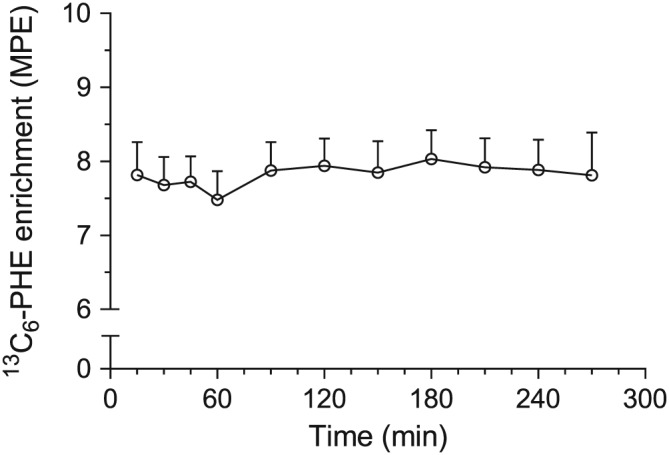
Plasma L‐[ring‐13C6]phenylalanine enrichments prior to and during surgery. Plasma phenylalanine enrichments remained stable throughout surgery (P = 0.325, Friedman ANOVA) for all patients. Values are displayed as mean + standard error of the mean. MPE, mole per cent excess.
Post‐absorptive skeletal muscle protein synthesis rates averaged 0.035 ± 0.005%/h, with rates ranging from 0.019 to 0.044%/h. Calculations using the successive biopsy approach resulted in similar muscle protein synthesis rates (0.039 ± 0.025%/h; P = 0.575). Quantification of the organ protein synthesis rates demonstrated much higher rates in organs compared with skeletal muscle (Figure 3). In comparison with skeletal muscle, protein synthesis rates in liver and pancreas were 13‐fold and 20‐fold higher, respectively, with mean values of 0.448 ± 0.043%/h (P = 0.012) and 0.694 ± 0.228%/h (P = 0.028). Liver protein synthesis rates were significantly lower when calculated using the successive biopsy approach (0.310 ± 0.055%/h; P = 0.012), indicating a possible inhibitory effect on protein synthesis during surgery.
Figure 3.
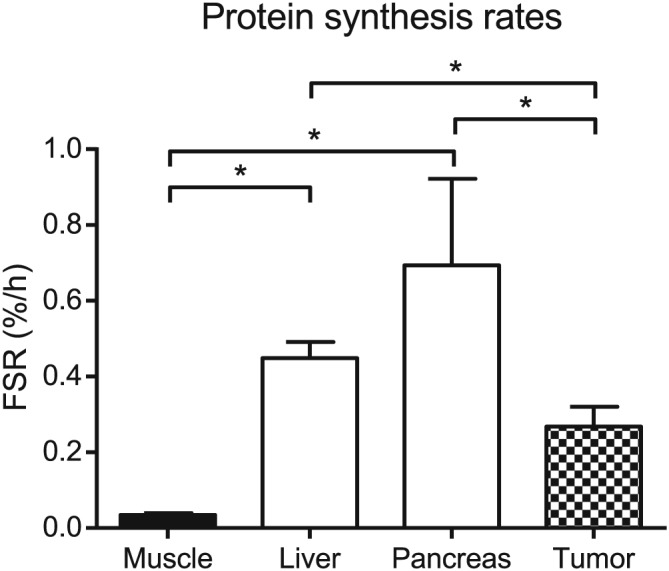
Muscle, organ, and tumour tissue protein synthesis rates. Liver and pancreas protein synthesis rates were 13‐fold and 20‐fold higher than those observed in skeletal muscle (* P = 0.012 and * P = 0.028, respectively, Wilcoxon signed‐rank). Protein synthesis rates in pancreatic tumour tissue were 2.5‐fold and 1.6‐folder lower, respectively, compared with those of the surrounding healthy pancreas and liver (* P = 0.028 and * P = 0.046, respectively, Wilcoxon signed‐rank). Data are presented as mean ± standard error of the mean. FSR, fractional synthetic rate.
Compared with healthy pancreas and liver tissue, samples from pancreatic tumours had up to 2.6‐fold lower protein synthesis rates (0.268 ± 0.053%/h; P = 0.028; Figure 3). Histological examination confirmed the presence of tumour tissue in all (n = 6) tumour biopsies (Figure 4).
Figure 4.
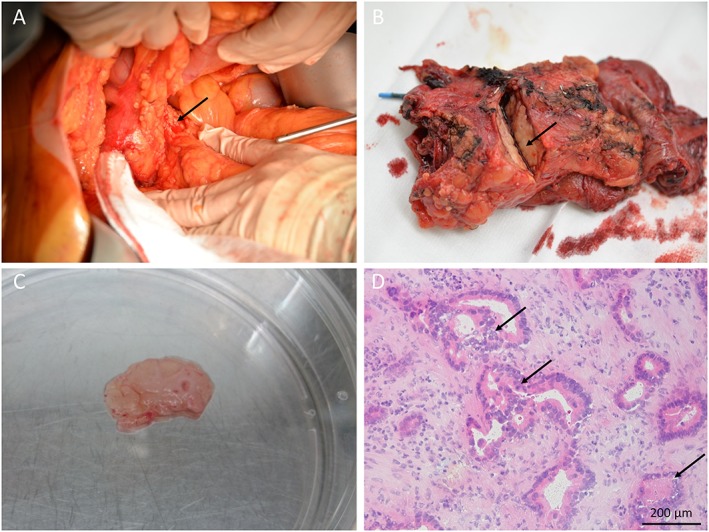
Pancreatic tumour biopsy. (A) The pancreas head (arrow) is visible during pancreatic surgery. (B) The pancreas specimen is incised by the pathologist revealing the pancreatic tumour (arrow). (C) Tumour containing tissue slice. (D) Frozen section of the tissue slice confirming the presence of adenocarcinoma (arrows) (haematoxylin/eosin staining).
Discussion
In this study, we demonstrated that organ and tumour protein synthesis rates are much higher than muscle protein synthesis rates, with liver and pancreas tissue protein synthesis rates exceeding tumour protein synthesis rates.
Although there are no studies that have compared human muscle and organ tissue FSRs, the observed liver protein synthesis rates are in line with liver protein synthesis rates of 0.32%/h13 and 0.62%/h14 reported previously in cancer patients using a 15N‐glycine tracer. Heys and colleagues reported even higher liver protein synthesis rates (0.96%/h),15 but this finding is likely attributable to the flooding‐dose technique used, which may have led to an overestimation of FSR due to the limited time frame of the experiment (<90 min).16 Liver FSRs of the current study translate into turnover rates of 11 ± 1% per day, implying that liver tissue is highly dynamic. These data are important in the context of liver regeneration after partial hepatectomy, where regrowth of the resected tissue has been observed at a rate of 0.8% of original liver volume per day.17 In the current patient cohort, the elevated CRP, IL‐1β, and IL‐6 levels suggest an (ongoing) acute phase response, which could be partly driving the high liver FSRs. However, although liver protein synthesis rates of secreted acute phase proteins are elevated in patients with high CRP,18 Fearon et al. demonstrated that fixed hepatic protein synthesis is actually reduced in patients with colorectal liver metastases and systemic inflammation when compared with non‐oncologic controls.13 However, as liver metastases could influence liver protein metabolism, future studies comparing liver FSRs in metastases‐free cancer patients with and without ongoing acute phase response are warranted. Interestingly, we found that liver protein synthesis rates were significantly lower when calculated using the successive biopsy approach. This may indicate a possible inhibitory effect of anaesthesia and/or surgery on liver tissue protein synthesis. The successive biopsy approach only uses the incorporation time during surgery (and anaesthesia), while the single biopsy approach uses both the periods before and during surgery to calculate the tracer incorporation. Therefore, effects of surgery and anaesthesia might be more noticeable in the successive biopsy approach. In contrast, skeletal muscle protein synthesis rates did not seem to be affected by anaesthesia as FSRs assessed by single vs. successive biopsy approaches were not different (see earlier discussion) and were consistent with those reported previously by our laboratory12 and the vast amount of published literature as shown in a systematic review.3 This shows that stable isotope tracer infusion studies requiring steady state conditions are feasible even during such complex abdominal surgery.
The high protein synthesis rates we observed in healthy pancreas are consistent with the pronounced secretory protein production by acinar cells.19 Pancreatic tumour protein synthesis rates were more than 2.5‐fold lower compared with healthy pancreas and liver tissue, in contrast to earlier findings that found relatively high tumour protein synthesis rates.14 Ductal pancreatic adenocarcinomas consist of epithelial tumour cells and stromal tissue that are not specialized in protein secretion. Considering the small size of pancreatic tumours (~2 cm in diameter20) and their relatively low protein synthesis rates as observed here, pancreatic tumours are unlikely to contribute substantially to whole‐body protein metabolism and are, therefore, unlikely a major driver of protein loss in wasting disorders such as cancer cachexia. However, tumour protein synthesis rates of 0.268 ± 0.053%/h corresponding to 6 ± 1% per day could theoretically enable a tumour to double in size within weeks, eventually leading to a mass that could affect whole‐body protein synthesis. The fact that this does not occur in the majority of pancreatic cancer patients further supports that protein breakdown rates in tumours are (more) important for regulating (and restricting) tumour growth, which is in line with previous observations in rats.6 Although we did not aim to assess protein breakdown rates within our study design, the fact that pancreatic tumours grow in size over time implies that tumour protein breakdown rates do not exceed tumour tissue protein synthesis rates.
It should be noted that although by itself the tumour FSR might not influence whole‐body protein metabolism directly, the specific proteins produced by the tumour may represent mediators altering whole‐body protein metabolism. Many tumours are capable of producing cytokines like IL‐1β, IL‐6, and TNF‐α, which can drive systemic inflammation and, as such, contribute to muscle wasting.21 This might be a possible explanation for the elevated levels of IL‐1β and IL‐6 in some patients in the present study. Several potential mediators of cancer cachexia produced by the tumour such as proteolysis‐inducing factor22 have been identified before, although their significance on stimulating protein breakdown in vivo in humans remains uncertain. Assessing synthesis rates of specific proteins could provide much more insight in the role of tumour protein synthesis in (muscle) wasting.
This is the first study to use contemporary isotope tracer methodology to determine protein synthesis rates across muscle, organ, and tumour tissues in vivo in humans. Two earlier in vivo studies in colon and breast cancer patients found relatively high tumour protein synthesis rates compared with liver tissue.23, 24 Potential explanations for the differences between our study and previous studies could be the advances in tracer methodology in recent years. Mass spectrometry resolving power has substantially improved, and we used a continuous tracer infusion instead of a flooding dose. Furthermore, biopsy sampling differences may account for different results. Earlier high protein synthesis rates reported for tumour tissue may have inadvertently included healthy tissue.14, 23, 24 Generally, tumour biopsies are taken from the edge of the tumour or by needle biopsy. This way, healthy tissue is easily mistaken for tumour tissue, especially in case of oedema or inflammation around the tumour. In our optimized tumour resection technique, we took a transverse section of the entire tumour that was identified by the pathologist macroscopically and confirmed microscopically thereafter, ensuring the sample analysed contained exclusively tumour tissue instead of healthy tissue.
In conclusion, pancreatic tumour protein synthesis rates are relatively low compared with protein synthesis rates in healthy tissue surrounding the tumour. Organ tissue protein synthesis rates by far exceed muscle protein synthesis rates and, as such, have the potential to strongly influence whole‐body protein metabolism in both health and disease. Therefore, the focus of protein metabolism research should be expanded to include both muscle and organ tissues in order to fully explore the contribution of organ protein synthesis rates in wasting disorders including various types of cancers, inflammatory bowel disease, and chronic infections.
Conflict of interest
None declared.
Funding
D.v.D. is supported as a PhD candidate by the Netherlands Organization for Scientific Research (NWO grant 022.003.011).
Acknowledgements
We thank Sofie Broen, Irene Fleur Kramer, Rianne Vaes, and Bas van de Valk for their help and assistance during the experimental test days as well as Dr Ronald van Dam, Prof. Laurens Stassen, and the Maastricht University Medical Centre surgery and anaesthesiology team for their help in taking biopsies and blood samples. Many thanks go out to Annemie Gijsen, Joy Goessens, Joan Senden, and Annemarie van Bijnen for their help in the laboratory analyses. Finally, we thank Hang Nguyen for editing our paper. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.25
van Dijk D. P. J., Horstman A. M. H., Smeets J. S. J., den Dulk M., Grabsch H. I., Dejong C. H. C., Rensen S. S., Olde Damink S. W. M., and van Loon L. J. C. (2019) Tumour‐specific and organ‐specific protein synthesis rates in patients with pancreatic cancer, Journal of Cachexia, Sarcopenia and Muscle, 10: 549–556. 10.1002/jcsm.12419.
References
- 1. Waterlow JC. Protein turnover with special reference to man. Q J Exp Physiol (Cambridge, England) 1984;69:409–438. [DOI] [PubMed] [Google Scholar]
- 2. Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 1975;35:609–616. [PubMed] [Google Scholar]
- 3. Smith GI, Patterson BW, Mittendorfer B. Human muscle protein turnover—why is it so variable? J Appl Physiol (1985) 2011;110:480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burd N, Hamer H, Pennings B, Pellikaan W, Senden J, Gijsen A, et al. Substantial differences between organ and muscle specific tracer incorporation rates in a lactating dairy cow. PLoS ONE 2013;8:e68109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wagenmakers A. Tracers to investigate protein and amino acid metabolism in human subjects. Proc Nutr Soc 1999;58:987–1000. [DOI] [PubMed] [Google Scholar]
- 6. Tayek JA, Blackburn GL, Bistrian BR. Alterations in whole body, muscle, liver, and tumor tissue protein synthesis and degradation in Novikoff hepatoma and Yoshida sarcoma tumor growth studied in vivo. Cancer Res 1988;48:1554–1558. [PubMed] [Google Scholar]
- 7. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 8. Hasselgren PO. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care 1999;2:201–205. [DOI] [PubMed] [Google Scholar]
- 9. Savary I, Debras E, Dardevet D, Sornet C, Capitan P, Prugnaud J, et al. Effect of glucocorticoid excess on skeletal muscle and heart protein synthesis in adult and old rats. Br J Nutr 1998;79:297–304. [DOI] [PubMed] [Google Scholar]
- 10. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 11. Lack EE. Pathology of the Pancreas, Gallbladder, Extrahepatic Biliary Tract, and Ampullary Region. Oxford: Oxford University Press; 2003. [Google Scholar]
- 12. Burd NA, Groen BB, Beelen M, Senden JM, Gijsen AP, van Loon LJ. The reliability of using the single‐biopsy approach to assess basal muscle protein synthesis rates in vivo in humans. Metabolism: Clin Exp 2012;61:931–936. [DOI] [PubMed] [Google Scholar]
- 13. Fearon KC, McMillan DC, Preston T, Winstanley FP, Cruickshank AM, Shenkin A. Elevated circulating interleukin‐6 is associated with an acute‐phase response but reduced fixed hepatic protein synthesis in patients with cancer. Ann Surg 1991;213:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stein T, Mullen J, Oram‐Smith J, Rosato E, Wallace H, Hargrove W. Relative rates of tumor, normal gut, liver, and fibrinogen protein synthesis in man. Am J Physiol 1978;234:52. [DOI] [PubMed] [Google Scholar]
- 15. Heys SD, Park KG, McNurlan MA, Keenan RA, Miller JD, Eremin O, et al. Protein synthesis rates in colon and liver: stimulation by gastrointestinal pathologies. Gut 1992;33:976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rennie MJ, Smith K, Watt PW. Measurement of human tissue protein synthesis: an optimal approach. Am J Physiol 1994;266:307. [DOI] [PubMed] [Google Scholar]
- 17. Haga J, Shimazu M, Wakabayashi G, Tanabe M, Kawachi S, Fuchimoto Y, et al. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver Transplant: Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc 2008;14:1718–1724. [DOI] [PubMed] [Google Scholar]
- 18. Preston T, Slater C, McMillan D, Falconer J, Shenkin A, Fearon K. Fibrinogen synthesis is elevated in fasting cancer patients with an acute phase response. J Nutr 1998;128:1355–1360. [DOI] [PubMed] [Google Scholar]
- 19. Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc 1978;53:211–354. [DOI] [PubMed] [Google Scholar]
- 20. Furukawa H, Iwata R, Moriyama N. Growth rate of pancreatic adenocarcinoma: initial clinical experience. Pancreas 2001;22:366–369. [DOI] [PubMed] [Google Scholar]
- 21. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. [DOI] [PubMed] [Google Scholar]
- 22. Wigmore SJ, Todorov PT, Barber MD, Ross JA, Tisdale MJ, Fearon KC. Characteristics of patients with pancreatic cancer expressing a novel cancer cachectic factor. Br J Surg 2000;87:53–58. [DOI] [PubMed] [Google Scholar]
- 23. Heys SD, Park KG, McNurlan MA, Calder AG, Buchan V, Blessing K, et al. Measurement of tumour protein synthesis in vivo in human colorectal and breast cancer and its variability in separate biopsies from the same tumour. Clin Sci (London, England: 1979) 1991;80:587–593. [DOI] [PubMed] [Google Scholar]
- 24. Gore DC, Wolfe KA, Foxx‐Orenstein A, Hibbert JM. Assessment of human colon cancer protein kinetics in vivo. Surgery 1997;122:593–599. [DOI] [PubMed] [Google Scholar]
- 25. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


