Abstract
Preeclampsia is the leading cause of death and morbidity world-wide for the mother and fetus during pregnancy. Preeclampsia does not only effect the mother and the baby during pregnancy, but can also have long-term effects, such as the increase risk of hypertension and cardiovascular disease, on the offspring and the postpartum mother later in life. The exact cause of preeclampsia is unknown, but women with preeclampsia have elevated concentrations of agonistic autoantibodies against the angiotensin II type 1 receptor (AT1-AA). These AT1-AA’s through multiple studies have shown to play a significant role in the pathology and possible genesis of preeclampsia. This review will discuss the discovery of AT1-AAs and the role of AT1-AAs in the pathophysiology of preeclampsia. This review will also discuss future therapeutic approaches towards the AT1-AA to prevent adverse pregnancy outcomes. Furthermore, we will examine the relationship between AT1-AA induced hypertension associated with increase oxidative stress, antiangiogenic factors (such as soluble fms-related tyrosine kinase-1 (sFlt-1), endothelin-1 (ET-1), inflammation, endothelial dysfunction, and reduced renal function. Understanding the pathological role of AT1-AAs in hypertensive pregnancies is important as we search for novel therapies to manage preeclampsia.
Keywords: Preeclampsia, angiotensin II type 1 receptor autoantibody (AT1-AA), oxidative stress, renal function, endothelin
The Role of AT1-AA in Pathophysiology of Preeclampsia
Preeclampsia (PE) is defined as new onset hypertension usually occurring during the third trimester of pregnancy.1,2 The incidence of PE worldwide ranges from 2–10%, with a higher incidence in developing countries ranging from 1 case per 100 pregnancies to 1 case per 1700 pregnancies.3 The incidence in developed countries, such as those in North America and Europe, have a similar incidence of PE ranging from 5–7 cases per 10,000 deliveries.3 In the USA It is estimated that between 3–5% of births are affected every year by PE.1 The differences incidences of preeclampsia according to the world health organization (WHO) is due to the lack of access to health care.3 PE is the leading cause of death for both the mother and the fetus during pregnancy.1 Women with PE have elevated concentrations of angiotensin II type 1 receptor agonistic autoantibodies (AT1-AA), antiangiogenic factors (such as sFlt1 and s-endoglin), oxidative stress, inflammation, reduced renal function, endothelial dysfunction, and increased sensitivity to Angiotensin II (ANGII) (Figure 1).1,2,4–11 The initiating event in preeclampsia is believed to be reduced placental perfusion due to shallow trophoblast invasion of the uterine spiral arterioles.2 This reduction in blood flow to the fetus, decreases the flow of essential nutrients which can result in intrauterine growth restriction.1 Such restriction of the placenta contributes to placental ischemia, which may result in oxidative stress, inflammation, and antiangiogenic factors contributing to hypertension, maternal end organ damage, and fetal demise.2 As of today there is no cure for PE, except for the delivery of the fetal placental unit.2 Thus drug discovery examining new pathways in PE are warranted to help these women. One such pathway worth further exploration is the AT1-AA.
Figure 1. AT1-AA induced hypertension flowchart.
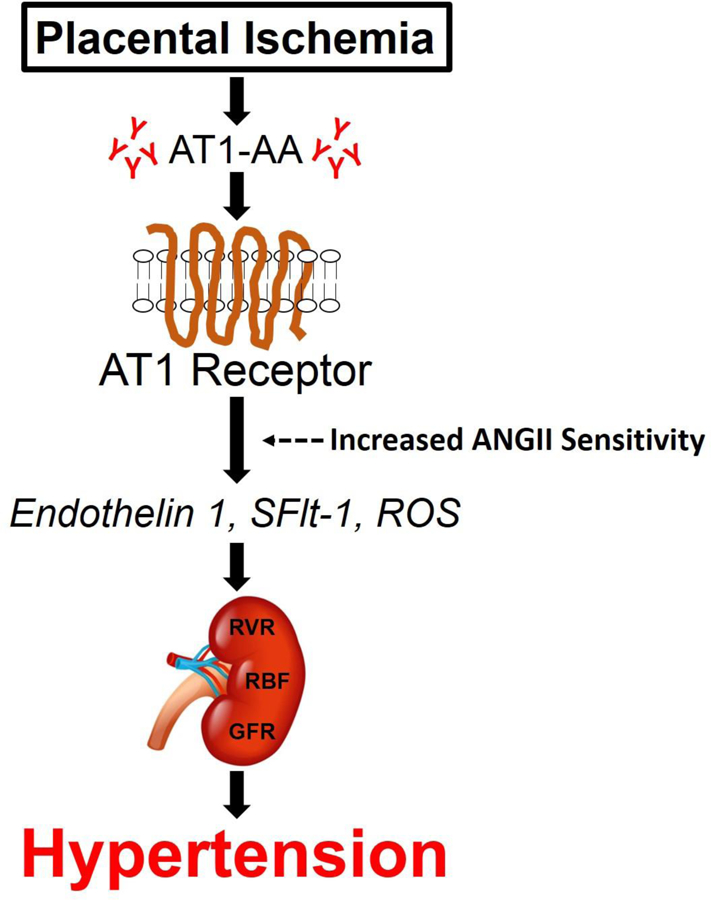
In hypertensive pregnancies, placental ischemia occurs, which increases the production of AT1-AAs. AT1-AAs bind and activate g-coupled AT1 receptors to increase ANGII sensitivity and to stimulate downstream pathways to elevate circulating concentrations endothelin 1, sFlt-1, and ROS that have a pathological effect on the kidney to increase RVR, decrease RBF, and thus decrease GFR (i.e. renal function). Thereby these changes in circulating factors and renal function contribute to hypertension.
Abbreviations: AT1-AA, angiotensin II type I receptor antibodies; ANGII, angiotensin II; AT1, angiotensin II type I receptor; sFlt-1, fms-like tyrosine kinase-1; ROS, reactive oxygen species; RVR, renal vascular resistance; RBF, renal blood flow; GFR, glomerular filtration rate.
AT1-AA Characterized
The angiotensin II type 1 receptor autoantibody (AT1-AA) was characterized in 1999 by a group of researchers in Germany.4 This group characterized the AT1-AA after isolation of total immunoglobulins from the serum of preeclamptic and normal pregnant women. After the isolation of these antibodies from the PE serum, they showed that these antibodies could stimulate the amount of beats per minute of eonatal rat cardiomyocytes similar to that seen with Angiotensin II (ANGII), which could be attenuated with losartan, an AT1 receptor blocker.4,5 After several other experiments with the AT1-AA, they realized that the antibody had the same affinity and ability to activate the AT1 receptor as ANG II.4 To understand the binding of the AT1-AA, they examined the number of beats per minute with the AT1-AA and small peptide sequences that corresponded to the AT1- receptor. From these experiments they identified a very specific seven amino acid (7AA) sequence on the 2nd extracellular loop of the receptor as the binding site of the AT1-AA. It is important to note that the AT1-AA does not compete for the AT1 receptor with ANG II, but rather the AT1-AA appears to enhance the binding and downstream effects of ANG II during pregnancy.6,9,10 Rather not the AT1-AA enhances ANG II binding and signaling in the absence of pregnancy is unknown, and is warranted for future research.
AT1-AA in Diseases
AT1-AAs are not only elevated in women with preeclampsia, but are also elevated in normotensive pregnancies with uterine growth-restricted fetuses, kidney transplant recipients undergoing renal rejection, patients with systemic sclerosis, vasculopathy, tissue fibrosis, hypertension, renovascular disease, and pregnant women with HELLP syndrome (a pregnancy disease characterized by Hemolysis, Elevated Liver enzymes, and Low Platelet count).12–16 AT1-AAs appear elevated in women with PE and women with abnormal uterine perfusion pregnancies as early as the second trimester of pregnancy.17 This suggests that AT1-AA may play a critical role in the pathogenesis of PE. Further studies by Hubel et al, show that some postpartum preeclamptic women have increased circulating concentrations of AT1-AA more than one year after delivery.18 The postpartum effects of AT1-AAs infused into rats during pregnancy caused an increase in blood pressure, heart rate, decreased cardiac function, cellular hyperplasia, and increased in susceptibility to ischemia/reperfusion injury in rats 16 weeks postpartum.19,20 Furthermore, not only were pregnant rats effected postpartum, but also the rat’s fetuses, which exhibited an increase in apoptosis of myocardial cells and susceptibility to ischemia-reperfusion injury.19,20 In women, it is known that preeclamptic women are at a 5–12 fold risk of end-stage renal disease, 2 fold risk of stroke, and 2 fold risk of cardiovascular disease later in life.21–24 Therefore it is possible that the sustained level of AT1-AAs in PE women, which first appeared during their PE episode during pregnancy, could be a risk factor for the development of cardiovascular disease, renal disease, and stroke for women later in life.
AT1-AA Induced hypertension- a model for PE
AT1-AAs cause an increase in blood pressure as well as other factors associated with PE, thereby making AT1-AAs administered to rats a model of PE. This model can be referred to as the AT1-AA induced hypertensive model in pregnant rats. One of the more prevalent current models of PE is the reduced uterine perfusion pressure (RUPP) model. The major characteristics of this model are hypertension, intrauterine growth restriction, proteinuria, increase in inflammation, increase in oxidative stress, endothelial dysfunction, and an increase in sflt-1.25 As shown in a study done by LaMarca et al, infusion of AT1-AA from serum of RUPP rats into pregnant rats increases blood pressure, as well as tissue concentrations of preproendothelin, a precursor for a vasoconstrictive factor ET-1, which is associated with late pregnancy PE.9,26,27 Furthermore, there is significant evidence of endothelial dysfunction as a result of AT1- AA infusion.9,26,28,29 AT1-AAs increase production of reactive oxygen species causing oxidative stress in the placenta during pregnancy.8–10 It is hypothesized that PE patients having increased AT1-AAs and abnormal uterine Doppler ultrasounds, that AT1-AAs can contribute to the impairment of placenta and fetal development due to increased placental vasoconstriction.17,30 Not only is there a decrease in placental blood flow due to placental vasoconstriction, but also an increase in renal vascular resistance.10 In our in-vivo and ex-vivo experiments, we have shown an increase in renal artery resistance index and vasoconstriction of isolated renal afferent arterioles from normal pregnant rats, incubated with the AT1-AAs and further constricted with ANG II and the AT1-AA 9. Studies conducted by Cunningham et al, demonstrated that the increase in renal vascular resistance (RVR) observed in ANG II and AT1-AA rats could be attributed to the systemic 3 fold increase in plasma isoprostane concentrations, a marker for oxidative stress.10 Moreover, there is also evidence that AT1-AA causes increases in sFlt-1 which can in turn increase proteinuria and is a major cause of hypertension during pregnancy.31,32 Tumor necrosis factor alpha (TNF-α) is elevated in AT1-AA infused pregnant rats.31 TNF-α is slightly elevated in a normal pregnancy, but is increased by twofold in preeclamptic pregnancies.33,34 While it has recently been shown that TNF- α is increased in pregnant mice with an injection of AT1-AAs31, we have shown AT1-AA to be produced in response to TNF alpha induced hypertension during pregnancy. Moreover, multiple studies have shown that TNF- α and AT1-AA contributes to increases in soluble fms-like tyrosine kinase (sFlt-1) all of which are strongly implicated in the pathology of PE during pregnancy in the rat. 31,32
It has been shown previously in our lab that infusion of AT1-AA causes increased placental oxidative stress9 partly due to activation of NADPH oxidase, which produces superoxide.35,36 Superoxide is a potent reactive oxygen species that is elevated in preeclamptic patients, where it can cause vascular dysfunction, poor invasion of trophoblast cells for placentation, placental hypoxia, release inflammatory cytokines, decease nitric oxide bioavailability, and increase antiangiogenic factors, such as sFlt-1.37 Administration of the antioxidant, tempol, attenuated the rise in hypertension in response to AT1-AA administered to pregnant rats along with decreasing the renal artery resistance index.8,9 Therefore these studies suggests that oxidative stress is a major contributor to the pathology of hypertension due to AT1-AAs in PE. To understand the role of AT1-AA’s activation of the AT1 receptor to increase oxidative stress and lead to hypertension; administration of losartan attenuated the increased oxidative stress and hypertension in AT1-AA induced hypertension rats.8 Thus supporting the link between AT1 receptor activation and increased oxidative stress.8,10 Therefore blockade of AT1-AAs to improve blood pressure through a decrease in reactive oxygen species, such as superoxide, could be a potential therapy for PE patients.
AT1-AA Renal Function
Studies from our lab have shown that AT1-AA alone deceases glomerular filtration rate (GFR), renal function, by decreasing renal blood flow (RBF) and renal vascular resistance (RVR) in pregnant rats.10 These studies also show a greater and significant 3 fold increase in RVR with pregnant rats infused with both ANG II and AT1- AA together.9,10 The increase in RVR caused a significant decrease in RBF and GFR in these studies.10 Renal afferent arterioles from pregnant rats subjected to a dose of either ANG II or AT1-AA alone did not cause vasoconstriction, but with both ANG II and AT1-AA there was an increase in vasoconstriction.10 Therefore these data taken together suggest a synergistic effect with ANG II and the AT1-AA especially in the kidney and suggest that AT1-AAs in pregnancy enhance ANG II mediated RVR, which leads to a decrease in renal function.
AT1-AA Endothelial Function
One pathway by which AT1-AAs increase blood pressure is by activating the endothelin-1(ET-1) system. In a previous study from our lab, we found that AT1-AA treated rats had an increase in blood pressure and preproendothelin (PPET-1) mRNA expression in the placenta, kidney, and the aorta.9,26,36 This increase in blood pressure was eliminated with the infusion of ETA receptor antagonist. In a more recent study it was demonstrated that AT1-AA and ANG II both stimulate endothelial cells to secrete ET-1, and that a combination of the two increases the secretion to greater magnitude.38 Furthermore we have shown that PPET-1 mRNA was blunted in AT1-AA induced hypertensive rats with AT1 receptor antagonist.26 PPET-1 is increased in the renal cortices and placentas of rats with an infusion of both ANG II and AT1-AA. (Brewer et al.).9 Furthermore, studies by Roberts et al, showed that human umbilical vein endothelial cells (HUVECs) incubated with the serum from RUPP rats had a 2 fold increase in ET-1 production after 6 hours of exposure.39 They also showed that HUVECs incubated with RUPP serum had a 3 fold increase in ET-1 after 18 hours of exposure with RUPP serum. 39 Thus these results taken together suggest that circulating factors in the RUPP serum causes an increase in ET-1.39 HUVECS cells treated with RUPP serum and pretreated with losartan, an AT-1 receptor antagonist, ET-1 production was normalized to concentrations of HUVECS incubated with normal pregnant rat serum.39
AT1-AA future directions and therapy
It has been shown that inhibition of the AT1 receptor by angiotensin II receptor blockers (ARB), improves blood pressure, renal function, and circulating sFlt-1.40–42 However ARBs are not good during pregnancy due to their well-documented association with birth defects.43–46 Thus another therapeutic option to inhibit the actions of AT1-AAs directly is warranted. Recently, we have shown that AT1-AA inhibition by a specific modified peptide sequence which binds AT1-AAs and prevent them from binding to the AT1 receptor during pregnancy improved the PE symptoms associated with placental ischemic rats. Specifically, we showed improvement in maternal blood pressure, secretion of anti-angiogenic and vasoconstrictive factors (such as pre- proendothelin, sFlt-1), renal function, systemic NO bioavailability, and oxidative stress in RUPP (rat model of PE) rats. We also showed a decrease in cytolytic natural killer cells, which are associated with PE in women and mediators of oxidative stress and secrete inflammatory cytokines that can cause tissue damage.47 Our data did not show any improvements in birth weight from placental ischemic pregnant rats, but also did not show a further decline in pup weight, suggesting that AT1-AA inhibition is not toxic to the offspring.
Summary
Understanding the role of the AT1-AA in the pathophysiology of preeclampsia is important, because it’s evident that the AT1-AA plays a critical role in the pathophysiology of this disease and that it could be a potential therapeutic target (Figure 1). The studies collected in this review highlight the roles that the autoantibody to increase several factors associated with PE that can lead to hypertension. In conclusion we believe that blocking the autoantibody could serve as a major treatment for the disease and should be further explored.
Acknowledgments
Funding Sources
This work was supported by NIH grants HL78147 and HL51971 and HD067541 awarded to BL. Funding was also supported by P20GM121334.
References
- 1.Amaral LM, Cunningham MW Jr., Cornelius DC, LaMarca B. Preeclampsia: long-term consequences for vascular health. Vascular health and risk management 2015;11:403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension December 2005;46(6):1243–1249. [DOI] [PubMed] [Google Scholar]
- 3.Osungbade KO, Ige OK. Public health perspectives of preeclampsia in developing countries: implication for health system strengthening. Journal of pregnancy 2011;2011:481095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallukat G, Neichel D, Nissen E, Homuth V, Luft FC. Agonistic autoantibodies directed against the angiotensin II AT1 receptor in patients with preeclampsia. Canadian journal of physiology and pharmacology February 2003;81(2):79–83. [DOI] [PubMed] [Google Scholar]
- 5.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. The Journal of clinical investigation April 1999;103(7):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herse F, LaMarca B. Angiotensin II type 1 receptor autoantibody (AT1-AA)- mediated pregnancy hypertension. American journal of reproductive immunology April 2013;69(4):413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller-Deile J, Schiffer M. Preeclampsia from a renal point of view: Insides into disease models, biomarkers and therapy. World journal of nephrology November 6 2014;3(4):169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parrish MR, Wallace K, Tam Tam KB, et al. Hypertension in response to AT1- AA: role of reactive oxygen species in pregnancy-induced hypertension. American journal of hypertension July 2011;24(7):835–840. [DOI] [PubMed] [Google Scholar]
- 9.Brewer J, Liu R, Lu Y, et al. Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody- enhanced renal and blood pressure response during pregnancy. Hypertension November 2013;62(5):886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham MW Jr., Williams JM, Amaral L, et al. Agonistic Autoantibodies to the Angiotensin II Type 1 Receptor Enhance Angiotensin II-Induced Renal Vascular Sensitivity and Reduce Renal Function During Pregnancy. Hypertension November 2016;68(5):1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmon AC, Cornelius DC, Amaral LM, et al. The role of inflammation in the pathology of preeclampsia. Clinical science March 2016;130(6):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu ML, Herlitz H, Schulze W, et al. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. Journal of hypertension July 2000;18(7):945–953. [DOI] [PubMed] [Google Scholar]
- 13.Dragun D The role of angiotensin II type 1 receptor-activating antibodies in renal allograft vascular rejection. Pediatric nephrology July 2007;22(7):911–914. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Cui Z, Zhang F, Chang W, Chen L, Liu L. Angiotensin II and cAMP regulate AT(1)-mRNA expression in rat cardiomyocytes by transcriptional mechanism. European journal of pharmacology July 12 2002;448(1):1–9. [DOI] [PubMed] [Google Scholar]
- 15.Liao YH, Wei YM, Wang M, Wang ZH, Yuan HT, Cheng LX. Autoantibodies against AT1-receptor and alpha1-adrenergic receptor in patients with hypertension. Hypertension research : official journal of the Japanese Society of Hypertension July 2002;25(4):641–646. [DOI] [PubMed] [Google Scholar]
- 16.Fischer T, Wallukat G, Schneider MP, Schlembach D, Munz W, Homuth V. HELLP syndrome in the 18th week of gestation in association with elevated angiotensin AT(1)-receptor autoantibodies. European journal of obstetrics, gynecology, and reproductive biology August 2001;97(2):255–257. [DOI] [PubMed] [Google Scholar]
- 17.Walther T, Wallukat G, Jank A, et al. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension December 2005;46(6):1275–1279. [DOI] [PubMed] [Google Scholar]
- 18.Hubel CA, Wallukat G, Wolf M, et al. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension March 2007;49(3):612–617. [DOI] [PubMed] [Google Scholar]
- 19.Wang HP, Zhang WH, Wang XF, et al. Exposure to AT1 receptor autoantibodies during pregnancy increases susceptibility of the maternal heart to postpartum ischemia-reperfusion injury in rats. International journal of molecular sciences June 27 2014;15(7):11495–11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin Z, Zhang W, Yang H, et al. Maternal treatment with agonistic autoantibodies against type-1 angiotensin II receptor in late pregnancy increases apoptosis of myocardial cells and myocardial susceptibility to ischemia-reperfusion injury in offspring rats. PloS one 2013;8(11):e80709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. The New England journal of medicine September 12 1996;335(11):768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. Bmj April 19 2003;326(7394):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. The New England journal of medicine August 21 2008;359(8):800–809. [DOI] [PubMed] [Google Scholar]
- 24.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta- analysis. Bmj November 10 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. American journal of physiology. Heart and circulatory physiology July 2012;303(1):H1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaMarca B, Parrish M, Ray LF, et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin- 1. Hypertension October 2009;54(4):905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension December 2008;52(6):1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parrish MR, Ryan MJ, Glover P, et al. Angiotensin II type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gender medicine June 2011;8(3):184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Wang F, Lau WB, et al. Autoantibodies isolated from preeclamptic patients induce endothelial dysfunction via interaction with the angiotensin II AT1 receptor. Cardiovascular toxicology March 2014;14(1):21–29. [DOI] [PubMed] [Google Scholar]
- 30.Irani RA, Zhang Y, Blackwell SC, et al. The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia. The Journal of experimental medicine November 23 2009;206(12):2809–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irani RA, Zhang Y, Zhou CC, et al. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension May 2010;55(5):1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parrish MR, Murphy SR, Rutland S, et al. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. American journal of hypertension August 2010;23(8):911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen HL, Yang YP, Hu XL, Yelavarthi KK, Fishback JL, Hunt JS. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. The American journal of pathology August 1991;139(2):327–335. [PMC free article] [PubMed] [Google Scholar]
- 34.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. American journal of reproductive immunology August 1998;40(2):102–111. [DOI] [PubMed] [Google Scholar]
- 35.Dechend R, Viedt C, Muller DN, et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation April 1 2003;107(12):1632–1639. [DOI] [PubMed] [Google Scholar]
- 36.Dhillion P, Wallace K, Herse F, et al. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. American journal of physiology. Regulatory, integrative and comparative physiology August 15 2012;303(4):R353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. International journal of molecular sciences March 2 2015;16(3):4600–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenzel K, Rajakumar A, Haase H, et al. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension July 2011;58(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension March 2006;47(3):615–618. [DOI] [PubMed] [Google Scholar]
- 40.Dechend R, Homuth V, Wallukat G, et al. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation May 23 2000;101(20):2382–2387. [DOI] [PubMed] [Google Scholar]
- 41.Regal JF, Strehlke ME, Peterson JM, et al. Role of IgM and angiotensin II Type I receptor autoantibodies in local complement activation in placental ischemia- induced hypertension in the rat. Molecular immunology October 2016;78:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy SR, Cockrell K. Regulation of soluble fms-like tyrosine kinase-1 production in response to placental ischemia/hypoxia: role of angiotensin II. Physiological reports February 1 2015;3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korkes HA, de Oliveira LG, Berlinck L, et al. Human fetal malformations associated with the use of an angiotensin II receptor antagonist: case report. Jornal brasileiro de nefrologia : ‘orgao oficial de Sociedades Brasileira e Latino- Americana de Nefrologia July-September 2014;36(3):410–413. [DOI] [PubMed] [Google Scholar]
- 44.Alwan S, Polifka JE, Friedman JM. Angiotensin II receptor antagonist treatment during pregnancy. Birth defects research. Part A, Clinical and molecular teratology February 2005;73(2):123–130. [DOI] [PubMed] [Google Scholar]
- 45.Saji H, Yamanaka M, Hagiwara A, Ijiri R. Losartan and fetal toxic effects. Lancet February 3 2001;357(9253):363. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez SI, Seltzer AM, Fuentes LB, Forneris ML, Ciuffo GM. Inhibition of Angiotensin II receptors during pregnancy induces malformations in developing rat kidney. European journal of pharmacology June 24 2008;588(1):114–123. [DOI] [PubMed] [Google Scholar]
- 47.Elfarra J, Amaral LM, McCalmon M, et al. Natural Killer Cells Mediate Pathophysiology in Response to Reduced Uterine Perfusion Pressure. Clinical science October 17 2017. [DOI] [PMC free article] [PubMed]


