Abstract
The human retina is a complex tissue responsible for detecting photons of light and converting information from these photons into the neurochemical signals interpreted as vision. Such visual signaling not only requires sophisticated interactions between multiple classes of neurons, but also spatially-dependent molecular specialization of individual cell types. In this study, we performed single-cell RNA sequencing on neural retina isolated from both the fovea and peripheral retina in three human donors. We recovered a total of 8,217 cells, with 3,578 cells originating from the fovea and 4,639 cells originating from the periphery. Expression profiles for all major retinal cell types were compiled, and differential expression analysis was performed between cells of foveal versus peripheral origin. Globally, mRNA for the serum iron binding protein transferrin (TF), which has been associated with age-related macular degeneration pathogenesis, was enriched in peripheral samples. Cone photoreceptor cells were of particular interest and formed two predominant clusters based on gene expression. One cone cluster had 96% of cells originating from foveal samples, while the second cone cluster consisted exclusively of peripherally isolated cells. A total of 148 genes were differentially expressed between cones from the fovea versus periphery. Interestingly, peripheral cones were enriched for the gene encoding Beta-Carotene Oxygenase 2 (BCO2). A relative deficiency of this enzyme may account for the accumulation of carotenoids responsible for yellow pigment deposition within the macula. Overall, this data set provides rich expression profiles of the major human retinal cell types and highlights transcriptomic features that distinguish foveal and peripheral cells.
Keywords: retina, cones, fovea, single-cell, transferrin
Graphical Abstract

1. Introduction
The human retina is a complex sensory tissue responsible for detecting photons of light and converting such photic patterns into neurochemical information that can ultimately be registered in the brain as vision. This elaborate physiologic process requires the coordinated function of a multitude of neurons and supporting cell types. In the adult retina, unique photoreceptor cells (rods and three classes of cones) convert light into an electrical signal, and this electrical signal is propagated to bipolar cells and subsequently retinal ganglion cells. The photoreceptor-bipolar cell-ganglion cell vertical pathway is modulated by additional classes of interneurons known as horizontal and amacrine cells. Local structural and metabolic support is provided by astrocytes and MÜller glia. In addition, the neural retina has its own blood supply, with continuous type capillaries that predominantly support the inner retina, and a population of microglia. Based on the distribution of cell surface markers, neurotransmitter composition, size, structure, and synaptic targets, each class of retinal neurons has several subclasses (Jo et al., 2018).
Adding to this complexity, populations of different cell types vary to an enormous degree based upon location in the retina. Humans and other primates possess a fovea centralis, a structure characterized by an excavated pit of approximately 0.65 – 0.7 mm in diameter that lacks rods, bipolar cells, ganglion cells, and vascular elements and is dominated by morphologically distinct cones and Müller cells (Bringmann et al., 2018; Yuodelis and Hendrickson, 1986). Foveal cones differ from extrafoveal cones as they possess elongated inner and outer segments and relatively long axons that form Henle’s nerve fiber layer, whereas peripheral cones exhibit the characteristic teardrop shaped “cone” inner segment and short outer segment, as originally described by Cajal (Ramón y Cajal, 1972).
Characterizing molecular differences that exist between the central and peripheral retinal is of high interest. Gene expression comparisons between macular versus peripheral retina performed with SAGE (Sharon et al., 2002) and RNA sequencing (Li et al., 2014b; Whitmore et al., 2014) have largely demonstrated regionally distinct retinal transcriptomes. Recently, single-cell RNA sequencing has been performed in Macaca fascicularis on foveal versus peripheral primate retina (Peng et al., 2019). This study concluded that most cell types exist in both foveal and peripheral retina, but cell types differ regionally in both proportion and gene expression.
In addition to basic science interest, a better understanding of the regional differences between cell classes has potential clinical importance. A number of human retinal diseases can either selectively spare or selectively damage foveal cones (Querques et al., 2016; Schindler et al., 2010), and even between patients with the same genetic cause of disease, modifying genes can alternatively prevent or allow damage to the foveal cones (Mullins et al., 2012). Foveal cones are responsible for visual acuity, and the proper function of this small region of the retina represents the difference between 20/20 vision and legal blindness. Moreover, patients who have lost their photoreceptor cells due to inherited or acquired diseases of the retina can potentially benefit greatly from autologous, induced pluripotent stem cell derived photoreceptor cell replacement therapy (Burnight et al., 2018). A more thorough understanding of the molecular architecture of different cell types will therefore be useful in directing, identifying and selecting cells that will be most beneficial for transplantation.
In order to both generate a thorough catalog of cell type specific transcripts in human retina and to identify region specific differences in these cell types, we performed a series of experiments using single-cell RNA sequencing on human donor retina of three individuals from both the foveal and peripheral retina. A total of 17 clusters of cells were identified, representing most expected retinal cell populations. Regional differences, particularly in foveal versus peripheral cones, are highlighted. Original and processed data for this project have been uploaded to NCBI’s Gene Expression Omnibus (GSE130636).
2. Materials and Methods
2.1. Donor eyes
Human donor eyes (n=3) were obtained through the Iowa Lions Eye Bank after full consent by family members and in accordance with the Declaration of Helsinki. Donor information is presented in Table 1. All donor eyes were received in the laboratory within 5.5 hours after death and were processed immediately. A 2-mm foveal-centered punch and a 4-mm peripheral punch from the inferotemporal region (approximately 9 mm from the foveal center, see Figure 1A) were used to acquire neural retina, which was separated from the underlying retinal pigment epithelium (RPE) and choroid. One set of donor eyes was received in the laboratory at 6 hours and 36 minutes post mortem. Due to the longer time post mortem, this set of eyes was not used for RNA-sequencing experiments but instead underwent morphological analysis with transmission electron microscopy. Neural retina from this set of eyes was dissociated and cryopreserved in the same manner as the retina used for single-cell RNA sequencing, as described below (2.2 Cell Dissociation). Thawed, dissociated cells from this donor were fixed in one half strength Karnovsky fixative overnight. The cell pellet subsequently underwent osmication, dehydration and processing for transmission electron microscopy, using methods described previously (Songstad et al., 2017; Tucker et al., 2013).
Table 1:
Sample information from three grossly normal human donors.
| Age | Sex | Cause of death | Time to preservation | Eye | Ocular history notes | |
|---|---|---|---|---|---|---|
| Donor-1 | 89 | M | Cancer | 5:21 | OD | Early stage glaucoma documented |
| Donor-2 | 54 | M | Cardiac arrest | 5:29 | OD | Records not received |
| Donor-3 | 82 | F | Cardiopulmonary arrest | 4:18 | OD | No ophthalmic records |
Figure 1. Experimental overview and classification of human retinal cell types.
A. A 2-mm foveal-centered punch (red) and a 4-mm peripheral punch (blue) were acquired from the retina of three grossly normal human donors. The 4-mm punch was acquired from the inferotemporal region approximately 9 mm in distance from the fovea. B-C. Transmission electron microscopy of dissociated and cryopreserved peripheral retina from an independent donor (see 2.1: Donor Eyes). Multiple morphologically distinct cell types were observed, including Müller cells (*) and neural cells. D. Unsupervised tSNE clustering of the 8,217 cells yielded 17 clusters. Subpopulation classification of horizontal cells and amacrine cells (insert) based on expression of cell specific transcripts was manually performed (SI Figure 3). E. Schematic of the diverse cellular subtypes in the human retina. F. For each cluster, the average RNA expression of each gene was calculated. The top 100 most enriched genes (average logFC) from each cluster were compiled and used as features for hierarchical clustering analysis. Violin expression plots of genes previously associated with retinal cell types were plotted alongside the dendrogram.
2.2. Cell Dissociation
Neural retina was dissociated using the Papain Dissociation System (Worthington Biochemical Corporation, Lakewood NJ) according to manufacturer's instructions with the following modifications. Cell suspensions were incubated at 37°C on a shaker, and gently mechanically agitated with a pipette every 15 minutes for a total of 1.25 hours. Dissociated cells were resuspended in DMSO-based Recovery Cell Culture Freezing Media (Life Technologies Corporation, Grand Island NY). Suspensions were placed in a Cryo-Safe cooler (CryoSafe, Summerville SC) to cool at 1°C/minute in a −80°C freezer for 3-8 hours before storage in liquid nitrogen.
2.3. Single-cell RNA sequencing
Cryopreserved cells were thawed in a 37°C water bath and resuspended in PBS + 0.04% non-acetylated bovine serum albumin (New England Biolabs, Ipswich MA) at a concentration of 1000 cells/μL. Viability analysis on peripheral samples was performed with Annexin V/Dead Cell Apoptosis Kit (Life Technologies Corporation, Eugene OR) with viability > 85% using the Countess II FL Automated Cell Counter (ThermoFisher Scientific, Waltham MA). Single cells were captured and barcoded using the Chromium System with the v3 single cell-reagent kit (10x Genomics, Pleasanton CA). Sequencing was performed on pooled libraries using the Illumina HiSeq 4000 platform (San Diego, CA), generating 150 base pair paired-end reads.
2.4. Computation
FASTQ files were generated from base calls converted with bcl2fastq software by the University of Iowa Genomic Division. FASTQ files were mapped to the human genome (hg19) and libraries were aggregated to the same effective sequencing depth with the CellRanger v3.0.1 pipeline (Zheng et al., 2017). Cells with unique gene counts of less than 200 were filtered, as were cells with greater than 2,500 unique genes per cell to eliminate potential doublets. Additionally, cells with greater than 60% of reads originating from mitochondrial genes were excluded. A total of 21,649 distinct genes were detected (SI Figure 2). Aggregated reads were log-normalized with Seurat (v2.3.4) using a scale factor of 10,000 (Butler et al., 2018). Cell-to-cell variation regression was performed to minimize batch effect introduced in sample preparation by regressing out unique molecular identifiers (nUMI) and percentage of reads from mitochondrial genes. The first ten principal components were used as inputs for t-distributed stochastic neighborhood embedding (tSNE) graph-based cell clustering. A granularity resolution value of 0.6 was used to discriminate clusters. Hierarchical clustering was performed on the normalized average gene expression for each cluster, and a dendrogram of relationships between the clusters was constructed with the Canberra distance metric and complete linkage method. Differential gene expression analysis was performed using the Wilcoxon rank sum test for all generated comparisons. Genes were considered to be differentially expressed if they (A) exhibited an absolute log fold change greater than 1.0, (B) had an adjusted p-value less than 1.0 × 10−3, and (C) were expressed above background in at least 25% of cells in either population being compared. In order to more specifically explore gene expression differences beyond metabolic state, ribosomal genes were removed from differential expression analysis.
2.5. Computational Comparisons
Genes identified to be differentially expressed between foveal and peripheral regions were explored in two additional regional RNAseq datasets. In the first additional dataset, log normalized expression data from monkey foveal versus peripheral retina cell types (Peng et al., 2019) was downloaded (accession number GEO: GSE118480). Expression values of cone photoreceptor cells and Müller glial cells were extracted and unlogged, and the mean expression of each gene originating from the fovea and periphery was computed. As average log fold change computations in Seurat use natural log, the natural log of the fold change was computed. We manually evaluated the transcripts for each gene against the Macaque genome (macFas5) on the UCSC genome browser. We identified several transcripts which were judged to better represent the gene’s expression level than the transcript in the Macaque GTF. Specifically, we identified LOC101866521 (TF), LOC102118176 (MUM1), LOC102146485 (RP11-39E3.3), LOC102136451 (MT1G), and LOC101866646 (MEG3).
In the second additional dataset, raw fastqs were downloaded (accession number dbGaP: phs001151.v1.p1) for four paired macular and temporal retinal samples (Whitmore et al., 2014). Fastqs were mapped to the human genome build hg19 with STAR and quantified with featureCounts. The natural log fold change (macular retina versus temporal retina) for each gene of interest was calculated.
3. Results
3.1. Experimental approach
Two regions of neural retina were acquired from three grossly normal human donors (Table 1). In order to assess regional differences in gene expression, a 2-mm fovea-centered punch and a 4-mm peripheral punch were obtained from each retina (Figure 1A). Although gene expression differences between the superior, inferior, nasal, and temporal retina are minimal (Whitmore et al., 2014), each peripheral punch was acquired from the inferotemporal retina to minimize potentially confounding regional expression differences. Each isolated retinal sample was gently dissociated into a suspension of individual cells. In order to assess the dissociation process, cell suspensions from an additional donor were pelleted and transmission electron microscopy was performed (see 2.1: Donor Eyes), and multiple well-preserved and morphologically distinct cell types were visualized including Müller cells (Figure 1B-C, asterisk) and neural cells (Figure 1C). Additional transmission electron microscopy images are also provided (SI Figure 1).
Independent single-cell RNA libraries were prepared using dissociated foveal and peripheral samples from each donor. After sequencing, a total of 8,217 cells passed filtering and quality control criteria, with 3,578 cells originating from the fovea and 4,639 cells originating from the periphery. Principal component (PC) analysis was performed, and a graph-based clustering approach using the first 10 principal components allowed for visualization of cells with similar transcriptomic profiles in two-dimensional space with t-distributed stochastic neighborhood embedding (t-SNE) (Figure 1D). A total of 17 cell clusters were identified, ranging in size from 68 cells to 1,384 cells. All clusters had cell contributions from each of the three donors (SI Table 1), although some clusters consisted exclusively of cells from either foveal or peripheral regions of the retina (SI Figure 2).
3.2. Cell cluster classification
The retina is composed of a diverse collection of neuronal cell types (Figure 1E). In order to classify each of the clusters to a specific cell type, average expression profiles for each of the 17 clusters were computed and used to construct a dendrogram of transcriptomically similar cell populations (Figure 1F). Next, we compared the relationship between cell clusters from this single-cell analysis and previously reported transcriptional profiles of retinal cell types (Macosko et al., 2015; Siegert et al., 2012).
Photoreceptor cells can be subclassified into rods, which provide vision in low light settings, and cones, which operate in bright light and are responsible for color and high acuity vision. Clusters 1–2 were interpreted as being derived from rod photoreceptor cells, with high expression of the rod-specific opsin rhodopsin (RHO) as well as the rod-specific phosphodiesterase PDE6A. Clusters 3-4 were proposed to consist of cone photoreceptor cells, which were enriched for the cone-specific phosphodiesterase PDE6H and arrestin ARR3. Interestingly, the vast majority of cones in Cluster 3 (96%) arose from foveal samples, while 100% of the cones in cluster 4 were of peripheral origin (SI Table 1), indicating that central and peripheral cones are transcriptionally dissimilar.
Clusters 5-6 were interpreted as being derived from bipolar cells, with strong expression of TRPM1, a cation channel involved in bipolar cell depolarization. Cluster 7 was interpreted as retinal ganglion cells, with high expression of THY1 and NRN1, which regulate axonal architecture and neurofilament integrity.
Interestingly, Cluster 8 demonstrated transcriptomic evidence of two populations of interneurons, horizontal cells, which expresses ONECUT2, and amacrine cells, which express NRXN2 (SI Figure 3). Consequently, subpopulations corresponding to horizontal cells and amacrine cells were manually characterized (Figure 1D, insert).
Cells in Cluster 9 did not express any of our selected cell-specific genes (Figure 1F). Of all 494 cells in Cluster 9, 80% originated from the foveal sample of donor 2. In attempt to classify this population, differential expression analysis was performed to identify enriched transcripts in this cluster (SI Table 2). The two most enriched genes in this population were MIAT and MEG3, which have been associated with diabetic retinopathy pathogenesis (Qiu et al., 2016; Zhang et al., 2017). Health records from this donor could not be obtained, and therefore these cells were designated with an unknown classification.
The inner retina receives its blood supply from the central retinal artery. Pericytes and smooth muscle cells, proposed Cluster 10, regulate blood flow through ICAM2 expressing retinal endothelial cells, which are interpreted as Cluster 11. Microglia, resident immune cells of the retina, were restricted to Cluster 12 and are noted for strong expression of complement component C1QA.
Clusters 13-17 were interpreted as being derived from glial cells (Müller cells and/or astrocytes). All five glial clusters also showed elevated expression of RLBP1, a gene with high expression in Müller cells. Glial Clusters 13, 14, and 17 were comprised predominantly of cells isolated from the peripheral retina, while glial Clusters 15 and 16 had an overwhelming majority of cells from the fovea (SI Table 1).
Additional expression of several previously-reported cell-specific genes (Macosko et al., 2015; Siegert et al., 2012) were overlaid with the tSNE plot in the form of heatmaps, and largely corroborate our proposed cell type classifications (Figure 2).
Figure 2: Cell specific genes overlaid with tSNE clustering.
tSNE clustering overlaid with heatmaps of genes previously reported to be enriched in cones (PDE6H, ARR3), rods (RHO, NRL), Müller cells (GLUL, RLBP1), bipolar cells (TRPM1, GNG13), retinal ganglion cells (SNCG, NEFM), and endothelial cells (FLT1, VWF). Cells colored in red demonstrate high expression of the identified gene, while cells colored in blue have low expression of the identified gene.
3.3. Transcriptomic profiles of human retinal cell types.
In order to assess transcriptomic signatures of each cell population, differential expression was performed between cells in each respective cluster versus all other cells (SI Table 2). Differentially expressed genes were denoted if in the cluster of interest, the gene (A) exhibited an absolute log fold change greater than 1.0 compared to all other combined clusters, (B) had an adjusted p-value less than 1.0 × 10−3, and (C) were expressed above background in at least 25% of cells in either population being compared. The seven most enriched transcripts were selected for each cluster, and the scaled average expression of each gene was displayed in a heatmap (SI Figure 4). To provide a central resource for comparing gene expression patterns, the differential expression results from all genes (not only those that fit the above criteria) were identified and merged with cluster-specific expression data (SI Table 3).
3.4. Differential expression: subpopulation analysis
As multiple clusters of cones, rods, bipolar cells, and glial cells were identified, differential expression analysis was performed to identify features unique to potential subpopulations. Comparisons were performed between the two proposed rod clusters (SI Table 4, SI Figure 5), the two proposed bipolar clusters (SI Table 4, SI Figure 5), the two proposed glial derived clusters originating from predominantly foveal cells (Clusters 15-16; SI Table 4, SI Figure 5), and the three proposed glial clusters originating from predominantly peripheral samples (Cluster 13, 14, 17; SI Table 4, SI Figure 6).
3.5. Regional differential expression analysis
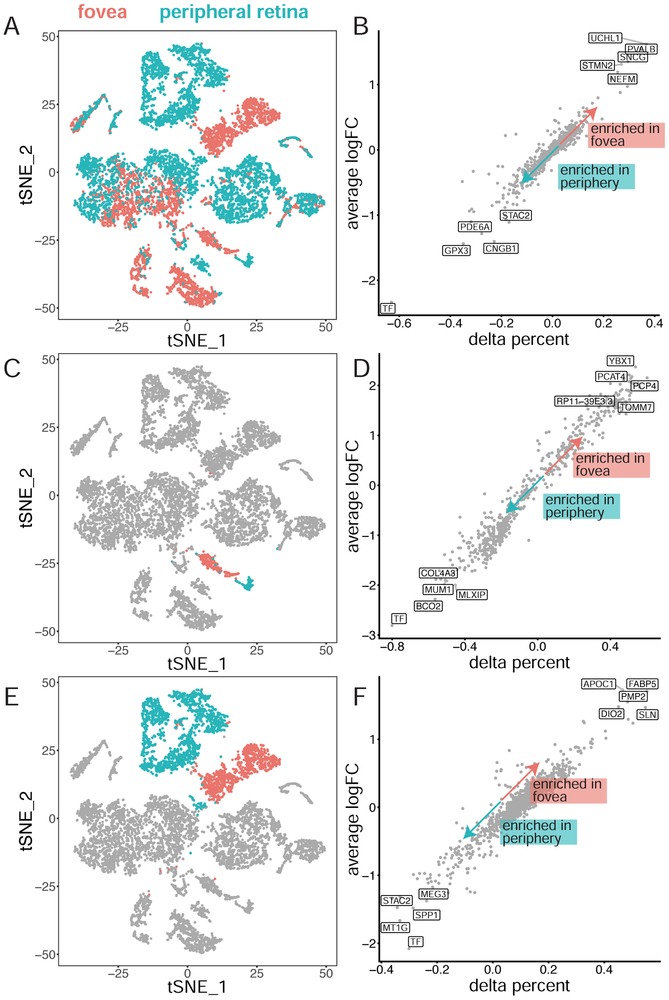
In addition to comparing populations of similar cell types based on tSNE clustering, we assessed differences in gene expression between cells originating from the fovea and the peripheral retina. First, differential expression was performed on all 8,217 cells based on location of origin. A total of 14 genes were differentially expressed between all cells of foveal versus peripheral origin regardless of cluster, with 6 genes enriched in foveal samples and 8 genes enriched in peripheral samples (Figure 3A-B, SI Table 5). Notably, the most upregulated gene in peripheral samples was the serum iron-binding protein transferrin (TF).
Figure 3: Differential expression analysis of cell populations by region.
For each comparison, the cells being compared are depicted (left). Cells colored in red originate from the fovea, whereas cells colored in blue originate from the peripheral retina. For each gene, the average log fold change and the percent of cells that express the gene above background are compared between the two clusters (right). For example, a delta percent of 0.4 indicates that 40% more cells in the lowered-number cluster express the gene above background than in the higher number cluster. Comparisons are made for (A-B) all cells based on location of origin, (C-D) cells in the presumed cone Clusters (3-4) based on location of origin, and (E-F) cells from proposed glial clusters (13-17) based on location of origin.
Both cone photoreceptors and glial cells formed distinct foveal and peripheral clusters (Figure 3A). Therefore, differential expression was performed between both cone photoreceptors and glial cells originating from the fovea and the periphery. Within the two proposed clusters of cone photoreceptor cells, a total of 148 differentially expressed genes were identified (SI Table 6), of which 125 were enriched in peripheral cells while 23 were enriched in foveal cells (Figure 3C-D). Interestingly, compared to foveal cones, peripheral cones were noted for significantly higher expression of the gene encoding Beta-Carotene Oxygenase 2 (BCO2), which is responsible for cleaving xanthophyllic compounds (Li et al., 2014a). In the five proposed clusters of glial cells, 34 differentially expressed genes were identified (SI Table 6), of which 22 were enriched in peripheral cells and 12 were enriched in foveal cells (Figure 3E-F). As in the global analysis and cone photoreceptor cells, the most peripherally enriched gene within glial cells was transferrin.
3.6. Comparison to previously published datasets
Several genes were identified to be differentially expressed between foveal and peripheral samples. In order to validate the regional differences, these genes were compared to expression patterns from two previously published retinal gene expression datasets. First, Peng et al recently performed single-cell RNA sequencing between foveal and peripheral retinal cell types in M. fascicularis (Peng et al., 2019). This study permitted the comparison of our results to spatially localized expression patterns specific to cone photoreceptors and glial cells in a primate model. Second, Whitmore et al performed bulk RNA sequencing between an 8-mm macular and peripheral retina (Whitmore et al., 2014). This study allowed for comparison of our results to regional gene expression patterns in human retina at the bulk RNA level. For both cone photoreceptors and glial cells, the ten most enriched genes in the fovea and the ten most enriched genes in the periphery were identified, and regional gene expression was compared between the three datasets (Figure 4).
Figure 4: Comparison to regional retinal RNA sequencing datasets.
The top ten differentially expressed genes between fovea and peripheral retina were determined for both cone photoreceptor cells and glial cells. The average log fold-change was determined for each gene between (1) fovea and periphery in the current study, (2) fovea and periphery in single cell sequencing of isolated foveal versus peripheral retina in M. fascicularis, and (3) bulk human RNA sequencing of 8-mm macular versus peripheral retina.
Within cone photoreceptors, transferrin (TF) was the most peripherally enriched gene in our study (logFC = −2.81). Similarly, cone photoreceptor cells in M. fascicularis demonstrated profound peripheral enrichment for transferrin (logFC = −5.53), as did bulk human peripheral retina (logFC = −0.80). Interestingly, transferrin was arguably the most differentially expressed gene in cone photoreceptors from M. fascicularis (SI Figure 7), although it was originally annotated only as LOC101866521 in the M. fascicularis GTF. However BCO2, our second-most peripherally enriched gene, demonstrated miniscule enrichment in peripheral M. fascicularis cone enrichment (logFC = −0.03) but modest human peripheral enrichment at the bulk RNA level (logFC = −0.35), suggesting that such RNA enrichment may be both species and cell type specific. The majority of the remaining evaluated cone photoreceptor cell differentially expressed genes demonstrated similar spatial patterns of expression between human and M. fascicularis. PCAT4 and LINC00969 could not be compared due to lack of M. fascicularis annotation. Discrepancies between our single cell results and the human bulk RNA results may be attributed to a dilution of cone-specific expression signals between a macular punch sixteen-times larger in area than the foveal punches acquired for this study.
Similar to cone photoreceptor cells, peripheral glial cells were enriched for TF expression across all three datasets, particularly in M. fascicularis single cell data (logFC = −6.36). Interestingly, TRH demonstrated foveal glial cell enrichment in our study (logFC = 1.29) and macular bulk enrichment in humans (logFC = 0.69) but peripheral glial enrichment in M. fascicularis (logFC = −4.70). The majority of the remaining evaluated differentially expressed genes between foveal and peripheral glial cells demonstrated concordant expression patterns across all three datasets. MT1G could not be compared due to lack of M. fascicularis annotation, while PMP2 and SLN had near zero expression in both foveal and peripheral regions of M. fascicularis retina.
4. Discussion
The recent advances in transcriptomic analysis at the single-cell level of resolution offers unprecedented power to understand gene expression (Hedlund and Deng, 2018). In contrast to bulk RNA sequencing, in which aggregate transcripts from thousands or millions of individual cells are pooled and analyzed in toto, single-cell RNA sequencing allows for interrogation of cell specific gene expression and regulatory relationships within heterogenous systems. While even cells that appear relatively homogeneous, such as clonal populations of B cells, show surprising levels of cell-to-cell variability (Marinov et al., 2014), single-cell analysis is especially beneficial in complex tissues such as the mammalian retina, which is comprised of five principal classes of neurons with dozens of subclasses and several supporting cell types (Wassle and Boycott, 1991).
One of the first single-cell RNA sequencing studies leveraged the technology to globally characterize retinal cell populations in the mouse (Macosko et al., 2015). Subsequent single-cell RNA sequencing studies have been performed on mouse retina in combination with cell sorting for focused characterization of cell subtypes, such as bipolar cells (Shekhar et al., 2016) and retinal ganglion cells (Rheaume et al., 2018). These reports characterized relationships between several novel neuronal subtypes and proposed gene expression patterns specific for each. Morphologic characteristics were further explored through lentiviral labeling of cell subtypes, allowing for powerful genotypic-phenotypic investigations. Most recently, single-cell RNA sequencing has been performed on paired foveal and peripheral samples immediately post mortem from Macaca fascicularis (Peng et al., 2019). This study identified approximately 160,000 cells and characterized both region-specific expression patterns and cellular subclasses of photoreceptor cells, bipolar cells, retinal ganglion cells, and interneurons.
In the current report, we performed a series of experiments with single-cell RNA sequencing to generate a catalog of cell type and region-specific transcripts in the human retina. In contrast to mouse and primate retina, procurement of suitable human retina is particularly unpredictable. Thus, cryopreservation of dissociated samples was logistically paramount. Cryopreservation permitted independent processing of all three pairs of human tissue, allowing for the stringent inclusion criteria of commencing dissociation within six hours post mortem. Thawing preserved cells empowered the aggregation of multiple samples acquired at different times with sufficient viability for one single-cell RNA sequencing run. Within this dataset, we identified all of the major cell types that exist within the human retina; clusters corresponding to rod and cone photoreceptor cells, inner retinal neurons, glia, and vascular cells were all readily discriminated (Figure 1F). Expression profiles for these cell types will be of high utility for both optimizing retinal cell differentiation protocols and selection of disease relevant cell populations for autologous cell replacement (Burnight et al., 2018).
For many classes of retinal neurons, several cellular subclasses have been reported and molecularly characterized (Jo et al., 2018; Macosko et al., 2015; Rheaume et al., 2018; Shekhar et al., 2016). In the current report, not every subclass of retinal cell could be discriminated. For example, glial cells provide important homeostatic roles in the retina, and can be broadly subdivided into Müller cells and astrocytes. While five clusters of glial cells were identified, we could not confidently subdivide neuroglia subpopulations beyond the spatial region from which they were derived. Expression of ALDH1L1 and GFAP, both reported markers specific for retinal astrocytes (Yang et al., 2011), were low in all clusters (SI Figure 8). However, all five glia clusters also showed high expression of RLBP1, a gene with highest retinal expression in Müller cells (Figure 1F). Of note, astrocytes were not discriminated from Müller glia in the recentM. fascicularis dataset (Peng et al., 2019). Likewise, we could not ascertain the multiple previously described subpopulations of bipolar cells (Shekhar et al., 2016), retinal ganglion cells (Rheaume et al., 2018) nor amacrine (Macosko et al., 2015) cells with gene expression patterns previously reported in the murine retina. Identification of these different subclasses of neurons may be enhanced by pre-enrichment procedures such as immunopanning (Rheaume et al., 2018; Shekhar et al., 2016).
In addition to characterizing cell type specific expression profiles, we performed a regional characterization of cell types originating from different areas of the retina at the single-cell level. In contrast to rodents, human and nonhuman primates possess striking regional specialization within the retina. Most notably, at its center the fovea centralis (Yuodelis and Hendrickson, 1986) consists entirely of non-tapering cone photoreceptor cells that are tightly packed with supporting Müller glia (Yamada, 1969). Foveal cones make one-to-one synaptic connections with bipolar cells, whereas in the periphery each bipolar cell receives inputs from multiple photoreceptor cells. While the distribution and anatomical differences between central and peripheral photoreceptor cells are well appreciated, the molecular distinctions between these cell types have not been well characterized. To address this, we acquired paired foveal and peripheral retina samples from each human donor and generated independent sequencing libraries based on cellular location of origin. The use of 2 mm punches centered on the fovea provides samples highly enriched for foveal cells, allowing for regional gene expression to be evaluated. As in the M. fascicularis regional single cell study (Peng et al., 2019), a small percentage of vascular cells and rod photoreceptors cells were recovered from our foveal punches due inclusion of a small amount of perifoveal retina.
Across all cells, the most peripherally enriched gene globally and within cone photoreceptor and glial cell populations was the serum iron binding protein transferrin (TF). Transferrin was also remarkably increased in peripheral cones and Müller cells in the M. fascicularis study, as well as in human peripheral retina at the bulk RNA level. Iron dyshomeostasis has been previously implicated in the development of age-related macular degeneration (AMD) (Chowers et al., 2006; Ueda et al., 2018) with Müller glial cells playing a central role in retinal iron metabolism (Baumann et al., 2017; Picard et al., 2008). In extrapolating our results to this model, the relative absence of foveal transferrin gene expression may make the central retina more susceptible to iron-mediated oxidative damage.
The glial cells and cone photoreceptor cells formed unique clusters of cells originating from foveal and peripheral regions (Figure 3A). One interesting finding from these data is that peripheral cones exhibited significantly higher expression of the gene encoding Beta-Carotene Oxygenase 2 (BCO2) compared to foveal cones. BCO2 is the major xanthophyll cleaving enzyme in humans (Li et al., 2014a). A relative deficiency of this enzyme in the fovea would presumably result in the accumulation of carotenoids such as lutein that contribute to the yellow-pigment observed in macular retina. Overall, this collection of foveal cone-specific expression profiles is of high utility for unraveling the molecular basis of foveal sparing in some inherited degenerative diseases of the retina (Querques et al., 2016; Schindler et al., 2010).
There are limitations to this study. Although all eyes were obtained within 5.5 hours of death, very short-lived transcripts may still be degraded in this time scale. Although we detected the majority of expected retinal cell types in this analysis, it is possible that papain dissociation and subsequent cryopreservation selectively influences the survival of certain cell types. In addition, genetic and environmental variability between different humans can artificially drive the formation of cell clusters from a single donor. However, in our dataset all clusters contained cells from each donor, and cell-type differences were more powerful in clustering than donor-to-donor differences. Finally, compared to whole tissue RNA-Seq, the single-cell approach we employed is limited to sequencing from the 3 ‘ end of the transcript. Thus, splicing isoforms and transcripts with very similar 3’ ends (such as the cone opsin genes OPN1MWand OPN1LW) can be challenging to discriminate.
Future studies are required to confirm gene expression patterns at the protein level and to further discriminate how gene expression at the single cell level changes in different disease states. However, this data set provides a rich resource for studying gene expression in a multitude of human retinal cell types. In addition to providing a single-cell transcriptomic characterization of human retina, the above regional comparisons highlight novel molecular features that distinguish foveal from peripheral cell types. Such characterizations will aid the search for novel disease-causing mutations with regional, cell-specific predilections.
Supplementary Material
Supplemental Figure 1: Transmission Electron Microscopy. Transmission electron microscopy was performed on a dissociated and cryopreserved retinal sample from an independent donor (see 2.1: Donor Eyes). Dissociated cells isolated from the fovea (A-B) and the periphery (C-D) are provided.
Supplemental Figure 2: Library characteristics. A-C. Unique genes (nGene) (A), molecular identifiers (nUMI) (B), and percent of mitochondrial genes (percent.mito) (C) in each of the six libraries. D. tSNE clustering overlaid with library of origin. All clusters contain contributions from all three human donors, although some clusters are restricted to cells isolated from either foveal or peripheral retina.
Supplemental Figure 3: Subpopulation analysis of horizontal and amacrine cell populations within Cluster 8. A. Violin plot of expression of ONECUT2, a gene previously reported to be enriched in horizontal cells, shows signal originating from Cluster 8. B. Violin plot of expression of NRXN2, a gene previously reported to be enriched in amacrine cells, also shows signal originating from Cluster 8. C. tSNE clustering and manual characterization of subpopulations 8A and 8B (insert). D. Heatmaps of genes previously reported to be enriched in horizontal cells. Expression of horizontal cell associated transcripts originates from a subpopulation within cluster 8. E. Heatmaps of genes previously reported to be enriched in amacrine cells. Expression of amacrine cell associated transcripts originates from a unique subpopulation within cluster 8.
Supplemental Figure 4: Differential expression analysis between each of the cell clusters. The seven most enriched genes in each cluster were plotted in a heatmap. Gene names (right) are demarcated with color to indicate which cell population each gene is enriched in. Genes designated with an asterisk indicate the transcript was reported to be enriched in the identified cell type in mouse retina at the single-cell level (Macosko et al., 2015).
SI Figure 5: Differential Expression between clusters of similar cell types. For each comparison, the clusters being compared are depicted (left). For each gene, the average log fold change and the percent of cells that express the gene above background are compared between the two clusters (right). For example, a delta percent of 0.4 indicates that 40% more cells in the lowered-number cluster express the gene above background than in the higher number cluster. Comparisons are made for (A-B) cells in the presumed rod Clusters (1-2), (C-D) cells from proposed bipolar cell Clusters (5–6), and (E-F) cells from proposed glial cell Clusters (15-16) predominantly originating from foveal samples.
SI Figure 6: Differential Expression between clusters of similar cell types. For each comparison, the clusters being compared are depicted (left). For each gene, the percent of cells that express the gene above background are compared between the two clusters. For example, a delta percent of 0.4 indicates that 40% more cells in the lowered-number cluster express the gene above background than in the higher number cluster. Comparisons are made for glial clusters consisting of predominantly peripherally isolated cells in (A-B) Clusters 13 vs 14, (C-D) Clusters 13 vs 17, and (E-F) Clusters 14 vs 17.
SI Figure 7: Differential expression of M. fascicularis cone genes in fovea versus periphery. The natural log fold change of fovea versus peripheral gene expression is plotted for each cone-expressed gene in M. fascicularis. For each gene, the percent of cells that express the gene above background are compared between the fovea and periphery clusters. For example, a delta percent of 0.4 indicates that 40% more foveal cones cluster express the gene above background than in peripheral cones. The most peripherally enriched gene in this study is LOC101866521, which was judged to be better represented by transferrin (TF) after analysis in the UCSC genome browser.
SI Figure 8: Expression of ALDH1L1 and GFAP, markers previously associated with astrocytes. All GFAP expressing clusters showed high expression of RLBP1, suggesting that the glial cells from which sequence was obtained are Müller glia.
Supplementary Table 1: Library composition of the 17 clusters.
Supplementary Table 2: Differential expression identification of genes most up- and down-regulated in each of the 17 clusters.
Supplementary Table 3: Differential expression of all genes between all clusters. For each cluster, the average log fold change and associated adjusted p-value are calculated by comparing average expression of the gene in the denoted cluster versus all other cluster. The delta percent column indicates the percentage of cells that express the gene above background in the denoted cluster minus the percent of cells that express the gene above background in all other cluster. In addition, the average expression value of the gene in each cluster is provided. NAs indicate that differential expression could not be performed due to the gene not being detected in at least one population being compared.
Supplementary Table 4: Differential expression between the two identified rod clusters (Clusters 1 vs 2), bipolar clusters (Clusters 5 vs 6), glial clusters of predominantly foveal origin (Clusters 15 vs 16), and pairwise comparisons for identified glial clusters of predominantly peripheral origin (Clusters 13 vs 14 vs 17).
Supplementary Table 5: Differential expression between all cells based on location of origin (fovea vs periphery).
Supplementary Table 6: Differential expression between the two identified cone clusters (Clusters 1 & 2: fovea vs periphery) and between the five identified glial clusters (Clusters 13-17: fovea vs periphery).
Highlights.
Gene expression characterization of human retinal cell types
Foveal and peripheral retinal cell types have distinct gene expression patterns
Transferrin is enriched in peripheral retina cell types
Acknowledgments:
We thank the donors, their families, and the Iowa Lions Eye Bank for their generous and essential role in this research. This work was supported by National Institutes of Health grants EY027038 and EY025580, MSTP training grant T32 GM007337, the Roy J. Carver, Jr. Chair in Bioinformatics and Computational Biology (TES), and support from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: none
References:
- Baumann B, Sterling J, Song Y, Song D, Fruttiger M, Gillies M, Shen W, Dunaief JL, 2017. Conditional Muller Cell Ablation Leads to Retinal Iron Accumulation. Investigative ophthalmology & visual science 58, 4223–4234 DOI: 10.1167/iovs.17-21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A, Syrbe S, Gorner K, Kacza J, Francke M, Wiedemann P, Reichenbach A, 2018. The primate fovea: Structure, function and development. Progress in retinal and eye research 66, 49–84 DOI: 10.1016/j.preteyeres.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Burnight ER, Giacalone JC, Cooke JA, Thompson JR, Bohrer LR, Chirco KR, Drack AV, Fingert JH, Worthington KS, Wiley LA, Mullins RF, Stone EM, Tucker BA, 2018. CRISPR-Cas9 genome engineering: Treating inherited retinal degeneration. Progress in retinal and eye research 65, 28–49 DOI: 10.1016/j.preteyeres.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E, Satija R, 2018. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nature Biotechnology 36, 411 DOI: 10.1038/nbt.4096 https://www.nature.com/articles/nbt.4096#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers I, Wong R, Dentchev T, Farkas RH, Iacovelli J, Gunatilaka TL, Medeiros NE, Presley JB, Campochiaro PA, Curcio CA, Dunaief JL, Zack DJ, 2006. The iron carrier transferrin is upregulated in retinas from patients with age-related macular degeneration. Investigative ophthalmology & visual science 47, 2135–2140 DOI: 10.1167/iovs.05-1135. [DOI] [PubMed] [Google Scholar]
- Hedlund E, Deng Q, 2018. Single-cell RNA sequencing: Technical advancements and biological applications. Molecular aspects of medicine 59, 36–46 DOI: 10.1016/j.mam.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Jo A, Xu J, Deniz S, Cherian S, DeVries SH, Zhu Y, 2018. Intersectional Strategies for Targeting Amacrine and Ganglion Cell Types in the Mouse Retina. Frontiers in neural circuits 12, 66 DOI: 10.3389/fncir.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, Bernstein PS, 2014a. Inactivity of human beta,beta-carotene-9',10'-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proceedings of the National Academy of Sciences of the United States of America 111, 10173–10178 DOI: 10.1073/pnas.1402526111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Jia C, Kazmierkiewicz KL, Bowman AS, Tian L, Liu Y, Gupta NA, Gudiseva HV, Yee SS, Kim M, Dentchev T, Kimble JA, Parker JS, Messinger JD, Hakonarson H, Curcio CA, Stambolian D, 2014b. Comprehensive analysis of gene expression in human retina and supporting tissues. Human molecular genetics 23, 4001–4014 DOI: 10.1093/hmg/ddu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA, 2015. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 161, 1202–1214 DOI: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinov GK, Williams BA, McCue K, Schroth GP, Gertz J, Myers RM, Wold BJ, 2014. From single-cell to cell-pool transcriptomes: stochasticity in gene expression and RNA splicing. Genome research 24, 496–510 DOI: 10.1101/gr.161034.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Kuehn MH, Radu RA, Enriquez GS, East JS, Schindler EI, Travis GH, Stone EM, 2012. Autosomal recessive retinitis pigmentosa due to ABCA4 mutations: clinical, pathologic, and molecular characterization. Investigative ophthalmology & visual science 53, 1883–1894 DOI: 10.1167/iovs.12-9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YR, Shekhar K, Yan W, Herrmann D, Sappington A, Bryman GS, van Zyl T, Do MTH, Regev A, Sanes JR, 2019. Molecular Classification and Comparative Taxonomics of Foveal and Peripheral Cells in Primate Retina. Cell 176, 1222–1237.e1222 DOI: 10.1016/j.cell.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard E, Fontaine I, Jonet L, Guillou F, Behar-Cohen F, Courtois Y, Jeanny JC, 2008. The protective role of transferrin in Muller glial cells after iron-induced toxicity. Molecular vision 14, 928–941. [PMC free article] [PubMed] [Google Scholar]
- Qiu GZ, Tian W, Fu HT, Li CP, Liu B, 2016. Long noncoding RNA-MEG3 is involved in diabetes mellitus-related microvascular dysfunction. Biochemical and biophysical research communications 471, 135–141 DOI: 10.1016/j.bbrc.2016.01.164. [DOI] [PubMed] [Google Scholar]
- Querques G, Kamami-Levy C, Georges A, Pedinielli A, Capuano V, Blanco-Garavito R, Poulon F, Souied EH, 2016. ADAPTIVE OPTICS IMAGING OF FOVEAL SPARING IN GEOGRAPHIC ATROPHY SECONDARY TO AGE-RELATED MACULAR DEGENERATION. Retina (Philadelphia, Pa.) 36, 247–254 DOI: 10.1097/iae.0000000000000692. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S, 1972. The Structure of the Retina. Charles C Thomas. [Google Scholar]
- Rheaume BA, Jereen A, Bolisetty M, Sajid MS, Yang Y, Renna K, Sun L, Robson P, Trakhtenberg EF, 2018. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat Commun 9, 2759 DOI: 10.1038/s41467-018-05134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler EI, Nylen EL, Ko AC, Affatigato LM, Heggen AC, Wang K, Sheffield VC, Stone EM, 2010. Deducing the pathogenic contribution of recessive ABCA4 alleles in an outbred population. Human molecular genetics 19, 3693–3701 DOI: 10.1093/hmg/ddq284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon D, Blackshaw S, Cepko CL, Dryja TP, 2002. Profile of the genes expressed in the human peripheral retina, macula, and retinal pigment epithelium determined through serial analysis of gene expression (SAGE). Proceedings of the National Academy of Sciences of the United States of America 99, 315–320 DOI: 10.1073/pnas.012582799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar K, Lapan SW, Whitney IE, Tran NM, Macosko EZ, Kowalczyk M, Adiconis X, Levin JZ, Nemesh J, Goldman M, McCarroll SA, Cepko CL, Regev A, Sanes JR, 2016. Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell 166, 1308–1323.e1330 DOI: 10.1016/j.cell.2016.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S, Cabuy E, Scherf BG, Kohler H, Panda S, Le Y-Z, Fehling HJ, Gaidatzis D, Stadler MB, Roska B, 2012. Transcriptional code and disease map for adult retinal cell types. Nature Neuroscience 15, 487 DOI: 10.1038/nn.3032 https://www.nature.com/articles/nn.3032#supplementary-information. [DOI] [PubMed] [Google Scholar]
- Songstad AE, Worthington KS, Chirco KR, Giacalone JC, Whitmore SS, Anfinson KR, Ochoa D, Cranston CM, Riker MJ, Neiman M, Stone EM, Mullins RF, Tucker A, 2017. Connective Tissue Growth Factor Promotes Efficient Generation of Human Induced Pluripotent Stem Cell-Derived Choroidal Endothelium. Stem cells translational medicine 6, 1533–1546 DOI: 10.1002/sctm.16-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Mullins RF, Streb LM, Anfinson K, Eyestone ME, Kaalberg E, Riker MJ, Drack AV, Braun TA, Stone EM, 2013. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. eLife 2, e00824 DOI: 10.7554/eLife.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Kim HJ, Zhao J, Song Y, Dunaief JL, Sparrow JR, 2018. Iron promotes oxidative cell death caused by bisretinoids of retina. Proceedings of the National Academy of Sciences of the United States of America 115, 4963–4968 DOI: 10.1073/pnas.1722601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Boycott BB, 1991. Functional architecture of the mammalian retina. Physiological reviews 71, 447–480 DOI: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Whitmore SS, Wagner AH, DeLuca AP, Drack AV, Stone EM, Tucker BA, Zeng S, Braun TA, Mullins RF, Scheetz TE, 2014. Transcriptomic analysis across nasal, temporal, and macular regions of human neural retina and RPE/choroid by RNA-Seq. Experimental eye research 129, 93–106 DOI: 10.1016/j.exer.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada E, 1969. Some structural features of the fovea centralis in the human retina. Archives of ophthalmology (Chicago, Ill. : 1960) 82, 151–159 DOI: 10.1001/archopht.1969.00990020153002. [DOI] [PubMed] [Google Scholar]
- Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, Frankl M, Rothstein JD, 2011. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia 59, 200–207 DOI: 10.1002/glia.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuodelis C, Hendrickson A, 1986. A qualitative and quantitative analysis of the human fovea during development. Vision research 26, 847–855 DOI: 10.1016/0042-6989(86)90143-4. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen M, Chen J, Lin S, Cai D, Chen C, Chen Z, 2017. Long non-coding RNA MIAT acts as a biomarker in diabetic retinopathy by absorbing miR-29b and regulating cell apoptosis. Bioscience reports 37 DOI: 10.1042/bsr20170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng GXY, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, Ziraldo SB, Wheeler TD, McDermott GP, Zhu J, Gregory MT, Shuga J, Montesclaros L, Underwood JG, Masquelier DA, Nishimura SY, Schnall-Levin M, Wyatt PW, Hindson BM, Bharadwaj R, Wong A, Ness KD, Beppu LW, Deeg HJ, McFarland C, Loeb KR, Valente WJ, Ericson NG, Stevens EA, Radich JP, Mikkelsen TS, Hindson BJ, Bielas JH, 2017. Massively parallel digital transcriptional profiling of single cells. Nature Communications 8, 14049 DOI: 10.1038/ncomms14049 https://www.nature.com/articles/ncomms14049#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Transmission Electron Microscopy. Transmission electron microscopy was performed on a dissociated and cryopreserved retinal sample from an independent donor (see 2.1: Donor Eyes). Dissociated cells isolated from the fovea (A-B) and the periphery (C-D) are provided.
Supplemental Figure 2: Library characteristics. A-C. Unique genes (nGene) (A), molecular identifiers (nUMI) (B), and percent of mitochondrial genes (percent.mito) (C) in each of the six libraries. D. tSNE clustering overlaid with library of origin. All clusters contain contributions from all three human donors, although some clusters are restricted to cells isolated from either foveal or peripheral retina.
Supplemental Figure 3: Subpopulation analysis of horizontal and amacrine cell populations within Cluster 8. A. Violin plot of expression of ONECUT2, a gene previously reported to be enriched in horizontal cells, shows signal originating from Cluster 8. B. Violin plot of expression of NRXN2, a gene previously reported to be enriched in amacrine cells, also shows signal originating from Cluster 8. C. tSNE clustering and manual characterization of subpopulations 8A and 8B (insert). D. Heatmaps of genes previously reported to be enriched in horizontal cells. Expression of horizontal cell associated transcripts originates from a subpopulation within cluster 8. E. Heatmaps of genes previously reported to be enriched in amacrine cells. Expression of amacrine cell associated transcripts originates from a unique subpopulation within cluster 8.
Supplemental Figure 4: Differential expression analysis between each of the cell clusters. The seven most enriched genes in each cluster were plotted in a heatmap. Gene names (right) are demarcated with color to indicate which cell population each gene is enriched in. Genes designated with an asterisk indicate the transcript was reported to be enriched in the identified cell type in mouse retina at the single-cell level (Macosko et al., 2015).
SI Figure 5: Differential Expression between clusters of similar cell types. For each comparison, the clusters being compared are depicted (left). For each gene, the average log fold change and the percent of cells that express the gene above background are compared between the two clusters (right). For example, a delta percent of 0.4 indicates that 40% more cells in the lowered-number cluster express the gene above background than in the higher number cluster. Comparisons are made for (A-B) cells in the presumed rod Clusters (1-2), (C-D) cells from proposed bipolar cell Clusters (5–6), and (E-F) cells from proposed glial cell Clusters (15-16) predominantly originating from foveal samples.
SI Figure 6: Differential Expression between clusters of similar cell types. For each comparison, the clusters being compared are depicted (left). For each gene, the percent of cells that express the gene above background are compared between the two clusters. For example, a delta percent of 0.4 indicates that 40% more cells in the lowered-number cluster express the gene above background than in the higher number cluster. Comparisons are made for glial clusters consisting of predominantly peripherally isolated cells in (A-B) Clusters 13 vs 14, (C-D) Clusters 13 vs 17, and (E-F) Clusters 14 vs 17.
SI Figure 7: Differential expression of M. fascicularis cone genes in fovea versus periphery. The natural log fold change of fovea versus peripheral gene expression is plotted for each cone-expressed gene in M. fascicularis. For each gene, the percent of cells that express the gene above background are compared between the fovea and periphery clusters. For example, a delta percent of 0.4 indicates that 40% more foveal cones cluster express the gene above background than in peripheral cones. The most peripherally enriched gene in this study is LOC101866521, which was judged to be better represented by transferrin (TF) after analysis in the UCSC genome browser.
SI Figure 8: Expression of ALDH1L1 and GFAP, markers previously associated with astrocytes. All GFAP expressing clusters showed high expression of RLBP1, suggesting that the glial cells from which sequence was obtained are Müller glia.
Supplementary Table 1: Library composition of the 17 clusters.
Supplementary Table 2: Differential expression identification of genes most up- and down-regulated in each of the 17 clusters.
Supplementary Table 3: Differential expression of all genes between all clusters. For each cluster, the average log fold change and associated adjusted p-value are calculated by comparing average expression of the gene in the denoted cluster versus all other cluster. The delta percent column indicates the percentage of cells that express the gene above background in the denoted cluster minus the percent of cells that express the gene above background in all other cluster. In addition, the average expression value of the gene in each cluster is provided. NAs indicate that differential expression could not be performed due to the gene not being detected in at least one population being compared.
Supplementary Table 4: Differential expression between the two identified rod clusters (Clusters 1 vs 2), bipolar clusters (Clusters 5 vs 6), glial clusters of predominantly foveal origin (Clusters 15 vs 16), and pairwise comparisons for identified glial clusters of predominantly peripheral origin (Clusters 13 vs 14 vs 17).
Supplementary Table 5: Differential expression between all cells based on location of origin (fovea vs periphery).
Supplementary Table 6: Differential expression between the two identified cone clusters (Clusters 1 & 2: fovea vs periphery) and between the five identified glial clusters (Clusters 13-17: fovea vs periphery).