Abstract
The association of sex hormone levels with mortality over a median of 16 years of follow-up was evaluated in a prospective cohort study. The study included 1,114 US men who participated in phase 1 (1988–1991) of the Third National Health and Nutrition Examination Survey Mortality Study and had no history of cardiovascular disease or cancer at baseline. Multivariable adjusted hazard ratios for all-cause mortality associated with a decrease in hormone concentration equal to the difference between the 90th and 10th percentiles of the sex hormone distributions were estimated by using proportional hazards regression. The hazard ratios associated with low free testosterone and low bioavailable testosterone levels were 1.43 (95% confidence interval (CI): 1.09, 1.87) and 1.52 (95% CI: 1.15, 2.02), respectively, for follow-up between baseline and year 9; they were 0.94 (95% CI: 0.51, 1.72) and 0.98 (95% CI: 0.56, 1.72), respectively, for follow-up between year 9 and year 18. Men with low free and bioavailable testosterone levels may have a higher risk of mortality within 9 years of hormone measurement. Future studies should be conducted to fully characterize the association of low free and bioavailable testosterone concentrations and mortality in men and to describe the mechanism underlying the association.
Keywords: androgens, cardiovascular diseases, estradiol, hormones, mortality, neoplasms, sex hormone-binding globulin, testosterone
Sex steroid hormones are major determinants of men's health status. Low testosterone levels have been associated with obesity, diabetes mellitus, renal disease, cardiovascular disease, and chronic obstructive pulmonary disease (1, 2). Whether testosterone levels predict premature mortality, however, is controversial (3–8). Furthermore, the association of sex steroid hormones other than testosterone with mortality has not been well studied in men (3).
The purpose of the current analysis was to evaluate the association of concentrations of serum total and free testosterone and estradiol, as well as their major carrier in circulation—sex hormone-binding globulin (SHBG)—with all-cause, cardiovascular, and cancer mortality in the general US adult male population by using follow-up data from the Third National Health and Nutrition Examination Survey (NHANES III) Mortality Study. Given that men with certain chronic diseases tend to have lower circulating testosterone levels, and many chronic diseases are linked with higher mortality, we excluded men with a history of cardiovascular disease or cancer.
MATERIALS AND METHODS
Study population
NHANES III was a stratified, multistage probability survey designed to be representative of the civilian, noninstitutionalized US population (9). NHANES III included 2 phases (phase I: October 1988–October 1991 and phase II: September 1991–October 1994), each capable of independently producing unbiased national estimates. Within each phase, participants were randomly assigned to either a morning or afternoon/evening examination. The present study (1988–2006) was conducted among men aged ≥20 years participating in the morning examination session of phase I (N = 1,967). The study was restricted to participants in the morning session to reduce extraneous variation due to diurnal production of sex hormones.
Serum for the sex steroid hormone assays was available for 1,470 men (75%) aged ≥20 years participating in the morning examination session of phase I. We excluded 179 participants with a self-reported history of cardiovascular disease or cancer. After we also excluded 21 with missing hormones data, 1 with missing follow-up data, and 155 with missing information on covariates, the final sample included 1,114 men.
The protocol for NHANES III was approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board. All participants gave written informed consent. The assay of stored serum specimens for the Hormone Demonstration Program was approved by the Institutional Review Boards at the Johns Hopkins Bloomberg School of Public Health and the National Center for Health Statistics, Centers for Disease Control and Prevention.
Baseline data collection
NHANES III baseline data were collected during an in-home interview and a subsequent visit to a mobile examination center (9). During the in-home interview, demographic information including age and race-ethnicity was obtained by using a standardized questionnaire. Additional data collected during the in-home interview included cigarette smoking, pack-years of smoking (for current and former smokers), household income, education, alcohol consumption, physical activity, and history of cardiovascular disease and/or cancer. Whole-body electrical resistance was measured by using a Valhalla Scientific Body Composition Analyzer (model 1990B; Valhalla Scientific, Inc., San Diego, California), and prediction equations were used to predict percent body fat (10).
Sex steroid hormones
Participants in the morning examination session fasted overnight before having blood drawn. Blood specimens were obtained by a trained phlebotomist according to a standardized protocol. After centrifugation, serum was aliquotted and stored at −70°C. Serum concentrations of total testosterone, total estradiol, androstanediol glucuronide (AAG; a metabolite of dihydrotestosterone), and SHBG were measured in 2005 at the laboratory of one of the authors (N. R.) at Children's Hospital, Boston, Massachusetts. Total testosterone, total estradiol, and SHBG concentrations were quantified by using competitive electrochemiluminescence immunoassays on the 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, Indiana). AAG was measured by an enzyme immunoassay (Diagnostics Systems Laboratories, Webster, Texas). The detection limits of the assays were 0.02 ng/mL for total testosterone, 5 pg/mL for total estradiol, 0.33 ng/mL for AAG, and 3 nmol/L for SHBG. The coefficients of variation for quality control specimens ranged from 5.8% to 5.9% for total testosterone, 2.5% to 6.7% for total estradiol, 5.0% to 9.5% for AAG, and 5.3% to 5.9% for SHBG. Serum albumin was measured by using the bromocresol purple method. Free testosterone, bioavailable testosterone, and free estradiol concentrations were calculated from measured total hormone, SHBG, and albumin concentrations (11, 12).
Mortality follow-up
Adult NHANES III participants were passively followed for mortality through December 31, 2006. Probabilistic matching was used to link NHANES III participants with the National Death Index to ascertain vital status and cause of death (13).
Follow-up for all study participants was calculated as the time between their NHANES III examination and the date of death, the date they became 90 years of age, or December 31, 2006, whichever occurred first. We censored follow-up at age 90 years because mortality was very high after this age and few participants contributed person-time in this age category.
Cause of death was determined by using the underlying cause listed on death certificates. The International Classification of Diseases, Ninth Revision, was used for deaths occurring between 1988 and 1998, and the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, was used for deaths from 1999 to 2006. Cause-specific mortality was ascertained for cardiovascular disease (Ninth Revision codes 390–434 and 436–459; Tenth Revision codes I00–I99) and cancer (Ninth Revision codes 140–208; Tenth Revision codes C00–C97). The numbers of deaths due to all causes, cardiovascular disease, and cancer were 206, 82, and 58, respectively.
Statistical methods
We categorized sex steroid hormones (total testosterone, total estradiol, AAG, free testosterone, bioavailable testosterone, and free estradiol), SHBG, and selected molar ratios (estradiol to testosterone, and testosterone to SHBG and estradiol to SHBG as indicators of bioavailable hormone) into tertiles based on the weighted population distribution. We used Cox proportional hazards regression to estimate the age- and multivariable-adjusted hazard ratios and 95% confidence intervals for all-cause mortality associated with each tertile of sex steroid hormones, SHBG, and molar ratios compared with the highest tertile in separate models. In this paper, results are presented separately for the first 9 years of follow-up and the last 9 years of follow-up; hormones change greatly as men age, and baseline sex steroid hormone concentrations might not be the relevant exposure for the later period of follow-up.
We adjusted for age, race-ethnicity, smoking status and pack-years of smoking, household income, education, alcohol consumption, exercise, and percent body fat in the multivariable models because these common chronic disease risk factors are also associated with hormone levels in NHANES III. Because total testosterone and total estradiol are correlated (0.38 for men in NHANES III) and because SHBG is the major carrier of testosterone and estradiol in circulation, in the multivariable model we also mutually adjusted for total testosterone, total estradiol, and SHBG. We computed tests for linear trend across tertiles by including the median of each tertile as a continuous variable in the models. Additional models included sex steroid hormones, SHBG, and molar ratios modeled as continuous, natural logarithm-transformed variables, and in this paper we present results for a difference in hormone concentration equal to the difference between the 90th and 10th percentiles of each sex steroid hormone, SHBG, or molar ratio. To assess the shape of the hormone and all-cause mortality associations, we evaluated the association of free testosterone and free estradiol with mortality using restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles of the hormone distributions.
To evaluate the association of sex steroid hormones with risk of death from cardiovascular disease and cancer, as well as from all causes, we entered each hormone, SHBG, and molar ratio as a continuous, natural logarithm-transformed variable in separate models and after mutual adjustment for other hormones (total testosterone, total estradiol, and SHBG adjusted for each other). Here, we present the age- and multivariable-adjusted hazard ratios for a decrease in concentration equal to the difference between the 90th and 10th percentiles. Data were analyzed by using SUDAAN software (version 9.0; Research Triangle Institute, Research Triangle Park, North Carolina) to account for the complex NHANES III sampling design, including unequal probabilities of selection, oversampling, and nonresponse.
RESULTS
Correlation coefficients between each of the sex steroid hormones, SHBG, and molar ratios are presented in Web Table 1. (This information is described in the first of 4 supplementary tables; each is referred to as “Web table” in the text and is posted on the Journal’s website (http://aje.oupjournals.org/).) The mean age of participants at baseline was 40 years, and the median length of follow-up was 16 years (maximum, 18). Geometric mean concentrations of sex steroid hormones and SHBG are shown in Web Figure 1 (also posted on the Journal’s website). Participants with higher total, free, and bioavailable testosterone concentrations were younger and more likely to be current smokers, to have an annual household income of <$20,000, to have a high school education, to consume alcohol, to exercise, and to have <25% body fat (Web Figure 1 and Web Table 2). Participants with higher total and free estradiol concentrations were younger and more likely to be non-Hispanic black, to be current smokers, to have smoked for more pack-years, and to have an annual household income of <$20,000. Participants with higher SHBG concentrations were older, were less likely to be Mexican American, and were more likely to be current smokers, to have smoked for more pack-years, to not have a high school education, and to have <25% body fat.
Table 1.
Hazard Ratios and 95% Confidence Intervals for All-Cause Mortality, by Tertile of Hormones, in Adult Men in NHANES III Followed up Between Study Baseline and Year 9, United States, 1988–2006
| Tertile 1 |
Tertile 2 |
Tertile 3 | P-Trend | |||
| HR | 95% CI | HR | 95% CI | HR | ||
| Testosterone, ng/mL | <4.5 | 4.5–6.2 | ≥6.3 | |||
| Age and race-ethnicity adjusted | 0.52 | 0.28, 0.98 | 0.53 | 0.22, 1.29 | 1.00 | 0.06 |
| Multivariable adjusteda | 0.54 | 0.28, 1.07 | 0.55 | 0.25, 1.24 | 1.00 | 0.09 |
| Multivariable and hormone adjustedb | 0.96 | 0.48, 1.90 | 0.92 | 0.44, 1.94 | 1.00 | 0.90 |
| Estradiol, pg/mL | <32.0 | 32.0–40.7 | ≥40.8 | |||
| Age and race-ethnicity adjusted | 0.91 | 0.47, 1.76 | 1.12 | 0.56, 2.24 | 1.00 | 0.77 |
| Multivariable adjusteda | 1.17 | 0.58, 2.36 | 1.36 | 0.66, 2.81 | 1.00 | 0.62 |
| Multivariable and hormone adjustedb | 1.23 | 0.63, 2.42 | 1.18 | 0.61, 2.29 | 1.00 | 0.52 |
| AAG, ng/mL | <9.5 | 9.5–15.2 | ≥15.3 | |||
| Age and race-ethnicity adjusted | 0.59 | 0.28, 1.25 | 0.96 | 0.40, 2.30 | 1.00 | 0.18 |
| Multivariable adjusteda | 0.57 | 0.23, 1.41 | 0.97 | 0.36, 2.63 | 1.00 | 0.22 |
| Free testosterone, ng/mL | <0.09 | 0.09–0.13 | ≥0.14 | |||
| Age and race-ethnicity adjusted | 2.16 | 0.54, 8.63 | 2.23 | 0.50, 9.96 | 1.00 | 0.23 |
| Multivariable adjusteda | 1.37 | 0.78, 2.41 | 1.34 | 0.97, 1.84 | 1.00 | 0.13 |
| Bioavailable testosterone, ng/mL | <2.1 | 2.1–3.0 | ≥3.1 | |||
| Age and race-ethnicity adjusted | 3.10 | 0.65, 14.8 | 1.85 | 0.32, 10.7 | 1.00 | 0.07 |
| Multivariable adjusteda | 1.27 | 0.58, 2.78 | 1.10 | 0.79, 1.54 | 1.00 | 0.48 |
| Free estradiol, pg/mL | <0.81 | 0.81–1.05 | ≥1.06 | |||
| Age and race-ethnicity adjusted | 2.37 | 1.13, 4.95 | 2.72 | 1.19, 6.22 | 1.00 | 0.03 |
| Multivariable adjusteda | 1.71 | 0.79, 3.68 | 1.60 | 0.80, 3.19 | 1.00 | 0.14 |
| SHBG, nmol/L | <27.7 | 27.7–42.4 | ≥42.5 | |||
| Age and race-ethnicity adjusted | 0.24 | 0.05, 1.24 | 0.60 | 0.28, 1.31 | 1.00 | 0.04 |
| Multivariable adjusteda | 0.67 | 0.31, 1.47 | 0.78 | 0.33, 1.83 | 1.00 | 0.34 |
| Multivariable and hormone adjustedb | 0.68 | 0.30, 1.53 | 0.78 | 0.33, 1.81 | 1.00 | 0.37 |
| Estradiol:testosterone ratio | <6.3 | 6.3–8.2 | ≥8.3 | |||
| Age and race-ethnicity adjusted | 1.58 | 0.78, 3.19 | 1.78 | 1.13, 2.81 | 1.00 | 0.07 |
| Multivariable adjusteda | 1.69 | 0.84, 3.40 | 1.97 | 1.00, 3.86 | 1.00 | 0.06 |
| Testosterone:SHBG ratio | <0.45 | 0.45–0.64 | ≥0.65 | |||
| Age and race-ethnicity adjusted | 2.57 | 0.54, 12.3 | 0.88 | 0.19, 4.05 | 1.00 | 0.21 |
| Multivariable adjusteda | 1.23 | 0.50, 3.04 | 1.02 | 0.64, 1.61 | 1.00 | 0.65 |
| Estradiol:SHBG ratio | <3.0 | 3.0–4.7 | ≥4.8 | |||
| Age and race-ethnicity adjusted | 4.57 | 1.10, 19.0 | 3.58 | 0.90, 14.3 | 1.00 | 0.03 |
| Multivariable adjusteda | 1.65 | 0.73, 3.74 | 1.30 | 0.79, 2.15 | 1.00 | 0.19 |
Abbreviations: AAG, androstanediol glucuronide; CI, confidence interval; HR, hazard ratio; NHANES III, Third National Health and Nutrition Examination Survey; SHBG, sex hormone-binding globulin.
Adjusted for age (spline), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), smoking status (never, former, current), tertile of pack-years of smoking, household income (</≥$20,000), education (</≥high school), alcohol consumption (</≥1 drink a week), exercise (none/1–2/≥3 times a week), and percent body fat (continuous).
Models for testosterone, estradiol, and SHBG were further mutually adjusted (categorized in tertiles).
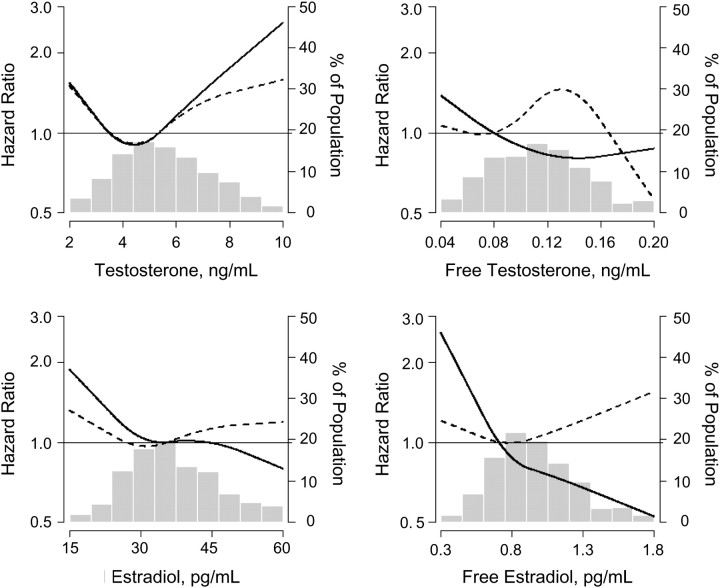
Figure 1.
Multivariable-adjusted relative hazard of all-cause mortality associated with testosterone, free testosterone, estradiol, and free estradiol, using restricted quadratic splines, for men in the Third National Health and Nutrition Examination Survey, United States, 1988–2006. Adjusted for age (spline), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), smoking status (never, former, current), tertile of pack-years of smoking, household income (</≥$20,000), education (</≥high school), alcohol consumption (</≥1 drink a week), exercise (none/1–2/≥3 times a week), and percent body fat (continuous). Solid line: follow-up between baseline and year 9; dashed line: follow-up between year 9 and year 18. A histogram of the hormone is superimposed in the background and is displayed on the right axis.
Table 2.
Hazard Ratios and 95% Confidence Intervals for All-Cause, Cardiovascular Disease, and Cancer Mortality Associated With a Decrease in Hormone Level Equal to the Difference Between the 90th and 10th Percentilesa for Follow-up Between Study Baseline and Year 9, NHANES III, United States, 1988–2006
| All-Cause Mortality (n =
103 Events) |
Cardiovascular Disease Mortality
(n = 42 Events) |
Cancer Mortality (n = 28
Events) |
||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Testosterone | ||||||
| Age and race-ethnicity adjusted | 0.97 | 0.61, 1.56 | 1.26 | 0.79, 2.03 | 0.42 | 0.05, 3.25 |
| Multivariable adjustedb | 1.13 | 0.73, 1.76 | 1.41 | 0.77, 2.55 | 0.89 | 0.26, 2.99 |
| Multivariable and hormone adjustedc | 1.18 | 0.68, 2.03 | 1.07 | 0.76, 1.53 | 1.08 | 0.65, 1.80 |
| Estradiol | ||||||
| Age and race-ethnicity adjusted | 1.20 | 0.69, 2.08 | 2.11 | 1.42, 3.14 | 0.54 | 0.13, 2.28 |
| Multivariable adjustedb | 1.52 | 0.99, 2.33 | 2.82 | 1.62, 4.90 | 0.96 | 0.34, 2.74 |
| Multivariable and hormone adjustedc | 1.21 | 0.72, 2.01 | 2.40 | 1.46, 3.95 | 0.97 | 0.39, 2.44 |
| AAG | ||||||
| Age and race-ethnicity adjusted | 0.67 | 0.32, 1.42 | 0.97 | 0.36, 2.64 | 0.86 | 0.38, 1.91 |
| Multivariable adjustedb | 0.60 | 0.23, 1.54 | 0.93 | 0.32, 2.75 | 0.68 | 0.21, 2.19 |
| Free testosterone | ||||||
| Age and race-ethnicity adjusted | 1.33 | 1.11, 1.59 | 1.45 | 1.13, 1.87 | 1.19 | 0.76, 1.86 |
| Multivariable adjustedb | 1.43 | 1.09, 1.87 | 1.53 | 1.05, 2.23 | 1.07 | 0.72, 1.58 |
| Bioavailable testosterone | ||||||
| Age and race-ethnicity adjusted | 1.41 | 1.19, 1.67 | 1.54 | 1.18, 2.00 | 1.31 | 0.93, 1.83 |
| Multivariable adjustedb | 1.52 | 1.15, 2.02 | 1.63 | 1.12, 2.37 | 1.12 | 0.79, 1.60 |
| Free estradiol | ||||||
| Age and race-ethnicity adjusted | 1.80 | 1.24, 2.59 | 2.51 | 1.85, 3.41 | 1.42 | 0.48, 4.21 |
| Multivariable adjustedb | 1.41 | 0.75, 2.64 | 3.22 | 2.30, 4.51 | 1.77 | 0.66, 4.71 |
| SHBG | ||||||
| Age and race-ethnicity adjusted | 0.22 | 0.07, 0.71 | 0.24 | 0.10, 0.54 | 0.13 | 0.01, 1.95 |
| Multivariable adjustedb | 0.49 | 0.17, 1.41 | 0.23 | 0.09, 0.57 | 0.26 | 0.00, 44.3 |
| Multivariable and hormone adjustedc | 0.65 | 0.24, 1.79 | 0.22 | 0.10, 0.49 | 0.21 | 0.01, 4.61 |
| Estradiol:testosterone ratio | ||||||
| Age and race-ethnicity adjusted | 1.14 | 0.73, 1.78 | 1.08 | 0.54, 2.19 | 1.48 | 0.54, 4.08 |
| Multivariable adjustedb | 1.10 | 0.65, 1.87 | 1.01 | 0.44, 2.34 | 1.08 | 0.48, 2.42 |
| Testosterone:SHBG ratio | ||||||
| Age and race-ethnicity adjusted | 1.53 | 1.25, 1.87 | 1.66 | 1.33, 2.07 | 1.45 | 1.01, 2.08 |
| Multivariable adjustedb | 1.61 | 0.97, 2.66 | 1.73 | 1.22, 2.43 | 1.51 | 1.05, 2.17 |
| Estradiol:SHBG ratio | ||||||
| Age and race-ethnicity adjusted | 3.41 | 1.92, 6.06 | 4.70 | 3.01, 7.32 | 3.19 | 0.68, 14.91 |
| Multivariable adjustedb | 1.55 | 0.66, 3.68 | 5.97 | 3.63, 9.81 | 2.83 | 0.44, 18.10 |
Abbreviations: AAG, androstanediol glucuronide; CI, confidence interval; HR, hazard ratio; NHANES III, Third National Health and Nutrition Examination Survey; SHBG, sex hormone-binding globulin.
The 10th and 90th percentile values—total testosterone: 3.2 ng/mL, 8.0 ng/mL; estradiol: 24.8 pg/mL, 52.0 pg/mL; AAG: 5.5 ng/mL, 22.9 ng/mL; free testosterone: 0.06 ng/mL, 0.16 ng/mL; bioavailable testosterone: 1.4 ng/mL, 3.9 ng/mL; free estradiol: 0.6 pg/mL, 1.4 pg/mL; SHBG: 19.8 nmol/L, 59.1 nmol/L; estradiol to testosterone ratio: 4.8, 11.7; testosterone to SHBG ratio: 0.3, 0.9; and estradiol to SHBG ratio: 1.9, 7.4.
All-cause mortality models were adjusted for age (spline), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), smoking status (never, former, current), tertile of pack-years of smoking, household income (</≥$20,000), education (</≥high school), alcohol consumption (</≥1 drink a week), exercise (none/1–2/≥3 times a week), and percent body fat (continuous). Cardiovascular disease and cancer mortality models were adjusted for age (spline), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), smoking status (never, former, current), tertile of pack-years of smoking, and percent body fat (continuous).
Models for testosterone, estradiol, and SHBG were further mutually adjusted.
Total mortality
Between baseline and year 9, total testosterone, total estradiol, AAG, free estradiol, SHBG, and the molar ratios of estradiol to testosterone, testosterone to SHBG, and estradiol to SHBG were not statistically significantly associated with all-cause mortality in multivariable-adjusted models or in multivariable models with mutual adjustment for the other hormones (Tables 1 and 2). However, between baseline and year 9, the multivariable-adjusted hazard ratios for a decrease in free testosterone and bioavailable testosterone equal to the difference between the 90th and 10th percentiles were 1.43 (95% confidence interval (CI): 1.09, 1.87) and 1.52 (95% CI: 1.15, 2.02), respectively (Table 2). Between year 9 and year 18, none of the sex steroid hormones, SHBG, or molar ratios was associated with all-cause mortality after multivariable adjustment (Tables 3 and 4). The multivariable-adjusted relative hazards of all-cause mortality associated with testosterone, free testosterone, total estradiol, and free estradiol, using restricted quadratic splines, are shown in Figure 1; none of the nonlinear terms in the spline models was statistically significant. The association of sex steroid hormones with mortality over the entire 18 years of follow-up is presented in Web Tables 3 and 4.
Table 3.
Hazard Ratios and 95% Confidence Intervals for All-Cause Mortality, by Tertile of Hormones, in Adult Men in NHANES III Followed up Between Study Year 9 and Year 18, United States, 1988–2006
| Tertile 1 |
Tertile 2 |
Tertile 3 | P-Trend | |||
| HR | 95% CI | HR | 95% CI | HR | ||
| Testosterone, ng/mL | <4.5 | 4.5–6.2 | ≥6.3 | |||
| Age and race-ethnicity adjusted | 0.69 | 0.30, 1.61 | 0.34 | 0.19, 0.60 | 1.00 | 0.57 |
| Multivariable adjusteda | 0.74 | 0.35, 1.60 | 0.36 | 0.19, 0.68 | 1.00 | 0.64 |
| Multivariable and hormone adjustedb | 0.72 | 0.26, 2.00 | 0.35 | 0.15, 0.81 | 1.00 | 0.65 |
| Estradiol, pg/mL | <32.0 | 32.0–40.7 | ≥40.8 | |||
| Age and race-ethnicity adjusted | 0.69 | 0.34, 1.39 | 0.98 | 0.56, 1.73 | 1.00 | 0.26 |
| Multivariable adjusteda | 0.85 | 0.41, 1.77 | 1.14 | 0.59, 2.18 | 1.00 | 0.67 |
| Multivariable and hormone adjustedb | 0.85 | 0.33, 2.17 | 1.14 | 0.51, 2.55 | 1.00 | 0.90 |
| AAG, ng/mL | <9.5 | 9.5–15.2 | ≥15.3 | |||
| Age and race-ethnicity adjusted | 1.24 | 0.60, 2.54 | 1.60 | 0.79, 3.21 | 1.00 | 0.56 |
| Multivariable adjusteda | 1.10 | 0.57, 2.13 | 1.37 | 0.71, 2.63 | 1.00 | 0.81 |
| Free testosterone, ng/mL | <0.09 | 0.09–0.13 | ≥0.14 | |||
| Age and race-ethnicity adjusted | 0.74 | 0.26, 2.10 | 1.50 | 0.63, 3.57 | 1.00 | 0.25 |
| Multivariable adjusteda | 1.00 | 0.40, 2.51 | 1.94 | 0.96, 3.91 | 1.00 | 0.55 |
| Bioavailable testosterone, ng/mL | <2.1 | 2.1–3.0 | ≥3.1 | |||
| Age and race-ethnicity adjusted | 0.77 | 0.21, 2.84 | 1.57 | 0.61, 4.07 | 1.00 | 0.32 |
| Multivariable adjusteda | 1.12 | 0.38, 3.35 | 1.98 | 0.93, 4.22 | 1.00 | 0.68 |
| Free estradiol, pg/mL | <0.81 | 0.81–1.05 | ≥1.06 | |||
| Age and race-ethnicity adjusted | 0.51 | 0.25, 1.05 | 0.60 | 0.36, 0.99 | 1.00 | 0.04 |
| Multivariable adjusteda | 0.71 | 0.37, 1.36 | 0.69 | 0.33, 1.45 | 1.00 | 0.27 |
| SHBG, nmol/L | <27.7 | 27.7–42.4 | ≥42.5 | |||
| Age and race-ethnicity adjusted | 1.31 | 0.60, 2.85 | 1.18 | 0.61, 2.25 | 1.00 | 0.48 |
| Multivariable adjusteda | 1.02 | 0.53, 1.98 | 1.10 | 0.62, 1.94 | 1.00 | 0.86 |
| Multivariable and hormone adjustedb | 1.11 | 0.53, 2.33 | 1.18 | 0.58, 2.40 | 1.00 | 0.57 |
| Estradiol:testosterone ratio | <6.3 | 6.3–8.2 | ≥8.3 | |||
| Age and race-ethnicity adjusted | 0.74 | 0.36, 1.51 | 0.72 | 0.41, 1.27 | 1.00 | 0.34 |
| Multivariable adjusteda | 0.88 | 0.49, 1.57 | 0.79 | 0.44, 1.45 | 1.00 | 0.50 |
| Testosterone:SHBG ratio | <0.45 | 0.45–0.64 | ≥0.65 | |||
| Age and race-ethnicity adjusted | 0.44 | 0.16, 1.22 | 0.77 | 0.27, 2.20 | 1.00 | 0.06 |
| Multivariable adjusteda | 0.64 | 0.28, 1.46 | 0.94 | 0.39, 2.27 | 1.00 | 0.22 |
| Estradiol:SHBG ratio | <3.0 | 3.0–4.7 | ≥4.8 | |||
| Age and race-ethnicity adjusted | 0.61 | 0.28, 1.35 | 0.66 | 0.40, 1.11 | 1.00 | 0.23 |
| Multivariable adjusteda | 0.87 | 0.48, 1.59 | 0.82 | 0.51, 1.33 | 1.00 | 0.66 |
Abbreviations: AAG, androstanediol glucuronide; CI, confidence interval; HR, hazard ratio; NHANES III, Third National Health and Nutrition Examination Survey; SHBG, sex hormone binding globulin.
Adjusted for age (spline), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), smoking status (never, former, current), tertile of pack-years of smoking, household income (</≥$20,000), education (</≥high school), alcohol consumption (</≥1 drink a week), exercise (none/1–2/≥3 times a week), and percent body fat (continuous).
Models for testosterone, estradiol, and SHBG were further mutually adjusted (categorized in tertiles).
Table 4.
Hazard Ratios and 95% Confidence Intervals for All-Cause, Cardiovascular Disease, and Cancer Mortality Associated With a Decrease in Hormone Level Equal to the Difference Between the 90th and 10th Percentilesa for Follow-up Between Study Year 9 and Year 18, NHANES III, United States, 1988–2006
| All-Cause Mortality (n =
103 Events) |
Cardiovascular Disease Mortality
(n = 40 Events) |
Cancer Mortality (n = 30
Events) |
||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Testosterone | ||||||
| Age and race-ethnicity adjusted | 0.94 | 0.47, 1.90 | 1.26 | 0.80, 1.99 | 0.20 | 0.06, 0.68 |
| Multivariable adjustedb | 1.00 | 0.54, 1.85 | 1.32 | 0.77, 2.28 | 0.31 | 0.07, 1.41 |
| Multivariable and hormone adjustedc | 0.99 | 0.54, 1.83 | 0.59 | 0.27, 1.30 | 0.31 | 0.04, 2.57 |
| Estradiol | ||||||
| Age and race-ethnicity adjusted | 0.63 | 0.31, 1.29 | 0.76 | 0.23, 2.54 | 0.62 | 0.22, 1.76 |
| Multivariable adjustedb | 0.88 | 0.45, 1.75 | 0.92 | 0.25, 3.38 | 0.92 | 0.40, 2.12 |
| Multivariable and hormone adjustedc | 0.88 | 0.45, 1.75 | 1.01 | 0.26, 4.02 | 1.20 | 0.35, 4.12 |
| AAG | ||||||
| Age and race-ethnicity adjusted | 0.94 | 0.57, 1.56 | 0.54 | 0.30, 1.00 | 1.24 | 0.69, 2.22 |
| Multivariable adjustedb | 0.86 | 0.53, 1.37 | 0.49 | 0.25, 0.95 | 1.07 | 0.58, 1.98 |
| Free testosterone | ||||||
| Age and race-ethnicity adjusted | 0.80 | 0.33, 1.93 | 0.57 | 0.25, 1.33 | 0.28 | 0.09, 0.91 |
| Multivariable adjustedb | 0.94 | 0.51, 1.72 | 0.58 | 0.24, 1.40 | 0.49 | 0.16, 1.51 |
| Bioavailable testosterone | ||||||
| Age and race-ethnicity adjusted | 0.85 | 0.37, 1.97 | 0.61 | 0.24, 1.57 | 0.25 | 0.06, 0.96 |
| Multivariable adjustedb | 0.98 | 0.56, 1.72 | 0.62 | 0.24, 1.61 | 0.44 | 0.12, 1.66 |
| Free estradiol | ||||||
| Age and race-ethnicity adjusted | 0.56 | 0.25, 1.25 | 0.38 | 0.12, 1.22 | 1.02 | 0.28, 3.70 |
| Multivariable adjustedb | 0.82 | 0.41, 1.64 | 0.49 | 0.13, 1.83 | 1.33 | 0.61, 2.91 |
| SHBG | ||||||
| Age and race-ethnicity adjusted | 1.27 | 0.56, 2.88 | 4.80 | 1.86, 12.38 | 0.36 | 0.08, 1.66 |
| Multivariable adjustedb | 1.08 | 0.58, 2.02 | 4.40 | 1.41, 13.76 | 0.51 | 0.11, 2.38 |
| Multivariable and hormone adjustedc | 1.09 | 0.60, 1.99 | 6.12 | 1.79, 20.84 | 0.91 | 0.11, 7.79 |
| Estradiol:testosterone ratio | ||||||
| Age and race-ethnicity adjusted | 0.88 | 0.59, 1.34 | 0.76 | 0.55, 1.06 | 2.93 | 0.72, 12.0 |
| Multivariable adjustedb | 0.95 | 0.60, 1.51 | 0.78 | 0.49, 1.24 | 2.32 | 0.67, 7.96 |
| Testosterone:SHBG ratio | ||||||
| Age and race-ethnicity adjusted | 0.74 | 0.27, 2.04 | 0.22 | 0.08, 0.59 | 0.59 | 0.14, 2.48 |
| Multivariable adjustedb | 0.94 | 0.53, 1.65 | 0.24 | 0.08, 0.79 | 0.76 | 0.29, 1.96 |
| Estradiol:SHBG ratio | ||||||
| Age and race-ethnicity adjusted | 0.59 | 0.24, 1.43 | 0.21 | 0.08, 0.59 | 1.87 | 0.28, 12.6 |
| Multivariable adjustedb | 0.86 | 0.46, 1.62 | 0.26 | 0.07, 0.91 | 1.75 | 0.38, 7.97 |
Abbreviations: AAG, androstanediol glucuronide; CI, confidence interval; HR, hazard ratio; NHANES III, Third National Health and Nutrition Examination Survey; SHBG, sex hormone binding globulin.
The 10th and 90th percentile values—total testosterone: 3.2 ng/mL, 8.0 ng/mL; estradiol: 24.8 pg/mL, 52.0 pg/mL; AAG: 5.5 ng/mL, 22.9 ng/mL; free testosterone: 0.06 ng/mL, 0.16 ng/mL; bioavailable testosterone: 1.4 ng/mL, 3.9 ng/mL; free estradiol: 0.6 pg/mL, 1.4 pg/mL; SHBG: 19.8 nmol/L, 59.1 nmol/L; estradiol to testosterone ratio: 4.8, 11.7; testosterone to SHBG ratio: 0.3, 0.9; and estradiol to SHBG ratio: 1.9, 7.4.
All-cause mortality models were adjusted for age (spline), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), smoking status (never, former, current), tertile of pack-years of smoking, household income (</≥$20,000), education (</≥high school), alcohol consumption (</≥1 drink a week), exercise (none/1–2/≥3 times a week), and percent body fat (continuous). Cardiovascular disease and cancer mortality models were adjusted for age (spline), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), smoking status (never, former, current), tertile of pack-years of smoking, and percent body fat (continuous).
Models for testosterone, estradiol, and SHBG were further mutually adjusted.
Cardiovascular mortality
Between baseline and year 9, total testosterone, AAG, and the molar ratio of estradiol to testosterone were not statistically significantly associated with cardiovascular disease mortality in multivariable-adjusted models (Table 2). However, between baseline and year 9, men with low total estradiol, low free testosterone, low bioavailable testosterone, low free estradiol, and low molar ratios of testosterone to SHBG and estradiol to SHBG had a higher risk of death from cardiovascular disease than men with high levels, whereas men with low SHBG levels had a lower risk of death from cardiovascular disease than men with high levels. The multivariable-adjusted hazard ratios of cardiovascular death for a decrease in hormone concentration equal to the difference between the 90th and 10th percentiles were 2.82 (95% CI: 1.62, 4.90) for total estradiol, 1.53 (95% CI: 1.05, 2.23) for free testosterone, 1.63 (95% CI: 1.12, 2.37) for bioavailable testosterone, 3.22 (95% CI: 2.30, 4.51) for free estradiol, 0.23 (95% CI: 0.09, 0.57) for SHBG, 1.73 (95% CI: 1.22, 2.43) for the molar ratio of testosterone to SHBG, and 5.97 (95% CI: 3.63, 9.81) for the molar ratio of estradiol to SHBG.
Between year 9 and year 18, total testosterone, total estradiol, free testosterone, bioavailable testosterone, free estradiol, and the molar ratio of estradiol to testosterone were not statistically significantly associated with cardiovascular disease mortality in multivariable-adjusted models (Table 4). However, between year 9 and year 18, men with low AAG levels and low molar ratios of testosterone to SHBG and estradiol to SHBG had a lower risk of death from cardiovascular disease than men with high levels, whereas men with low SHBG levels had a higher risk of death from cardiovascular disease than men with high levels. The multivariable-adjusted hazard ratios of cardiovascular death for a decrease in hormone concentration equal to the difference between the 90th and 10th percentiles were 0.49 (95% CI: 0.25, 0.95) for AAG, 4.40 (95% CI: 1.41, 13.76) for SHBG, 0.24 (95% CI: 0.08, 0.79) for the molar ratio of testosterone to SHBG, and 0.26 (95% CI: 0.07, 0.91) for the molar ratio of estradiol to SHBG.
Cancer mortality
Between baseline and year 9, total testosterone, total estradiol, AAG, free testosterone, bioavailable testosterone, free estradiol, SHBG, and the molar ratios of estradiol to testosterone and estradiol to SHBG were not statistically significantly associated with cancer mortality in multivariable-adjusted models (Table 2). However, between baseline and year 9, the multivariable-adjusted hazard ratio for a decrease in the molar ratio of testosterone to SHBG equal to the difference between the 90th and 10th percentiles was 1.51 (95% CI: 1.05, 2.17). Between year 9 and year 18, none of the sex steroid hormones, SHBG, or molar ratios was associated with cancer mortality after multivariable adjustment (Table 4).
Sensitivity analyses
We calculated multivariable-adjusted hazard ratios of all-cause death associated with a decrease in free testosterone concentration equal to the difference between the 90th and 10th percentiles in 3 separate sensitivity analyses. When we excluded 1) participants (n = 336) with hypertension, chronic kidney disease, or diabetes mellitus (hazard ratio = 1.81, 95% CI: 1.33, 2.45 for follow-up between baseline and year 9 and hazard ratio = 0.73, 95% CI: 0.09, 5.72 for follow-up between year 9 and year 18) or 2) men aged <45 years (n = 602) (hazard ratio = 1.51, 95% CI: 1.22, 1.87 for follow-up between baseline and year 9 and hazard ratio = 0.85, 95% CI: 0.41, 1.77 for follow-up between year 9 and year 18), these associations were not appreciably different from those in the primary analysis. However, when we excluded 3) current and former smokers (n = 699) (hazard ratio = 0.82, 95% CI: 0.54, 1.23 for follow-up between baseline and year 9 and hazard ratio = 0.42, 95% CI: 0.05, 3.68 for follow-up between year 9 and year 18), free testosterone was not associated with all-cause death.
DISCUSSION
In this US representative, prospective study of adult men followed for up to 18 years, low serum levels of free testosterone and bioavailable testosterone were associated with an increased risk of all-cause and cardiovascular mortality, and low estradiol and free estradiol were associated with an increased risk of cardiovascular mortality during follow-up between baseline and year 9, but these associations did not persist during follow-up between year 9 and year 18. Low SHBG concentration was independently associated with a decreased risk of cardiovascular death during follow-up between baseline and year 9 and an increased risk of cardiovascular death during follow-up between year 9 and year 18. Total testosterone was not significantly associated with mortality in our analysis; however, there was a nonsignificant U-shaped pattern for both time periods. Free testosterone remained associated with all-cause mortality after we excluded men with other chronic diseases and young men, but not after we excluded men who had ever smoked. Sex steroid hormones were not associated with risk of cancer death except for a positive association for a low ratio of testosterone to SHBG.
Despite the importance of sex steroid hormones for male health, relatively few studies have evaluated their association with risk of death. Low total or bioavailable testosterone was associated with a higher risk of all-cause and cardiovascular death in several prior prospective studies. Among 794 men 50–91 years of age participating in the Rancho Bernardo study, low total and bioavailable testosterone was associated with higher all-cause and cardiovascular death after a mean of 12 years of follow-up (5). In a nested case-control study including 825 deceased men and 1,489 living men 40–79 years of age who participated in the EPIC-Norfolk study, which included up to 10 years of follow-up, low testosterone was associated with an increased risk of all-cause and cardiovascular mortality (4). Among 187 men a mean age of 71–72 years from Turku, Finland, low testosterone was associated with an increased risk of all-cause mortality over 10 years of follow-up (6). These associations persisted after excluding men with certain prevalent chronic diseases from these studies (4, 5) and from ours.
Our study, with extended follow-up, showed that the association between free or bioavailable testosterone and mortality does not extend after one decade. One possible explanation is that hormones change as men age; therefore, baseline, when hormone concentrations were obtained, may not be the relevant exposure period for mortality more than 9 years later. Alternatively, it is possible that the associations observed in the first half of the study were due to reverse causation by conditions already present at baseline, but not yet diagnosed, that may alter hormone concentrations and increase the risk of death. Indeed, men with acute illness and injury (14–16) and chronic disease (1, 2) often have reduced testosterone levels compared with similarly aged men. We took several steps to minimize the potential influence of reverse causation. First, a priori we excluded men with a self-reported history of cardiovascular disease or cancer. Second, we adjusted for major chronic disease risk factors. Third, we repeated the analysis excluding participants with hypertension, chronic kidney disease, or diabetes mellitus at baseline, and the results were remarkably similar.
Our findings regarding low testosterone and all-cause and cardiovascular disease risk are not consistent with those of other studies (8, 17–19), although some of these studies were small and thus subject to substantial random variability. Among men in the Massachusetts Male Aging Study (1,709 men aged 40–70 years) (3), low SHBG was associated with a lower risk of all-cause mortality but was not associated with ischemic heart disease mortality. We found that the association between SHBG and mortality depended on time since measurement of SHBG. Prospective studies with repeated measures of SHBG are needed to better characterize the relation between SHBG and all-cause and cardiovascular disease mortality.
With respect to cancer death, the EPIC-Norfolk Study (4), the Rancho Bernardo study (5), and the Massachusetts Male Aging Study (3) found that men with low total, free, or SHBG-adjusted testosterone concentrations had a nonsignificantly higher risk of cancer death. In NHANES III, we did not find any significant associations with cancer mortality, except that men with a low molar ratio of testosterone to SHBG had a higher risk of cancer mortality between baseline and year 9 than men with a higher ratio. It is important to note that fatal cancers are a unique subset of all cancers, and the association of sex steroid hormones with cancer may differ for fatal as opposed to incident cancer. Most previous studies of sex steroid hormones and cancer, however, have focused on prostate cancer incidence. In a meta-analysis of 8 prospective studies, testosterone and non-SHBG bound testosterone were not associated with the risk of prostate cancer (20). We did not have sufficient power to examine prostate cancer mortality separately.
Because sex steroid hormones affect multiple biologic systems, it is likely that the mechanisms underlying an inverse association between free hormones and mortality are complex. Nevertheless, our findings suggest that the level of androgens and estrogens available to enter cells to initiate signaling cascades may be a more important determinant of premature death than total hormone levels. Lower testosterone levels are associated with an increased risk of developing atherosclerosis (21), abdominal obesity (22), and diabetes (23) in men, and these conditions are associated with an increased risk of death. Furthermore, independent of its role as a hormone-binding protein, SHBG may affect hormone signaling (24) and thus influence risk of death.
Several additional aspects of our study warrant discussion. Our analysis was based on a single measurement of sex steroid hormones, which are subject to within-person variability over the long term and to laboratory error. The serum used to measure sex steroid hormones was collected from all participants during the morning, which should limit within-person diurnal variation. Because serum was available at only one point in time, we were not able to assess whether the slope of change in levels was associated with mortality. Free and bioavailable testosterone and free estradiol were estimated instead of directly measured. In NHANES III, causes of death were assigned on the basis of death certificates, which may contain inaccurate information, although we do not expect the accuracy to differ by hormone level. The mean age of participants at baseline was 40 years and the number of deaths was limited after stratifying follow-up to two 9-year periods, possibly limiting power to detect associations. There were 103 total deaths, 42 cardiovascular deaths, and 28 cancer deaths between baseline and year 9 and 103 total deaths, 40 cardiovascular deaths, and 30 cancer deaths between year 9 and year 18.
NHANES III collected data on a variety of health exposures and outcomes by using a rigorous protocol with extensive quality control procedures, which enabled us to adjust for potentially confounding factors in the analysis, in particular, age and cigarette smoking. We investigated multiple hormones and reported molar ratios of hormones to SHBG. It is reassuring that each expression of available testosterone—free, bioavailable, or the molar ratio of testosterone to SHBG—yielded very similar results. Importantly, NHANES III findings are generalizable to the US noninstitutionalized civilian population.
In summary, adult men from the general US population with no history of cardiovascular disease or cancer but with low free and bioavailable testosterone concentrations may have a higher risk of all-cause and cardiovascular death than men with high levels within 9 years of hormone measurement. In addition, we found that men with low estradiol and free estradiol concentrations had a higher risk of cardiovascular disease mortality than men with higher levels within 9 years of hormone measurement. Screening of the general population of adult men for low testosterone levels or testosterone replacement therapy to reduce mortality in men with low serum testosterone levels cannot be recommended at this time (25), but our findings provide the impetus to conduct future studies to fully characterize the association between sex hormones and mortality and to characterize the mechanisms underlying these associations.
Supplementary Material
Acknowledgments
Author affiliations: Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Andy Menke, Eliseo Guallar, Manning Feinleib, Elizabeth A. Platz); Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Norma Kanarek); Division of General Internal Medicine, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland (Eliseo Guallar); Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland (Erin D. Michos); Division of Endocrinology and Metabolism, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland (Adrian Dobs); Department of Pathology, Johns Hopkins School of Medicine, Baltimore, Maryland (William G. Nelson); Department of Pharmacology and Molecular Sciences, Johns Hopkins School of Medicine, Baltimore, Maryland (William G. Nelson); The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Medical Institutions, Baltimore, Maryland (William G. Nelson, Norma Kanarek, Adrian Dobs, Elizabeth A. Platz); The James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, Maryland (William G. Nelson, Elizabeth A. Platz); Department of Cardiovascular Epidemiology and Population Genetics, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain (Eliseo Guallar); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (Sabine Rohrmann); and Department of Laboratory Medicine, Harvard Medical School and Children's Hospital, Boston, Massachusetts (Nader Rifai).
This study is the 14th manuscript from the Hormone Demonstration Program, which is supported by the Maryland Cigarette Restitution Fund Research Grant Program at the Johns Hopkins University.
Conflict of interest: none declared.
Glossary
Abbreviations
- AAG
androstanediol glucuronide
- CI
confidence interval
- NHANES III
Third National Health and Nutrition Examination Survey
- SHBG
sex hormone-binding globulin
References
- 1.Maggi M, Schulman C, Quinton R, et al. The burden of testosterone deficiency syndrome in adult men: economic and quality-of-life impact. J Sex Med. 2007;4(4 pt 1):1056–1069. doi: 10.1111/j.1743-6109.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Feinleib M, Zhang L, et al. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30(2):234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 3.Araujo AB, Kupelian V, Page ST, et al. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med. 2007;167(12):1252–1260. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- 4.Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 5.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehtonen A, Huupponen R, Tuomilehto J, et al. Serum testosterone but not leptin predicts mortality in elderly men. Age Ageing. 2008;37(4):461–464. doi: 10.1093/ageing/afn048. [DOI] [PubMed] [Google Scholar]
- 7.Shores MM, Matsumoto AM, Sloan KL, et al. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166(15):1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 8.Smith GD, Ben-Shlomo Y, Beswick A, et al. Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation. 2005;112(3):332–340. doi: 10.1161/CIRCULATIONAHA.104.489088. [DOI] [PubMed] [Google Scholar]
- 9.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat. 1994;32:1–407. [PubMed] [Google Scholar]
- 10.Chumlea WC, Guo SS, Kuczmarski RJ, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26(12):1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- 11.Rinaldi S, Geay A, Déchaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002;11(10 pt 1):1065–1071. [PubMed] [Google Scholar]
- 12.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. The Third National Nutrition and Health Survey Linked Mortality File: Matching Methodology. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 14.Nierman DM, Mechanick JI. Hypotestosteronemia in chronically critically ill men. Crit Care Med. 1999;27(11):2418–2421. doi: 10.1097/00003246-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Dong Q, Hawker F, McWilliam D, et al. Circulating immunoreactive inhibin and testosterone levels in men with critical illness. Clin Endocrinol (Oxf) 1992;36(4):399–404. doi: 10.1111/j.1365-2265.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 16.Woolf PD, Hamill RW, McDonald JV, et al. Transient hypogonadotropic hypogonadism caused by critical illness. J Clin Endocrinol Metab. 1985;60(3):444–450. doi: 10.1210/jcem-60-3-444. [DOI] [PubMed] [Google Scholar]
- 17.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78(3):539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 18.Haffner SM, Moss SE, Klein BE, et al. Sex hormones and DHEA-SO4 in relation to ischemic heart disease mortality in diabetic subjects. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 1996;19(10):1045–1050. doi: 10.2337/diacare.19.10.1045. [DOI] [PubMed] [Google Scholar]
- 19.Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167(20):2249–2254. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton NE, Reeves GK, Appleby PN, et al. Endogenous sex hormones and prostate cancer: a quantitative review of prospective studies. Br J Cancer. 1999;80(7):930–934. doi: 10.1038/sj.bjc.6690445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87(8):3632–3639. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 22.Khaw KT, Barrett-Connor E. Lower endogenous androgens predict central adiposity in men. Ann Epidemiol. 1992;2(5):675–682. doi: 10.1016/1047-2797(92)90012-f. [DOI] [PubMed] [Google Scholar]
- 23.Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 24.Kahn SM, Hryb DJ, Nakhla AM, et al. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175(1):113–120. doi: 10.1677/joe.0.1750113. [DOI] [PubMed] [Google Scholar]
- 25.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91(6):1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.