Almost two decades ago, the first vaccine against Alzheimer's disease (AD), the most common form of dementia, reached clinical trials (Gilman et al., 2005). Its goal was to induce an adaptive immune response against aggregated amyloid-β peptide (Aβ), a main component of Alzheimer's hallmark amyloid plaques, which accumulate in affected brains (Hardy and Higgins, 1992). In its Phase II trial, the vaccine, known as AN1792, was administered to 300 patients with mild to moderate AD. But although almost 20% of patients developed the expected humoral response, producing antibodies against Aβ, the trial was prematurely canceled, as 6% of vaccinated participants showed symptoms of meningoencephalitis. This disappointing outcome was the result of an exacerbated proinflammatory response, likely caused by the vaccine's adjuvant (Wisniewski and Goñi, 2015).
Passive immunotherapies later emerged as an alternative; by injecting antibodies, hyperinflammation related to either antigens or adjuvants could be circumvented. By then, focus in the AD field had shifted substantially toward oligomeric forms of Aβ (AβOs), soluble toxins that attack synapses, now seen as more relevant targets than amyloid plaques for improving AD symptoms. Several clinical trials are currently ongoing, and most antibodies have at least some affinity for AβOs or other soluble Aβ species. Unfortunately, however, many promising candidates, the latest being the humanized monoclonal antibody solanezumab, have failed to meaningfully improve AD symptoms, despite reducing brain amyloid load (Doody et al., 2014). Among possible reasons for the hitherto failure of passive approaches is the fact that excessive brain inflammation remains a serious concern, which limits the safe dosage of antibodies and hinder cognitive improvement. Additionally, vascular amyloid deposits (known as cerebral amyloid angiopathy), an important feature of AD, have not been effectively cleared by immunotherapies. Although AN1792 did not alter vascular amyloid at all (Gilman et al., 2005), more current approaches have reduced it, but too often causing microhemorrhages and vasogenic edema (Sperling et al., 2011).
Indeed, when applied to AD, immunotherapies have unique challenges to overcome. Although inflammation is an aspect of virtually all pathologies, as many authors have suggested, inflammatory mediators and cytokines may have a causal role in AD. This “inflammatory hypothesis” explains certain puzzling aspects of the disease, such as its association with head trauma, depression, and diabetes, conditions where neuroinflammation is perhaps the only connecting thread (Minter et al., 2016). Because of this, immunotherapies targeting Aβ, an endogenous antigen accumulating in the brain parenchyma and vasculature, have a high risk of adverse effects, and any overt proinflammatory response is prone to worsening AD symptoms and patient outcomes.
At the center of these problems are microglia, specialized macrophages of the CNS. Although their role in AD etiology remains a matter of debate, microglial activation is a well-established event in AD pathogenesis (Minter et al., 2016). Activation profiles (defined by cytokines produced and levels of phagocytic behavior) fluctuate in AD microglia, in a disease-stage-dependent manner. An anti-inflammatory profile, with enhanced phagocytic activity, may be more abundant in preclinical stages of AD, whereas proinflammatory states peak with disease progression (Fan et al., 2017). Given that the balance of these microglial phenotypes can be a driver of either amyloid clearance or neurodegeneration, managing microglial activation will likely be essential if any immunotherapies for AD are to succeed (Delrieu et al., 2012).
In a recent issue of The Journal of Neuroscience, Scholtzova et al. (2017) provide evidence in support of an immunomodulatory approach against AD, focusing precisely on microglial and macrophage responses. The authors targeted CpG motifs, which are common features of bacterial and viral DNA, but rare in the vertebrate genome. These motifs function as toll-like receptor 9 (TLR9) agonists, typically activating innate immune responses, and enhancing adaptive responses (Krieg, 2002). Using CpG oligodeoxynucleotides (CpG ODN), Scholtzova et al. (2017) were able to improve several biochemical and behavioral traits of Tg-SwDI mice, a triple transgenic AD mouse model, noted for its extensive vascular amyloid pathology. These mice start developing Aβ deposits, particularly of the cerebral amyloid angiopathy type, at 3–4 months of age.
To assess therapeutic potential at different stages of disease progression, Scholtzova et al. (2017) administered intraperitoneal injections of CpG ODN monthly to 4-month-old and 8-month-old Tg-SwDI mice, for 10 months. For most experiments, transgenic mice receiving vehicle were used as controls. Both age groups benefitted from CpG ODN injections: the treatments prevented or reversed short-term and working memory deficits expected of Tg-SwDI mice. Furthermore, histological analyses showed significant reductions in fibrillar Aβ associated with the brain vasculature. Most notably, this reduction was not accompanied by an increase in microhemorrhages, which were indeed significantly decreased. Soluble Aβ levels were also reduced in both treatment groups, possibly as a direct consequence of the loss of fibrils, as these species likely exist at an equilibrium. At the end of the 10 month treatment period, microglial and macrophage markers were unchanged or even somewhat lower in treated mice than in controls, indicating that no significant proliferation or activation occurred. Wild-type mice subject to CpG ODN treatments also had no indication of activated microglia.
To test whether transient immune activation occurred, Scholtzova et al. (2017) used an acute administration protocol and evaluated cytokine levels in the plasma of Tg-SwDI mice 4 h after injection. Although a more complete time course might have allowed for better tracking of peak cytokine values, the analysis revealed that a CpG ODN injection induced a “mixed” response in AD mice. Several proinflammatory and anti-inflammatory mediators, including IL-10, IFN-γ, IL1-β, and TNF-α, were increased at the 4 h time point; these subsided after 30 d.
Scholtzova et al. (2017) did not elaborate on the role of these transient and relatively mild (e.g., lower than lipopolysaccharide-stimulated increases) cytokine upregulations in the cognitive effects of CpG ODN. However, it must be noted that these cytokines are able to cross the blood–brain barrier (BBB), and even an unsustained activation such as the one described by Scholtzova et al. (2017) may cause long-term, cumulative effects, by means of microglial priming (sensitization or preactivation). TNF-α in particular has been shown to induce lasting memory deficits after a single inflammatory event, while blocking its action with a neutralizing antibody restored the normal phenotype (Ledo et al., 2016). On the other hand, in a model of ischemic injury, Stevens et al. (2008) described a neuroprotective effect of CpG ODN via TLR9 stimulation, which required an increase in TNF-α levels. Notably, in this context, mice lacking TNF-α did not benefit from CpG ODN treatments. These apparently conflicting results suggest that TNF-α may promote neuronal survival or dysfunction, depending on the nature of the stimulus and the intensity and duration of the response. Likewise, the mild and transient nature of the CpG ODN-induced inflammation used by Scholtzova et al. (2017) may be key to the benefits they report in Tg-SwDI mice.
This work is the latest in a series of papers by the same group, describing the effects of TLR9 activation in three different transgenic mouse models of AD, each focused on a distinct aspect of pathophysiology (Scholtzova et al., 2009, 2014, 2017). In the previous two, Tg2576 and 3xTg mice were similarly treated with monthly intraperitoneal CpG ODN injections. Both were protected from cognitive impairment and saw decreasing levels of soluble and insoluble Aβ fractions in brain parenchyma. In 3xTg mice, this was accompanied by a reduction in the Tau pathology characteristic of this model. Doi et al. (2009), an independent group, also showed, using in vitro experiments and an AD mouse model based on intracerebroventricular AβO injections, that CpG ODN treatments markedly increase Aβ uptake by microglia and restore normal cognitive behavior. Not unlike Scholtzova et al. (2017), this group noted that the dose of CpG ODN they used had to be adjusted so as not to elicit cytotoxicity (Doi et al., 2009).
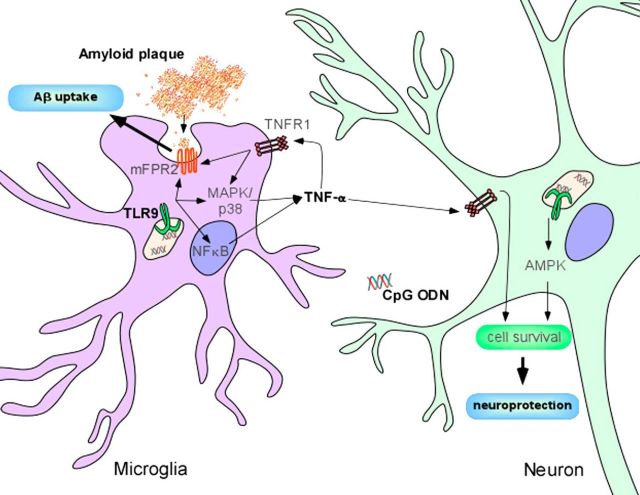
One important difference between the reports described above, however, is the route used for treatment, a relevant variable when considering the question of what cell types mediate the effects induced by CpG ODN. Whereas Doi et al. (2009) administered CpG ODN via intracerebroventricular injection, in the paper by Scholtzova et al. (2017), it was administered intraperitoneally. Because CpG ODN will not cross an intact BBB, Scholtzova et al. (2017) credited the reduced Aβ load they measured primarily to clearance by peripheral macrophages, an observation coherent with the reported lack of histological markers of microglial activation. Nevertheless, aging and vascular amyloid pathology itself are known to disrupt the BBB (Marques et al., 2013), and Scholtzova et al. (2017) recognize the possibility of a direct or indirect effect on microglia. Indeed, CpG ODN were previously shown to enhance mRNA levels of mFRP2, a transmembrane receptor that recognizes aggregated Aβ and promotes microglial migration and clearance of amyloid plaques by phagocytosis (Iribarren et al., 2005). Importantly, even without entering the brain, CpG ODN may boost peripherally derived TNF-α, which in turn would activate TNFR1 receptors present in microglia. As shown in Figure 1, this signaling initiates both p38/MAPK and NFκB responses, resulting in enhanced transcription and activation of mFPR2 (Cui et al., 2002).
Figure 1.
Possible protective mechanisms of CpG ODN in Alzheimer's disease transgenic mouse models. As reported by Scholtzova et al. (2017), treatment of Tg-SwDI mice with CpG ODN causes a mild and transient increase in TNF-α levels. Concurrently, soluble and deposited Aβ levels are reduced, and normal behavior is restored. Despite being a classic proinflammatory cytokine, TNF-α may mediate neuroprotection and survival, via NFkB and p38/MAPK activation. Additionally, mFPR2, a transmembrane receptor involved in Aβ phagocytosis and clearance, may be stimulated by TNF-α and CpG ODN. Another interesting (albeit still controversial) possibility is a direct effect of TNF-α and CpG ODN on neurons. Notably, TLR9 is expressed in neurons, where it was shown to activate AMPK, promoting stress resistance.
Although TLR9 is typically found in immune cell populations, including B cells, dendritic cells, and cells of the monocyte/macrophage lineage, such as microglia, where it localizes to endosomal vesicles and is exposed to phagocytosed debris (Chaturvedi and Pierce, 2009), it has also been detected at the cell surface (Eaton-Bassiri et al., 2004) and in certain nonimmune cells, including neurons. Recently, Shintani et al. (2014) showed that TLR9 activation in neurons can induce a response that protects them from stress. They suggest that, while in microglia, the TLR type adaptor MyD88 activates an inflammatory pathway in response to TLR9 agonists; in neurons, lower expression of MyD88 leads to an alternative response, culminating in phosphorylation of AMPK, a key stress response enzyme (Shintani et al., 2014). Although still poorly understood, a direct neuronal self-protective response could have a role in explaining the results obtained by Scholtzova et al. (2017) (Fig. 1).
CpG ODN is already an approved vaccine adjuvant. Indeed, an AD vaccine named UB-311, consisting of Aβ peptide immunogens associated with CpG ODN, is currently undergoing Phase II trials (NCT02551809). Interestingly, in this context, CpG ODN is thought to stimulate primarily a proinflammatory response. However, Scholtzova et al. (2017) show that, at least in AD mouse models, CpG ODN may produce a transient, balanced, and ultimately beneficial response, with increased phagocytic activity and upregulation of anti-inflammatory cytokines, such as IL-10. As these and other authors have shown, dose appears to be key. These results suggest that CpG ODN may also be a promising cotherapy in passive immunotherapies, an immunomodulator capable of shifting microglia and macrophages to a more neuroprotective activation profile, driving clearance of parenchymal and vascular amyloid.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/preparing-manuscript#journalclub.
This work was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro. M.C.S. and J.T.S.F. are graduate students in the Institute of Medical Biochemistry of the Federal University of Rio de Janeiro. L.E.S. is a postdoctoral fellow at the Institute of Medical Biochemistry of the Federal University of Rio de Janeiro.
The authors declare no competing financial interests.
References
- Chaturvedi A, Pierce SK (2009) How location governs toll-like receptor signaling. Traffic 10:621–628. 10.1111/j.1600-0854.2009.00899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YH, Le Y, Zhang X, Gong W, Abe K, Sun R, Van Damme J, Proost P, Wang JM (2002) Up-regulation of FPR2, a chemotactic receptor for amyloid beta 1–42 (A beta 42), in murine microglial cells by TNF alpha. Neurobiol Dis 10:366–377. 10.1006/nbdi.2002.0517 [DOI] [PubMed] [Google Scholar]
- Delrieu J, Ousset PJ, Caillaud C, Vellas B (2012) Clinical trials in Alzheimer's disease: immunotherapy approaches. J Neurochem 120:186–193. 10.1111/j.1471-4159.2011.07458.x [DOI] [PubMed] [Google Scholar]
- Doi Y, Mizuno T, Maki Y, Jin S, Mizoguchi H, Ikeyama M, Doi M, Michikawa M, Takeuchi H, Suzumura A (2009) Microglia activated with the toll-like receptor 9 ligand CpG attenuate oligomeric amyloid β neurotoxicity in in vitro and in vivo models of Alzheimer's disease. Am J Pathol 175:2121–2132. 10.2353/ajpath.2009.090418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med 370:311–321. 10.1056/NEJMoa1312889 [DOI] [PubMed] [Google Scholar]
- Eaton-Bassiri A, Dillon SB, Cunningham M, Rycyzyn MA, Mills J, Sarisky RT, Mbow ML (2004) Toll-like receptor 9 can be expressed at the cell surface of distinct populations of tonsils and human peripheral blood mononuclear cells. Infect Immun 72:7202–7211. 10.1128/IAI.72.12.7202-7211.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Brooks DJ, Okello A, Edison P (2017) An early and late peak in microglial activation in Alzheimer's disease trajectory. Brain 140:792–803. 10.1093/brain/aww349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM (2005) Clinical effect of AB immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64:1553–1562. 10.1212/01.WNL.0000159740.16984.3C [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256:184–185. 10.1126/science.1566067 [DOI] [PubMed] [Google Scholar]
- Iribarren P, Chen K, Hu J, Gong W, Cho EH, Lockett S, Uranchimeg B, Wang JM (2005) CpG-containing oligodeoxynucleotide promotes microglial cell uptake of amyloid beta 1–42 peptide by up-regulating the expression of the G-protein-coupled receptor mFPR2. FASEB J 19:2032–2034. 10.1096/fj.05-4578fje [DOI] [PubMed] [Google Scholar]
- Krieg AM. (2002) CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20:709–760. 10.1146/annurev.immunol.20.100301.064842 [DOI] [PubMed] [Google Scholar]
- Ledo JH, Azevedo EP, Beckman D, Ribeiro FC, Santos LE, Razolli DS, Kincheski GC, Melo HM, Bellio M, Teixeira AL, Velloso LA, Foguel D, De Felice FG, Ferreira ST (2016) Cross talk between brain innate immunity and serotonin signaling underlies depressive-like behavior induced by Alzheimer's amyloid-oligomers in mice. J Neurosci 36:12106–12116. 10.1523/JNEUROSCI.1269-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques F, Sousa JC, Sousa N, Palha JA (2013) Blood–brain-barriers in aging and in Alzheimer's disease. Mol. Neurodegener 8:38. 10.1186/1750-1326-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter MR, Taylor JM, Crack PJ (2016) The contribution of neuroinflammation to amyloid toxicity in Alzheimer's disease. J Neurochem 136:457–474. 10.1111/jnc.13411 [DOI] [PubMed] [Google Scholar]
- Scholtzova H, Kascsak RJ, Bates KA, Boutajangout A, Kerr DJ, Meeker HC, Mehta PD, Spinner DS, Wisniewski T (2009) Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease-related pathology. J Neurosci 29:1846–1854. 10.1523/JNEUROSCI.5715-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtzova H, Chianchiano P, Pan J, Sun Y, Goñi F, Mehta PD, Wisniewski T (2014) Amyloid β and Tau Alzheimer's disease related pathology is reduced by Toll-like receptor 9 stimulation. Acta Neuropathol Commun 2:101. 10.1186/s40478-014-0101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtzova H, Do E, Dhakal S, Sun Y, Liu S, Mehta PD, Wisniewski T (2017) Innate immunity stimulation via toll-like receptor 9 ameliorates vascular amyloid pathology in Tg-SwDI mice with associated cognitive benefits. J Neurosci 37:936–959. 10.1523/JNEUROSCI.1967-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Drexler HC, Kioka H, Terracciano CM, Coppen SR, Imamura H, Akao M, Nakai J, Wheeler AP, Higo S, Nakayama H, Takashima S, Yashiro K, Suzuki K (2014) Toll-like receptor 9 protects non-immune cells from stress by modulating mitochondrial ATP synthesis through the inhibition of SERCA2. EMBO Rep 15:438–445. 10.1002/embr.201337945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Jack CR, Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, Black RS, Brashear HR, Grundman M, Siemers ER, Feldman HH, Schindler RJ (2011) Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimer's Dement 7:367–385. 10.1016/j.jalz.2011.05.2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP (2008) Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab 28:1040–1047. 10.1038/sj.jcbfm.9600606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Goñi F (2015) Immunotherapeutic approaches for Alzheimer's disease. Neuron 85:1162–1176. 10.1016/j.neuron.2014.12.064 [DOI] [PMC free article] [PubMed] [Google Scholar]