Injuries to the CNS lead to severe and often irreversible deficits in sensorimotor and autonomous function. In spinal cord injury (SCI), mechanical damage results in the death of local neurons and glia at the lesion site within minutes to hours. This is followed by a delayed secondary damage phase characterized by neuronal and glial apoptosis (Liu et al., 1997), an increase in blood–spinal cord barrier (BSB) permeability (Popovich et al., 1996) and a neuroinflammatory response (Fleming et al., 2006) that remains poorly understood. This secondary damage process worsens the outcome and thus should serve as an important target for therapy (Oyinbo, 2011).
The neuroinflammatory response after SCI is mediated by various cell types, including astrocytes, resident microglia, infiltrating immune cells, and endothelial cells, which form the linings of the blood vessels (Hausmann, 2003). The spinal cord has a more pronounced inflammatory response to injury than the brain, with twice as many neutrophils infiltrating within 24 h, a sustained macrophage infiltration, and enhanced lymphocyte infiltration (Schnell et al., 1999). This special property may make the spinal cord particularly vulnerable to secondary lesion processes.
In recent years, the understanding of the functional role of the inflammatory response after SCI has developed significantly, with support for both beneficial and detrimental effects that promote tissue repair or degeneration, respectively (David and Kroner, 2011). On the one hand, activated microglia in the injured spinal cord produce various proinflammatory cytokines, including IL-1β, TNF-α, proteases, and other cytotoxic factors. Infusions of IL-1 antagonists 72 h after SCI in rats markedly reduced injury-induced apoptosis, indicating that the early expression of IL-1β is detrimental (Nesic et al., 2001). Moreover, immunobased neutralization of TNF-α improves functional recovery and reduces apoptosis and tissue loss after SCI (Genovese et al., 2008). On the other hand, macrophages and microglia have also been implicated in protection after injury. Beside the detrimental effects of TNF-α, appropriate TNF-α signaling seems to be required for proper remyelination by promoting the proliferation of oligodendrocyte progenitors (Arnett et al., 2001). Additionally, specific parts of the astrocytic scar were recently shown to have a supportive function for axon regeneration following SCI (Anderson et al., 2016) and might be induced by activated microglia (Liddelow et al., 2017). This Janus-faced inflammation control of immune cells depends on many factors, such as environmental cues and intracellular and intercellular signaling, and it is incompletely understood.
In a study recently published in The Journal of Neuroscience, Cohen et al. (2017) revealed a new mechanism of inflammation control after SCI. It is orchestrated by the CD200 ligand (CD200L) in endothelial cells and delivers an inhibitory signal for infiltrating macrophages and microglia. Under normal conditions, CD200L is expressed in the CNS at the blood–CSF barrier and in the spinal cord meninges, but not in the endothelium of the BSB. After SCI, however, proliferating endothelial cells located in the core of the lesion upregulate CD200L (Cohen et al., 2017, their Fig. 2A). Immunohistological analysis of the BSB showed coexpression of CD200L with the endothelium-specific antigen CD31 (Cohen et al., 2017, their Fig. 2B) and the proliferation marker Ki67, suggesting that newly formed endothelial cells are the source of CD200L (Cohen et al., 2017, their Fig. 2F). To investigate whether CD200L is essential for functional recovery, CD200L−/− and wild-type (WT) control animals were subjected to a mild SCI and analyzed for hindlimb motor function in an open field using a crude scoring test (Basso Mouse Scale). Spontaneous recovery of hindlimb function was impaired in CD200L−/− mice compared with controls (Cohen et al., 2017, their Fig. 2G). Because bone marrow-derived cells also express CD200L and infiltrate the lesion site, CD200L−/− mice were injected with WT bone marrow cells (WT>CD200L−/− mice) and vice versa (CD200L−/−>WT mice) to rule out the role of bone marrow cells in functional recovery. Only the deletion of CD200L in CNS-resident cells impaired recovery following SCI (Cohen et al., 2017, their Fig. 2H), emphasizing the crucial role of CNS-resident endothelial cells in this process.
Expression of the CD200L receptor (CD200R) was highly upregulated in isolectin B4-activated myeloid cells in the spinal cord parenchyma at the lesion site as early as 3 d after SCI (Cohen et al., 2017, their Fig. 3A,B). Further analysis by fluorescent-activated cell sorting of CD200R expression revealed different subpopulations of myeloid cells including activated microglia (CD11blowCD45.2low) and monocyte-derived macrophages (CD11bhighCD45.2high; Cohen et al., 2017, their Fig. 3C–G). A direct interaction between CD200L+ endothelial cells and CD200R+ activated myeloid cells was confirmed by both in vitro and in vivo assays. Initially, isolated bone marrow-derived cells were differentiated into macrophages and cultured with or without endothelial cells. An inflammation response was induced by lipopolysaccharide, and the gene expression profile was analyzed by quantitative PCR. In the presence of endothelial cells, expression levels of proinflammatory cytokines decreased in macrophages (Cohen et al., 2017, their Fig. 5C). This effect was blocked by adding a CD200L inhibitor to the coculture, suggesting that endothelial cells directly reduce macrophage-derived inflammation through CD200 signaling. To confirm the functional relevance of CD200L, infiltrating macrophages were isolated from the lesion site of WT and CD200L−/− animals. Flow cytometric analysis of proinflammatory (TNF-α and IL-1β) and anti-inflammatory factors (Dectin-1, CD206, IL-4R) confirmed increased inflammation levels in CD200L−/− mice relative to WT (Cohen et al., 2017, their Fig. 6G,H).
Overall, these results support the key finding about a new cellular interaction among newly formed CD200L+ endothelial cells, which negatively control the inflammatory response in CD200R+ immune cells and thereby enhance functional recovery following a spinal cord injury.
In contrast to studies focusing on neuron–microglia interaction as an important mediator of the inflammatory response in CNS diseases such as experimental autoimmune encephalomyelitis (EAE) (Chitnis et al., 2007), Cohen et al. (2017) provide evidence for another cell type to interact with immune cells: proliferating endothelial cells. Their work indicates that angiogenesis is not only enhanced by immune cells, as previously shown (for review, see Ribatti and Crivellato, 2009) but, conversely, that angiogenesis can regulate immune cells. This is in agreement with previous results indicating that endothelial growth factors enhance immune cell recruitment and the interaction of immune cells with the vessel wall (Zittermann and Issekutz, 2006).
Newly generated blood vessels can grow in the developing body via several modes, which include angiogenesis from pre-existing blood vessels and vasculogenesis from infiltrating endothelial precursor cells. Although the major mechanism of vascular regeneration after injury is thought to be angiogenesis, there is emerging evidence that endothelial precursor cells are also able to enter the lesion site (Popa-Wagner et al., 2010). However, there is a debate about whether these new vessels are functional or just serve to facilitate macrophage infiltration and removal of cellular debris from necrotic tissue. Cohen et al. (2017) provide evidence that the newly formed blood vessels also function as immune modulators through CD200L/CD200R.
Deletion of the ligand was shown here to attenuate spontaneous functional recovery in a selected motor task after SCI. However, in addition to proliferating endothelial cells, CD200L is expressed in a variety of cells, including neurons, oligodendrocytes, and astrocytes (Koning et al., 2009). Therefore, the worse outcome in the behavior of CD200L-KO mice after SCI might also be explained by a difference in neuronal, oligodendrial, or astrocytic responses. The generation of an endothelial cell-specific conditional CD200L KO would give additional evidence for the suggested positive, damage-restricting, or proregenerative role of immune cells after SCI. Analysis of lesion size, neuronal sprouting, and revascularization levels after SCI in WT and CD200L−/− animals would, in the meantime, answer further questions about the mechanisms of recovery that are seen in this study.
From a clinical point of view, it would be of great interest to investigate whether overexpression of endothelial CD200L can be beneficial for functional recovery after SCI. Several experimental studies have been conducted to enhance spontaneous recovery using neuroprotective and anti-inflammatory treatments, promotion of axonal regeneration and compensatory sprouting, transplantation of stem cells into the lesion site, electrophysiological stimulation of supralesional or sublesional spinal networks, and rehabilitative training, respectively (Hawryluk et al., 2008; Marsh et al., 2011). Cohen et al. (2017) might provide a new treatment option using CD200 signaling for spinal cord injury by specifically targeting neovascularization and the endothelial cell-associated regulation of inflammation (Fig. 1).
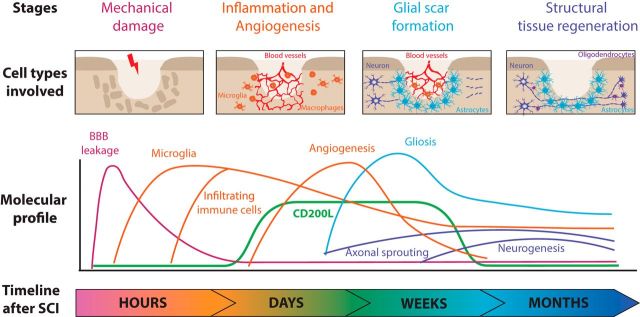
Figure 1.
Molecular processes after SCI. The following four sequential stages follow spinal cord injury: within the first hours, mechanical injury is associated with opening of the blood–brain barrier (BBB); over the next several days, immune cells infiltrate the injured area through the vascular system; next, activated astrocytes form a glial scar to protect the surrounding of the injured area; and, finally, limited levels of structural tissue regeneration occur several weeks up to months following SCI. The CD200 ligand is upregulated several days after SCI and is therefore temporally coexpressed with newly formed endothelial cells and the inflammatory response. Time points are reviewed in the study by Shechter and Schwartz (2013).
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/preparing-manuscript#journalclub.
The authors declare no competing financial interests.
References
- Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200. 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP (2001) TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4:1116–1122. 10.1038/nn738 [DOI] [PubMed] [Google Scholar]
- Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, Raddassi K, Bronson RT, Khoury SJ (2007) Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am J Pathol 170:1695–1712. 10.2353/ajpath.2007.060677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Ben-Yehuda H, Porat Z, Raposo C, Gordon S, Schwartz M (2017) Newly formed endothelial cells regulate myeloid cell activity following spinal cord injury via expression of CD200 ligand. J Neurosci 37:972–985. 10.1523/JNEUROSCI.2199-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388–399. 10.1038/nrn3053 [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC (2006) The cellular inflammatory response in human spinal cords after injury. Brain 129:3249–3269. 10.1093/brain/awl296 [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muià C, Esposito E, Bramanti P, Cuzzocrea S (2008) TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock 29:32–41. 10.1097/shk.0b013e318059053a [DOI] [PubMed] [Google Scholar]
- Hausmann ON. (2003) Post-traumatic inflammation following spinal cord injury. Spinal Cord 41:369–378. 10.1038/sj.sc.3101483 [DOI] [PubMed] [Google Scholar]
- Hawryluk GW, Rowland J, Kwon BK, Fehlings MG (2008) Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus 25:E14. 10.3171/FOC.2008.25.11.E14 [DOI] [PubMed] [Google Scholar]
- Koning N, Swaab DF, Hoek RM, Huitinga I (2009) Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol 68:159–167. 10.1097/NEN.0b013e3181964113 [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW (1997) Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci 17:5395–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh BC, Astill SL, Utley A, Ichiyama RM (2011) Movement rehabilitation after spinal cord injuries: emerging concepts and future directions. Brain Res Bull 84:327–336. 10.1016/j.brainresbull.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R (2001) IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma 18:947–956. 10.1089/089771501750451857 [DOI] [PubMed] [Google Scholar]
- Oyinbo CA. (2011) Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 71:281–299. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Pirici D, Petcu EB, Mogoanta L, Buga AM, Rosen CL, Leon R, Huber J (2010) Pathophysiology of the vascular wall and its relevance for cerebrovascular disorders in aged rodents. Curr Neurovasc Res 7:251–267. 10.2174/156720210792231813 [DOI] [PubMed] [Google Scholar]
- Popovich PG, Horner PJ, Mullin BB, Stokes BT (1996) A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol 142:258–275. 10.1006/exnr.1996.0196 [DOI] [PubMed] [Google Scholar]
- Ribatti D, Crivellato E (2009) Immune cells and angiogenesis. J Cell Mol Med 13:2822–2833. 10.1111/j.1582-4934.2009.00810.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Fearn S, Klassen H, Schwab ME, Perry VH (1999) Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci 11:3648–3658. 10.1046/j.1460-9568.1999.00792.x [DOI] [PubMed] [Google Scholar]
- Shechter R, Schwartz M (2013) CNS sterile injury: just another wound healing? Trends Mol Med 19:135–143. 10.1016/j.molmed.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Zittermann SI, Issekutz AC (2006) Endothelial growth factors VEGF and bFGF differentially enhance monocyte and neutrophil recruitment to inflammation. J Leukoc Biol 80:247–257. 10.1189/jlb.1205718 [DOI] [PubMed] [Google Scholar]