Abstract
Studies of activity-driven nervous system plasticity have primarily focused on the gray matter. However, MRI-based imaging studies have shown that white matter, primarily composed of myelinated axons, can also be dynamically regulated by activity of the healthy brain. Myelination in the CNS is an ongoing process that starts around birth and continues throughout life. Myelin in the CNS is generated by oligodendrocytes and recent evidence has shown that many aspects of oligodendrocyte development and myelination can be modulated by extrinsic signals including neuronal activity. Because modulation of myelin can, in turn, affect several aspects of conduction, the concept has emerged that activity-regulated myelination represents an important form of nervous system plasticity. Here we review our increasing understanding of how neuronal activity regulates oligodendrocytes and myelinated axons in vivo, with a focus on the timing of relevant processes. We highlight the observations that neuronal activity can rapidly tune axonal diameter, promote re-entry of oligodendrocyte progenitor cells into the cell cycle, or drive their direct differentiation into oligodendrocytes. We suggest that activity-regulated myelin formation and remodeling that significantly change axonal conduction properties are most likely to occur over timescales of days to weeks. Finally, we propose that precise fine-tuning of conduction along already-myelinated axons may also be mediated by alterations to the axon itself. We conclude that future studies need to analyze activity-driven adaptations to both axons and their myelin sheaths to fully understand how myelinated axon plasticity contributes to neuronal circuit formation and function.
Keywords: axons, myelin, oligodendrocytes, plasticity
Introduction
Approximately half of the volume of the human CNS is white matter (WM), which is largely composed of myelinated axons. The presence of concentric wraps of myelin membrane around axons in our nervous system can greatly increase their conduction velocity (CV) compared with unmyelinated axons of the same size (Waxman and Bennett, 1972). Furthermore, variation in myelin sheath length and thickness has predictable effects on CV (Hursh, 1939; Smith and Koles, 1970; Waxman, 1980; Seidl, 2014; Arancibia-Cárcamo et al., 2017). Additionally, myelin enables energetically efficient impulse propagation by restricting the regeneration of action potentials to the unmyelinated gaps between consecutive sheaths called nodes of Ranvier (Sherman and Brophy, 2005; Hartline and Colman, 2007; Chiu, 2011). However, the generation of myelin itself is costly, and it is thought that it takes months to recoup the initial energy invested in making a myelin sheath from savings in conduction efficiency (Harris and Attwell, 2012). Indeed, not all axons in our CNS are myelinated, and those that are, can be myelinated at different times in life. For example, histological studies have indicated that spinal cord axons, essential for basic motor functions, are myelinated around birth in humans; whereas cortical axons, involved in executive functions, may be myelinated decades later (Flechsig, 1896; Yakovlev and Lecours, 1967; Benes et al., 1994; Miller et al., 2012). This is supported by magnetic resonance imaging (MRI) analyses, which show ongoing growth and development of WM tracts well into adulthood (Giedd et al., 1999; Sowell et al., 2003; Lebel et al., 2008; Glasser and Van Essen, 2011; Krogsrud et al., 2016). The life-long importance of myelin for circuit formation and function is underscored by the severity of neurodevelopmental, neurodegenerative, and neuropsychiatric diseases associated with its disruption, such as leukodystrophies, schizophrenia, multiple sclerosis, and amyotrophic lateral sclerosis, among an increasing number of others (Compston and Coles, 2008; Fields, 2008; Y. Lee et al., 2012; Philips and Rothstein, 2014; Pouwels et al., 2014; Zeidán-Chuliá et al., 2014; Huang et al., 2015; Mighdoll et al., 2015; Miyata et al., 2015; Olmos-Serrano et al., 2016).
In the CNS, myelin is made by oligodendrocytes, which can make numerous myelin sheaths on multiple axons (Sherman and Brophy, 2005). Oligodendrocytes derive from oligodendrocyte progenitor cells (OPCs), also known as NG2 cells, which are present throughout our CNS from birth through death (Bergles and Richardson, 2015; Nishiyama et al., 2016). This persistence of OPCs allows not only the generation of new myelinating oligodendrocytes in the healthy adult brain, but also the regeneration of myelin following damage or disease (Zawadzka et al., 2010; Dimou and Götz, 2014). Recent evidence has indicated that myelin made in adulthood in humans arises from a combination of the production of new oligodendrocytes and the remodeling of existing myelin (Yeung et al., 2014). Supporting the possibility that myelin sheath remodeling may take place is evidence that mature myelin sheaths can be stimulated to renew growth in the adult, long after their initial formation (Flores et al., 2008; Snaidero et al., 2014; Jeffries et al., 2016). It is now clear that neuronal activity regulates many aspects of CNS myelination (Demerens et al., 1996; Makinodan et al., 2012; Gibson et al., 2014; Mensch et al., 2015). Indeed, the concept has recently emerged that activity-regulated myelination might play an important role in dynamically modulating neuronal circuit function. Supporting evidence has derived from two principal lines of investigation: MRI-based studies of how physiological brain activity relates to WM structure, and mechanistic investigations of myelination in vitro and in vivo. Here we review recent insights into how neuronal activity regulates WM, the oligodendrocyte lineage, and myelinated axons in the healthy nervous system from development through adulthood. We then focus on how such interactions might affect the formation and function of neuronal circuits in vivo.
The healthy WM is more dynamic than previously thought
Many MRI-based studies of neural plasticity in humans have focused on gray matter (GM) and revealed significant structural and functional plasticity in response to neuronal activity, now thought to underlie cognitive functions such as learning and memory (Zatorre et al., 2012). Attention to WM has been more recent, and to date has focused on structural MRI-based analysis. Over the past decade or so, we have begun to understand that WM structure is significantly dynamic and responsive to physiological experience, and that WM adaptations in the healthy brain may represent a hitherto unappreciated form of neural plasticity (Fields, 2005, 2015; Wang and Young, 2014).
Most MRI studies on WM to date have used diffusion tensor imaging (DTI), a method that provides quantitative measures of the directionality of water diffusion. In WM, water does not diffuse unconstrained in all directions; instead it occurs preferentially along myelinated axons (anisotropically). Myelin contributes to anisotropy because it prevents water diffusion transversally to the axon. Thus, an increase in anisotropy can be inferred to reflect an increase in myelination (Zatorre et al., 2012; Roberts et al., 2013); although modulation of numerous components of WM can influence anisotropy, as will be discussed below. A seminal cross-sectional WM diffusion MRI study found that expert pianists had significantly increased anisotropy in important tracts mediating bimanual motor coordination and connecting auditory regions (Bengtsson et al., 2005). Long-term practice in other cognitive modalities, such as attention and working memory, has also been associated with anisotropy changes in relevant WM tracts (B. Lee et al., 2010; Hu et al., 2011).
Given the years-long duration of repetitive training-induced neuronal activity, such cross-sectional studies are not informative about physiological alterations occurring on shorter timescales. Furthermore, these analyses cannot disentangle whether physiological activity actually causes structural WM changes or whether prior WM structural differences facilitate learning and performance. In contrast, longitudinal studies examine brain structure before and after learning a task to study shorter-term activity-induced structural changes. Seminal longitudinal studies used juggling, a complex visuomotor skill that requires bimanual coordination, grasping, and visual tracking, and showed that a week of training induces an increase in volume in cortical GM (Draganski et al., 2004; Boyke et al., 2008; Driemeyer et al., 2008). Interestingly 6 weeks of training increased anisotropy in the underlying WM, which lasted for at least 4 weeks after training stopped (Scholz et al., 2009). Learning a computer-based task that required similar skills also increased anisotropy in the same region (Lakhani et al., 2016). Training in other cognitive modalities, such as working memory (Takeuchi et al., 2010), spatial learning (Hofstetter et al., 2013), reading ability (Keller and Just, 2009), or language acquisition (Schlegel et al., 2012), also elicited WM changes after days to weeks of training.
A remarkable finding of some longitudinal studies is that structural WM plasticity can also occur in response to brief stimuli and over short timescales. For instance, two 45 min sessions of training in a whole-body balancing task, spaced a week apart, induced changes in volume in frontal and parietal brain areas and in the adjacent WM regions after the second session (Taubert et al., 2010). In other studies, subjects scanned just before and just after 2 h of training in a computer game that stimulates spatial learning showed changes in the hippocampus (Sagi et al., 2012), and in its main WM projection, the fornix (Hofstetter et al., 2013). Similar changes occurred in rats trained for 1 d in the Morris water maze (Hofstetter et al., 2013). In both humans and rats, the extent of WM changes correlated with GM changes in associated regions (Hofstetter et al., 2013), suggesting that activity-regulated adaptations take place in connected regions, potentially along specific circuits, which span GM and WM.
Collectively, these studies highlight how dynamic the healthy WM can be: responsive within hours, following even moderate stimuli. If WM changes were due solely to myelin dynamics, this would imply a very high rate of myelin synthesis and/or turnover, which is not easy to reconcile with the high energetic demand of myelin biosynthesis and with the timing of myelination (discussed below). This begs the question: what are the cellular correlates of WM structural changes?
From WM to cells
In addition to allowing inference on the myelination status of WM tracts, changes in anisotropy can also, in principle, be caused by alterations to axonal diameter, axon density, and to WM components beyond myelinated axons, such as astrocytes, OPCs, microglia, and the vasculature (Zatorre et al., 2012; Walhovd et al., 2014). MRI analyses provide only a low resolution signature that includes all of these components: for instance, a WM volume of a typical human DTI voxel size (2 mm3) has been approximately estimated to contain up to 5 million axons (which can be quite diverse in morphological and functional properties); 700,000 oligodendrocytes; 180,000 astrocytes (whose processes may occupy as much volume as myelin); 52,000 OPCs; and 76,000 microglia (Walhovd et al., 2014). Furthermore, essentially all of these respond to changes in neuronal activity (Hawrylak and Greenough, 1995; Ishibashi et al., 2006; Braun et al., 2009; Schafer et al., 2012; Yuen et al., 2014; Sun et al., 2016; Hasel et al., 2017). Thus, it remains challenging to define what the precise underlying cellular correlates of these MRI changes occurring in response to brain activity are. Nevertheless, studies in animal models, which enable histological analyses to follow-up on specific MRI changes, have supported the premise that alteration to myelin does indeed occur following stimulus-induced neuronal activity. Rats scanned the day before and the day after a 5-d-long spatial learning task show increased anisotropy in the corpus callosum (Blumenfeld-Katzir et al., 2011). Similarly, rats trained in a skilled reaching task over the course of 11 d also show increased anisotropy in the relevant subcortical WM region (Sampaio-Baptista et al., 2013). In both cases, histological follow-up show increased myelin basic protein staining in the relevant areas. These studies suggest that broad changes in myelination are indeed likely to represent one component of WM plasticity, at least over days-long timescales. How might activity-regulated myelination be mediated at a cellular level to regulate nervous system function?
Neuronal activity regulates multiple stages of oligodendrocyte development and myelination
In parallel to MRI-based approaches, mechanistic studies have now revealed that neuronal activity can regulate many aspects of oligodendrocyte lineage behavior and myelination (Baraban et al., 2016; Mount and Monje, 2017). These studies encompass those performed in vitro and in vivo, in developing systems and in adulthood, and with both physiological and nonphysiological manipulations of activity. Together these analyses reveal many cellular interactions between axons and the oligodendrocyte lineage that could contribute to WM plasticity in the human brain.
OPCS
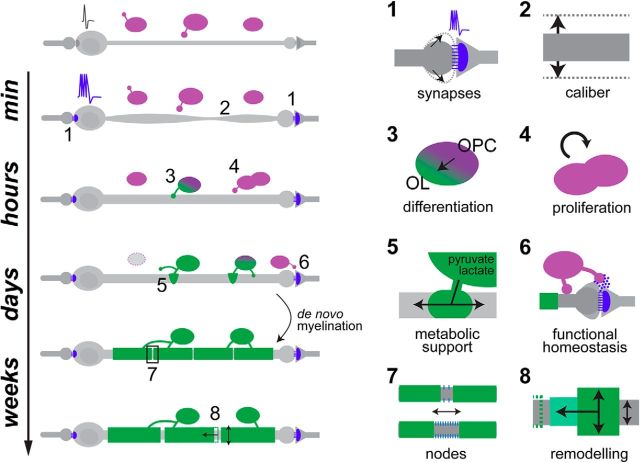
OPCs are specified during embryogenesis in discrete neural tube domains from where they migrate and proliferate to colonize the CNS (Rowitch, 2004; Richardson et al., 2006). OPCs remain present throughout life, representing 3–10% of total cells in the CNS (Dawson et al., 2003; Nishiyama et al., 2009; Richardson et al., 2011; Dimou and Götz, 2014). Interestingly, in vivo imaging based studies have indicated that the dynamic activity of OPCs appears conserved from the embryonic zebrafish spinal cord to the adult mammalian cortex (Kirby et al., 2006; Hughes et al., 2013), suggesting that developmental mechanisms regulating their lineage progression may be similar not only between species, but also at distinct times of life. Extrinsic factors, including neuronal activity, regulate OPC development (Barres and Raff, 1999; Bergles and Richardson, 2015). The ability of OPCs to sense and respond to neuronal activity is mediated by their expression of a variety of neurotransmitter receptors (Káradóttir and Attwell, 2007) and the formation of functional synapses between their processes and axons, observed from early stages of development through to adulthood (for review, see Paukert and Bergles, 2006; Sakry et al., 2011; Almeida and Lyons, 2014; Dimou and Gallo, 2015). OPCs appear to remain responsive to activity not only in development, but also in the mature CNS. For example, high-frequency electrical stimulation of corticospinal neurons in adult rats induced OPC proliferation and differentiation in the spinal cord (Li et al., 2010). More recently, optogenetic activation of motor cortex projection neurons both in juvenile and in adult mice promoted proliferation of OPCs and neural precursors in the premotor cortex and associated subcortical WM in the corpus callosum (Gibson et al., 2014). Remarkably, a 30 min stimulation paradigm was sufficient to cause a significant number of OPCs to re-enter the cell cycle, fourfold over unstimulated controls, detectable just 3 h later. Given estimates of cell cycle time for OPCs at a similar stage (Young et al., 2013), this response would be predicted to lead to a significant increase in OPC number over the course of the following days. A more protracted period of stimulation additionally drove oligodendrocyte differentiation over a period of weeks, which was accompanied by increased myelin protein expression and myelin sheath thickness in the corpus callosum (Gibson et al., 2014). This suggests that distinct responses of oligodendrocyte-lineage cells to neuronal activity may occur with different timelines: a rapid re-entry of OPCs into the cell cycle within hours, and a more protracted oligodendrocyte differentiation-myelination response occurring over days to weeks (Fig. 1).
Figure 1.
Potential timeline of activity-related changes to GM and WM. Minutes: functional synaptic adaptations (e.g., potentiation and depression) as well as structural adaptations can occur within milliseconds to minutes of stimulus onset in GM (1). Axons can grow in diameter within tens of minutes, potentially both in GM and WM (2). Hours: new oligodendrocytes can differentiate rapidly in the WM (3), and OPCs can also re-enter the cell cycle within several hours (4). MRI-detected WM changes likely reflect changes in non-myelin components, e.g., axon diameter and OPCs. Days: dividing OPCs differentiate over days, and together with rapidly differentiated oligodendrocytes may provide important metabolic support to axons (5). In parallel, OPCs can contribute to functional homeostasis at synapses (6). MRI-detected WM changes may reflect an increase in cell number following OPC proliferation, and/ or myelination. To be determined: it remains unclear over what timescales dynamic changes to nodes of Ranvier (7), axon diameter and myelin remodeling (8) take place along myelinated axons or how such changes affect one another or corresponding MRI signatures.
Additional roles of OPCs
Emerging evidence suggests that at least some OPCs perform additional functions that are independent of generating differentiated oligodendrocytes. For instance, OPCs can contact nodes of Ranvier (Butt et al., 1999; Serwanski et al., 2017), as well as axon-dendritic synapses, where they may help maintain potassium homeostasis in the extracellular space during periods of high-frequency firing (Maldonado et al., 2013), and indirectly regulate glutamate homeostasis at synapses by modulating astrocytic glutamate uptake (Birey et al., 2015). OPCs have also been implicated in regulating neuronal long-term potentiation and postsynaptic neuron AMPA receptor (AMPAR) composition via activity-driven cleavage of the NG2 proteoglycan (Sakry et al., 2014), or secretion of neuromodulatory factors (Sakry et al., 2015). Axon-OPC synapses may allow OPCs to perform their additional functions with high temporal and spatial precision, independent of or before differentiation and myelination itself. For instance, in the corpus callosum, additional OPCs generated by stimulus-induced proliferation may take days to start differentiating, but could in the meantime help buffer ion or neurotransmitter homeostasis near more actively firing axons (Fig. 1). Alternatively, it is possible that a subset of OPCs contribute to generating myelinating oligodendrocytes and other(s) to mediating these additional roles.
Oligodendrocyte differentiation
The differentiation of OPCs into oligodendrocytes is regulated by intrinsic and extrinsic factors (Zuchero and Barres, 2013). Because oligodendrocytes have a default intrinsic propensity to differentiate, both in vitro (Zeller et al., 1985; Dubois-Dalcq et al., 1986; Kachar et al., 1986; Knapp et al., 1987; Tang et al., 2000) and in vivo (Ueda et al., 1999; Almeida and Lyons, 2016), extrinsic signals are generally considered as regulators of differentiation, rather than being completely required for differentiation. A lineage-tracing study in the young and juvenile rodent CNS identified a window of 3–8 d after OPC division in which each cell initiates differentiation (Hill et al., 2014), in line with the protracted period of differentiation following optogenetic stimulation of OPC proliferation. During this window, newly differentiating oligodendrocytes appear particularly sensitive to extrinsic regulation, including by neuronal activity: sensory-deprivation, for instance, reduced the survival of newly differentiating oligodendrocytes (Hill et al., 2014). A role for activity in regulating survival of newly differentiating oligodendrocytes has recently been supported by analysis of animals in which glutamate-mediated signaling through AMPARs was ablated in the oligodendrocyte lineage. This led to a transient 20–25% reduction in differentiated oligodendrocyte and myelinated axon number in the corpus callosum at postnatal days (P)14–P21, following increased apoptosis of newly differentiating oligodendrocytes (Kougioumtzidou et al., 2017). Interestingly, the converse manipulation of increasing neuronal activity in the corpus callosum by electrical stimulation in young adults elicited distinct responses by oligodendrocytes according to firing frequency-promoting differentiation at lower frequencies and promoting OPC proliferation at higher frequencies (Nagy et al., 2017). Given that myelination can take place throughout life, it will be important to determine how cells of the oligodendrocyte lineage respond to different patterns of activity in yet other circuits at distinct stages.
In complement to studies that directly manipulate neuronal activity by genetic, optogenetic or electrophysiological approaches, behavior-driven manipulations provide the most physiologically relevant way to assess the effect of activity on myelinated axons. Two recent behavior-driven studies examined how motor learning affects oligodendrocyte lineage behavior in vivo. McKenzie et al. (2014) studied adult mice learning how to run in a complex wheel with irregularly spaced rungs. Initially, mice have great difficulty running in a complex wheel, but become proficient over a week with voluntary training. Remarkably, mice that are genetically prevented from differentiating new myelinating oligodendrocytes in adulthood, with no disruption of developmental myelination or locomotor abilities, were impaired in their performance on the complex wheel (McKenzie et al., 2014). Thus, oligodendrocyte differentiation may underlie some aspects of the motor learning process. In an important follow-up study, Xiao et al. (2016) showed that these mice had impaired performance on the complex wheel within a matter of hours. Using a novel marker of newly differentiating oligodendrocytes, Xiao et al. (2016) showed that when control mice learn to run in a complex wheel, G1-paused OPCs rapidly transition into newly differentiating oligodendrocytes without proliferation, specifically in task-relevant regions, e.g., within 2.5 h in the subcortical WM. Over the course of the training week, the OPC population did exhibit an increase in proliferation, which generated a later secondary wave of oligodendrocyte differentiation, in line with the previously noted days-long timeline of differentiation following OPC division. Importantly, myelination remains to be assessed in the context of such motor training, both in the hours-long response mediated by G1-paused OPCs that undergo rapid differentiation (Fig. 1), and in the more protracted days-long proliferation–differentiation response. It will be interesting to determine what proportion of OPCs exists in a G1-paused state (potentially poised to differentiate), in different WM tracts, and over the life-course.
Additional roles for differentiated oligodendrocytes
Although differentiated oligodendrocytes produce myelin sheaths that regulate conduction, recent evidence suggests that they may also perform additional roles. In the mouse GM, for instance, a significant proportion of myelin is deposited on inhibitory interneurons in small discontinuous patches (Micheva et al., 2016; Stedehouder et al., 2017), and it remains to be determined whether or how such myelin would impact conduction. A fundamental emerging concept is that oligodendrocytes also provide significant metabolic support to the axons they myelinate. Oligodendrocytes are thought to shuttle the glycolytic byproducts lactate and pyruvate to the associated axon, via oligodendroglial monocarboxylate transporters MCT1 and axonal MCT2, where they serve as substrates for aerobic ATP production (Fünfschilling et al., 2012). Indeed, MCT1 loss-of-function causes axonal pathology while sparing oligodendrocytes (Y. Lee et al., 2012). Remarkably, a recent study uncovered a link between axonal activity and metabolic support, whereby NMDA receptor (NMDAR) activation in oligodendrocytes stimulates glucose uptake by promoting surface localization of the glucose transporter Glut1, increasing glycolysis (Saab et al., 2016). The authors propose that the resulting lactate and pyruvate are then shuttled to axons via MCT1/2 to maintain energy supplies. Interestingly, axonal tracts with NMDAR-deficient oligodendrocytes have essentially normal myelination (De Biase et al., 2011), but they recover poorly from energy deprivation or increased energy demand (e.g., in response to high-frequency firing) and also develop age-related axonopathy (Saab et al., 2016). These results suggested the hypothesis that NMDARs in oligodendrocytes serve to sense glutamate to regulate the transfer of metabolic substrates to the axon. Further additional functions for oligodendrocytes have been suggested by two recent studies, namely regulating potassium homeostasis near the somas of pyramidal neurons (Battefeld et al., 2016), and inducing clustering of sodium channels along axons into “pre-nodes”, which can speed up conduction independently of myelination (Freeman et al., 2015). It will be important in future studies to consider all of these additional roles. For instance, in the corpus callosum, oligodendrocytes that differentiate rapidly following motor learning may not fully myelinate entire axons within hours, but their initial interactions with axons may provide important metabolic support (Fig. 1) to help facilitate a higher firing rate (Krasnow and Attwell, 2016; Saab et al., 2016; Trevisiol et al., 2017), or begin clustering ion channels to accelerate conduction.
Myelin sheath formation, growth, and remodeling
Oligodendrocytes exhibit a default propensity to make myelin: in vitro, they can extend flat sheets with extensive myelin protein expression (Bradel and Prince, 1983; Rome et al., 1986; Knapp et al., 1987), or even actual myelin sheaths around inert axon-shaped plastic fibers in the absence of specific molecular cues (S. Lee et al., 2012; Bechler et al., 2015). In vivo, the environment is more complex: only some axons are myelinated, and axons of distinct neuronal subtypes can be myelinated by different mechanisms (Koudelka et al., 2016) and at very different times. The fact that axons are myelinated in a stereotyped manner over time, and that WM structure appears responsive to neuronal activity suggests that extrinsic axonal cues are likely to coordinate if, when, and to what extent specific axons should be myelinated, both during early development and in the adult. Newly differentiated oligodendrocytes extend numerous highly dynamic processes that interact with multiple prospective axons in their environment. Live imaging studies in the developing zebrafish spinal cord have shown that individual oligodendrocytes initially overproduce short (∼5 μm long) myelin sheaths, some of which become stabilized and others retracted during a critical dynamic window of ∼5 h (Czopka et al., 2013), a similar timescale to initial sheath generation by individual mammalian oligodendrocytes in vitro (Watkins et al., 2008). After this period of axonal selection, no new sheaths are made by individual oligodendrocytes and very few are retracted. There is now good evidence that vesicular release of neurotransmitters (and possibly other signals) can bias myelin sheath formation and axon selection by oligodendrocytes. For example, preventing vesicular release from individual neurons can reduce the number of sheaths made on their axons in zebrafish (Hines et al., 2015; Koudelka et al., 2016), which was also observed in mammalian neurons in vitro (Wake et al., 2015). Interestingly, this functional regulation of myelination by activity appears specific to only some neuronal subtypes in vivo (Koudelka et al., 2016). In addition to biasing myelination to certain axons, activity-driven vesicular release may also regulate the total amount of myelin made by individual oligodendrocytes, at least during the initial period of myelin sheath formation. For example, global abrogation of vesicular release in zebrafish embryos reduces the number of sheaths made by individual oligodendrocytes during this period, whereas promoting neuronal activity increases myelin sheath number per cell (Mensch et al., 2015). However, it remains unclear whether these observations simply reflect a role of activity in regulating the local dynamics of myelinating processes, or whether activity can also influence a central program in the oligodendrocyte that sets the overall gain of myelin production. Future studies that can accurately assess myelin sheath number, length, and thickness and thus total myelin production of individual cells over time will be required to investigate these possible roles of activity. Although the studies noted here focus on developmental myelination, we suggest that the basic principles of activity-regulated myelination may apply throughout life, regardless of an individual oligodendrocyte's date of birth (Fig. 1); although further studies in adults will be required to test this prediction.
Once formed, stabilized myelin sheaths grow around and along axons, with sheath growth occurring at the direct interface with the axon (Snaidero et al., 2014). In zebrafish, the formation and growth of myelin sheaths along the length of individual axons can now be followed over time using a novel reporter that indicates the position and length of myelin sheaths on axons (Koudelka et al., 2016). The analyses of the first axons myelinated in the zebrafish CNS have revealed myelination along the entire length of axons just 2–3 mm long took several days (Koudelka et al., 2016). Although these are developmental timelines, de novo myelination of entire axons in adult is likely to take as long, or even longer, given that cellular processes tend to slow with age, as evidenced by the greatly increased cell cycle times of OPCs in adulthood (Young et al., 2013). Faster myelination of entire axons would require synchronous oligodendrocyte differentiation and myelination along the length of the axons, e.g., if G1-paused OPCs were poised to differentiate along a specific tract, but such a scenario has not been observed in vivo.
In addition to de novo myelination, changes to already myelinated axons, e.g., in myelin sheath length or thickness, may also affect circuit function. High-resolution 3D reconstruction of growing myelin sheaths revealed the presence of a network of cytoplasmic channels during myelination, which may be the transport routes for myelin components from the cell to the myelin sheath. These channels are not detected in mature sheaths, suggesting that they close as sheaths stop growing (Snaidero et al., 2014). Interestingly, forced activation of the Akt signaling pathway in adult myelinating oligodendrocytes in mice resulted in the reopening of cytoplasmic channels and the subsequent renewed growth of mature myelin sheaths (Snaidero et al., 2014). This occurred over days to weeks, and may very well be a mechanism co-opted by neuronal activity to induce sheath regrowth and remodeling. Myelin sheaths retain neurotransmitter receptors in the innermost layer at the site of interaction with the axon, where they have been proposed to enable mature oligodendrocytes to sense neurotransmitter release (Micu et al., 2016). Indeed, abrogation of vesicular release from individual axons results in shorter myelin sheaths both in developing zebrafish (Hines et al., 2015; Koudelka et al., 2016) and rodents (Wake et al., 2015; Etxeberria et al., 2016), suggesting a second independent role of activity in regulating myelin growth, after formation. However, the dynamics of myelin remodeling in vivo are unclear, and it remains to be demonstrated whether mature sheaths are indeed responsive to neuronal activity and how any such changes would actually affect conduction properties or WM signatures.
Two recent behavior-driven studies have indicated that physiological activity can also regulate myelin sheath formation and growth, and importantly that such changes in myelination impact circuit function. Makinodan et al. (2012) studied how social isolation affects CNS structure and function in juvenile mice. They identified a period from P21 to P35 during which social isolation led to pronounced behavioral defects and disruption to oligodendrocyte morphology in the prefrontal cortex (PFC). Although their number was normal, oligodendrocytes in the PFC of socially isolated mice had simpler morphologies, with fewer, shorter, and thinner myelin sheaths, and a corresponding decreased expression of myelin genes (Makinodan et al., 2012). These socially isolated mice had impairments in sociability and working memory, two PFC-dependent behaviors. Interestingly, the myelin alterations preceded the behavioral impairments, suggesting that patterns of myelination can affect neural circuit function. This suggestion was supported by a phenocopy experiment wherein conditional ablation of the receptor tyrosine kinase gene erbb3 specifically in oligodendrocytes from P19 phenocopied both the PFC myelination defects and behavioral impairments of socially isolated animals (Makinodan et al., 2012). Interestingly, the ligand for the erbb3 receptor, neuregulin1, is known to be regulated by neuronal activity and is downregulated following social isolation (Liu et al., 2011; Makinodan et al., 2012). Furthermore, neuregulin is capable of switching the myelination of oligodendrocytes to being responsive to neuronal activity in vitro (Lundgaard et al., 2013), suggesting a possible molecular basis for these observations.
A parallel study of how social isolation affects myelination showed that in adult animals, protracted isolation for 8 weeks also leads to alterations of myelin gene expression and myelination in the PFC (Liu et al., 2012). Remarkably, these phenotypes could be rescued by rehousing in a social environment, or, as shown in a follow-up study, by treating animals with the promyelinating drug clemastine (Liu et al., 2016). These studies indicate that activity regulates myelination in juveniles and adults in a similar manner, but over different timescales. Future studies that monitor the myelination status of specific axons and circuits over time will be required to determine to what extent social isolation, or indeed any form of neuronal activity, affects de novo myelination or remodeling of already-myelinated axons. Nonetheless, these studies of social isolation and myelination lend further support to the idea that neuronal activity dynamically modulates myelination; that this, in turn, affects neuronal circuit function, and thus that activity-regulated myelination represents a form of functional plasticity.
Neuronal activity also regulates axon structure
In addition to the fact that neuronal activity can regulate oligodendrocytes and myelination, there is now emerging evidence that the structure and molecular composition of the axon itself is responsive to experience. For example, unmyelinated axons were recently observed to be dynamically regulated by both high-frequency and physiological firing ex vivo, wherein increased activity led to a progressive enlargement of axons in diameter over tens of minutes (Chéreau et al., 2017; Fig. 1). Interestingly, axon diameter is now known to be a core determinant of myelination in the CNS (Almeida et al., 2011; S. Lee et al., 2012; Goebbels et al., 2017), as has long been known in the peripheral nervous system (Voyvodic, 1989). Thus, primary and rapid regulation of axon diameter in response to neuronal activity may in fact trigger later de novo myelination (Fig. 1), which will be important to investigate in the future.
In addition to the observation that neuronal activity can regulate the diameter of unmyelinated axons, an increase in axonal diameter has also been observed along myelinated axons of the auditory brainstem, coincident with the onset of hearing. Indeed, when the onset of auditory stimuli is experimentally delayed, the growth in diameter of the same myelinated axons is prevented, until later restoration of sensory input (Sinclair et al., 2017), demonstrating a role for activity in regulating the diameter of myelinated axons as well. Thus, activity may contribute to the dynamic regulation of both de novo myelination and myelinated axon remodeling via modulation of axon diameter (Fig. 1).
Indeed, alterations to axonal diameter may also contribute to the WM signatures observed by MRI following physiological brain activity in humans. For example, in a longitudinal study of WM plasticity following meditation, anisotropy-based measures thought to reflect an increase in axon diameter were observed before those reflecting an increase in myelination (Tang et al., 2012). However, how changes in axonal organization and diameter affect various aspects of MRI-based signatures is complex and context dependent (Beaulieu, 2002). Furthermore, if changes in axon diameter lead to subsequent changes in myelination along WM tracts, the anisotropy-based signatures reflecting such changes are likely to dynamically change over time. Given the importance of dynamic changes in WM structure in both the healthy nervous system and in disease (Beaulieu, 2002), there is an important drive in the community to develop increasingly refined structural MRI analyses (Stikov et al., 2015; Lerch et al., 2017; Wu and Miller, 2017) and to better correlate MRI signatures with actual cellular alterations. Furthermore, the emergence of functional MRI analysis of WM tracts (Gawryluk et al., 2014; Peer et al., 2017; Warbrick et al., 2017) will further reveal the full extent of WM dynamics and the relative contribution of myelin and non-myelin adaptations.
How does regulation of myelination and axonal structure and composition affect neuronal circuit function?
In principle, myelinated axon structure and composition can regulate neuronal circuit function in several ways. Myelination is primarily thought to regulate CV. For instance, de novo myelination of previously unmyelinated axons accelerates CV. In addition, regulation of the number, distribution, length, and thickness of myelin sheaths along myelinated axons could be used to fine-tune CV. This is because regulation of the geometric properties of myelin sheaths also regulate CV (Hursh, 1939; Smith and Koles, 1970; Waxman, 1980; Wu et al., 2012; Seidl, 2014; Arancibia-Cárcamo et al., 2017). Recent studies have indicated surprising diversity in the pattern of myelination along the length of at least some axons, whereby myelin sheaths are irregularly spaced and often interspersed by very large unmyelinated stretches (Tomassy et al., 2014). How such a pattern of myelination relates to the axons' function remains to be determined. However, there is evidence that precise regulation of myelination occurs in at least some other specific circuits in vivo to meet specific conduction requirements (Lang and Rosenbluth, 2003; Salami et al., 2003; Ford et al., 2015; Seidl and Rubel, 2016). For instance, encoding the spatial location of an auditory stimulus requires uniform conduction times along the two main branches of individual cochlear neuron axons to coincidentally deliver action potentials to distinct target neurons in opposite hemispheres. To compensate for the different lengths of the collateral branches projecting to each hemisphere, longer myelin sheaths are found along the longer collateral, which is thought to help increase its CV, equalize conduction times along each collateral, and thus facilitate coincident impulse arrival (Seidl et al., 2010; Seidl and Rubel, 2016). Thus, dynamically changing CV along specific axons by refining myelination may alter the coincident arrival of action potentials in postsynaptic neurons. Changing the arrival of impulses at postsynaptic neurons may also change the balance between excitation and inhibition, in essence, regulating the firing probability of a neuron. In some circuits, the order and precise timing of presynaptic and postsynaptic potentials determine whether potentiation or depression is induced (Feldman, 2012; Markram et al., 2012). In addition to regulating the speed and timing of conduction, myelinated axons may also better sustain high-frequency firing compared with unmyelinated axons (Perge et al., 2012). This could be due to the possibility that myelin may help support metabolically demanding high-frequency firing of action potentials (Saab et al., 2016). Additionally, myelin restricts the regeneration of action potentials to the very small nodes of Ranvier, which enables rapid repetitive cycles of axolemma depolarization and repolarization (Fields, 2008). At the network level, precise regulation of both conduction timing and firing frequency may be necessary between neuron populations to generate synchronous or time-locked firing patterns and oscillations, which have been associated with numerous higher cognitive functions such as attention, sleep, or memory (Pajevic et al., 2014). Future studies that combine high-resolution 3D analyses of anatomy with functional assessment of neurophysiology will provide important information to allow informed modeling of the role of myelin in regulating emergent properties of neural circuits.
In addition to myelin-driven changes in conduction, activity-driven regulation of the axon itself can also affect function. In fact, to modulate conduction and circuit function, it is arguably simpler, faster, and energetically cheaper to regulate the structure or composition of the axon, than to remodel myelin made by numerous independent cells along its length. For example, CV increases with axon diameter (Hursh, 1939; Matsumoto and Tasaki, 1977; Waxman, 1980). Furthermore, fine-tuning CV could be achieved by changing axonal domains. For example, in axons of the auditory brainstem, the diameter of nodes of Ranvier increases along the axon, which has been predicted to contribute to regulation of precise conduction times (Ford et al., 2015). Indeed, further anatomically-informed modeling studies have indicated that nodal size and composition can be regulated along myelinated axons to achieve comparable CV alterations to those of myelin changes, but at a fraction of the energetic cost (Arancibia-Cárcamo et al., 2017). Dynamic alteration of node of Ranvier structure remains to be visualized in vivo, but nodal length actually can be fine-tuned by the axon via its own cytoskeleton during development, and not necessarily by the flanking myelin sheaths (Brivio et al., 2017). The axon initial segment, where the action potential is initiated, and which is similar in composition to nodes, can, in fact, be structurally remodeled in response to activity (Grubb and Burrone, 2010; Kuba et al., 2010; Yamada and Kuba, 2016), to control action potential firing. Thus, modulation of many aspects of myelinated axons are well poised to have profound effects on nervous system function (Fig. 1).
To understand how dynamic alterations to myelin and myelinated axons regulate neuronal circuit function it will be necessary to concomitantly interrogate the morphological and functional development of entire individual axons over time in the context of de novo myelination and remodeling. Ongoing technical developments will allow detailed reconstruction of the morphology and ultrastructure of individual myelinated axons over time (Wang et al., 2005; Schain et al., 2014; Tomassy et al., 2014; de Vito et al., 2014), which will help bridge this gap, when integrated with detailed functional studies.
Final remarks and perspectives
In summary, mechanistic studies have provided numerous insights into the dynamic and adaptive nature of the oligodendrocyte lineage and myelination, particularly in response to neuronal activity. In parallel, MRI studies have provided compelling evidence that brain activity can regulate WM structure in a circuit-specific manner that implies a role in functional plasticity. In Figure 1 we provide an overview of the timelines of prospective activity-driven changes to axonal morphology, the oligodendrocyte lineage, and myelinated axon subdomains. We propose that the effects that occur acutely on the order of minutes to hours are most likely to represent initial changes to the axon and non-myelin related changes to OPCs and differentiating oligodendrocytes, and those that occur over longer timescales will represent de novo myelination of axons, myelin remodeling, and further dynamic alteration to the myelinated axon (Fig. 1). It is essential to note that myelination is not restricted to WM. Many axons in the GM are myelinated (Tomassy et al., 2014; Micheva et al., 2016; Stedehouder et al., 2017). Neurons with myelinated axons that project through WM tracts will typically have their cell body, some of their axon and also their distal synaptic terminals in GM regions. Therefore, future analyses will need to focus on entire myelinated axons that traverse both GM and WM. Furthermore, a complete understanding of myelinated axon function will require study of the structure and composition of the domains of the axon itself. Therefore, we suggest that the term myelinated axon plasticity more completely conveys the range of potential adaptations within these functional units. Given that neuronal activity can regulate multiple stages of oligodendrocyte lineage behavior through myelination as well as axonal structure, it is likely that myelinated axon plasticity plays a central role in many aspects of the formation and function of neuronal circuits that remain to be discovered.
Numerous fundamental questions remain to be addressed, for example:
1. How does myelinated axon plasticity affect axonal conduction and function?
Changes to axonal diameter, myelination, and the formation of associated axonal domains changes conduction from graded to saltatory, but we know little about how the functional properties of individual axons change throughout these processes. For example, does partial myelination already affect conduction or the ability to sustain high-frequency firing? Similarly, our knowledge of the functional impact of subsequent myelin remodeling and refinement of axonal domains along single axons remains unclear. Emerging technologies to map and manipulate individual neurons and their connections coupled with the ability to interrogate function in vivo will be essential to bridge this gap (Fosque et al., 2015; Joesch et al., 2016; Wanner et al., 2016; Förster et al., 2017; Hildebrand et al., 2017).
2. Which neuronal subtypes and circuits exhibit myelinated axon plasticity?
In developing zebrafish, activity-regulated myelination has been shown to be a property of only specific neuronal subtypes (Koudelka et al., 2016). It will be important to define which neurons and circuits exhibit myelinated axon plasticity throughout life, and in response to experience. In addition to neuronal diversity, there is increasing evidence of diversity in the oligodendrocyte lineage, which will be important to consider from the point of view of circuit formation and function in future studies (Butt et al., 2005; Káradóttir et al., 2008; Nishiyama et al., 2009; Viganò et al., 2013; Bechler et al., 2015; Marques et al., 2016).
3. How does myelinated axon plasticity relate to nervous system growth?
Myelination of certain axons occurs very early in life, long before the nervous system has grown to its mature adult size. This begs the question: how is function maintained along individual axons over time? Do axons grow in both length and diameter in-step with animal growth, and if so, do individual myelin sheaths follow suit? Or, do individual axons need to be actively remodeled over time to sustain function, e.g., by addition of new myelin sheaths as the animal grows? Again, longitudinal live imaging will address these questions.
4. How flexible is myelinated axon plasticity?
Could adaptations to the structure and function of specific myelinated axons during development or following training in a specific task facilitate the subsequent learning or execution of a related task? For instance, could fine-motor skills acquired when learning to play piano also benefit subsequent learning of another musical instrument? Also, how stable are activity-regulated structural modifications to myelinated axons? Once made, are they stable for an indefinite period, or do they require continuous activity to be maintained, such that myelin sheaths may shrink or be retracted from axons in disuse (e.g., following social isolation)?
5. How relevant is myelinated axon plasticity to disease?
It is now clear that disruption to myelinated axons is a feature of many CNS diseases. To what extent could myelinated axon plasticity be used to maintain function during the disease course? For instance, if axons become demyelinated (e.g., in multiple sclerosis), could adaptations to that axon or to other axons in the circuit compensate to help maintain function? Furthermore, could disruption to the mechanisms underlying the plasticity of myelinated axons underlie defects in circuit-level communication that characterize neuropsychiatric conditions?
Future studies that bridge scales of analyses from ultrastructure to circuit, from molecule to behavior, and from fish to man will illuminate how myelinated axon plasticity affects neuronal circuit formation and higher-order function.
Footnotes
The authors declare no competing financial interests.
References
- Almeida R, Lyons D (2016) Oligodendrocyte development in the absence of their target axons in vivo. PloS One 11:e0164432. 10.1371/journal.pone.0164432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RG, Lyons DA (2014) On the resemblance of synapse formation and CNS myelination. Neuroscience 276:98–108. 10.1016/j.neuroscience.2013.08.062 [DOI] [PubMed] [Google Scholar]
- Almeida RG, Czopka T, Ffrench-Constant C, Lyons DA (2011) Individual axons regulate the myelinating potential of single oligodendrocytes in vivo. Development 138:4443–4450. 10.1242/dev.071001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia-Cárcamo IL, Ford MC, Cossell L, Ishida K, Tohyama K, Attwell D (2017) Node of Ranvier length as a potential regulator of myelinated axon conduction speed. Elife 6:e23329. 10.7554/eLife.23329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban M, Mensch S, Lyons DA (2016) Adaptive myelination from fish to man. Brain Res 1641:149–161. 10.1016/j.brainres.2015.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Raff MC (1999) Axonal control of oligodendrocyte development. J Cell Biol 147:1123–1128. 10.1083/jcb.147.6.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battefeld A, Klooster J, Kole MH (2016) Myelinating satellite oligodendrocytes are integrated in a glial syncytium constraining neuronal high-frequency activity. Nat Commun 7:11298. 10.1038/ncomms11298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. (2002) The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 15:435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Bechler ME, Byrne L, Ffrench-Constant C (2015) CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr Biol 25:2411–2416. 10.1016/j.cub.2015.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P (1994) Myelination of a key relay zone in the hippocampal-formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiat 51:477–484. 10.1001/archpsyc.1994.03950060041004 [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F (2005) Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8:1148–1150. 10.1038/nn1516 [DOI] [PubMed] [Google Scholar]
- Bergles DE, Richardson WD (2015) Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol 8:a020453. 10.1101/cshperspect.a020453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F, Kloc M, Chavali M, Hussein I, Wilson M, Christoffel DJ, Chen T, Frohman MA, Robinson JK, Russo SJ, Maffei A, Aguirre A (2015) Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron 88:941–956. 10.1016/j.neuron.2015.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y (2011) Diffusion MRI of structural brain plasticity induced by a learning and memory task. PloS One 6:e20678. 10.1371/journal.pone.0020678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Büchel C, May A (2008) Training-induced brain structure changes in the elderly. J Neurosci 28:7031–7035. 10.1523/JNEUROSCI.0742-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradel EJ, Prince FP (1983) Cultured neonatal rat oligodendrocytes elaborate myelin membrane in the absence of neurons. J Neurosci Res 9:381–392. 10.1002/jnr.490090404 [DOI] [PubMed] [Google Scholar]
- Braun K, Antemano R, Helmeke C, Büchner M, Poeggel G (2009) Juvenile separation stress induces rapid region- and layer-specific changes in S100ss- and glial fibrillary acidic protein-immunoreactivity in astrocytes of the rodent medial prefrontal cortex. Neuroscience 160:629–638. 10.1016/j.neuroscience.2009.02.074 [DOI] [PubMed] [Google Scholar]
- Brivio V, Faivre-Sarrailh C, Peles E, Sherman DL, Brophy PJ (2017) Assembly of CNS nodes of Ranvier in myelinated nerves is promoted by the axon cytoskeleton. Curr Biol 27:1068–1073. 10.1016/j.cub.2017.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M (1999) Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia 26:84–91. [DOI] [PubMed] [Google Scholar]
- Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M (2005) Synantocytes: the fifth element. J Anat 207:695–706. 10.1111/j.1469-7580.2005.00458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chéreau R, Saraceno GE, Angibaud J, Cattaert D, Nägerl UV (2017) Superresolution imaging reveals activity-dependent plasticity of axon morphology linked to changes in action potential conduction velocity. Proc Natl Acad Sci U S A 114:1401–1406. 10.1073/pnas.1607541114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SY. (2011) Matching mitochondria to metabolic needs at nodes of Ranvier. Neuroscientist 17:343–350. 10.1177/1073858410393740 [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517. 10.1016/S0140-6736(08)61620-7 [DOI] [PubMed] [Google Scholar]
- Czopka T, Ffrench-Constant C, Lyons DA (2013) Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev cell 25:599–609. 10.1016/j.devcel.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24:476–488. 10.1016/S1044-7431(03)00210-0 [DOI] [PubMed] [Google Scholar]
- De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE (2011) NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci 31:12650–12662. 10.1523/JNEUROSCI.2455-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C (1996) Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A 93:9887–9892. 10.1073/pnas.93.18.9887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vito G, Tonazzini I, Cecchini M, Piazza V (2014) RP-CARS: label-free optical readout of the myelin intrinsic healthiness. Opt Express 22:13733–13743. 10.1364/OE.22.013733 [DOI] [PubMed] [Google Scholar]
- Dimou L, Gallo V (2015) NG2-glia and their functions in the central nervous system. Glia 63:1429–1451. 10.1002/glia.22859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Götz M (2014) Glial cells as progenitors and stem cells: new roles in the healthy and diseased brain. Physiol Rev 94:709–737. 10.1152/physrev.00036.2013 [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004) Neuroplasticity: changes in grey matter induced by training. Nature 427:311–312. 10.1038/427311a [DOI] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A (2008) Changes in gray matter induced by learning: revisited. PloS One 3:e2669. 10.1371/journal.pone.0002669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Behar T, Hudson L, Lazzarini RA (1986) Emergence of three myelin proteins in oligodendrocytes cultured without neurons. J Cell Biol 102:384–392. 10.1083/jcb.102.2.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria A, Hokanson KC, Dao DQ, Mayoral SR, Mei F, Redmond SA, Ullian EM, Chan JR (2016) Dynamic modulation of myelination in response to visual stimuli alters optic nerve conduction velocity. J Neurosci 36:6937–6948. 10.1523/JNEUROSCI.0908-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. (2012) The spike-timing dependence of plasticity. Neuron 75:556–571. 10.1016/j.neuron.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. (2005) Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist 11:528–531. 10.1177/1073858405282304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31:361–370. 10.1016/j.tins.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. (2015) A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci 16:756–767. 10.1038/nrn4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig PE. (1896) Gehirn und seele: Rede, gehalten am 31. October 1894 in der Universitätskirche zu Leipzig. Leipzig: Verlag von Veit. [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB (2008) Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci 28:7174–7183. 10.1523/JNEUROSCI.0150-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C, Pecka M, Attwell D, Grothe B (2015) Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat Commun 6:8073. 10.1038/ncomms9073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster D, Dal Maschio M, Laurell E, Baier H (2017) An optogenetic toolbox for unbiased discovery of functionally connected cells in neural circuits. Nat Commun 8:116. 10.1038/s41467-017-00160-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosque BF, Sun Y, Dana H, Yang CT, Ohyama T, Tadross MR, Patel R, Zlatic M, Kim DS, Ahrens MB, Jayaraman V, Looger LL, Schreiter ER (2015) Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science 347:755–760. 10.1126/science.1260922 [DOI] [PubMed] [Google Scholar]
- Freeman SA, Desmazières A, Simonnet J, Gatta M, Pfeiffer F, Aigrot MS, Rappeneau Q, Guerreiro S, Michel PP, Yanagawa Y, Barbin G, Brophy PJ, Fricker D, Lubetzki C, Sol-Foulon N (2015) Acceleration of conduction velocity linked to clustering of nodal components precedes myelination. Proc Natl Acad Sci U S A 112:E321–E328. 10.1073/pnas.1419099112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521. 10.1038/nature11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryluk JR, Mazerolle EL, D'Arcy RC (2014) Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front Neurosci 8:239. 10.3389/fnins.2014.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304. 10.1126/science.1252304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999) Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2:861–863. 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC (2011) Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci 31:11597–11616. 10.1523/JNEUROSCI.2180-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Wieser GL, Pieper A, Spitzer S, Weege B, Yan K, Edgar JM, Yagensky O, Wichert SP, Agarwal A, Karram K, Renier N, Tessier-Lavigne M, Rossner MJ, Káradóttir RT, Nave KA (2017) A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte precursor recruitment and myelination. Nat Neurosci 20:10–15. 10.1038/nn.4425 [DOI] [PubMed] [Google Scholar]
- Grubb MS, Burrone J (2010) Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465:1070–1074. 10.1038/nature09160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Attwell D (2012) The energetics of CNS white matter. J Neurosci 32:356–371. 10.1523/JNEUROSCI.3430-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline DK, Colman DR (2007) Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol 17:R29–R35. 10.1016/j.cub.2006.11.042 [DOI] [PubMed] [Google Scholar]
- Hasel P, Dando O, Jiwaji Z, Baxter P, Todd AC, Heron S, Márkus NM, McQueen J, Hampton DW, Torvell M, Tiwari SS, McKay S, Eraso-Pichot A, Zorzano A, Masgrau R, Galea E, Chandran S, Wyllie DJA, Simpson TI, Hardingham GE (2017) Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat Commun 8:15132. 10.1038/ncomms15132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylak N, Greenough WT (1995) Monocular deprivation alters the morphology of glial fibrillary acidic protein-immunoreactive astrocytes in the rat visual cortex. Brain Res 683:187–199. 10.1016/0006-8993(95)00374-Y [DOI] [PubMed] [Google Scholar]
- Hildebrand DG, Cicconet M, Torres RM, Choi W, Quan TM, Moon J, Wetzel AW, Scott Champion A, Graham BJ, Randlett O, Plummer GS, Portugues R, Bianco IH, Saalfeld S, Baden AD, Lillaney K, Burns R, Vogelstein JT, Schier AF, Lee WA, et al. (2017) Whole-brain serial-section electron microscopy in larval zebrafish. Nature 545:345–349. 10.1038/nature22356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A (2014) Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat Neurosci 17:1518–1527. 10.1038/nn.3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B (2015) Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci 18:683–689. 10.1038/nn.3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter S, Tavor I, Tzur Moryosef S, Assaf Y (2013) Short-term learning induces white matter plasticity in the fornix. J Neurosci 33:12844–12850. 10.1523/JNEUROSCI.4520-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Wei W, Wang G, Gaertig MA, Feng Y, Wang W, Li XJ, Li S (2015) Mutant huntingtin downregulates myelin regulatory factor-mediated myelin gene expression and affects mature oligodendrocytes. Neuron 85:1212–1226. 10.1016/j.neuron.2015.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Geng F, Tao L, Hu N, Du F, Fu K, Chen F (2011) Enhanced white matter tracts integrity in children with abacus training. Hum Brain Mapp 32:10–21. 10.1002/hbm.20996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE (2013) Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci 16:668–676. 10.1038/nn.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh JB. (1939) Conduction velocity and the diameter of nerve fibers. Am J Physiol 127:131–139. [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD (2006) Astrocytes promote myelination in response to electrical impulses. Neuron 49:823–832. 10.1016/j.neuron.2006.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries MA, Urbanek K, Torres L, Wendell SG, Rubio ME, Fyffe-Maricich SL (2016) ERK1/2 activation in preexisting oligodendrocytes of adult mice drives new myelin synthesis and enhanced CNS function. J Neurosci 36:9186–9200. 10.1523/JNEUROSCI.1444-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joesch M, Mankus D, Yamagata M, Shahbazi A, Schalek R, Suissa-Peleg A, Meister M, Lichtman JW, Scheirer WJ, Sanes JR (2016) Reconstruction of genetically identified neurons imaged by serial-section electron microscopy. Elife 5:e15015. 10.7554/eLife.15015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B, Behar T, Dubois-Dalcq M (1986) Cell shape and motility of oligodendrocytes cultured without neurons. Cell Tissue Res 244:27–38. [DOI] [PubMed] [Google Scholar]
- Káradóttir R, Attwell D (2007) Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience 145:1426–1438. 10.1016/j.neuroscience.2006.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, Hamilton NB, Bakiri Y, Attwell D (2008) Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci 11:450–456. 10.1038/nn2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Just MA (2009) Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron 64:624–631. 10.1016/j.neuron.2009.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B (2006) In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci 9:1506–1511. 10.1038/nn1803 [DOI] [PubMed] [Google Scholar]
- Knapp PE, Bartlett WP, Skoff RP (1987) Cultured oligodendrocytes mimic in vivo phenotypic characteristics: cell shape, expression of myelin-specific antigens, and membrane production. Dev Biol 120:356–365. 10.1016/0012-1606(87)90238-7 [DOI] [PubMed] [Google Scholar]
- Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, Lyons DA (2016) Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr Biol 26:1447–1455. 10.1016/j.cub.2016.03.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kougioumtzidou E, Shimizu T, Hamilton NB, Tohyama K, Sprengel R, Monyer H, Attwell D, Richardson WD (2017) Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. Elife 6:e28080. 10.7554/eLife.28080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow AM, Attwell D (2016) NMDA receptors: power switches for oligodendrocytes. Neuron 91:3–5. 10.1016/j.neuron.2016.06.023 [DOI] [PubMed] [Google Scholar]
- Krogsrud SK, Fjell AM, Tamnes CK, Grydeland H, Mork L, Due-Tønnessen P, Bjørnerud A, Sampaio-Baptista C, Andersson J, Johansen-Berg H, Walhovd KB (2016) Changes in white matter microstructure in the developing brain: a longitudinal diffusion tensor imaging study of children from 4 to 11years of age. Neuroimage 124:473–486. 10.1016/j.neuroimage.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Oichi Y, Ohmori H (2010) Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature 465:1075–1078. 10.1038/nature09087 [DOI] [PubMed] [Google Scholar]
- Lakhani B, Borich MR, Jackson JN, Wadden KP, Peters S, Villamayor A, MacKay AL, Vavasour IM, Rauscher A, Boyd LA (2016) Motor skill acquisition promotes human brain myelin plasticity. Neural Plast 2016:7526135. 10.1155/2016/7526135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Rosenbluth J (2003) Role of myelination in the development of a uniform olivocerebellar conduction time. J Neurophysiol 89:2259–2270. 10.1152/jn.00922.2002 [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008) Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055. 10.1016/j.neuroimage.2007.12.053 [DOI] [PubMed] [Google Scholar]
- Lee B, Park JY, Jung WH, Kim HS, Oh JS, Choi CH, Jang JH, Kang DH, Kwon JS (2010) White matter neuroplastic changes in long-term trained players of the game of “Baduk” (GO): a voxel-based diffusion-tensor imaging study. Neuroimage 52:9–19. 10.1016/j.neuroimage.2010.04.014 [DOI] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, Feng ZQ, Corey JM, Chan JR (2012) A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods 9:917–922. 10.1038/nmeth.2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448. 10.1038/nature11314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, van der Kouwe AJ, Raznahan A, Paus T, Johansen-Berg H, Miller KL, Smith SM, Fischl B, Sotiropoulos SN (2017) Studying neuroanatomy using MRI. Nat Neurosci 20:314–326. 10.1038/nn.4501 [DOI] [PubMed] [Google Scholar]
- Li Q, Brus-Ramer M, Martin JH, McDonald JW (2010) Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci Lett 479:128–133. 10.1016/j.neulet.2010.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P (2012) Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 15:1621–1623. 10.1038/nn.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dupree JL, Gacias M, Frawley R, Sikder T, Naik P, Casaccia P (2016) Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J Neurosci 36:957–962. 10.1523/JNEUROSCI.3608-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bates R, Yin DM, Shen C, Wang F, Su N, Kirov SA, Luo Y, Wang JZ, Xiong WC, Mei L (2011) Specific regulation of NRG1 isoform expression by neuronal activity. J Neurosci 31:8491–8501. 10.1523/JNEUROSCI.5317-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJ, Charles FC, Attwell D, Káradóttir RT (2013) Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol 11:e1001743. 10.1371/journal.pbio.1001743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G (2012) A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337:1357–1360. 10.1126/science.1220845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado PP, Vélez-Fort M, Levavasseur F, Angulo MC (2013) Oligodendrocyte precursor cells are accurate sensors of local K+ in mature gray matter. J Neurosci 33:2432–2442. 10.1523/JNEUROSCI.1961-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Gerstner W, Sjöström PJ (2012) Spike-timing-dependent plasticity: a comprehensive overview. Front Synaptic Neurosci 4:2. 10.3389/fnsyn.2012.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcão A, Xiao L, Li H, Häring M, Hochgerner H, Romanov RA, Gyllborg D, Muñoz Manchado A, La Manno G, Lönnerberg P, Floriddia EM, Rezayee F, Ernfors P, Arenas E, Hjerling-Leffler J, Harkany T, et al. (2016) Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352:1326–1329. 10.1126/science.aaf6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto G, Tasaki I (1977) A study of conduction velocity in nonmyelinated nerve fibers. Biophys J 20:1–13. 10.1016/S0006-3495(77)85532-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD (2014) Motor skill learning requires active central myelination. Science 346:318–322. 10.1126/science.1254960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA (2015) Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci 18:628–630. 10.1038/nn.3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Wolman D, Mensh BD, Pax E, Buchanan J, Smith SJ, Bock DD (2016) A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife 5:e15784. 10.7554/eLife.15784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micu I, Plemel JR, Lachance C, Proft J, Jansen AJ, Cummins K, van Minnen J, Stys PK (2016) The molecular physiology of the axo-myelinic synapse. Exp Neurol 276:41–50. 10.1016/j.expneurol.2015.10.006 [DOI] [PubMed] [Google Scholar]
- Mighdoll MI, Tao R, Kleinman JE, Hyde TM (2015) Myelin, myelin-related disorders, and psychosis. Schizophr Res 161:85–93. 10.1016/j.schres.2014.09.040 [DOI] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC (2012) Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A 109:16480–16485. 10.1073/pnas.1117943109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Hattori T, Shimizu S, Ito A, Tohyama M (2015) Disturbance of oligodendrocyte function plays a key role in the pathogenesis of schizophrenia and major depressive disorder. BioMed Res Int 2015:492367. 10.1155/2015/492367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount CW, Monje M (2017) Wrapped to adapt: experience-dependent myelination. Neuron 95:743–756. 10.1016/j.neuron.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B, Hovhannisyan A, Barzan R, Chen TJ, Kukley M (2017) Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLoS Biol 15:e2001993. 10.1371/journal.pbio.2001993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X (2009) Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10:9–22. 10.1038/nrn2495 [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Boshans L, Goncalves CM, Wegrzyn J, Patel KD (2016) Lineage, fate, and fate potential of NG2-glia. Brain Res 1638:116–128. 10.1016/j.brainres.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Kang HJ, Tyler WA, Silbereis JC, Cheng F, Zhu Y, Pletikos M, Jankovic-Rapan L, Cramer NP, Galdzicki Z, Goodliffe J, Peters A, Sethares C, Delalle I, Golden JA, Haydar TF, Sestan N (2016) Down syndrome developmental brain transcriptome reveals defective oligodendrocyte differentiation and myelination. Neuron 89:1208–1222. 10.1016/j.neuron.2016.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajevic S, Basser PJ, Fields RD (2014) Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276:135–147. 10.1016/j.neuroscience.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukert M, Bergles DE (2006) Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol 16:515–521. 10.1016/j.conb.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Peer M, Nitzan M, Bick AS, Levin N, Arzy S (2017) Evidence for functional networks within the human brain's white matter. J Neurosci 37:6394–6407. 10.1523/JNEUROSCI.3872-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perge JA, Niven JE, Mugnaini E, Balasubramanian V, Sterling P (2012) Why do axons differ in caliber? J Neurosci 32:626–638. 10.1523/JNEUROSCI.4254-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, Rothstein JD (2014) Glial cells in amyotrophic lateral sclerosis. Exp Neurol 262:111–120. 10.1016/j.expneurol.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels PJ, Vanderver A, Bernard G, Wolf NI, Dreha-Kulczewksi SF, Deoni SC, Bertini E, Kohlschütter A, Richardson W, Ffrench-Constant C, Köhler W, Rowitch D, Barkovich AJ (2014) Hypomyelinating leukodystrophies: translational research progress and prospects. Ann Neurol 76:5–19. 10.1002/ana.24194 [DOI] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, Pringle N (2006) Oligodendrocyte wars. Nat Rev Neurosci 7:11–18. 10.1038/nrn1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WD, Young KM, Tripathi RB, McKenzie I (2011) NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron 70:661–673. 10.1016/j.neuron.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Anderson EJ, Husain M (2013) White matter microstructure and cognitive function. Neuroscientist 19:8–15. 10.1177/1073858411421218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome LH, Bullock PN, Chiappelli F, Cardwell M, Adinolfi AM, Swanson D (1986) Synthesis of a myelin-like membrane by oligodendrocytes in culture. J Neurosci Res 15:49–65. 10.1002/jnr.490150106 [DOI] [PubMed] [Google Scholar]
- Rowitch DH. (2004) Glial specification in the vertebrate neural tube. Nat Rev Neurosci 5:409–419. 10.1038/nrn1389 [DOI] [PubMed] [Google Scholar]
- Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Möbius W, Goetze B, Jahn HM, Huang W, Steffens H, Schomburg ED, Pérez-Samartín A, Pérez-Cerdá F, Bakhtiari D, Matute C, Löwel S, Griesinger C, Hirrlinger J, Kirchhoff F, et al. (2016) Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91:119–132. 10.1016/j.neuron.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y (2012) Learning in the fast lane: new insights into neuroplasticity. Neuron 73:1195–1203. 10.1016/j.neuron.2012.01.025 [DOI] [PubMed] [Google Scholar]
- Sakry D, Karram K, Trotter J (2011) Synapses between NG2 glia and neurons. J Anat 219:2–7. 10.1111/j.1469-7580.2011.01359.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D, Neitz A, Singh J, Frischknecht R, Marongiu D, Binamé F, Perera SS, Endres K, Lutz B, Radyushkin K, Trotter J, Mittmann T (2014) Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol 12:e1001993. 10.1371/journal.pbio.1001993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D, Yigit H, Dimou L, Trotter J (2015) Oligodendrocyte precursor cells synthesize neuromodulatory factors. PloS One 10:e0127222. 10.1371/journal.pone.0127222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami M, Itami C, Tsumoto T, Kimura F (2003) Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc Natl Acad Sci U S A 100:6174–6179. 10.1073/pnas.0937380100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C, Khrapitchev AA, Foxley S, Schlagheck T, Scholz J, Jbabdi S, DeLuca GC, Miller KL, Taylor A, Thomas N, Kleim J, Sibson NR, Bannerman D, Johansen-Berg H (2013) Motor skill learning induces changes in white matter microstructure and myelination. J Neurosci 33:19499–19503. 10.1523/JNEUROSCI.3048-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain AJ, Hill RA, Grutzendler J (2014) Label-free in vivo imaging of myelinated axons in health and disease with spectral confocal reflectance microscopy. Nat Med 20:443–449. 10.1038/nm.3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel AA, Rudelson JJ, Tse PU (2012) White matter structure changes as adults learn a second language. J Cogn Neurosci 24:1664–1670. 10.1162/jocn_a_00240 [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H (2009) Training induces changes in white-matter architecture. Nat Neurosci 12:1370–1371. 10.1038/nn.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH. (2014) Regulation of conduction time along axons. Neuroscience 276:126–134. 10.1016/j.neuroscience.2013.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW (2016) Systematic and differential myelination of axon collaterals in the mammalian auditory brainstem. Glia 64:487–494. 10.1002/glia.22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW, Harris DM (2010) Mechanisms for adjusting interaural time differences to achieve binaural coincidence detection. J Neurosci 30:70–80. 10.1523/JNEUROSCI.3464-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwanski DR, Jukkola P, Nishiyama A (2017) Heterogeneity of astrocyte and NG2 cell insertion at the node of ranvier. J Comp Neurol 525:535–552. 10.1002/cne.24083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Brophy PJ (2005) Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci 6:683–690. 10.1038/nrn1743 [DOI] [PubMed] [Google Scholar]
- Sinclair JL, Fischl MJ, Alexandrova O, Heβ M, Grothe B, Leibold C, Kopp-Scheinpflug C (2017) Sound-evoked activity influences myelination of brainstem axons in the trapezoid body. J Neurosci 37:8239–8255. 10.1523/JNEUROSCI.3728-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Koles ZJ (1970) Myelinated nerve fibers: computed effect of myelin thickness on conduction velocity. Am J Physiol 219:1256–1258. [DOI] [PubMed] [Google Scholar]
- Snaidero N, Möbius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave KA, Simons M (2014) Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156:277–290. 10.1016/j.cell.2013.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003) Mapping cortical change across the human life span. Nat Neurosci 6:309–315. 10.1038/nn1008 [DOI] [PubMed] [Google Scholar]
- Stedehouder J, Couey JJ, Brizee D, Hosseini B, Slotman JA, Dirven CMF, Shpak G, Houtsmuller AB, Kushner SA (2017) Fast-spiking parvalbumin interneurons are frequently myelinated in the cerebral cortex of mice and humans. Cereb Cortex 27:5001–5013. 10.1093/cercor/bhx203 [DOI] [PubMed] [Google Scholar]
- Stikov N, Campbell JS, Stroh T, Lavelée M, Frey S, Novek J, Nuara S, Ho MK, Bedell BJ, Dougherty RF, Leppert IR, Boudreau M, Narayanan S, Duval T, Cohen-Adad J, Picard PA, Gasecka A, Côté D, Pike GB (2015) In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage 118:397–405. 10.1016/j.neuroimage.2015.05.023 [DOI] [PubMed] [Google Scholar]
- Sun W, Matthews EA, Nicolas V, Schoch S, Dietrich D (2016) NG2 glial cells integrate synaptic input in global and dendritic calcium signals. Elife 5:e16262. 10.7554/eLife.16262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R (2010) Training of working memory impacts structural connectivity. J Neurosci 30:3297–3303. 10.1523/JNEUROSCI.4611-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DG, Tokumoto YM, Raff MC (2000) Long-term culture of purified postnatal oligodendrocyte precursor cells: evidence for an intrinsic maturation program that plays out over months. J Cell Biol 148:971–984. 10.1083/jcb.148.5.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Fan M, Yang Y, Posner MI (2012) Mechanisms of white matter changes induced by meditation. Proc Natl Acad Sci U S A 109:10570–10574. 10.1073/pnas.1207817109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Müller K, Horstmann A, Villringer A, Ragert P (2010) Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci 30:11670–11677. 10.1523/JNEUROSCI.2567-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P (2014) Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344:319–324. 10.1126/science.1249766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisiol A, Saab AS, Winkler U, Marx G, Imamura H, Möbius W, Kusch K, Nave KA, Hirrlinger J (2017) Monitoring ATP dynamics in electrically active white matter tracts. Elife 6:e24241. 10.7554/eLife.24241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Levine JM, Miller RH, Trapp BD (1999) Rat optic nerve oligodendrocytes develop in the absence of viable retinal ganglion cell axons. J Cell Biol 146:1365–1374. 10.1083/jcb.146.6.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viganò F, Möbius W, Götz M, Dimou L (2013) Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci 16:1370–1372. 10.1038/nn.3503 [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. (1989) Target size regulates calibre and myelination of sympathetic axons. Nature 342:430–433. 10.1038/342430a0 [DOI] [PubMed] [Google Scholar]
- Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD (2015) Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Commun 6:7844. 10.1038/ncomms8844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Johansen-Berg H, Káradóttir RT (2014) Unraveling the secrets of white matter: bridging the gap between cellular, animal and human imaging studies. Neuroscience 276:2–13. 10.1016/j.neuroscience.2014.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Fu Y, Zickmund P, Shi R, Cheng JX (2005) Coherent anti-stokes Raman scattering imaging of axonal myelin in live spinal tissues. Biophys J 89:581–591. 10.1529/biophysj.105.061911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Young KM (2014) White matter plasticity in adulthood. Neuroscience 276:148–160. 10.1016/j.neuroscience.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Wanner AA, Genoud C, Masudi T, Siksou L, Friedrich RW (2016) Dense EM-based reconstruction of the interglomerular projectome in the zebrafish olfactory bulb. Nat Neurosci 19:816–825. 10.1038/nn.4290 [DOI] [PubMed] [Google Scholar]
- Warbrick T, Rosenberg J, Shah NJ (2017) The relationship between BOLD fMRI response and the underlying white matter as measured by fractional anisotropy (FA): a systematic review. Neuroimage 153:369–381. 10.1016/j.neuroimage.2016.12.075 [DOI] [PubMed] [Google Scholar]
- Watkins TA, Emery B, Mulinyawe S, Barres BA (2008) Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 60:555–569. 10.1016/j.neuron.2008.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. (1980) Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 3:141–150. 10.1002/mus.880030207 [DOI] [PubMed] [Google Scholar]
- Waxman SG, Bennett MV (1972) Relative conduction velocities of small myelinated and non-myelinated fibres in the central nervous system. Nat New Biol 238:217–219. 10.1038/newbio238217a0 [DOI] [PubMed] [Google Scholar]
- Wu LM, Williams A, Delaney A, Sherman DL, Brophy PJ (2012) Increasing internodal distance in myelinated nerves accelerates nerve conduction to a flat maximum. Curr Biol 22:1957–1961. 10.1016/j.cub.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Miller KL (2017) Image formation in diffusion MRI: a review of recent technical developments. J Magn Reson Imaging 46:646–662. 10.1002/jmri.25664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD (2016) Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci 19:1210–1217. 10.1038/nn.4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR (1967) The myelogenetic cycles of regional maturation of the brain. In: Regional development of the brain in early life (Minkowsky A, ed), pp 3–70. Philadelphia: F.A. Davis. [Google Scholar]
- Yamada R, Kuba H (2016) Structural and functional plasticity at the axon initial segment. Front Cell Neurosci 10:250. 10.3389/fncel.2016.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, Druid H, Frisén J (2014) Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159:766–774. 10.1016/j.cell.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD (2013) Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77:873–885. 10.1016/j.neuron.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SP, Zahed H, Maltepe E, Rowitch DH (2014) Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158:383–396. 10.1016/j.cell.2014.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H (2012) Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536. 10.1038/nn.3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ (2010) CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6:578–590. 10.1016/j.stem.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidán-Chuliá F, Salmina AB, Malinovskaya NA, Noda M, Verkhratsky A, Moreira JC (2014) The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev 38:160–172. 10.1016/j.neubiorev.2013.11.008 [DOI] [PubMed] [Google Scholar]
- Zeller NK, Behar TN, Dubois-Dalcq ME, Lazzarini RA (1985) The timely expression of myelin basic protein gene in cultured rat brain oligodendrocytes is independent of continuous neuronal influences. J Neurosci 5:2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Barres BA (2013) Intrinsic and extrinsic control of oligodendrocyte development. Curr Opin Neurobiol 23:914–920. 10.1016/j.conb.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]