Abstract
The K+ channel pore-forming subunit Kv4.3 is expressed in a subset of nonpeptidergic nociceptors within the dorsal root ganglion (DRG), and knockdown of Kv4.3 selectively induces mechanical hypersensitivity, a major symptom of neuropathic pain. K+ channel modulatory subunits KChIP1, KChIP2, and DPP10 are coexpressed in Kv4.3+ DRG neurons, but whether they participate in Kv4.3-mediated pain control is unknown. Here, we show the existence of a Kv4.3/KChIP1/KChIP2/DPP10 complex (abbreviated as the Kv4 complex) in the endoplasmic reticulum and cell surface of DRG neurons. After intrathecal injection of a gene-specific antisense oligodeoxynucleotide to knock down the expression of each component in the Kv4 complex, mechanical hypersensitivity develops in the hindlimbs of rats in parallel with a reduction in all components in the lumbar DRGs. Electrophysiological data further indicate that the excitability of nonpeptidergic nociceptors is enhanced. The expression of all Kv4 complex components in DRG neurons is downregulated following spinal nerve ligation (SNL). To rescue Kv4 complex downregulation, cDNA constructs encoding Kv4.3, KChIP1, and DPP10 were transfected into the injured DRGs (defined as DRGs with injured spinal nerves) of living SNL rats. SNL-evoked mechanical hypersensitivity was attenuated, accompanied by a partial recovery of Kv4.3, KChIP1, and DPP10 surface levels in the injured DRGs. By showing an interdependent regulation among components in the Kv4 complex, this study demonstrates that K+ channel modulatory subunits KChIP1, KChIP2, and DPP10 participate in Kv4.3-mediated mechanical pain control. Thus, these modulatory subunits could be potential drug targets for neuropathic pain.
SIGNIFICANCE STATEMENT Neuropathic pain, a type of moderate to severe chronic pain resulting from nerve injury or disorder, affects 6.9%–10% of the global population. However, less than half of patients report satisfactory pain relief from current treatments. K+ channels, which act to reduce nociceptor activity, have been suggested to be novel drug targets for neuropathic pain. This study is the first to show that K+ channel modulatory subunits KChIP1, KChIP2, and DPP10 are potential drug targets for neuropathic pain because they form a channel complex with the K+ channel pore-forming subunit Kv4.3 in a subset of nociceptors to selectively inhibit mechanical hypersensitivity, a major symptom of neuropathic pain.
Keywords: DPP10, DRG, KChIP1, KChIP2, Kv4.3
Introduction
Neuropathic pain is a type of moderate to severe chronic pain that results from nerve injury or disorder, and the major symptoms are mechanical and thermal hypersensitivity (Basbaum et al., 2009; Gold and Gebhart, 2010; Basbaum and Jessel, 2012; von Hehn et al., 2012). Neuropathic pain affects 6.9%–10% of the global population (van Hecke et al., 2014), but less than half of patients report satisfactory pain relief from current treatments (O'Connor and Dworkin, 2009). Voltage-gated K+ (Kv) channels, which act to reduce nociceptor activity, have been suggested to be novel drug targets for neuropathic pain (Tsantoulas and McMahon, 2014; Waxman and Zamponi, 2014).
Kv4 channels generate subthreshold A-type K+ currents (ISAs), which can inhibit neuronal excitability below the threshold of action potential (Jerng et al., 2004). Accumulating evidence indicates that native Kv4 channels function in complexes comprising Kv4 pore-forming subunits (Kv4.1-Kv4.3) and two types of modulatory subunits: cytosolic K+ channel interacting proteins (KChIP1–4) and transmembrane dipeptidyl peptidase-like proteins (DPP6 and DPP10) (Maffie and Rudy, 2008; Pongs and Schwarz, 2010; Jerng and Pfaffinger, 2014; Wang et al., 2015; Cheng et al., 2016). When expressed alone, Kv4 subunits are poorly transported to the cell surface; coexpression with KChIP (except KChIP4) or DPP promotes the trafficking and surface expression of Kv4, resulting in enhanced Kv4 current density. Only coexpression of Kv4, KChIP, and DPP can fully reconstitute all of the characteristics of ISAs (An et al., 2000; Holmqvist et al., 2002; Nadal et al., 2003; Foeger et al., 2010; Pongs and Schwarz, 2010; Foeger et al., 2012; Jerng and Pfaffinger, 2014). The effects of KChIP and DPP in Kv4 currents have been elucidated in vitro, but their functions in vivo remain unclear.
Small- and medium-diameter neurons in the DRG are the primary sensory neurons responsible for pain sensation, also known as nociceptors. There are two types of nociceptors: one with lightly myelinated Aδ fibers and the other with unmyelinated C-fibers. C-fiber nociceptors can be divided into peptidergic and nonpeptidergic groups, which are identified by the expression of neuropeptides and the binding of isolectin B4 (IB4), respectively (Le Pichon and Chesler, 2014). In rat lumbar DRGs, Kv4.3 is expressed in some small- and medium-diameter neurons, which constitute ∼26% of total DRG neurons, and all Kv4.3+ DRG neurons are IB4+ nociceptors (Chien et al., 2007). Furthermore, knockdown of Kv4.3 in rat lumbar DRGs selectively induces mechanical hypersensitivity in the hindlimbs (Chien et al., 2007).
Similar to Kv4.3, K+ channel modulatory subunits KChIP1, KChIP2, and DPP10 have been detected in some small- and medium-diameter DRG neurons that also bind IB4 (Cheng et al., 2016). In contrast, KChIP3 appears mainly in medium- and large-diameter neurons, and none of them express Kv4.3 (Cheng et al., 2016). In addition, there is no visible Kv4.2-, KChIP4-, or DPP6-immunoreactivity (IR) in the DRG (Huang et al., 2005; Cheng et al., 2016). The existence of Kv4 complexes in the DRG is supported by the detection of ISAs in small- to medium-diameter DRG neurons (Phuket and Covarrubias, 2009) and the immunohistochemical colocalization of KChIP1, KChIP2, and DPP10 in Kv4.3+ DRG neurons (Cheng et al., 2016). However, coimmunoprecipitation evidence is still lacking. Particularly, whether and how KChIP1, KChIP2, and DPP10 participate in Kv4.3-mediated pain control are unknown.
In this study, using immunoprecipitation analysis, we detected the Kv4.3/KChIP1/KChIP2/DPP10 complex in rat DRGs. Next, to investigate whether KChIP1, KChIP2, and DPP10 participate in Kv4.3-mediated pain control, loss-of-function and gain-of-function assays were performed. For the loss-of-function assay, we asked whether pain behaviors were induced after knockdown of each component in the Kv4 complex. For the gain-of-function assay, because the expression of all components in the Kv4 complex was downregulated in the injured DRGs of spinal nerve ligation (SNL) rats (an animal model of neuropathic pain), we tested whether rescue of Kv4 complex downregulation could relieve pain.
Materials and Methods
Animals.
Adult male Sprague Dawley rats (6 weeks) were provided by the Animal Center of National Yang-Ming University. National guidelines of animal care and handling were followed. Rats were housed individually in plastic cages and maintained on a 12 h light/dark cycle with free access to food and water. All the experiments were approved by the local ethics committee. Behavioral tests for pain assessment were performed in accordance with policies and recommendations of the International Association for the Study of Pain.
Intrathecal catheterization.
The method of Yaksh and Rudy (1976) was used. After a rat was anesthetized with inhalational isoflurane, the occipital magnus in the dorsal side of neck was opened and the dura mater was incised. A catheter (PE-5 polyethylene tubing) was inserted intrathecally and advanced caudally 8.5–9 cm, and the neck incision was closed with sutures. Two days later, rats displaying normal grooming, ambulation, and weight gain were used for intrathecal injection. Catheter placement was verified by visual inspection after the animal was killed.
Antisense oligodeoxynucleotide (ASO).
Unmodified ASO (21–24 nucleotides in length; see Figs. 345-6) was synthesized by Mission Biotech. Kv4.3 ASO sequence was 5′-TCATCTTGCCGCTTGTTCTTGTCG-3′, complementary to nucleotides 96–119 of the rat Kv4.3 coding region (GenBank L48619.1). KChIP1 ASO sequence was 5′-ATCTCCTCTTTGTTTATGTAG-3′, complementary to nucleotides 420–440 of rat KChIP1 coding region (GenBank AY082658.1). KChIP2 ASO sequence was 5′-ATTGTCAAAGAGCTGCATGGA-3′, complementary to nucleotides 634–654 of rat KChIP2 coding region (GenBank AF269285.1). DPP10 ASO sequence was 5′-TATCGGTGTCAATAAGAACTG-3′, complementary to nucleotides 1757–1777 of rat DPP10 coding region (GenBank NM001012205.1). LacZ ASO sequence was 5′-ACTCGGGTGATTACGATCGCG-3′, complementary to a sequence only found in Escherichia coli lacZ gene (GenBank CP012633.1) and was used as a negative control. ASO with FAM (6-carboxyfluorescein) tagged on the 5′ end, without (see Fig. 2) or with (see Fig. 7) phosphorothioate bond modifications between the last four nucleotides at both the 5′ and 3′ ends to increase nuclease resistance, was produced by Integrated DNA Technologies. ASO was dissolved in normal saline to a concentration of 9 μg/μl. For each intrathecal injection, 5 μl of ASO was used followed by a 15 μl normal saline flush.
Figure 3.
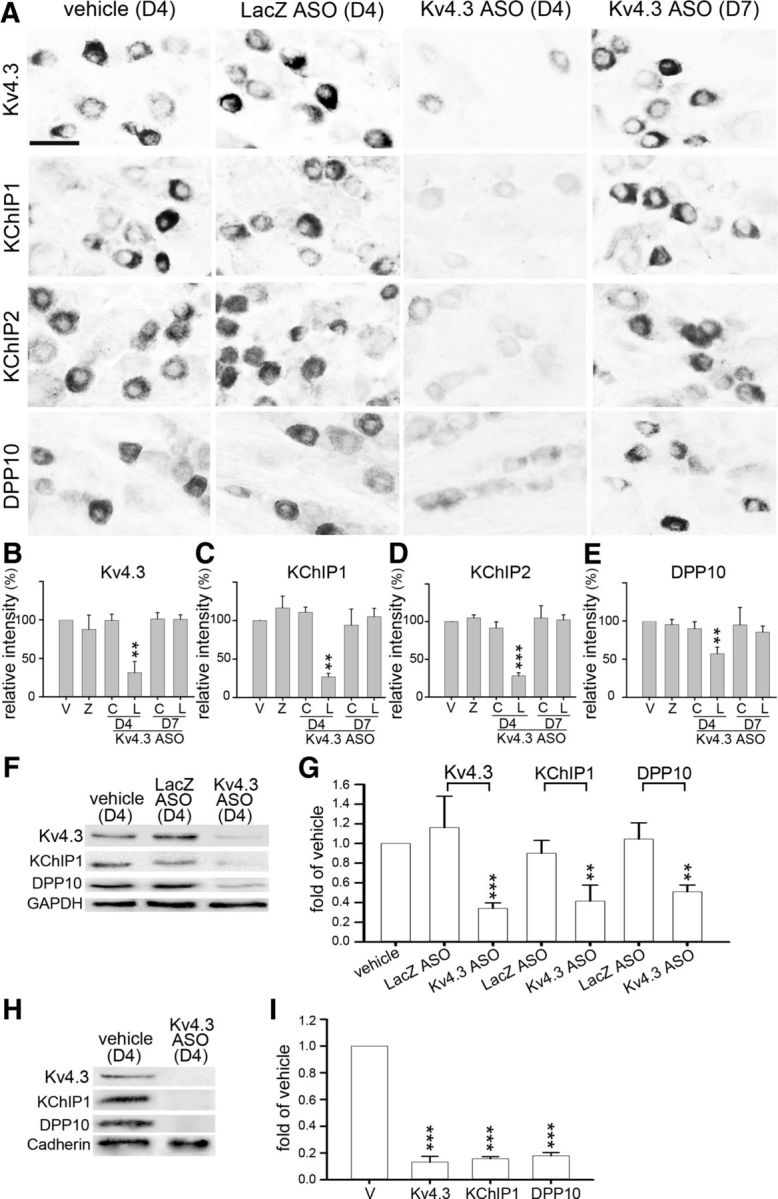
Intrathecal injection of Kv4.3 ASO reduces all Kv4 complex components in the L4–L6 DRGs. Rats were intrathecally injected with Kv4.3 ASO twice daily for 3 consecutive days (D1–D3) and killed on day 4 (D4) or D7. Rats injected with vehicle (V) or LacZ ASO (Z) were used as controls. A, In the L5 DRG of rats treated with Kv4.3 ASO, Kv4.3-, KChIP1-, KChIP2-, and DPP10-IR were greatly reduced on D4 but returned to the normal levels on D7. Scale bar, 40 μm. B–E, Quantitative data of A. C, C7 DRG. L, L5 DRG. F–I, Western blotting was performed using total proteins (F, G) or plasma membrane proteins (H, I) isolated from the bilateral L4–L6 DRGs on D4. Kv4.3 ASO treatment reduces Kv4.3, KChIP1, and DPP10 in both protein preparations. Bands of GAPDH and cadherin were loading controls for total proteins and plasma membrane proteins, respectively. Data are mean ± SD (n = 3). **p < 0.01, compared with vehicle (normalized to 1; Student's t test). ***p < 0.001, compared with vehicle (normalized to 1; Student's t test).
Figure 4.
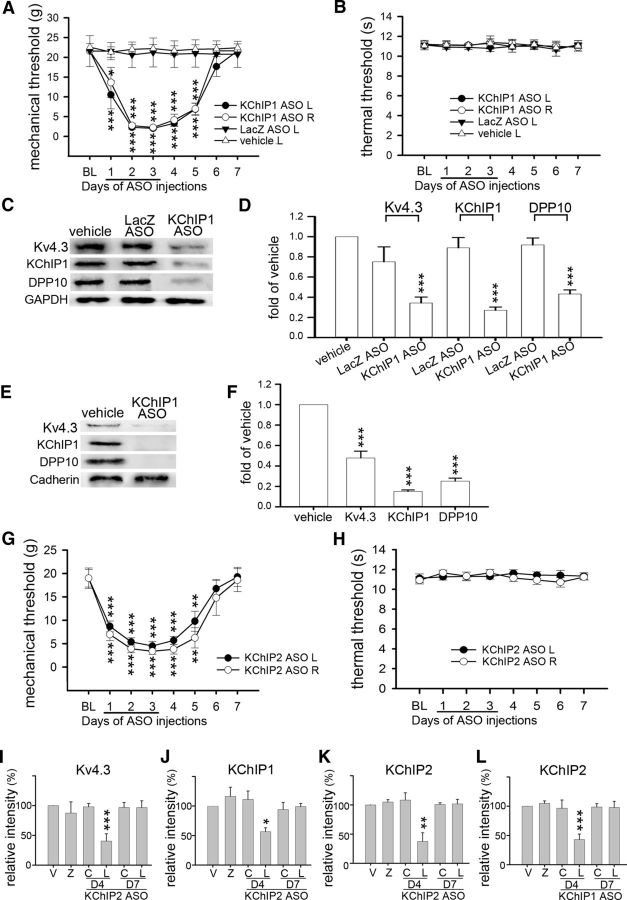
KChIP1 or KChIP2 ASO reduces all Kv4 complex components in the DRGs and induces mechanical hypersensitivity. Rats were intrathecally injected with vehicle, LacZ ASO, KChIP1 ASO, or KChIP2 ASO during D1–D3. A, B, KChIP1 ASO induces bilateral mechanical hypersensitivity during D1–D5. Thermal hypersensitivity was not detected. L, Left side; R, right side. Data are mean ± SEM (n = 6). *p < 0.05, compared with the baseline (BL) on the corresponding side (Tukey's post hoc test after one-way ANOVA). **p < 0.05, compared with the baseline (BL) on the corresponding side (Tukey's post hoc test after one-way ANOVA). ***p < 0.001, compared with the baseline (BL) on the corresponding side (Tukey's post hoc test after one-way ANOVA). C–F, Rats were killed on D4 after ASO treatment. Western blotting was performed using total proteins (C, D) or plasma membrane proteins (E, F) isolated from the bilateral L4–L6 DRGs on D4. KChIP1 ASO treatment reduces Kv4.3, KChIP1, and DPP10 in both protein preparations. G, H, KChIP2 ASO induces bilateral mechanical hypersensitivity during D1–D5. Thermal hypersensitivity was not detected. **p < 0.05, compared with the baseline (BL) on the corresponding side by Tukey's post hoc test after one-way ANOVA. ***p < 0.001, compared with the baseline (BL) on the corresponding side by Tukey's post hoc test after one-way ANOVA. I–K, In rats treated with KChIP2 ASO, Kv4.3-, KChIP1- and KChIP2-IR in L5 DRG neurons were reduced on D4. L, In rats treated with KChIP1 ASO, KChIP2-IR in L5 DRG neurons was also reduced on D4. Data are mean ± SD (n = 3). *p < 0.05, compared with vehicle (normalized to 1; Student's t test). **p < 0.01, compared with vehicle (normalized to 1; Student's t test). ***p < 0.001, compared with vehicle (normalized to 1; Student's t test).
Figure 5.
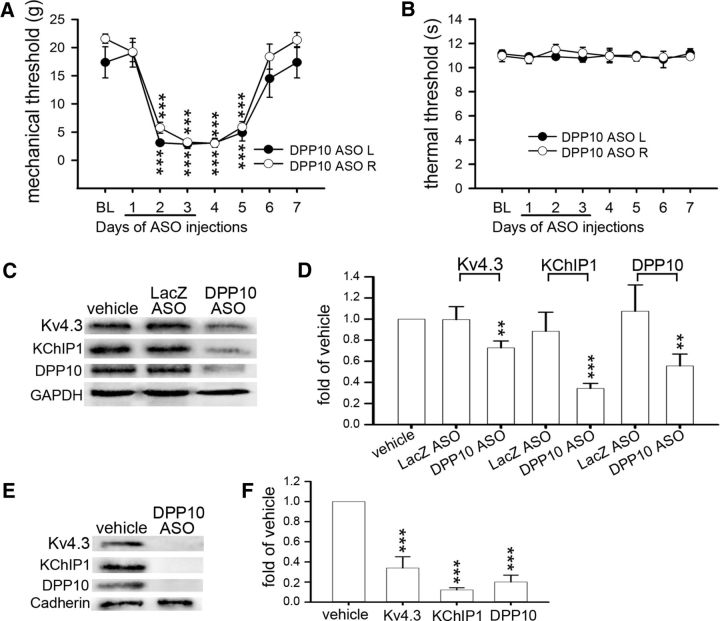
DPP10 ASO reduces all Kv4 complex components in the DRGs and induces mechanical hypersensitivity. Rats were intrathecally injected with vehicle, LacZ ASO, or DPP10 ASO during D1–D3. A, B, DPP10 ASO induces bilateral mechanical hypersensitivity during D2–D5. Thermal hypersensitivity was not detected. L, Left side; R, right side. Data are mean ± SEM (n = 6). ***p < 0.001, compared with BL on the corresponding side (Tukey's post hoc test after one-way ANOVA). C–F, Western blotting was performed using total proteins (C, D) or plasma membrane proteins (E, F) isolated from the bilateral L4–L6 DRGs on D4. DPP10 ASO treatment reduces Kv4.3, KChIP1, and DPP10 in both protein preparations. Data are mean ± SD (n = 3). **p < 0.01, compared with vehicle (normalized to 1; Student's t test). ***p < 0.001, compared with vehicle (normalized to 1; Student's t test).
Figure 6.
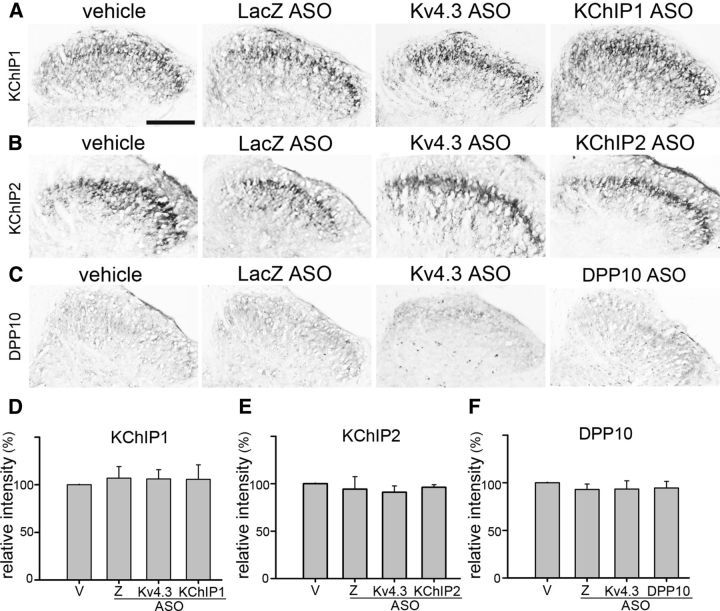
Intrathecal ASO injections do not affect KChIP1, KChIP2, and DPP10 expression in the spinal cord. Rats were intrathecally injected with ASO for LacZ (Z), Kv4.3, KChIP1, KChIP2, or DPP10 during D1–D3 and killed on D4. Sections of the L5 spinal cord segment were immunostained. A–C, KChIP1-, KChIP2-, or DPP10-IR in the dorsal spinal cord (on either side) is similar between each ASO group and the vehicle (V) group. Scale bar, 210 μm. D–F, Quantitative data of A–C. Data are mean ± SD (n = 3), compared with vehicle by Student's t test.
Figure 2.
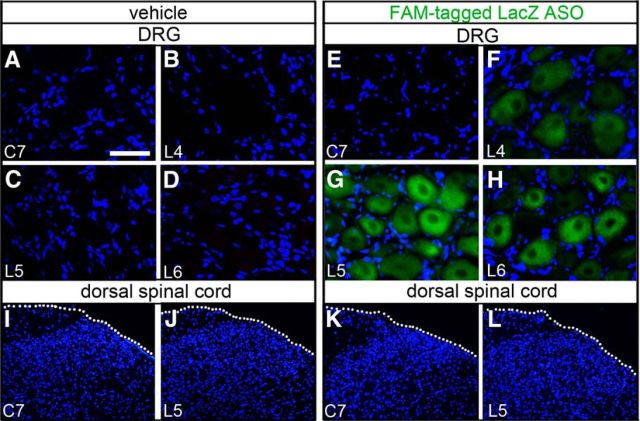
Uptake of ASO by DRG neurons near the intrathecal injection site. Rats were intrathecally injected with vehicle (A–D, I, J) or FAM-tagged LacZ ASO (E–H, K, L) into the lower lumbar region twice daily for 3 consecutive days and killed on day 4. Images were taken from sections of the cervical (C)7, lumbar (L)4, L5, and L6 DRGs (A–H) as well as transverse sections of the C7 and L5 spinal cord segments (I–L). DAPI (blue color) labels cell nuclei. A–D, Absence of fluorescence in the C7 and L4–L6 DRGs of the vehicle-injected rats. E–H, Green fluorescence was evident in many neuronal somata in the bilateral L4–L6 DRGs but not in the C7 DRGs. I–L, Absence of fluorescence in the dorsal spinal cord of rats injected with vehicle (I, J) or ASO (K, L). White dotted lines indicate the surface of spinal cord. Scale bar (in A): A–H, 25 μm; I–L, 220 μm.
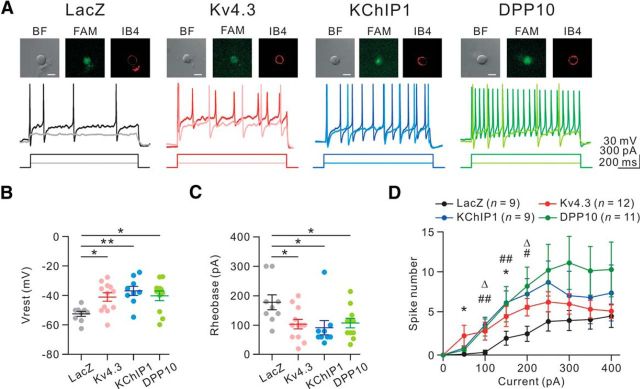
Figure 7.
The excitability of IB4+ nociceptors is enhanced after knockdown of Kv4.3, KChIP1, or DPP10. FAM-tagged ASO for LacZ, Kv4.3, KChIP1, or DPP10 was intrathecally injected into the rat by lumbar puncture, and the bilateral L4–L6 DRGs were isolated 5 h later. DRG neurons were dissociated and cultured for 20–24 h before whole-cell patch-clamp recording. A, Top, Only small DRG neurons (soma diameter ≤ 30 μm; bright-field, BF) with both FAM (green) and Alexa594-conjugated IB4 (red) signals were analyzed. Scale bar, 20 μm. Bottom, Responses to the depolarizing current pulses (100 and 300 pA; 1 s). B, The resting membrane potential (Vrest) in each ASO group. n = 9–12 as indicated in D. *p < 0.05 (Tukey's post hoc test after one-way ANOVA). **p < 0.01 (Tukey's post hoc test after one-way ANOVA). C, The rheobase of each ASO group. *p < 0.05 (Tukey's post hoc test after one-way ANOVA). D, Plot of spike number versus injected current pulse. *p < 0.05, Kv4.3 ASO versus LacZ ASO. #p < 0.05, KChIP1 ASO versus LacZ ASO. ##p < 0.01, KChIP1 ASO versus LacZ ASO. Δp < 0.05, DPP10 ASO versus LacZ ASO (Tukey's post hoc test after one-way ANOVA).
L5/L6 spinal nerve ligation.
The method was similar to that described previously (Kim and Chung, 1992). Rats (200–250 g on the day of surgery) were anesthetized with inhalational isoflurane (induced with 3% isoflurane in O2 at 2 L/min and maintained with 0.5% isoflurane in O2) and placed under a microsurgical apparatus in a prone position. To perform unilateral spinal nerve ligation on the right side, an incision was made near the midline of spinal cord, and the right paraspinal muscles were separated from the spinous processes between the lumbar (L)4 and sacral (S)2 levels. After the L6 transverse process was removed, the L4 and L5 spinal nerves were identified. We ligated the L5 spinal nerve with a 6-0 silk thread at a site 1–2 cm distal to the L5 DRG and kept holding tight for 3 s before making another knot upon the first knot. The L6 spinal nerve on the right side, located caudally and medially to the sacroiliac junction, was ligated similarly. Rats were allowed to recover after the closure of incision. Sham-operated controls were rats receiving the same operation but without nerve ligation. Animals with motor dysfunction after surgery were excluded from additional experimental procedures.
cDNA constructs.
The cDNA construct encoding the M splicing form of rat Kv4.3 was a gift from Dr. Bernardo Rudy (New York University). The cDNA constructs encoding rat KChIP1a and rat DPP10a were kindly provided by Dr. Koichi Takimoto (University of Pittsburgh). The plasmid pCAGGS-enhanced yellow fluorescence protein (EYFP) encoding EYFP was a gift from Drs. Tetsuichiro Saito (Chiba University) and Jun-ichi Miyazaki (Osaka University). A total of 8 μg of plasmid DNA was used for each injection, including pEYFP alone (8 μg), pEYFP/pKv4.3 (1.6 μg and 6.4 μg, respectively), or a combination of pEYFP (0.8 μg)/pKv4.3 (2.4 μg)/pKChIP1a (2.4 μg)/pDPP10a (2.4 μg). DNA/polyethyleneimine (PEI) mix in 5% glucose solution was prepared according to the manufacturer's protocol (in vivo jetPEI; Polyplus-transfection). After incubation at room temperature for 15 min, 20 μl of DNA/PEI mix was intrathecally injected into a rat via lumbar puncture. Following injection, the needle remained in situ for 30 s before being withdrawn.
Lumbar puncture.
The method was modified from Papir-Kricheli et al. (1987). After lightly anesthetized with isoflurane inhalation, a rat was placed in a prone position on a Styrofoam board (20 cm × 17 cm × 3 cm), the forelimbs were extended toward the front, and the hindlimbs were left to hang off the board. The rat's vertebral column was therefore flexed around the L3-L6 levels to widen the dorsal intervertebral space, and the midpoint between the tips of the iliac crest was a tactile landmark for the L5-L6 intervertebral space. A Hamilton syringe with 27 gauge needle was inserted into the subarachnoid space of spinal cord between the L5 and L6 spinous processes, and a short tail flick was observed upon needle insertion.
Behavioral tests.
Two types of tests (mechanical and thermal sensitivity) were performed 1 d before spinal nerve ligation or intrathecal ASO injection to get the baseline values. Tests were performed on both rat hindpaws once daily at 2–4 pm by an examiner blinded to the treatment groups.
Mechanical sensitivity was assessed by von Frey filaments (Stoelting). Rats were placed in a transparent plastic dome with a metal-mesh floor allowing access to the plantar surface of hindpaws, with a habituation time ∼30 min before testing. The filament was pressed perpendicular to the plantar surface of the hindpaw with sufficient force to cause a slight buckling for 6 s. A positive response was noted when the hindpaw was sharply withdrawn. Flinching immediately after the removal of the filament was also considered as a positive response. The force (in grams) producing a 50% likelihood of withdrawal was determined by the “up-down” method (Chaplan et al., 1994). Each trial was repeated twice at 2 min intervals, and the mean value represented the paw withdrawal threshold.
Thermal sensitivity was measured by an Analgesia Meter apparatus (IITC/Life Science Instruments) as described by Hargreaves et al. (1988). Rats were placed on a temperature-controlled, 3-mm-thick glass floor and habituated for 30 min before testing. A movable light box was located beneath the glass floor, and radiant heat was focused on the plantar surface of the hindpaw. A cutoff time was set at 20 s to avoid tissue damage. Light intensity was adjusted to obtain a baseline value within 10–12 s. Three withdrawal latencies were collected with at least 5 min intervals and the mean represented the thermal latency.
Isolation of total proteins.
Rats were anesthetized with inhalational isoflurane and decapitated. DRGs were frozen on dry ice immediately after isolation and kept frozen at −72°C before use. Tissues were cut into small pieces and homogenized in ice-cold lysis buffer (20 mm Tris, pH 8.0, 137 mm NaCl, 1% NP40, and 10% glycerol) containing 0.5 mm sodium orthovanadate, 1.15 mm PMSF, 0.07 mm calpain inhibitor I, 0.06 mm calpain inhibitor II, and 1% protease inhibitor mixture [1.04 mm 4-(2-aminoethyl)-benzenesulfonyl fluoride, 800 nm aproptinin, 40 μm bestatin hydrochloride, 14 μm N-(trans-epoxysuccinyl)-L-leucine-guanidinobutylamide, 20 μm leupeptin hemisulfate salt, and 15 μm pepstatin A] (Sigma). After centrifugation at 14,000 rpm for 30 min at 4°C, the supernatant was collected, and its protein concentration was determined by the BCA protein assay (Pierce). Aliquots of the supernatant were stored at −72°C before use.
Isolation of plasma membrane proteins.
DRGs were homogenized in the same lysis buffer as described in Isolation of total proteins. The homogenate was centrifuged at 700 × g for 10 min at 4°C to remove debris. After the suspension was centrifuged at 10,000 × g for 30 min at 4°C, both the plasma and organelle membrane proteins were in the pellet. Using the Plasma Membrane Protein Extraction Kit (Abcam), the pellet was resuspended in 200 μl of a fresh upper phase solution. The suspension was mixed with 200 μl of a fresh lower phase solution, incubated on ice for 5 min, centrifuged at 1000 × g for 5 min at 4°C, and the upper phase solution was collected. To maximize the yield, the lower phase solution was extracted after adding 100 μl of a fresh upper phase solution. The upper phase solution of the first and second extractions was combined and extracted again with a fresh lower phase solution. Then, the upper phase solution was collected, diluted in 5 volume of sterilized water, and kept on ice for 5 min. Following the diluted upper phase solution was centrifuged at 21,130 × g for 10 min at 4°C, the plasma membrane proteins, which were in the pellet, were dissolved in PBS (137 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, 2 mm KH2PO4, pH 7.4) containing 0.5% Triton X-100 and stored at −72°C before use.
Isolation of ER proteins.
Rough ER proteins were prepared by using the Endoplasmic Reticulum Isolation Kit (Sigma) with a procedure modified from that of the manufacturer. DRGs were homogenized in an isotonic extraction buffer (10 mm HEPES, pH 7.8, 0.25 m sucrose, 1 mm EGTA, 25 mm KCl). The homogenate was centrifuged at 1000 × g for 10 min at 4°C, and the supernatant was collected and centrifuged again at 12,000 × g for 15 min at 4°C. Then, the supernatant was collected, mixed with 8 mm calcium chloride, and centrifuged at 8000 × g for 10 min at 4°C to get the enriched rough ER microsomes in the pellet. The pellet was suspended in the isotonic extraction buffer, and aliquots of rough ER proteins were stored at −72°C before use.
Western blotting.
Approximately 20 μg of proteins was solubilized in a loading buffer (50 mm Tris, pH 6.8, 2% SDS, 0.2% bromophenol blue, and 10% glycerol). After denature by boiling for 5 min in the presence of 0.01 m dithiothreitol, proteins were separated by 10% SDS-PAGE, and electrophoretically transferred to polyvinylidene difluoride membrane (Millipore) using a semidry method (Bio-Rad). After a brief rinse in high salt Tris-buffered saline (HTBS, 25 mM Tris pH 7.5, 1.7% NaCl) containing 0.1% Tween 20 (HTBSW), membrane was blocked for nonspecific binding with 5% nonfat milk in HTBSW for 1 h, washed in HTBSW 5 min for 3 times, and incubated with primary antibody in HTBSW containing 1% BSA at 4°C overnight.
Primary antibodies and their dilution factors were mouse anti-cadherin (1:500; Sigma catalog #C1821, RRID:AB_476826), goat anti-calnexin (1:500; Santa Cruz Biotechnology catalog #sc6465, RRID:AB_2069146), rabbit anti-DPP10 (1:500; customer made by GeneTex, RRID:AB_236936), rabbit anti-GAPDH (1:5000; GeneTex catalog #GTX100118, RRID:AB_1080976), rabbit anti-KChIP1 (1:500; Alomone Labs catalog #APC-141, RRID:AB_10917754), mouse anti-KChIP2 (1:400; NeuroMab catalog #75-004, RRID:AB_2280942), and rabbit anti-Kv4.3 (1:500; customer made by GeneTex, RRID:AB_845371). Following washing, secondary antibody (donkey anti-goat, donkey anti-mouse, or donkey anti-rabbit) conjugated with HRP (1:10,000; Jackson ImmunoResearch Laboratories) was applied to membrane at 4°C for 1 h. In the presence of chemiluminescent reagent (Luminata Forte, Millipore), immunoreactive bands were visualized by exposing a membrane in a luminescent image analyzer (Fujifilm LAS-4000). Immunoreactivity of each band on the corresponding position of molecular weight in each image was circled and measured by the ImageJ 1.40c software (National Institutes of Health): cadherin (135 kDa), calnexin (90 kDa), DPP10 (97 kDa), GAPDH (36 kDa), KChIP1 (26 kDa), KChIP2 (35 kDa), and Kv4.3 (69 kDa).
Immunoprecipitation.
Immunoprecipitation matrix (Santa Cruz Biotechnology) was incubated with 4.1 μg of rabbit anti-Kv4.3 (same as for Western blotting) or rabbit IgG (Sigma) for 1.5 h at 4°C to form the antibody-matrix complex. The mixture was washed in PBS three times by repeated centrifugation at 14,000 rpm for 1 min before resuspension in ice-cold lysis buffer (same as for Western blotting). Approximately 100 μg of plasma membrane or rough ER proteins was added to the antibody-matrix complex and mixed on a rotator for 2 h at 4°C. The mixture was washed four times by repeated centrifugation at 14,000 rpm and resuspension in PBS. The pellet containing the antibody-protein complex was resuspended in a loading buffer and analyzed by Western blotting.
Tissue processing for immunohistochemistry.
Rats were anesthetized by intraperitoneal injection of sodium pentobarbital (100 mg/kg) and briefly perfused transcardially with normal saline, followed by cold 4% paraformaldehyde (PFA) in PBS for 20 min. DRGs and spinal cord were isolated and immersed in the same fixative at room temperature for 30 min (DRG) or 1 h (spinal cord). Following dehydration for cryo-protection in 0.1 m phosphate buffer (PB, 0.08 m K2HPO4, 0.02 m NaH2PO4, pH 7.4) containing 30% (w/v) sucrose, all specimens were kept at −72°C before sectioning.
Immunohistochemistry.
DRG sections (20 μm) were cut with a cryostat and mounted directly onto gelatin-coated slides, whereas transverse sections of spinal cord (20 μm) were processed in a floating manner. After washing in law salt Tris-buffered saline (LTBS, 25 mm Tris pH 7.5, 0.85% NaCl), followed by LTBS containing 0.1% Triton X-100 (LTBST) 10 min twice, sections were treated with 0.3% hydrogen peroxide in LTBS until no bubbles to exhaust endogenous hydrogen peroxidase. Nonspecific binding was blocked by 3% normal donkey serum plus 2% bovine serum albumin in LTBST for 1.5 h. Primary antibodies included rabbit anti-DPP10 (1:3000), rabbit anti-GFAP (1:8000; Dako catalog #Z0334, RRID:AB_2314535), rabbit anti-Iba1 (1:800; Wako catalog #019-19741, RRID:AB_839504), mouse anti-KChIP1 (1:100; NeuroMab catalog #75-003, RRID:AB_10673162), mouse anti-KChIP2 (1:50; NeuroMab catalog #75-004, RRID:AB_2280942), and mouse anti-Kv4.3 (1:4000; NeuroMab catalog #75-017, RRID:AB_2314723).
After overnight reaction with primary antibody, sections were washed by HTBS for 10 min three times. Then, sections were incubated with biotinylated donkey anti-mouse or anti-rabbit secondary antibody (1:500; Jackson ImmunoResearch Laboratories) for 1.5 h at room temperature. Following washing three times with HTBST, avidin-biotin HRP complex (1:160; Pierce) in LTBST was applied to sections for 1.5 h at room temperature. Antigens were visualized by combining equal volumes of an ammonium nickel sulfate solution (30 mg/ml in 0.1 m sodium acetate, pH 6.0) and a diaminobenzidine solution (4 mg/ml in LTBS) in the presence of 0.01% hydrogen peroxide. Floating sections were spread flat on slides and air dried. All sections on slide were rinsed with distill water for 1 min, and dehydrated through an ethanol gradient (70% once, 95% twice, and 100% twice) for 2 min each and then in xylene for 3 min twice, before coverslipped with mounting medium (Permount; Merck). Images were acquired with a DMX1200 digital camera connected to a BX51 microscope (Olympus) and processed with the Photoshop CS3 software (Adobe).
Quantitative measurements in DRG.
For the measurement of Kv4.3-, KChIP1-, KChIP2-, or DPP10-IR in DRG neurons, the C7 and L5 DRGs on either side were analyzed in the ASO experiments (see Figs. 3A–E, 4I–L), whereas the ipsilateral L5 DRG was analyzed in the SNL experiment (see Fig. 9A,B). Three sections with a distance of every 80 μm were sampled approximately in the middle of each DRG. Images were taken from 5 nonoverlapping areas (160 μm × 160 μm for each area) randomly selected from each DRG section after immunostaining. Only neurons with obvious nuclear localization at or near the center of cells were measured. IR in each neuron was circled, and the intensity within the circled area was measured by the software Image Beta 4.0.3.2 (Scion). The average of 50 positive neurons (10 in each area) was obtained from one DRG section, and the average of three sections in one rat was assigned as the sample value (S). To measure the background, the average of 10 negative cells (2 large neurons without obvious IR in each area) was obtained from one section, and the average of three sections in one rat was assigned as the background value (B). After dividing the S by the B, a ratio was obtained from each rat. Then, by normalization to the average value of the vehicle group (n = 3) (100% for the vehicle group), a percentage for each rat was obtained. The mean percentage of three rats was presented as mean ± SD.
Figure 9.
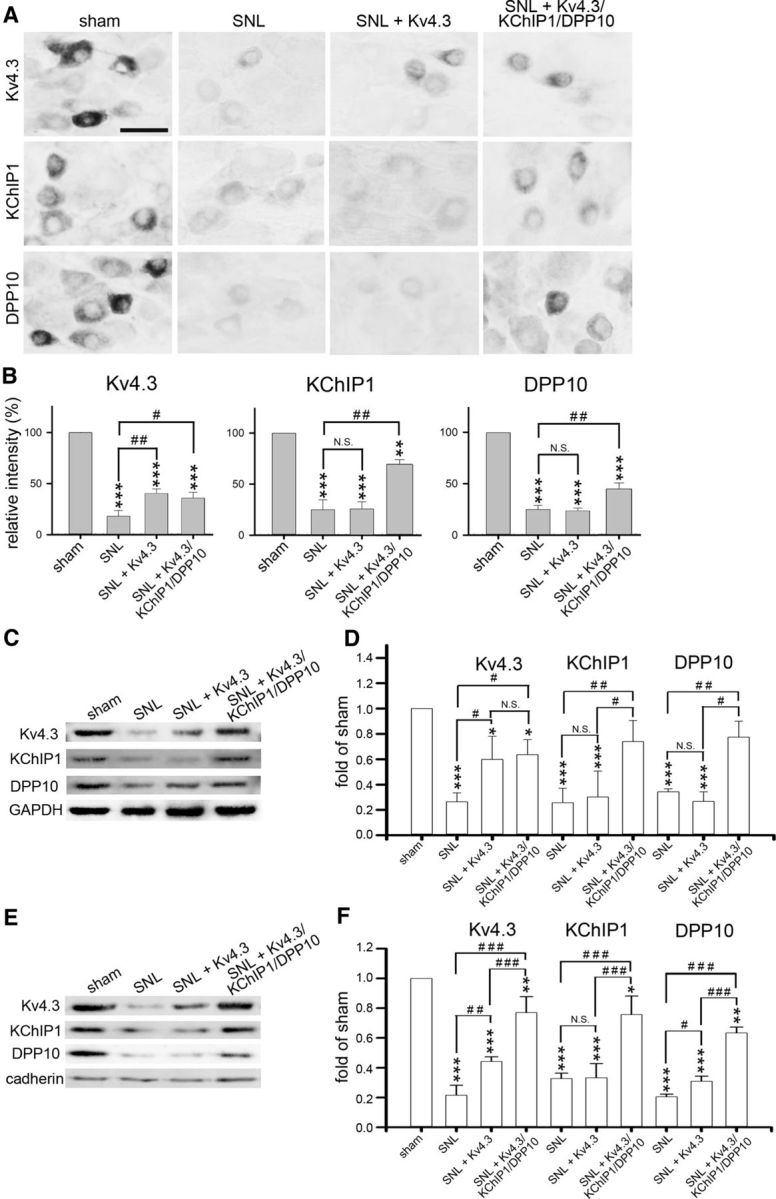
Intrathecal injection of Kv4.3/KChIP1/DPP10 cDNAs rescues SNL-evoked Kv4 complex downregulation in the injured DRGs. Rats were killed on D7 after sham operation, SNL, SNL with Kv4.3 cDNA injection (SNL + Kv4.3) on D4, or SNL with coinjection of Kv4.3/KChIP1/DPP10 cDNAs (SNL + Kv4.3/KChIP1/DPP10) on D4. A, The ipsilateral L5 DRG was immunostained for Kv4.3, KChIP1, or DPP10. Scale bar, 40 μm. B, Quantification of IR in A. C–F, Western blotting was performed using total proteins (C, D) or plasma membrane proteins (E, F) isolated from the ipsilateral L5/L6 DRGs, respectively. Data are mean ± SD (n = 3). *p < 0.05, compared with sham (normalized to 1). **p < 0.01, compared with sham (normalized to 1). ***p < 0.001, compared with sham (normalized to 1). #p < 0.05, comparison of the indicated pairs (Tukey's post hoc test after one-way ANOVA). ##p < 0.01, comparison of the indicated pairs (Tukey's post hoc test after one-way ANOVA). ###p < 0.001, comparison of the indicated pairs (Tukey's post hoc test after one-way ANOVA; N.S., no significant difference).
For the measurement of GFAP- or Iba1-IR in the ipsilateral L5 DRG (see Fig. 10), images were taken from 5 nonoverlapping areas (160 μm × 160 μm) randomly selected from each DRG section after immunostaining. Five large-diameter DRG neurons were randomly selected in each area. Using Scion Image analysis, the background intensity of IR in the cytoplasm of a large neuron was measured, and the average of 5 neurons was designated as the threshold. The GFAP+ area (in percentage of the entire area) with intensity above the threshold was measured, and the average of 5 images was obtained from each DRG section. Then, an average value was obtained from three DRG sections of one rat, and the average of 3 rats was presented as mean ± SD.
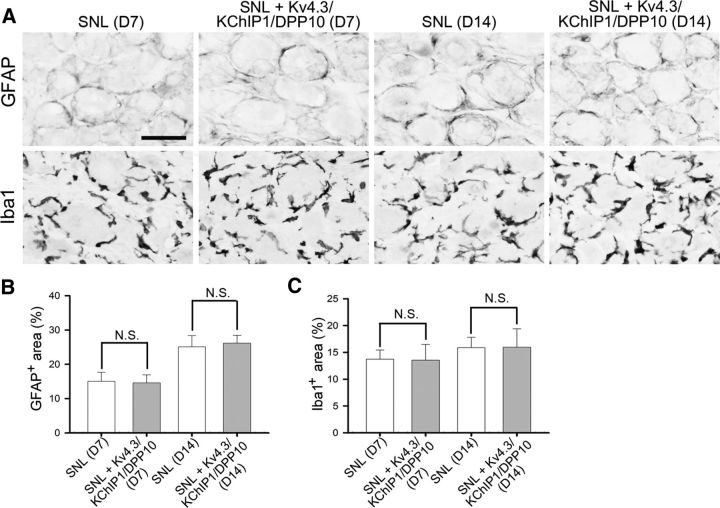
Figure 10.
Intrathecal cDNA injection does not suppress SNL-evoked glial activation and macrophage proliferation in the injured DRGs. Rats were intrathecally injected with Kv4.3/KChP1/DPP10 cDNAs on D4 and killed on D7 or D14 after SNL. A, Sections of the ipsilateral L5 DRG were immunostained for GFAP and Iba1, respectively. Scale bar, 48 μm. B, C, Quantitative data of A. GFAP+ or Iba1+ area shows no significant difference (N.S.) between SNL rats and the SNL rats treated with Kv4.3/KChIP1/DPP10 cDNAs. Data are mean ± SD (n = 3), compared with SNL on the same day by Student's t test.
Quantitative measurement in spinal cord.
Three rats per group were killed, and the L5 spinal cord segment was used. Three transverse sections with a distance of every 80 μm were sampled. Images were taken after immunohistochemistry. To quantify KChIP1-, KChIP2-, or DPP10-IR in the dorsal horn on either side (see Fig. 6A–F), three nonoverlapping regions (100 μm × 100 μm for each) with positive signal were randomly selected, and the intensity of IR in each region was measured by the software Scion Image Beta 4.0.3.2. The average of three sections was designated as the sample value (S) for each rat. To quantify the background, three different regions (100 μm × 100 μm for each), respectively, located in the dorsal, lateral, and ventral parts of the white matter were selected, and the intensity of IR in each region was measured. Three different regions were measured in one section, and the average of three sections was designated as the background value (B) for each rat. After dividing the S by the B, a ratio was obtained from each rat. Then, by normalization to the average value of the vehicle group (n = 3) (i.e., 100% for the vehicle group), a percentage for each rat was obtained. The mean percentage of three rats was presented as mean ± SD.
Electrophysiology.
LacZ, Kv4.3, KChIP1, or DPP10 ASO, which was modified for nuclease resistance and tagged with FAM, was intrathecally injected into the rat by lumbar puncture. Five hours later, the rat was decapitated after brief isoflurane anesthesia, and the bilateral L4-L6 DRGs were isolated aseptically. Following dissociation in DMEM containing 1 mg/ml Type IA collagenase (Sigma) for 1.5 h and then 0.25% trypsin (Invitrogen) for 30 min, cells were plated onto 10-mm-diameter coverglasses coated with poly-L-lysine (Sigma). DRG cells were cultured in DMEM (Invitrogen) containing 10% FBS (Invitrogen) and 100 units penicillin/100 μg streptomycin (Sigma) per milliliters, in a humid chamber with 5% CO2 at 37°C. All cells were cultured for 20–24 h before testing. The extracellular solution contained (in mM): 145 NaCl, 3 KCl, 10 glucose, 3 CaCl2, 2 MgCl2 and 10 HEPES (pH adjusted to 7.3 with KOH). Right before recording, a coverglass with cells was dipped in the extracellular solution containing 10 μg/ml AlexaFluor-594-conjugated IB4 (Sigma) for 5–10 min, and only the IB4+/FAM+ DRG neurons were chosen for recording. Then, the cells were mounted in the extracellular solution. The pipette internal solution for whole-cell patch-clamp recordings contained the following (in mm): 135 K-gluconate, 20 KCl, 0.1 EGTA, 2 MgCl2, 4 Na2ATP, and 10 HEPES (pH adjusted to 7.3 with KOH). IB4+/FAM+ small-diameter DRG neurons (≤30 μm) were visually selected for recording under bright-field and fluorescence optics (BX51WI, Olympus). Using a Multiclamp 700B amplifier (Molecular Devices), whole-cell patch-clamp recordings were made at 22°C–24°C as described previously (Lien and Jonas, 2003). Patch pipettes (2–5 mΩ) were pulled from borosilicate glass tubing (outer diameter 1.5 mm and inner diameter 0.86 mm; Harvard Apparatus) and heat polished. Pipette capacitances of both electrodes were carefully compensated and series resistance was compensated using the automatic bridge balance (readouts after compensation were 8–15 mΩ). Spikes were evoked in single neurons by depolarizing current pulse (50–400 pA; 1 s) injection in the current-clamp configuration. The spike number was calculated from the spike train elicited in response to current pulse injection. Signals were sampled at 10 kHz using the Digidata 1440 interface (Molecular Devices). Data acquisition and pulse generation were performed using pClamp 10.3 software (Molecular Devices).
Statistics.
SPSS version 18.0 (IBM) for Windows were used. Values obtained from the behavioral tests (n = 6), and the electrophysiological recordings (n = 9–12) were presented as mean ± SEM, and the differences between the experimental and control groups were compared by Tukey's post hoc test after one-way analysis of variance (ANOVA). Values obtained from the other experiments (n = 3) were presented as mean ± SD, and differences between two experimental groups were compared by Student's t test.
Results
Detection of a Kv4 complex in rat DRGs
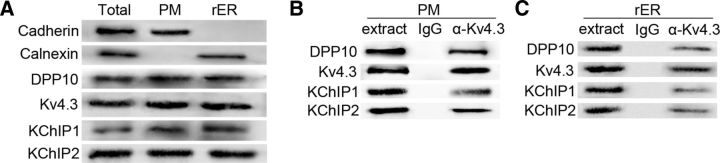
Western blotting showed Kv4.3, KChIP1, KChIP2, and DPP10 in the rough endoplasmic reticulum (ER) and plasma membrane isolated from rat DRGs (Fig. 1A). To examine whether Kv4 complexes exist in these two subcellular compartments, we performed immunoprecipitation. The Kv4.3 antibody not only immunoprecipitated Kv4.3 but also coimmunoprecipitated KChIP1, KChIP2, and DPP10 in both protein preparations (Fig. 1B,C). The immunoprecipitation data indicate that Kv4.3, KChIP1, KChIP2, and DPP10 form a Kv4 complex in rat DRGs. KChIP1, KChIP2, and DPP10 are coexpressed in rat Kv4.3+ DRG neurons (Cheng et al., 2016). Both findings suggest that the Kv4 complex exists in Kv4.3+ DRG neurons and is formed in the ER before it is trafficked to the plasma membrane.
Figure 1.
Detection of a Kv4.3/KChIP1/KChIP2/DPP10 complex in the DRG. Total proteins (Total), plasma membrane proteins (PM), and rough ER proteins (rER) were isolated from rat lumbar DRGs, respectively. A, Representative Western blot data show Kv4.3, KChIP1, KChIP2, and DPP10 in each protein preparation. Cadherin and calnexin are markers for the PM and rER, respectively. The purity of PM proteins was confirmed by the presence of cadherin and the absence of calnexin, whereas the purity of rER proteins was confirmed by the presence of calnexin and the absence of cadherin. B, C, In addition to immunoprecipitation of Kv4.3, rabbit anti-Kv4.3 antibody (α-Kv4.3) coimmunoprecipitates KChIP1, KChIP2, and DPP10 from the PM proteins (B) and rER proteins (C), respectively. Nonspecific rabbit IgG was used as a control of immunoprecipitation, and “extract” indicates a bead-only loading control.
Knockdown of Kv4.3 reduces all Kv4 complex components in DRG neurons
Selective knockdown of gene translation in DRG neurons has been achieved by intrathecal injection of a gene-specific ASO (Bourinet et al., 2005; Chien et al., 2007; Yu et al., 2011). Temporally, after a single intrathecal injection of a fluorescent dye-labeled ASO into the rat lumbar (L) region, the average fluorescence intensity in L5 DRG neurons is weak at 3 hours (h), strong at 5 h, moderate at 8 h, and weak at 12 h after injection (Chien et al., 2007). Here, we examined the spatial distribution of the ASO after repeated intrathecal injections. FAM-tagged LacZ ASO was injected into the rat via a pre-embedded intrathecal catheter with an opening at the lumbar region twice daily (8:00 A.M. and 8:00 P.M.) for 3 consecutive days (day 1–3). On day 4, green fluorescence was detected in neurons with variable somatic sizes in the bilateral L4-L6 DRGs, and the fluorescence intensity was also varied: strong in some neurons but weak in others within each DRG (Fig. 2A–H). There was no detectable fluorescence in the cervical (C)7 DRG and spinal cord at the C7 and L5 levels (Fig. 2E,I–L). Thus, the ASO permeates only into the DRGs near the site of intrathecal injection.
To examine whether knockdown of Kv4.3 affects KChIP1, KChIP2, and DPP10 expression in DRG neurons, a Kv4.3 ASO (without any modification) was intrathecally injected into rats twice daily for 3 consecutive days. In the bilateral L5 DRGs, immunohistochemistry showed that Kv4.3-, KChIP1-, KChIP2-, and DPP10-IR in DRG neurons decreased on day 4 but reversed to the normal levels on day 7 after stopping ASO injection during days 4–7 (Fig. 3A–E). Thus, the knockdown effect was reversible. In the C7 DRG, Kv4.3-, KChIP1-, KChIP2-, and DPP10-IR remained unchanged (Fig. 3A–E), consistent with no detection of the FAM-tagged ASO (Fig. 2).
KChIP1 and KCIP2 are coexpressed in the same population of DRG neurons in the lumbar DRGs, as 95% of KChIP2+ neurons express KChIP1 and 88% of KChIP1+ neurons have KChIP2 signal (Cheng et al., 2016). In addition, both KChIP1 and KChIP2 have positive modulatory effects on Kv4 currents (Pongs and Schwarz, 2010). Thus, we used KChIP1 as a representative in some of the experiments below. Western blotting revealed an evident reduction in Kv4.3, KChIP1, and DPP10 in the bilateral L4-L6 DRGs after Kv4.3 ASO treatment, not only in the total proteins synthesized but also in their surface levels, which correlate directly to Kv4 channel function (Fig. 3F–I). These results indicate that knockdown of Kv4.3 also reduces the other Kv4 complex components in DRG neurons.
Knockdown of any Kv4 complex component reduces all components in DRG neurons and induces mechanical hypersensitivity
To test whether knockdown of KChIP1 in nociceptors can induce pain, a KChIP1 ASO was intrathecally injected into rats. Mechanical hypersensitivity in the rat bilateral hindpaws developed on day 1, remained evident on days 2–4, partially subsided on day 5, and disappeared on days 6 and 7 (Fig. 4A). Thermal hypersensitivity was not detected throughout days 1–7 (Fig. 4B). There was a reduction in Kv4.3, KChIP1, and DPP10 in both total protein and plasma membrane protein levels isolated from the bilateral L4-L6 DRGs on day 4 (Fig. 4C–F). Knockdown of KChIP2 in DRG neurons showed the same effects as knockdown of KChIP1 (Fig. 4G–K). Although knock-out of individual KChIPs was compensated by a reciprocal increased expression of the other KChIP in the cortical pyramidal neurons (Norris et al., 2010), we observed no compensation between KChIP1 and KChIP2 after knockdown of either one in the DRG neurons (Fig. 4J,L). Furthermore, knockdown of DPP10 in DRG neurons also induced mechanical hypersensitivity (Fig. 5A,B) in parallel with a reduction in Kv4.3, KChIP1, and DPP10 (Fig. 5C–F). Thus, reducing any component of the Kv4 complex also reduces the other components in DRG neurons, which leads to mechanical hypersensitivity.
In the dorsal spinal cord, KChIP1 and KChIP2 are expressed in the somatodendritic domain of certain lamina II interneurons, whereas DPP10 appears mainly in the cell bodies of some projection neurons scattered in laminae I, II, and IV (Cheng et al., 2016). Immunohistochemical analysis in the L5 dorsal spinal cord showed no difference in KChIP1-, KChIP2-, and DPP10-IR between rats injected with vehicle and each Kv4 complex component ASO (Fig. 6A–F). The data confirm that the concentration of the gene-specific ASO used here is sufficient to suppress target protein expression in the DRG without affecting its expression in the spinal cord.
Knockdown of Kv4.3, KChIP1, or DPP10 enhances the excitability of IB4+ nociceptors
We tested whether the excitability of Kv4.3+ DRG neurons was enhanced after knockdown of Kv4.3, KChIP1, or DPP10 using the electrophysiological approach. There is no direct method to mark Kv4.3+ neurons, which comprise ∼26% of total DRG neurons (Chien et al., 2007). Here, we labeled DRG neurons with IB4 and then recorded from IB4+ cells. This approach could increase the proportion of Kv4.3+ neurons in the total recorded cells, because all Kv4.3+ DRG neurons bind IB4, and 44% of IB4+ nociceptors express Kv4.3 (Chien et al., 2007). It is also worth noting that DRG neurons showed no detectable currents after being cultured for <20 h (data not shown), and the efficacy of the ASO without modification for nuclease resistance was <16 h (Chien et al., 2007). To prolong the efficacy of the ASO, FAM-tagged ASO was further modified for nuclease resistance. Five hours after ASO injection into the rat by lumbar puncture, the bilateral L4-L6 DRGs were dissociated, and ∼70% of the neurons showed green fluorescence, indicating a high efficiency of in vivo transfection. Following 20–24 h in culture, neurons were labeled by Alexa-594-conjugated IB4 immediately before whole-cell patch-clamp recording. We only recorded small-diameter DRG neurons showing red fluorescence (IB4+) on their surface and green fluorescence (FAM) in their cytoplasm (Fig. 7A, top).
We first analyzed the intrinsic properties of ASO-transfected IB4+ neurons (Fig. 7A, bottom). The resting membrane potential (Vrest) of the three ASO groups (Kv4.3 ASO, −41.1 ± 2.9 mV; KChIP1 ASO, −36.9 ± 3.1 mV; DPP10 ASO, −40.5 ± 3.2 mV) was significantly more depolarized than that of the LacZ ASO group (−52.5 ± 1.7 mV; Fig. 7B). The rheobase is the minimal current injection required for a neuron to generate action potentials. As shown in Figure 7C, the rheobase of each ASO group (Kv4.3 ASO, 101.3 ± 15.9 pA; KChIP1 ASO, 91.1 ± 24.3 pA; DPP10 ASO, 92.7 ± 16.0 pA) was significantly lower than that of the LacZ ASO group (177.8 ± 25.3 pA). Furthermore, the effect of knocking down Kv4.3, KChIP1, and DPP10 on neuronal excitability was obviously observed from 50 to 200 pA current injections (Fig. 7D). To compare the slope change, we obtained the slopes by fitting the neuronal responses against current injections from 50 pA to 200 pA. The slopes of the frequency-current (f-I) relationship after knockdown of Kv4.3, KChIP1, and DPP10 were 0.032 ± 0.005 Hz/pA (n = 12), 0.058 ± 0.009 Hz/pA (n = 9), and 0.054 ± 0.016 Hz/pA (n = 11), respectively, which were significantly steeper than that (0.013 ± 0.001 Hz/pA, n = 9) of the LacZ group (LacZ ASO vs Kv4.3 ASO: p < 0.05; LacZ ASO vs KChIP1 ASO: p < 0.01; LacZ ASO vs DPP10 ASO: p < 0.05; Tukey's post hoc test after one-way ANOVA). Together, these results suggest that the excitability of Kv4.3+ DRG neurons is increased after knockdown of Kv4.3, KChIP1, or DPP10.
Rescue of Kv4 complex downregulation in injured DRGs attenuates SNL-evoked pain
SNL is a widely used animal model for neuropathic pain, and the two major symptoms are mechanical and thermal hypersensitivity (Kim and Chung, 1992). Because SNL downregulates Kv4.3 in the injured L5/L6 DRGs (Chien et al., 2007), we tested whether rescue of Kv4.3 downregulation could attenuate pain. Exogenous genes can be transfected into DRG neurons of living animals by intrathecal injection of PEI nanoparticles containing cDNA constructs (Wang et al., 2005). Several days after lumbar puncture of PEI nanoparticles containing EYFP cDNA, fluorescence was evident in lumbar DRG neurons but undetectable in the spinal cord (Cheng et al., 2015).
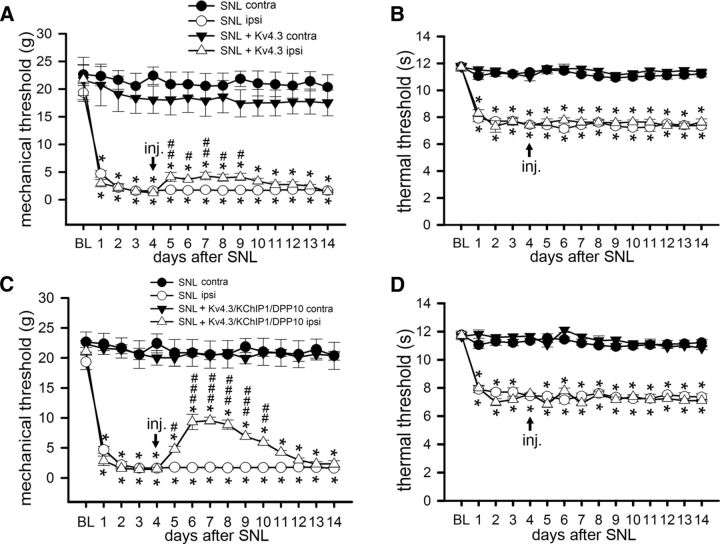
We intrathecally injected PEI nanoparticles containing Kv4.3 cDNA by lumbar puncture on day 4 after SNL. Mechanical hypersensitivity was slightly attenuated, and the weak analgesic effect lasted for 5 days (Fig. 8A). Because SNL downregulated not only Kv4.3 but also KChIP1 and DPP10 in the injured DRGs (Fig. 9A), we asked whether a rescue of Kv4 complex downregulation had a better analgesic effect. Indeed, mechanical hypersensitivity was greatly attenuated in SNL rats coinjected with Kv4.3, KChIP1, and DPP10 cDNAs, and the analgesic effect lasted for 6 days (Fig. 8C). Thermal hypersensitivity remained unchanged in SNL rats after injecting Kv4.3 cDNA or Kv4.3/KChIP1/DPP10 cDNAs (Fig. 8B,D).
Figure 8.
Intrathecal injection of Kv4.3/KChIP1/DPP10 cDNAs attenuates SNL-evoked pain. Rats received the SNL surgery following the baseline (BL) measurement on D0. Mechanical and thermal hypersensitivity developed in the hindpaw at the ipsilateral (ipsi) but not contralateral (contra) side (A, B). After intrathecal injection (inj.) of Kv4.3 cDNA (A, B) or Kv4.3/KChIP1/DPP10 cDNAs (C, D) at the ipsilateral side on D4, mechanical hypersensitivity was attenuated (A, C), but thermal hypersensitivity remained (B, D). Data are mean ± SEM (n = 6). *p < 0.05, compared with BL. #p < 0.05, compared with D4 (Tukey's post hoc test after one-way ANOVA). ##p < 0.01, compared with D4 (Tukey's post hoc test after one-way ANOVA). ###p < 0.001, compared with D4 (Tukey's post hoc test after one-way ANOVA).
In the injured DRGs, in vivo transfection of Kv4.3 cDNA into SNL rats recovered some of the Kv4.3-IR, and transfection of Kv4.3/KChIP1/DPP10 cDNAs partially restored the Kv4.3-, KChIP1-, and DPP10-IR (Fig. 9A,B). Using total proteins and plasma membrane proteins isolated from the injured DRGs, Western blotting showed an increase in Kv4.3 by Kv4.3 cDNA as well as a simultaneous enhancement of Kv4.3, KChIP1, and DPP10 expression by Kv4.3/KChIP1/DPP10 cDNAs (Fig. 9C–F). Thus, SNL-induced mechanical hypersensitivity can be greatly attenuated by reexpression of the Kv4 complex in the injured DRGs.
Finally, to elucidate whether the analgesic effect from cDNA injection is due to the suppression of SNL-induced glial activation and/or macrophage proliferation in the injured DRGs, we quantified the expression levels of glial fibrillary acidic protein (GFAP, for satellite glial cells) and ionized calcium-binding adaptor molecule (Iba1, for macrophages). On both day 7 and day 14 after SNL, GFAP- and Iba1-IR showed no significant difference between rats treated with Kv4.3/KChIP1/DPP10 cDNAs and those not treated (Fig. 10A–C). Thus, intrathecal cDNA injection does not suppress glial activation and macrophage proliferation in the injured DRGs.
Discussion
Five major findings arise from this study. First, a Kv4 complex, which contains the K+ channel pore-forming subunit Kv4.3 and three K+ channel modulatory subunits KChIP1, KChIP2, and DPP10, is detected in both the ER and plasma membrane isolated from rat DRGs (Fig. 1). Second, the total and surface protein levels of the Kv4 complex components in rat lumbar DRGs are interdependently regulated: reducing one causes the reduction of the others (Figs. 2–6). Third, mechanical hypersensitivity is induced in rat hindlimbs after knockdown of KChIP1, KChIP2, or DPP10 in lumbar DRGs (Figs. 4, 5). Fourth, knockdown of any Kv4 component in rat lumbar DRGs is sufficient to enhance the excitability of IB4+ nociceptors (Fig. 7). Fifth, peripheral nerve injury-evoked mechanical hypersensitivity can be attenuated by rescue of the Kv4 complex downregulation in the rat DRGs with injured nerves (Figs. 89-10). Because Kv4.3, KChIP1, KChIP2, and DPP10 are coexpressed in Kv4.3+ DRG neurons (Cheng et al., 2016), these findings suggest that the expression level of the Kv4 complex in Kv4.3+ DRG neurons is crucial for regulating pain sensitivity to mechanical stimuli.
KChIP1 or KChIP2 may confer calcium dependence on Kv4.3 in Kv4.3+ DRG neurons
Cerebellar stellate interneurons express K+ channel subunits Kv4.2, Kv4.3, KChIP2, KChIP3, DPP6, and DPP10, as well as T-type Ca2+ channel isoforms Cav3.2 and Cav3.3 (Anderson et al., 2010). Immunoprecipitation analysis in the cerebellum not only shows a Kv4 channel complex composed of Kv4.2, KChIP3, and DPP10 (Jerng et al., 2005) but also reveals a Cav3-Kv4 channel complex (Anderson et al., 2010). This Cav3-Kv4 channel complex efficiently couples calcium influx to KChIP3, which allows Kv4 channels to open at subthreshold potentials to regulate neuronal firing properties. The calcium-sensing effect mediated by KChIP3 cannot be replaced by KChIP1, KChIP2, or KChIP4, providing evidence for specific actions of different KChIPs (Anderson et al., 2010).
Kv4.3+ DRG neurons express Kv4.3, KChIP1, KChIP2, and DPP10 in their somata (Cheng et al., 2016), and immunoprecipitation analysis of the DRG further demonstrates that they form a Kv4 channel complex (Fig. 1). Interestingly, Cav3 channels also appear in DRG neurons and act as key players in pain control (Talley et al., 1999; Chen et al., 2015; Zamponi, 2016). Whether a Cav3-Kv4 channel complex exists in Kv4.3+ DRG neurons and whether KChIP1 or KChIP2 confers calcium dependence on Kv4.3 in these neurons require further investigation.
Interdependent regulation of expression levels among Kv4 complex components
Previous knockdown and knock-out experiments have shown an interdependent regulation among Kv4 complex components in their total protein levels. Knockdown of DPP6 reduces Kv4.2 and KChIP3 in cerebellar granule neurons (Nadin and Pfaffinger, 2010). Knock-out of Kv4.2 decreases KChIP2 and KChIP3 in the hippocampus, KChIP1 and KChIP3 in the cerebellum, and KChIP2 in the striatum (Menegola and Trimmer, 2006). Knock-out of DPP6 reduces Kv4.2 and KChIP2 in hippocampal CA1 pyramidal neurons (Sun et al., 2011). Knock-out of Kv4.2 and/or Kv4.3 decreases KChIP2, KChIP3, and KChIP4 in the posterior cortex (Norris et al., 2010), but DPP6 was not affected (Foeger et al., 2012). Because of a weak expression of DPP6 in the neocortex (Clark et al., 2008), it is likely that the difference was too small to be detected. In contrast, DPP10 is abundantly expressed in cortical layer 5 pyramidal neurons and colocalized with Kv4.2, Kv4.3, and KChIP3 (Wang et al., 2015), suggesting that DPP10 is the major DPP in the neocortex.
In addition to the total protein levels analyzed in the knockdown and knock-out studies described above, this report further demonstrates that the surface levels of the Kv4 complex components in DRG neurons are also interdependently regulated (Figs. 34-5). Subunits of the nicotinic acetylcholine receptor assemble into a functional α2βγδ pentamer in the ER through a series of interdependent folding and oligomerization events. However, only 20%–30% of synthesized subunits assemble into mature receptors in the ER, and the remaining unassembled subunits are degraded (Wanamaker et al., 2003). Similarly, Kv4 channel components are synthesized and assembled in the ER before transport to the plasma membrane to exert their function (Jerng and Pfaffinger, 2014). We speculate that reduced synthesis of one Kv4 complex component will result in fewer complexes assembled in the ER, leading to more degradation of the other unassembled components.
Kv4.3+ DRG neurons could be mechano-nociceptors
Several lines of evidence suggest that Kv4.3+ DRG neurons could be mechano-nociceptors. First, Kv4.3+ neurons respond selectively to mechanical stimuli, as revealed by the development of mechanical hypersensitivity after knockdown of each Kv4 complex component in DRG neurons (Chien et al., 2007) (Figs. 4, 5). Second, downregulation of Kv4.3 in Kv4.3+ neurons that innervate muscle fibers contributes to mechanical hyperalgesia in a rat model for hand-arm vibration syndrome in humans (Conner et al., 2016). Third, C-M nociceptors, a group of C-fiber nociceptors that constitutes ∼20% of DRG neurons, are high-threshold mechano-nociceptors (Lewin and Moshourab, 2004). Kv4.3 is expressed in 26% of DRG neurons (Chien et al., 2007), a population similar to that of C-M nociceptors. Fourth, reduction in the Kv4 complex in Kv4.3+ neurons enhances the excitability of IB4+ nociceptors (Fig. 7). The majority of IB4+ nociceptors express MrgprD, a sensory neuron-specific G-protein-coupled receptor. When MrgprD+ DRG neurons are ablated in adult mice, there is a significant reduction in the responses to noxious mechanical stimuli. Thus, MrgprD has been used as a marker for mechano-nociceptors (Le Pichon and Chesler, 2014). All Kv4.3+ neurons bind IB4, and 44% of IB4+ nociceptors express Kv4.3 (Chien et al., 2007). It is likely that Kv4.3+ and MrgprD+ DRG neurons largely overlap.
Peripheral nerve injury downregulates Kv4 complex expression in Kv4.3+ DRG neurons
Peripheral nerve injury downregulates Kv4.3 expression in Kv4.3+ DRG neurons (Chien et al., 2007), and the mechanism is described below. The Kv4.3 gene in the rat or human genome contains two conserved neuron-restrictive silencer elements (NRSEs) that can bind repressor element 1-silencing transcription factor (REST). Peripheral nerve injury increases REST expression and promotes the binding of REST to Kv4.3-NRSE to suppress Kv4.3 gene transcription in Kv4.3+ DRG neurons, and knockdown of REST by ASO can prevent Kv4.3 downregulation (Uchida et al., 2010). A putative NRSE has been detected in the human KChIP1 gene (Otto et al., 2007), but whether the KChIP2 and DPP10 genes contain NRSEs is unknown. Peripheral nerve injury also downregulates the expression of KChIP1, KChIP2, and DPP10 in Kv4.3+ DRG neurons (Fig. 9; KChIP2 data not shown). Further examination will be necessary to elucidate whether a reduction in each modulatory subunit also occurs at the transcriptional level.
Kv4 complexes in pain control
Kv4.1 mRNA has been detected in cultured small-diameter DRG neurons by single-cell RT-PCR (Phuket and Covarrubias, 2009), and in situ hybridization shows Kv4.1 mRNA in 59.5% of DRG neurons with various somatic sizes (Matsuyoshi et al., 2012). Kv4.3+ DRG neurons, which constitute 26% of total DRG neurons, are IB4+ (Chien et al., 2007). Because of the lack of a specific Kv4.1 antibody, whether Kv4.1 is expressed in Kv4.3+ DRG neurons remains unknown. In this study, because there is no direct way to identify Kv4.3+ neurons for electrophysiological recording, we analyzed IB4+ neurons instead (Fig. 7). It is likely that some IB4+ neurons express both Kv4.1 and Kv4.3, some express one or the other, and the others express neither. Because of the possible heterologous and/or compensatory expression of Kv4.1 and Kv4.3 in IB4+ neurons, delineating whether Kv4.3 currents are affected by the Kv4.3, KChIP1, or DPP10 ASO but not by the LacZ ASO would be difficult.
Although Kv4.2 is not expressed in the DRG, knock-out of Kv4.2 increases the excitability of dorsal spinal neurons, resulting in mechanical and thermal hypersensitivity (Hu et al., 2006). Kv4.2 is colocalized with Kv4.3, KChIP1, KChIP2, and DPP6 in certain spinal lamina II excitatory interneurons expressing the μ-opiate receptor (Huang et al., 2005; Cheng et al., 2016), which implies the existence of a Kv4 complex in pain modulatory interneurons.
K+ channel modulatory subunits are potential drug targets for neuropathic pain
Currently, the first-line drugs for neuropathic pain include voltage-gated Ca2+ channel blockers, antidepressants, local anesthetics, and opioid agonists (Baron et al., 2010). The analgesic effect of Ca2+ channel blockers involves its binding to the modulatory subunit α2δ to disrupt channel trafficking to the neuronal surface (Hendrich et al., 2008). The Kv4 activator NS5806, which increases Kv4 currents after binding to KChIP2 or KChIP3 (Lundby et al., 2010; Gonzalez et al., 2014), is a potential analgesic. By elucidating how KChIP1, KChIP2, and DPP10 participate in Kv4.3-mediated pain control, this study suggests that these K+ channel modulatory subunits can act as novel drug targets for neuropathic pain.
Footnotes
This work was supported by Ministry of Science and Technology Grants MOST 104-2320-B-010-010-MY3 and MOST 103-2311-B-010-012, Mackay Memorial Hospital Grants MMH104-099 and MMH-105-76, Brain Research Center of National Yang-Ming University, and Ministry of Education-Aim for the Top University Plan. We thank Drs. Chin-Cheng Chen and Sheng-Jie Shiue for technical support.
The authors declare no competing financial interests.
References
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ (2000) Modulation of A-type potassium channels by a family of calcium sensors. Nature 403:553–556. 10.1038/35000592 [DOI] [PubMed] [Google Scholar]
- Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JD, Hameed S, Zamponi GW, Turner RW (2010) Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci 13:333–337. 10.1038/nn.2493 [DOI] [PubMed] [Google Scholar]
- Baron R, Binder A, Wasner G (2010) Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 9:807–819. 10.1016/S1474-4422(10)70143-5 [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Jessell TM (2012) Pain. In: Principles of neural science (Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, eds), pp 531–534. New York: McGraw-Hill. [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284. 10.1016/j.cell.2009.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E, Alloui A, Monteil A, Barrère C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J (2005) Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J 24:315–324. 10.1038/sj.emboj.7600515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. 10.1016/0165-0270(94)90144-9 [DOI] [PubMed] [Google Scholar]
- Chen YL, Tsaur ML, Wang SW, Wang TY, Hung YC, Lin CS, Chang YF, Wang YC, Shiue SJ, Cheng JK (2015) Chronic intrathecal infusion of mibefradil, ethosuximide and nickel attenuates nerve ligation-induced pain in rats. Br J Anaesth 115:105–111. 10.1093/bja/aev198 [DOI] [PubMed] [Google Scholar]
- Cheng CF, Cheng JK, Chen CY, Rau RH, Chang YC, Tsaur ML (2015) Nerve growth factor-induced synapse-like structures in contralateral sensory ganglia contribute to chronic mirror-image pain. Pain 156:2295–2309. 10.1097/j.pain.0000000000000280 [DOI] [PubMed] [Google Scholar]
- Cheng CF, Wang WC, Huang CY, Du PH, Yang JH, Tsaur ML (2016) Coexpression of auxiliary subunits KChIP and DPPL in potassium channel Kv4-positive nociceptors and pain-modulating spinal interneurons. J Comp Neurol 524:846–873. 10.1002/cne.23876 [DOI] [PubMed] [Google Scholar]
- Chien LY, Cheng JK, Chu D, Cheng CF, Tsaur ML (2007) Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 27:9855–9865. 10.1523/JNEUROSCI.0604-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BD, Kwon E, Maffie J, Jeong HY, Nadal M, Strop P, Rudy B (2008) DPP6 localization in brain supports function as a Kv4 channel associated protein. Front Mol Neurosci 1:8. 10.3389/neuro.02.008.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner LB, Alvarez P, Bogen O, Levine JD (2016) Role of Kv4.3 in vibration-induced muscle pain in the rat. J Pain 17:444–450. 10.1016/j.jpain.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foeger NC, Marionneau C, Nerbonne JM (2010) Co-assembly of Kv4 α subunits with K+ channel-interacting protein 2 stabilizes protein expression and promotes surface retention of channel complexes. J Biol Chem 285:33413–33422. 10.1074/jbc.M110.145185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foeger NC, Norris AJ, Wren LM, Nerbonne JM (2012) Augmentation of Kv4.2-encoded currents by accessory dipeptidyl peptidase 6 and 10 subunits reflects selective cell surface Kv4.2 protein stabilization. J Biol Chem 287:9640–9650. 10.1074/jbc.M111.324574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Gebhart GF (2010) Nociceptor sensitization in pain pathogenesis. Nat Med 16:1248–1257. 10.1038/nm.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez WG, Pham K, Miksovska J (2014) Modulation of the voltage-gated potassium channel (Kv4.3) and the auxiliary protein (KChIP3) interactions by the current activator NS5806. J Biol Chem 289:32201–32213. 10.1074/jbc.M114.577528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88. 10.1016/0304-3959(88)90026-7 [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC (2008) Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc Natl Acad Sci U S A 105:3628–3633. 10.1073/pnas.0708930105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist MH, Cao J, Hernandez-Pineda R, Jacobson MD, Carroll KI, Sung MA, Betty M, Ge P, Gilbride KJ, Brown ME, Jurman ME, Lawson D, Silos-Santiago I, Xie Y, Covarrubias M, Rhodes KJ, Distefano PS, An WF (2002) Elimination of fast inactivation in Kv4 A-type potassium channels by an auxiliary subunit domain. Proc Natl Acad Sci U S A 99:1035–1040. 10.1073/pnas.022509299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RW 4th (2006) The Kv4.2 potassium channel subunit is required for pain plasticity. Neuron 50:89–100. 10.1016/j.neuron.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Huang HY, Cheng JK, Shih YH, Chen PH, Wang CL, Tsaur ML (2005) Expression of A-type K+ channel α subunits Kv4.2 and Kv4.3 in rat spinal lamina II excitatory interneurons and co-localization with pain-modulating molecules. Eur J Neurosci 22:1149–1157. 10.1111/j.1460-9568.2005.04283.x [DOI] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ (2014) Modulatory mechanisms and multiple functions of somatodendritic A-type K+ channel auxiliary subunits. Front Cell Neurosci 8:82. 10.3389/fncel.2014.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M (2004) Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci 27:343–369. 10.1016/j.mcn.2004.06.011 [DOI] [PubMed] [Google Scholar]
- Jerng HH, Kunjilwar K, Pfaffinger PJ (2005) Multiprotein assembly of Kv4.2, KChIP3 and DPP10 produces ternary channel complexes with ISA-like properties. J Physiol 568:767–788. 10.1113/jphysiol.2005.087858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM (1992) An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50:355–363. 10.1016/0304-3959(92)90041-9 [DOI] [PubMed] [Google Scholar]
- Le Pichon CE, Chesler AT (2014) The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat 8:21. 10.3389/fnana.2014.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Moshourab R (2004) Mechanosensation and pain. J Neurobiol 61:30–44. 10.1002/neu.20078 [DOI] [PubMed] [Google Scholar]
- Lien CC, Jonas P (2003) Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J Neurosci 23:2058–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby A, Jesperson T, Schmitt N, Grunnet M, Olesen SP, Cordeiro JM, Calloe K (2010) Effect of the I(to) activator NS5806 on cloned Kv4 channels depends on the accessory protein KChIP2. Br J Pharmacol 160:2028–2044. 10.1111/j.1476-5381.2010.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffie J, Rudy B (2008) Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J Physiol 586:5609–5623. 10.1113/jphysiol.2008.161620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyoshi H, Takimoto K, Yunoki T, Erickson VL, Tyagi P, Hirao Y, Wanaka A, Yoshimura N (2012) Distinct cellular distributions of Kv4 pore-forming and auxiliary subunits in rat dorsal root ganglion neurons. Life Sci 91:258–263. 10.1016/j.lfs.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegola M, Trimmer JS (2006) Unanticipated region- and cell-specific downregulation of individual KChIP auxiliary subunit isotypes in Kv4.2 knock-out mouse brain. J Neurosci 26:12137–12142. 10.1523/JNEUROSCI.2783-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, Rudy B (2003) The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 37:449–461. 10.1016/S0896-6273(02)01185-6 [DOI] [PubMed] [Google Scholar]
- Nadin BM, Pfaffinger PJ (2010) Dipeptidyl peptidase-like protein 6 is required for normal electrophysiological properties of cerebellar granule cells. J Neurosci 30:8551–8565. 10.1523/JNEUROSCI.5489-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Foeger NC, Nerbonne JM (2010) Interdependent roles for accessory KChIP2, KChIP3, and KChIP4 subunits in the generation of Kv4-encoded IA channels in cortical pyramidal neurons. J Neurosci 30:13644–13655. 10.1523/JNEUROSCI.2487-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor AB, Dworkin RH (2009) Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 122:S22–S32. 10.1016/j.amjmed.2009.04.007 [DOI] [PubMed] [Google Scholar]
- Otto SJ, McCorkle SR, Hover J, Conaco C, Han JJ, Impey S, Yochum GS, Dunn JJ, Goodman RH, Mandel G (2007) A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci 27:6729–6739. 10.1523/JNEUROSCI.0091-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papir-Kricheli D, Frey J, Laufer R, Gilon C, Chorev M, Selinger Z, Devor M (1987) Behavioral effects of receptor-specific substance P agonists. Pain 31:263–276. 10.1016/0304-3959(87)90041-8 [DOI] [PubMed] [Google Scholar]
- Phuket TR, Covarrubias M (2009) Kv4 channels underlie the subthreshold-operating A-type K-current in nociceptive dorsal root ganglion neurons. Front Mol Neurosci 2:3. 10.3389/neuro.02.003.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O, Schwarz JR (2010) Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev 90:755–796. 10.1152/physrev.00020.2009 [DOI] [PubMed] [Google Scholar]
- Sun W, Maffie JK, Lin L, Petralia RS, Rudy B, Hoffman DA (2011) DPP6 establishes the A-type K+ current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron 71:1102–1115. 10.1016/j.neuron.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA (1999) Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 19:1895–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsantoulas C, McMahon SB (2014) Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci 37:146–158. 10.1016/j.tins.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Sasaki K, Ma L, Ueda H (2010) Neuron-restrictive silencer factor causes epigenetic silencing of Kv4.3 gene after peripheral nerve injury. Neuroscience 166:1–4. 10.1016/j.neuroscience.2009.12.021 [DOI] [PubMed] [Google Scholar]
- van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N (2014) Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 155:654–662. 10.1016/j.pain.2013.11.013 [DOI] [PubMed] [Google Scholar]
- von Hehn CA, Baron R, Woolf CJ (2012) Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73:638–652. 10.1016/j.neuron.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanamaker CP, Christianson JC, Green WN (2003) Regulation of nicotinic acetylcholine receptor assembly. Ann N Y Acad Sci 998:66–80. 10.1196/annals.1254.009 [DOI] [PubMed] [Google Scholar]
- Wang WC, Cheng CF, Tsaur ML (2015) Immunohistochemical localization of DPP10 in rat brain supports the existence of Kv4/KChIP/DPPL ternary complex in neurons. J Comp Neurol 523:608–628. 10.1002/cne.23698 [DOI] [PubMed] [Google Scholar]
- Wang X, Wang C, Zeng J, Xu X, Hwang PY, Yee WC, Ng YK, Wang S (2005) Gene transfer to dorsal root ganglia by intrathecal injection: effects on regeneration of peripheral nerves. Mol Ther 12:314–320. 10.1016/j.ymthe.2005.03.032 [DOI] [PubMed] [Google Scholar]
- Waxman SG, Zamponi GW (2014) Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci 17:153–163. 10.1038/nn.3602 [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA (1976) Chronic catheterization of the spinal subarachnoid space. Physiol Behav 17:1031–1036. 10.1016/0031-9384(76)90029-9 [DOI] [PubMed] [Google Scholar]
- Yu YQ, Zhao F, Guan SM, Chen J (2011) Antisense-mediated knockdown of Nav1.8, but not Nav1.9, generates inhibitory effects on complete Freund's adjuvant-induced inflammatory pain in rat. PLoS One 6:e19865. 10.1371/journal.pone.0019865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW. (2016) Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Discov 15:19–34. 10.1038/nrd.2015.5 [DOI] [PubMed] [Google Scholar]