Each time we move our eyes or rotate our head, the whole visual field moves across our retinas. Such optic flow stimuli are contingent on the course of our movements and thus can be used to monitor self-movements. Many animals with image-forming eyes, from Drosophila to humans, seem to use optic flow information to guide their behavior (Götz, 1964; Prokop et al., 1997), and many visual neurons sensitive to optic flow have been identified (Hausen, 1976; Lagae et al., 1994). Tethered fruit flies that are presented with optic flow stimuli attempt to turn their heads and bodies in the same direction as the visual motion (Hecht and Wald, 1934; Götz, 1964). In freely behaving flies, this optomotor response would oppose the initial, aberrant body or head movement that had caused the optic flow stimulus, thereby acting to stabilize the locomotor heading or visual gaze of the animal.
In dipterans, a set of visual lobe output neurons, called horizontal system (HS) and vertical system (VS) cells, are sensitive to rotational optic flow, with each HS and VS cell being most sensitive to a distinct axis of head or body rotation (Krapp and Hengstenberg, 1996; Schnell et al., 2010). Rotational optic flow is described in relation to the following three primary axes of rotation: yaw, pitch, and roll. In Drosophila, three HS cells—HS North (HSN), HS Equatorial (HSE), and HS South—respond maximally to yaw-associated optic flow, VS1–3 cells respond maximally to pitch-associated optic flow, and VS4–6 cells respond maximally to roll-associated optic flow (Maimon et al., 2010; Schnell et al., 2010). Although HS and VS cells have been studied extensively, circuits downstream to HS/VS cells have not been identified in Drosophila. To understand how optomotor behavior is organized in the Drosophila brain, Suver et al. (2016) investigated how three pairs of descending neurons (DNs) represent visual information about the locomotor rotations of the animal.
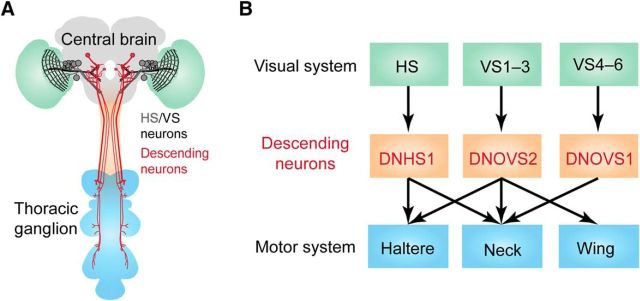
Suver et al. (2016) first identified three fly lines in which gene expression is targeted to distinct combinations of three DNs. They used these lines to study the three DNs reproducibly across animals and named these neurons DNOVS1 (descending neuron of the ocellar and vertical system 1), DNOVS2, and DNHS1 (descending neuron of the horizontal system 1; Fig. 1). DNOVS1 and DNOVS2 were named based on their anatomical similarity to previously identified blowfly neurons; DNHS1 is a newly identified cell type. To assess connectivity with neighboring neurons, they filled each DN with dye that can pass through gap junctions and discovered that DNOVS1 dye couples to VS4–6 cells, DNOVS2 couples to VS2–3 cells, and DNHS1 couples to HSN and HSE cells. They further analyzed projection patterns in the thoracic ganglion for these neurons. DNOVS1 projects to a neck motor region; DNOVS2 projects to neck, wing, and haltere regions; and DNHS1 projects to neck and haltere regions (Fig. 1B). These anatomical data suggested that the three DNs may be directly involved in the flight and neck motor control.
Figure 1.
A, Schematic of a fly brain highlights HS/VS cells and three descending neurons (adapted from Suver et al., 2016). B, A block diagram illustrates the functional connectivity of descending neurons with the visual system and the motor system.
The authors next examined the visual responses of DNs in relation to those of HS and VS cells. They designed 24 optic flow stimuli in which the axis of rotation shifted with 15° intervals around the azimuthal plane, simulating visual stimuli flies would experience during various roll and pitch maneuvers. They measured changes in membrane voltage in responses to each motion stimulus via the whole-cell patch clamp technique (Wilson et al., 2004; Maimon et al., 2010) and constructed a 24-element response vector for each DN on the right side of the brain. They examined these vectors and found that DNOVS1 was most sensitive to optic flow associated with body rotations intermediate between downward pitch and counterclockwise roll, whereas DNOVS2 was most sensitive to optic flow associated with body rotations intermediate between upward pitch and counterclockwise roll. DNHS1 responded most strongly to optic flow associated with clockwise roll, among the 24 roll–pitch optic flow patterns. Overall, the maximally tuned axes of rotation for the three DNs were separated by 120° from one another, suggesting that they reflect three descending channels with maximally distinct rotation information. Furthermore, the authors observed that visual responses of the DNs increased significantly during flight, akin to previous observations on HS and VS cells (Maimon et al., 2010; Schnell et al., 2014), suggesting that the entire flight visuomotor system is selectively “turned on” during flight.
To understand functional relationships between DNs and presynaptic visual neurons, Suver et al. (2016) measured visual responses of HS and VS cells in response to the same set of visual stimuli. They found that the response vectors of the DNs could be closely predicted by a weighted sum of HS and VS responses. Furthermore, the predictive ability of the model, as estimated in terms of residual prediction error with respect to the actual DN response vector, saturated after incorporating the two most significantly related HS/VS response vectors. Consequently, this analysis identified two HS/VS cells that are functionally associated with each DN, and this functional connectivity between HS/VS cells and DNs was consistent with the anatomical connectivity identified by the dye fill experiment.
Together, Suver et al. (2016) provide convincing evidence for the following model of the optomotor response. When an animal is caught by a gust of wind and rotated to a certain direction, the eyes of the fly will experience optic flow rotating to the opposite direction. This will depolarize or hyperpolarize a subset of HS and VS cells. The HS/VS cells will then collectively activate the neck motor system, both directly via gap junctions and indirectly via the downstream DNs, so as to revert unexpected gaze changes. In particular, HS/VS cells would activate DNHS1 for yaw rotations, and DNOVS1/2 for roll-pitch rotations (Fig. 1). During flight, these DNs will additionally recruit wing and haltere motor systems to maintain the stable body orientation. Note, however, the HS/VS pathway may not represent the only neural circuit for the optomotor behavior. A recent study (Kim et al., 2017) showed that the functional inactivation of HS and VS cells impaired only the early component of wing optomotor responses for yaw optic flow stimuli. This is consistent with the lack of wing motor neuropil innervation by DNHS1 and strongly suggests the existence of optomotor circuits independent of HS/VS cells. Suver et al. (2016) also noted that the three DNs described here may not be the only DNs that pass optic flow information to the thoracic ganglion. In blowflies, two additional DNOVS cells have been reported (Strausfeld and Bassemir, 1985; Gronenberg and Strausfeld, 1990). Finally, the three DNs may carry sensory information above and beyond that conveyed by HS and VS cells, as suggested by the diffusive pattern of DN dendrites. Specifically, the three DNs may also integrate optic flow information encoded by the non-HS/VS optomotor circuits or even inputs from other sensory cues, such as visual inputs from ocelli and mechanosensory inputs from halteres (Sherman and Dickinson, 2003).
DNs in insects are in a unique position, as they connect the following two major brain structures: the central brain, which is specialized for sensory processing and higher-order functions; and the thoracic ganglion, which generates motor programs (among other putative functions). In Drosophila, there are estimated to be ∼1000 DNs in total, <1% of the total number of neurons in the nervous system (Hsu and Bhandawat, 2016). Thus, DNs may represent an information bottleneck between the brain and thoracic ganglion. Using whole-cell patch recordings in Drosophila, Suver et al. (2016) characterized the visual properties of three descending visual neurons downstream of well known visual neurons (HS and VS cells). This study not only is an important step toward understanding how optic flow stimuli lead to optomotor behaviors in Drosophila, but also provides a template for studying neurons bridging the gap between sensory and motor systems in this important model organism.
A primary function of the brain is to encode physical changes in the external world and generate appropriate behaviors. Because of the complexity of the brain, most neuroscience studies focus on either sensory or motor systems. Less common are studies on neurons that bridge the gap between the two. By identifying three DNs in Drosophila, Suver et al. (2016) opened a new avenue for understanding how visual neurons encoding self-motion information feed into motor centers in Drosophila. The numerical simplicity of the Drosophila brain, along with the advanced genetic and physiological tools available for this organism, may permit further advances. For example, one can test the hypothesis that the DNs show increased optic flow selectivity and robust self-rotation coding under variable surrounding texture, compared with HS and VS cells (Wertz et al., 2009a, 2009b). In addition, it has been reported recently that HS/VS cells receive motor-related modulations during flight and walking (Kim et al., 2015, 2017; Fujiwara et al., 2017). It will be important to determine how these brief motor-related signals are reflected in the physiology of postsynaptic DNs.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/preparing-manuscript#journalclub.
Lisa Fenk, Gaby Maimon, and Vikram Vijayan provided helpful comments on the manuscript.
References
- Fujiwara T, Cruz TL, Bohnslav JP, Chiappe ME (2017) A faithful internal representation of walking movements in the Drosophila visual system. Nat Neurosci 20:72–81. 10.1038/nn.4435 [DOI] [PubMed] [Google Scholar]
- Götz K. (1964) Optomotorische untersuchung des visuellen systems einiger augenmutanten der fruchtfliege Drosophila. Biol Cybern 2:77–92. [DOI] [PubMed] [Google Scholar]
- Gronenberg W, Strausfeld NJ (1990) Descending neurons supplying the neck and flight motor of Diptera: physiological and anatomical characteristics. J Comp Neurol 302:973–991. 10.1002/cne.903020420 [DOI] [PubMed] [Google Scholar]
- Hausen K. (1976) Functional characterization and anatomical identification of motion sensitive neurons in the lobula plate of the blowfly Calliphora erythrocephala. Z Naturforsch C 31:629–633. [Google Scholar]
- Hecht S, Wald G (1934) The visual acuity and intensity discrimination of Drosophila. J Gen Physiol 17:517–547. 10.1085/jgp.17.4.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CT, Bhandawat V (2016) Organization of descending neurons in Drosophila melanogaster. Sci Rep 6:20259. 10.1038/srep20259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AJ, Fitzgerald JK, Maimon G (2015) Cellular evidence for efference copy in Drosophila visuomotor processing. Nat Neurosci 18:1247–1255. 10.1038/nn.4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AJ, Fenk LM, Lyu C, Maimon G (2017) Quantitative predictions orchestrate visual signaling in Drosophila. Cell 168:280–294.e12. 10.1016/j.cell.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp HG, Hengstenberg R (1996) Estimation of self-motion by optic flow processing in single visual interneurons. Nature 384:463–466. 10.1038/384463a0 [DOI] [PubMed] [Google Scholar]
- Lagae L, Maes H, Raiguel S, Xiao DK, Orban GA (1994) Responses of macaque STS neurons to optic flow components: a comparison of areas MT and MST. J Neurophysiol 71:1597–1626. [DOI] [PubMed] [Google Scholar]
- Maimon G, Straw AD, Dickinson MH (2010) Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci 13:393–399. 10.1038/nn.2492 [DOI] [PubMed] [Google Scholar]
- Prokop T, Schubert M, Berger W (1997) Visual influence on human locomotion. Modulation to changes in optic flow. Exp Brain Res 114:63–70. 10.1007/PL00005624 [DOI] [PubMed] [Google Scholar]
- Schnell B, Joesch M, Forstner F, Raghu SV, Otsuna H, Ito K, Borst A, Reiff DF (2010) Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J Neurophysiol 103:1646–1657. 10.1152/jn.00950.2009 [DOI] [PubMed] [Google Scholar]
- Schnell B, Weir PT, Roth E, Fairhall AL, Dickinson MH (2014) Cellular mechanisms for integral feedback in visually guided behavior. Proc Natl Acad Sci U S A 111:5700–5705. 10.1073/pnas.1400698111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman A, Dickinson MH (2003) A comparison of visual and haltere-mediated equilibrium reflexes in the fruit fly Drosophila melanogaster. J Exp Biol 206:295–302. 10.1242/jeb.00075 [DOI] [PubMed] [Google Scholar]
- Strausfeld N, Bassemir U (1985) Lobula plate and ocellar interneurons converge onto a cluster of descending neurons leading to neck and leg motor neuropil in Calliphora erythrocephala. Cell Tissue Res 240:617–640. 10.1007/BF00216351 [DOI] [Google Scholar]
- Suver MP, Huda A, Iwasaki N, Safarik S, Dickinson MH (2016) An array of descending visual interneurons encoding self-motion in Drosophila. J Neurosci 36:11768–11780. 10.1523/JNEUROSCI.2277-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz A, Gaub B, Plett J, Haag J, Borst A (2009a) Robust coding of ego-motion in descending neurons of the fly. J Neurosci 29:14993–15000. 10.1523/JNEUROSCI.3786-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz A, Haag J, Borst A (2009b) Local and global motion preferences in descending neurons of the fly. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195:1107–1120. 10.1007/s00359-009-0481-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G (2004) Transformation of olfactory representations in the Drosophila antennal lobe. Science 303:366–370. 10.1126/science.1090782 [DOI] [PubMed] [Google Scholar]