Abstract
SCRAPPER is an E3 ubiquitin ligase expressed in presynaptic terminals, neural cell body, and dendrites of the hippocampus and cortex, which is coded by the FBXL20 gene. SCRAPPER is known to regulate synaptic transmissions and long-term potentiation (LTP) in the hippocampus, but no report is available for the cortex. Here we show genetic evidence for critical roles of SCRAPPER in excitatory transmission and presynaptic LTP (pre-LTP) of the anterior cingulate cortex (ACC), a critical cortical region for pain, anxiety, and fear. Miniature and spontaneous releases, but not evoked release, of glutamate were significantly increased in SCRAPPER knock-out (SCR-KO) mice. Interestingly, SCRAPPER selectively contributes to the increases of frequency and amplitude. The pre-LTP in the ACC was completely blocked in SCR-KO mice. Our results thus provide direct evidence for SCRAPPER in both spontaneous release and pre-LTP in the ACC and reveal a potential novel target for treating anxiety-related disease.
SIGNIFICANCE STATEMENT The anterior cingulate cortex (ACC) plays critical roles in pain, anxiety, and fear. Peripheral injury induces long-term changes in synaptic transmission in the ACC. Our recent study found that a presynaptic form of LTP (pre-LTP) in the ACC contributes to chronic pain-induced anxiety. Here, we show that SCRAPPER plays a critical role in ACC pre-LTP as well as synaptic transmission.
Keywords: cortex, knock-out mice, LTP, presynaptic, scrapper, spontaneous release
Introduction
Human and animal studies indicate that the anterior cingulate cortex (ACC) plays a critical role in pain, anxiety, and fear (Frankland et al., 2004; Gross and Hen, 2004; Tang et al., 2005; Damsa et al., 2009; Kim et al., 2011; Descalzi et al., 2012; Steenland et al., 2012; Grupe and Nitschke, 2013). Glutamate is the major excitatory transmitter, and postsynaptic AMPA receptors mediate most postsynaptic responses in ACC neurons. Adult glutamatergic synapses in the ACC are highly plastic and can undergo long-term changes (Wei et al., 2001; Zhuo, 2008; Bliss et al., 2016).
Long-term potentiation (LTP) is a key cellular model for activity-dependent learning and memory, as well as chronic pain (Bliss and Collingridge, 1993, 2013; Zhuo, 2008, 2014; Kandel, 2012). There are at least two major forms of LTP: presynaptic (pre-) and postsynaptic (post-) LTP (Nicoll and Schmitz, 2005; Kerchner and Nicoll, 2008; Bliss and Collingridge, 2013). Both forms of LTP in the hippocampus have been suggested to play important roles in learning and memory (Tsien et al., 1996; Nakazawa et al., 2002). In the ACC, post-LTP and pre-LTP have been reported (Zhao et al., 2005; Zhuo, 2008; Koga et al., 2015a). Presynaptic changes of glutamate release in the ACC have been reported after peripheral tissue or nerve injury (Zhao et al., 2006; Xu et al., 2008; Koga et al., 2015a). Recently, we found that pre-LTP in the ACC may link to injury-related behavioral anxiety (Koga et al., 2015a).
The ubiquitin–proteasome system (UPS) is a major protein degradation pathway that modulates synaptic transmission and LTP (Hegde and DiAntonio, 2002; Fonseca et al., 2006; Karpova et al., 2006; Yao et al., 2007; Tai and Schuman, 2008; Cai et al., 2010; Rinetti and Schweizer, 2010). SCRAPPER is a major presynaptic E3 ubiquitin ligase that is enriched in the presynaptic hippocampal terminals (Dobie and Craig, 2007; Yao et al., 2007). Behavioral studies found that SCRAPPER transgenic mice display decreased anxiety-like behaviors (Yao et al., 2011), but the cellular mechanisms driving this reduction remain unknown. Based on our recent discovery of the role of pre-LTP, our present study tests the possible link between cortical pre-LTP and SCRAPPER-related signaling pathways in adult cortical neurons. We found that both a selective proteasome inhibitor and deletion of SCAPPER abolished pre-LTP in the ACC. Furthermore, we found that excitatory glutamatergic transmission is significantly enhanced in SCRAPPER knock-out (SCR-KO) mice.
Materials and Methods
Animals.
Adult male SCRAPPER wild-type (SCR-WT), Heterozygous (SCR-Hetero), and SCR-KO mice were used (8–12 weeks old; Yao et al., 2007, 2011; Takagi et al., 2012). For experiments using KO mice, SCRAPPER transgenic mice were maintained on a mixed 129Sv × C57BL/6 background. For some pharmacological experiments, we used adult male C57BL/6 mice (8–12 weeks) purchased from Charles River. All mice were maintained on a 12 h light/dark cycle with food and water provided ad libitum. The Animal Care and Use Committees at Xi'an Jiaotong University, the University of Toronto, and Hamamatsu University School of Medicine approved all experimental protocols. All experiments related to mutant mice were performed with experimenters blind to genotype.
In vitro whole-cell patch-clamp recordings.
Coronal brain slices (300 μm) at the level of the ACC were prepared using standard methods (Zhao et al., 2005; Li et al., 2010; Koga et al., 2015a,b). Brain slices were transferred to a submerged recovery chamber with oxygenated (95% O2 and 5% CO2) artificial CSF containing (in mm) 124 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 25 NaHCO3, 1 NaH2PO4, and 10 glucose at room temperature for at least 1 h. Experiments were performed in a recording chamber on the stage of an Axioskop 2FS microscope (Zeiss) with infrared differential interference contrast optics for visualization. For recording in the ACC, evoked excitatory postsynaptic currents (eEPSCs) were recorded from layer II/III neurons with an Axon 200B amplifier (Molecular Devices), and the stimulations were delivered by a bipolar tungsten stimulating electrode placed in layer V/VI of the ACC. AMPA/kainate receptor-mediated EPSCs were induced by repetitive stimulations at 0.02 Hz, and neurons were voltage clamped at −60 mV. The recording pipettes (3–5 MΩ) were filled with a solution containing (in mm) 124 K-gluconate, 5 NaCl, 1 MgCl2, 0.2 EGTA, 10 HEPES, 2 MgATP, 0.1 Na3GTP, and10 phosphocreatine disodium (adjusted to pH 7.2 with KOH). Picrotoxin (100 μm) was always present to block GABAA receptor-mediated inhibitory synaptic currents in all experiments. The amplitudes of eEPSCs were adjusted between 50 and 100 pA in baseline. Paired-pulse stimulations with a 50 ms interpulse interval were given during the recording every 30 s. We recorded from neurons for 4 h after making coronal brain slices (Zhao et al., 2005; Xu et al., 2008). For induction of pre-LTP, low-frequency stimulation, which is 240 paired presynaptic stimuli (with 50 ms interpulse intervals), was delivered at 2 Hz to the presynaptic fibers at a holding potential of −60 mV (Shin et al., 2010; Koga et al., 2015a,b). The initial access resistance was 15–30 MΩ, and it was monitored throughout the experiment. Data were discarded if the access resistance changed >15% during the experiment. Data were filtered at 1 kHz and digitized at 10 kHz.
Data analysis.
Statistical comparisons were made using unpaired and paired t tests and one-way or two-way ANOVAs (Student–Newman–Keuls or Tukey's test was used for post hoc comparisons). All data are presented as the mean ± SEM. In all cases, p < 0.05 was considered statistically significant.
Results
Facilitation of spontaneous and miniature EPSCs in the ACC
Spontaneous neurotransmitter release and evoked synaptic release may use distinct presynaptic signaling pathways and may have different functional implications (for review, see Kavalali, 2015). We thus decided to test whether the gene deletion of SCRAPPER could alter spontaneous EPSCs (sEPSCs) in the ACC (Fig. 1). We performed whole-cell patch-clamp recordings from layer II/III pyramidal neurons in the ACC. We recorded and analyzed the properties of sEPSCs in SCRAPPER transgenic mice (SCR-KO, n = 16 neurons/12 mice; SCR-WT, n = 14/10; Fig. 1). Neurons from SCR-KO mice showed enhanced sEPSC frequency and amplitude compared with SCR-WT mice (Fig. 1C–F). We also analyzed the frequency and amplitude of sEPSCs in SCR-Hetero mice (n = 14/10; Fig. 1C–E). Among all three groups, both frequency and amplitude of sEPSCs in SCR-KO mice were significantly enhanced compared with SCR-WT (Fig. 1E,F). These data suggest that SCRAPPER plays important roles in basal spontaneous excitatory synaptic transmission in the ACC. We also analyzed the kinetics of sEPSCs in SCR-KO, SCR-WT, and SCR-Hetero mice (Fig. 1B). sEPSCs from SCR-KO mice show a significant increase in the averaged decay time compared with SCR-WT mice (Fig. 1B). There was no difference in rise time among all groups (Fig. 1B).
Figure 1.
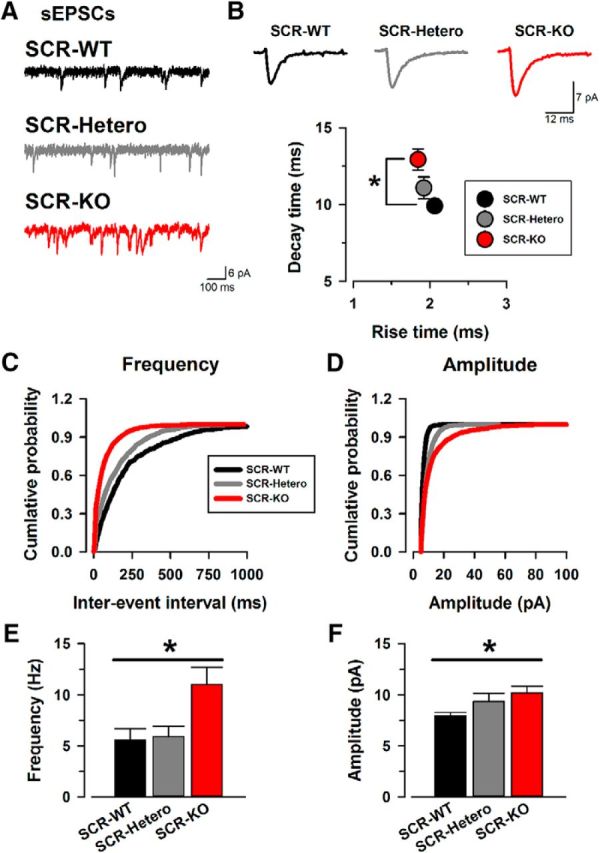
SCR-KO mice facilitate spontaneous excitatory transmission release in the ACC. A, Sample traces of sEPSCs from SCR-WT (top), SCR-Hetero (middle), and SCR-KO (bottom) mice. B, Sample traces of averaged sEPSCs in SCR-WT (left), SCR-Hetero (middle), and SCR-KO (right) mice. Also shown is the averaged rise time and decay time of sEPSCs in the three groups. There were significant differences in rise time and decay time between SCR-KO and SCR-WT mice (rise time: SCR-KO, 1.9 ± 0.1; SCR-Hetero, 1.9 ± 0.1; SCR-WT, 2.1 ± 0.1 ms; decay time: SCR-KO, 12.9 ± 0.7; SCR-Hetero, 11.1 ± 0.7; SCR-WT, 9.9 ± 0.2 ms). C, Cumulative probability of the frequencies in sEPSCs. The curve of SCR-KO mice shows a leftward shift compared with SCR-WT mice. D, Cumulative probability of the amplitudes in sEPSCs. The curves of all groups are similar. E, Average frequency of sEPSCs in SCR-WT, SCR-Hetero, and SCR-KO mice. SCR-KO mice showed significantly higher frequencies of sEPSCs compared with SCR-WT mice (SCR-KO, 11.0 ± 1.7 Hz; SCR-Hetero, 6.9 ± 1.0 Hz; SCR-WT, 5.6 ± 1.1 Hz). F, Average amplitude of sEPSCs in SCR-WT, SCR-Hetero, and SCR-KO mice. The averaged amplitudes of sEPSCs show a significant difference between SCR-KO and SCR-WT mice (SCR-KO, 10.2 ± 0.6; SCR-Hetero, 9.4 ± 0.8; SCR-WT, 7.9 ± 0.4 pA). *p < 0.05 compared with SCR-WT mice. Data are presented as mean ± SEM.
Next, we examined the effects of deletion of SCRAPPER on miniature EPSCs (mEPSCs; Fig. 2). In the presence of tetrodotoxin (1 μm) to block sodium channels, we recorded and analyzed the mEPSCs in ACC neurons from SCR-KO (n = 18/14) and SCR-WT (n = 14/10; Fig. 2A) mice. The frequency of SCR-KO mice showed a significant increase in the frequency of mEPSCs compared with SCR-WT mice (Fig. 2E). On the other hand, the amplitudes of mEPSCs were statistically similar among all groups (Fig. 2D).
Figure 2.
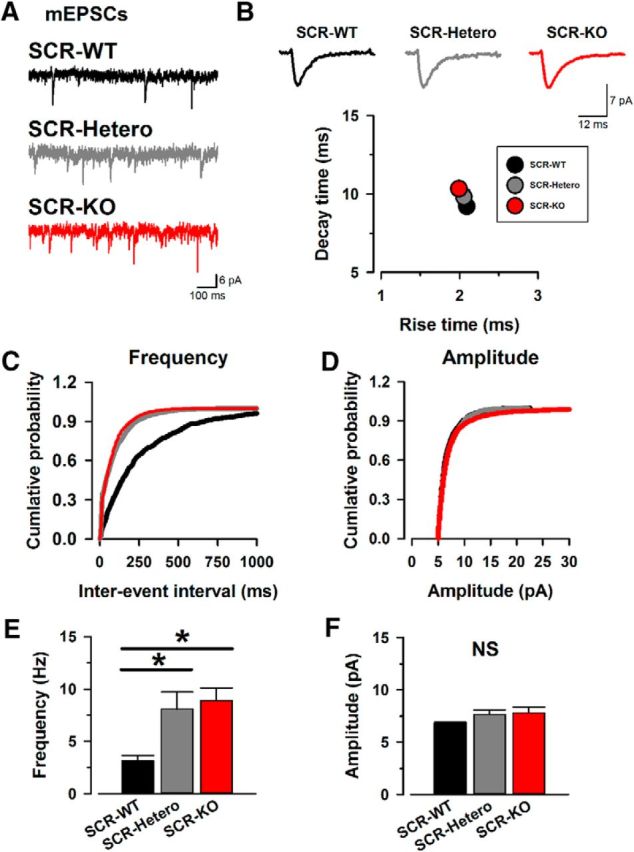
SCR-KO mice enhance miniature excitatory transmission release in the ACC. A, Sample traces of mEPSCs in SCR-WT (top), SCR-Hetero, (middle), and SCR-KO (bottom) mice. B, Sample traces of averaged mEPSCs in SCR-WT (left), SCR-Hetero (middle), and SCR-KO (right) mice. Also shown is the averaged rise time and decay time of mEPSCs in the three groups. Rise time and decay time did not change in all groups. C, Cumulative probability of the frequencies in mEPSCs. The curves of SCR-KO and SCR-Hetero mice show a leftward shift compared with SCR-WT mice. D, Cumulative probability of mEPSC amplitudes. There is no difference in the curves of all groups. E, SCR-KO and SCR-Hetero mice show a significant increase in the frequency of mEPSCs compared with SCR-WT mice (SCR-KO: 8.9 ± 1.2 Hz, n = 18 neurons/14 mice; SCR-Hetero: 8.1 ± 1.6 Hz, n = 14/10; SCR-WT: 3.2 ± 0.5 Hz, n = 14/10). F, There was no difference in mEPSC amplitudes among the three groups (SCR-KO: 7.8 ± 0.5 pA, n = 18/14; SCR-Hetero: 7.7 ± 0.4 pA, n = 14/10; SCR-WT: 6.8 ± 0.1 pA, n = 14/10). *p < 0.05 compared with SCR-WT mice. NS, No statistical difference. Data are presented as mean ± SEM.
We analyzed the averaged rise time and decay time of mEPSCs in SCR-KO and SCR-WT mice (Fig. 2B). The decay time was similar across all groups (Fig. 2B). Among all groups, the rise time of mEPSCs had no statistical difference (Fig. 2B). These data suggest that SCRAPPER regulates glutamatergic transmitter release in the ACC without affecting the kinetics of mEPSCs.
Evoked synaptic transmission in SCRAPPER KO mice
We recently showed a link between enhanced excitatory transmissions in the ACC to injury-induced anxiety (Koga et al., 2015a). Considering behavioral alteration in anxiety-like behavioral tests, we wanted to examine whether SCRAPPER regulates evoked excitatory transmissions in the ACC (Fig. 3A). We first analyzed the input–output (I-O) relationships between SCR-WT and SCR-KO mice (Fig. 3B,C) to measure EPSC amplitude (output) as a function of the afferent stimulus intensity (input). We recorded the I-O in SCR-WT (n = 9/7; Fig. 3B) and in SCR-KO (n = 8/6; Fig. 3C) mice. We also analyzed the I-O in SCR-Hetero mice (n = 9/7; Fig. 3C). We found that the EPSC amplitudes of I-O did not differ across all three groups (Fig. 3B,C), indicating that basal excitatory transmission in the ACC is not affected. To determine whether SCRAPPER may affect the kinetics of AMPA receptor-mediated responses, we analyzed the rise time and decay time of evoked EPSCs in SCR-WT, SCR-KO, and SCR-Hetero mice (Fig. 3D). Interestingly, the decay time in SCR-KO mice was statistically enhanced compared with SCR-WT mice (Fig. 3D). In contrast, no differences were observed in the rise time among all groups (Fig. 3D). These results suggest that SCRAPPER selectively regulates the decay time of evoked excitatory transmission in the ACC.
Figure 3.
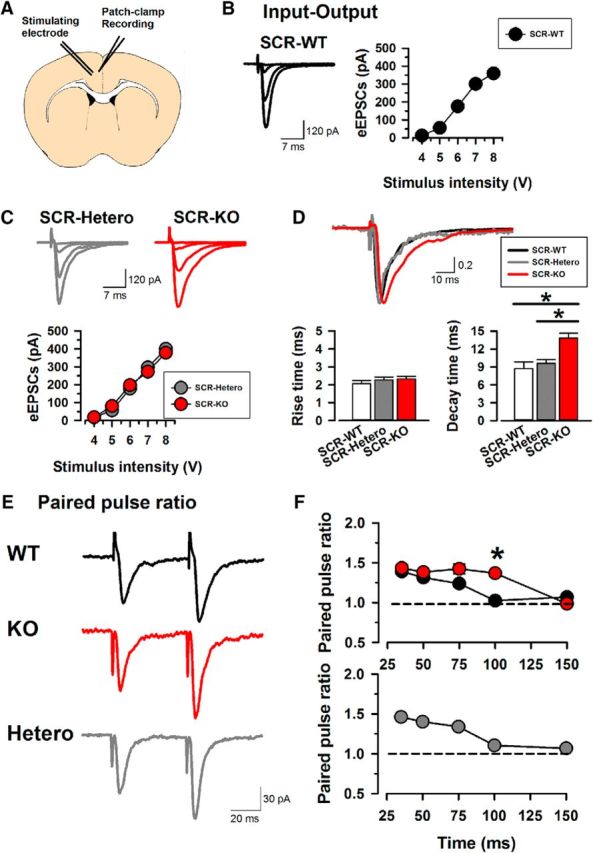
Evoked EPSCs in the ACC from SCR-KO mice. A, A scheme of the whole-cell patch-clamp recording in a layer II/III pyramidal neuron, with a stimulating electrode in layer V/VI of the adult mouse ACC. B, Left, Sample traces of input–output in SCR-WT. Right, Plots of input–output from 4–8 V. C, Sample traces of input–output in SCR-Hetero (left) and SCR-KO (right) mice. Plots of input–output (gray, SCR-Hetero; red, SCR-KO). The values in I–O did not change among the three groups. D, Normalized eEPSCs in SCR-WT, SCR-Hetero, and SCR-KO mice. SCR-KO mice show a significant increase in decay time of eEPSCs compared with SCR-WT and SCR-Hetero mice. The rise time did not differ among the three groups. E, Sample traces of the paired-pulse ratio at a 50 ms interval in SCR-WT (top), SCR-KO (middle), and SCR-Hetero (bottom) mice. F, Averaged paired-pulse ratio recorded from the three groups. Red, SCR-KO; gray, SCR-Hetero. Data are presented as mean ± SEM.
To test whether SCRAPPER may contribute to presynaptic plasticity, we measured the paired-pulse ratio (PPR) in the ACC. We gave paired pulses at the intervals 35, 50, 75, 100, and 150 ms and analyzed the PPR (second/first of eEPSCs) in SCRAPPER transgenic mice (Fig. 3E,F). SCR-KO mice showed a significantly altered PPR at 100 ms compared with SCR-WT (Fig. 3F). The PPR in SCR-Hetero mice was similar to SCR-WT mice (Fig. 3F).
Cortical pre-LTP requires SCRAPPER
Pre-LTP in the ACC has recently been reported (Koga et al., 2015a,b). We next investigated whether SCRAPPER is involved in pre-LTP (Figs. 4, 5). Using the pre-LTP protocol, we recorded eEPSCs with paired pulse (interval, 50 ms) delivered by a stimulating electrode placed in layer V/VI (adjusted amplitudes of eEPSCs to 50–100 pA; Fig. 4A) holding membrane potential at −60 mV. After a stable recording of eEPSCs was achieved for at least 10 min, we gave the low-frequency stimulation (2 Hz for 2 min, holding membrane potentials at −60 mV) and recorded at least for 60 min (Fig. 4). Similar to previous reports (Koga et al., 2015a,b), SCR-WT mice showed normal pre-LTP that lasted for at least 1 h (n = 8/6; Fig. 4A,B,D). The PPR was altered by the low-frequency stimulation (n = 8/6; Fig. 4C). In contrast, SCR-KO mice failed to produce pre-LTP in the ACC (n = 10/8; Fig. 4B) and showed no change in the PPR before or after the low-frequency stimulation (n = 10/8; Fig. 4A,C,E). SCR-Hetero mice showed pre-LTP levels comparable with SCR-WT mice (n = 8/7; Fig. 5A,C) and altered PPR (n = 8/7; Fig. 5B,D). Among the three groups, SCR-KO mice showed significantly reduced induction of cortical pre-LTP and altered PPR compared with SCR-WT mice (Fig. 4F,G). These results indicate that SCRAPPER is critical for pre-LTP in the ACC.
Figure 4.
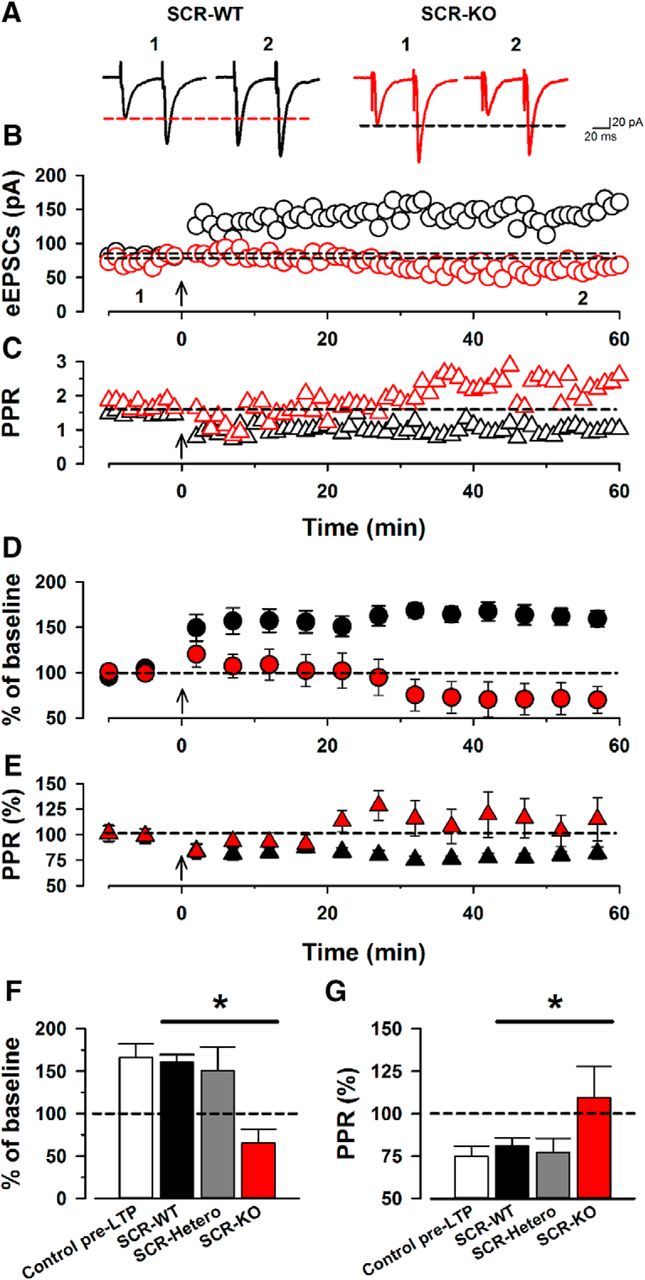
SCR-KO mice fail to produce the cortical pre-LTP. A, Single traces of eEPSCs with paired pulse before (1) and 60 min after (2) low-frequency stimulation in SCR-WT mice (left, black) and SCR-KO mice (right, red). B, Single plots of the amplitudes of eEPSCs from one neuron in a SCR-WT (black) and a SCR-KO (red) mouse. The arrow indicates the onset of low-frequency stimulation. C, Single plots of the paired-pulse ratio from the same neuron in a SCR-WT (black) and a SCR-KO (red) mouse. D, Averaged plots of eEPSCs before and after low-frequency stimulation in SCR-WT (black) and SCR-KO (red) mice. E, Averaged plots of paired-pulse ratios before and after the low-frequency stimulation in SCR-WT (black) and SCR-KO (red) mice. F, Summary of pre-LTP in control pre-LTP, SCR-WT, SCR-Hetero, and SCR-KO mice. SCR-KO mice (66 ± 16%) show a significant reduction of pre-LTP compared with SCR-WT or SCR-Hetero mice (SCR-WT, 160 ± 9%; SCR-Hetero, 150 ± 28%). G, Summary of paired-pulse ratios after low-frequency stimulation. SCR-KO mice did not alter the paired-pulse ratio (110 ± 18%) compared with SCR-WT mice (81 ± 5%). *p < 0.05 compared with SCR-WT and SCR-Hetero mice. Data are presented as mean ± SEM.
Figure 5.
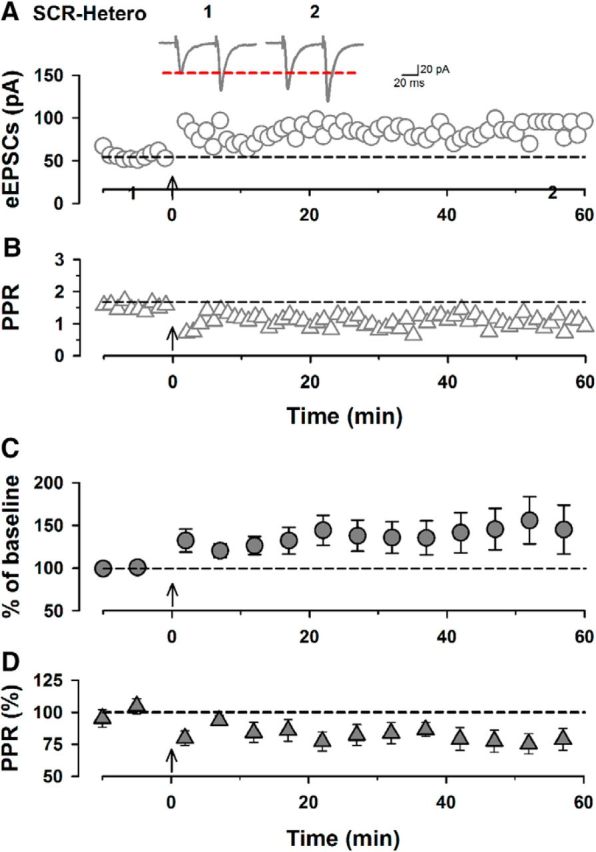
SCR-Hetero mice showed normal pre-LTP in the ACC. A, Single traces of eEPSCs in SCR-Hetero mice before (1) and 60 min after (2) low-frequency stimulation. Shown are single plots of eEPSC amplitudes from one neuron in a SCR-Hetero mouse. The arrow indicates the onset of low-frequency stimulation. B, Single plots of the paired-pulse ratio from the same neuron in the SCR-Hetero mouse. C, Averaged plots of eEPSCs before and after low-frequency stimulation in SCR-Hetero mice (150 ± 28% of baseline, n = 8/7). D, Averaged plots of paired-pulse ratios before and after low-frequency stimulation in SCR-Hetero mice (77 ± 8% of baseline, n = 8/7). Data are presented as mean ± SEM.
Cortical pre-LTP is reduced by a proteasome inhibitor
Results from SCRAPPER mutant mice suggest that proteasome activity is likely important for pre-LTP in ACC neurons. To test this possibility, we used the proteasome inhibitor MG-132 in brain slice recordings from WT mice. In slices of normal WT mice, we found that low-frequency stimulation produced LTP (n = 8/8; Fig. 6A,B,D). The PPR of eEPSCs was also altered by the stimulation (n = 8/8; Fig. 6A,C,E). We found that a proteasome inhibitor, MG-132 (10 μm), in a bath solution at least for 20 min did not affect the PPR compared with the control group (control, 1.39 ± 0.12; MG-132, 1.34 ± 0.08). Next, we tested whether proteasome activity could be involved in cortical pre-LTP. In the presence of MG-132 (10 μm) in the bath solution, we gave the low-frequency stimulation. MG-132 statistically reduced the induction of the cortical pre-LTP (n = 8/6; Fig. 6A,B,D). The PPR did not change before and after the low-frequency stimulation (Fig. 6A,C,E). These results suggest that proteasome activity is involved in ACC pre-LTP.
Figure 6.
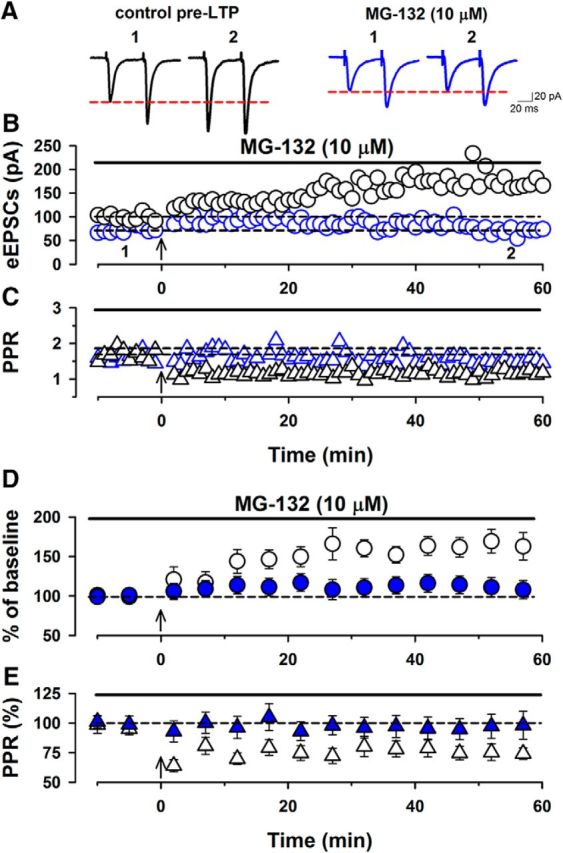
Proteasome is involved in the induction of pre-LTP in the ACC. A, Single traces of eEPSCs with paired pulse at 50 ms baseline (1) and 60 min after (2) low-frequency stimulation in control pre-LTP (left, black) and a proteasome inhibitor, MG-132 (10 μm) group (right, blue). B, Single plots from one neuron in control pre-LTP (black) and in the presence of MG-132 (blue). The arrow indicates the onset of low-frequency stimulation (2 Hz for 2 min). C, Single traces of the paired-pulse ratio from the same neuron in control pre-LTP (black) and in the presence of MG-132 (blue). D, Averaged plots of the amplitudes of eEPSCs before and after low-frequency stimulation. The low-frequency stimulation produced LTP (166 ± 16%, n = 8/8). MG-132 reduced the induction of pre-LTP (110 ± 12%, n = 8/6). E, Averaged plots of paired-pulse ratios before and after low-frequency stimulation. The low-frequency stimulation altered paired-pulse ratios (75 ± 6%, n = 8/6). MG-132 did not alter the PPR before and after the low-frequency stimulation (before the stimulation: 98 ± 11%, n = 8/6). Data are presented as mean ± SEM.
Discussion
In the present study, we provide strong evidence for critical roles of SCRAPPER in spontaneous release of glutamate in the adult mouse cortex. In contrast to spontaneous transmitter release, evoked responses triggered by focal stimulation do not require SCRAPPER. Our studies provide new genetic evidence that spontaneous releases and evoked release may use different presynaptic release mechanisms (Kavalali, 2015). Previous studies of SCRAPPER have been mainly performed in hippocampal cultured neurons or young brain slices (Yao et al., 2007; Takagi et al., 2012). Our results thus provide new evidence for an important role of SCRAPPER in adult cortical preparations. Considering critical roles of the ACC in chronic pain, anxiety, and fear, these results indicate that SCRAPPER-related signaling pathways may have important roles in the processing of sensory and emotional responses.
SCRAPPER regulates glutamatergic transmitter release and kinetics in the ACC
In the present study, we found that deletion of SCRAPPER enhanced mEPSCs in the ACC. The rise time and decay time of mEPSCs were not altered among the SCR-WT, SCR-Hetero, and SCR-KO groups. These results were similar to those in hippocampal data (Yao et al., 2007). Yao et al. (2007) reported that under the hippocampal primary culture condition, deletion of SCRAPPER facilitated the frequency of mEPSCs. The rise time and decay time of mEPSCs in the hippocampal neurons were not altered among all groups. Furthermore, we found that in the ACC, the kinetics of sEPSCs and eEPSCs were altered between the SCR-KO and SCR-WT groups. SCR-KO mice showed a significant longer decay time in sEPSCs and eEPSCs. The exact molecular mechanism remains to be determined in future studies. One possible explanation is the alteration of glutamate uptake. Previous reports show that inhibiting the glutamate transporter by l-trans-2,4-PDC produced a longer decay time of eEPSCs in cerebellar neurons (Takahashi et al., 1995; Overstreet et al., 1999). Blocking the uptake of glutamate could increase the amount of glutamatergic transmitter in synaptic cleft, resulting in affecting the decay time of eEPSCs. The longer decay time of eEPSCs in SCR-KO mice might be attributable to the increased level of glutamate in synaptic cleft by genetic deletion of SCRAPPER.
SCRAPPER plays different roles in spontaneous and evoked release of glutamate
SCR-KO mice enhanced spontaneous glutamatergic transmitter release, whereas SCR-KO mice showed a higher PPR at the 100 ms interval than that in SCR-WT mice. This finding provides new evidence that spontaneous neurotransmitter release may use different presynaptic and postsynaptic mechanisms than evoked transmitter release (see Kavalali, 2015). The mechanisms of spontaneous and evoked release of glutamate are likely to be different (Kavalali, 2015; see Kerchner et al., 2001a,b). There are at least two main reasons for such difference in the present study. First, different projection fibers may contribute to spontaneous and evoked release of glutamate. sEPSCs are caused by spontaneous release of glutamate from different sources such as thalamic projection cells, callosal projection, and subcortical projections, whereas local stimulation-induced eEPSCs are likely from the thalamic projection pathway and local projections (Lee et al., 2007). Second, different presynaptic vesicles may contribute to spontaneous versus evoked release of glutamate. Spontaneous and evoked neurotransmitter release may occur at different locations within a single synapse or into different synapses (Kavalali, 2015).
Although the probability of release was increased in SCR-KO mice, the input–output did not change among all groups in our studies. Synaptic efficacy is determined by both the probability of release and postsynaptic sensitivity to glutamate. In addition to possible difference in spontaneous versus evoked release, we cannot exclude the possible mechanisms of postsynaptic AMPA/kainate receptors. Indeed, ubiquitin ligase E3 including SCRAPPER expresses both in presynaptic and postsynaptic sites (Yao et al., 2007). AMPA receptors are subject to ubiquitination by neural precursor cells expressed developmentally down-regulated protein 4 (Nedd4) that is a ubiquitin ligase E3 (Schwarz et al., 2010; Lin et al., 2011). In addition, all AMPA receptors subunits are subject to ubiquitination, which is regulated by neuronal activity (Widagdo et al., 2015). Therefore, the postsynaptic regulation of AMPA receptors by ubiquitin ligases E3 including SCRAPPER may reflect the synaptic efficacy.
Mechanisms of the UPS including SCRAPPER for LTP
The UPS is emerging as a powerful modulator of synaptic function, acting at both presynaptic and postsynaptic sites (Hegde and DiAntonio, 2002; Tai and Schuman, 2008). Consistent with findings from the hippocampal CA1 region, where a proteasome inhibitor reduced LTP (Fonseca et al., 2006; Karpova et al., 2006; Cai et al., 2010), we found that proteasome was involved in cortical pre-LTP. Although NMDA receptors are important for hippocampal LTP induced by tetanic stimulation, pre-LTP in the ACC is NMDA receptor independent (Koga et al., 2015a). Thus, although UPS has been reported to regulate NMDA receptors (Ehlers, 2003; Yi and Ehlers, 2005), it is unlikely the case for pre-LTP in the ACC. Consistently, using genetic knock-out mice, we found that deletion of SCRAPPER failed to produce pre-LTP in the ACC. It is possible that SCRAPPER may play different roles in other types of LTP-induction protocols in brain areas. For example, tetanic stimulation produced larger magnitudes of LTP compared with SCR-WT mice in the CA1 region of the hippocampus (Takagi et al., 2012).
Our study is the first study to investigate the contribution of SCRAPPER to cortical pre-LTP. One possible explanation is that SCRAPPER deletion disrupts the precision of neural transmitter recycling mechanisms, which may be critical for pre-LTP (Südhof, 2004; Wu et al., 2014). SCRAPPER and the proteasome may maintain a fine tuning for vesicle recycling. In the hippocampus, pre-LTP enhances neurotransmitter release (Nicoll and Schmitz, 2005; Fourcaudot et al., 2008; Shin et al., 2010), and pre-LTP in the amygdala and cerebellar parallel fiber synapses requires RIM1 (Lonart et al., 2003; Fourcaudot et al., 2008). Protein kinase A (PKA) phosphorylates RIM1 and enhances neurotransmitter release (Lonart et al., 2003). Cortical pre-LTP also requires a PKA-dependent pathway via the activation of adenylyl cyclase 1 (Koga et al., 2015a). A cyclic AMP activator, forskolin increases the mRNA level of SCRAPPER directly or indirectly, suggesting that the PKA pathway regulates not only the activations of presynaptic molecules but also the UPS (Yao et al., 2007). Because SCRAPPER and RIM1 are strongly linked (Yao et al., 2007), pre-LTP may require the activation of both presynaptic molecules and the UPS including SCRAPPER protein. In support of this possibility, the blocking of protein degradation by a pharmacological inhibition of proteasome by MG-132 reduced the induction of cortical pre-LTP. Future studies need to determine how SCRAPPER contributes to vesicle recycling for pre-LTP.
Functional implications
The ACC plays important roles in both sensory and emotional processing such as chronic pain and anxiety (Zhuo, 2008; Kim et al., 2011; Zhuo, 2014). Recently, our group found that the ACC of mice exposed to an anxiety-inducing behavior, or to nerve injury, showed reduced cortical pre-LTP, suggesting that cortical pre-LTP is associated with both anxiety and chronic pain (Koga et al., 2015a; Yates, 2015). Furthermore, inhibiting hyperpolarization-activated cyclic nucleotide-gated channels, which erase cortical pre-LTP, also reduces behavioral anxiety (Koga et al., 2015a). In accordance, SCRAPPER transgenic mice show abnormal behaviors in the open field test (Yao et al., 2011). In the present study, we found that genetic deletion of SCRAPPER protein abolished the induction of pre-LTP in the ACC. These findings suggest that SCRAPPER-related signaling pathways may play important roles in chronic pain and its related emotional changes. Interestingly, a previous study of SCRAPPER transgenic mice found that footshock-induced fear memory was reduced (Yao et al., 2011). Inhibiting the SCRAPPER-related pathway may provide novel mechanisms to control emotional fear and anxiety.
Footnotes
This work was supported by grants from the EJLB-CIHR Michael Smith Chair in Neurosciences and Mental Health, the Canada Research Chair, the Canadian Institute for Health Research (operating grants CIHR66975 and 84256 and project grant PJT-148648), the Azrieli Neurodevelopmental Research Program, and Brain Canada (M.Z.). This work was supported in part by MEXT KAKENHI Grant JP24111547, JSPS KAKENHI Grant JP26460388, and CREST to I.Y. and M.S. K.K. was supported by a postdoctoral fellowship from Fragile X Research Foundation of Canada, the Nakatomi Foundation, Kato Memorial Bioscience Foundation, and GSK Japan Research Grant 2016. We thank Drs. H. Toyoda and G. Descalzi for technical assistance.
The authors declare no competing financial interests.
References
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39. 10.1038/361031a0 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (2013) Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol Brain 6:1–14. 10.1186/1756-6606-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, Kaang BK, Zhuo M (2016) Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 17:485–496. 10.1038/nrn.2016.68 [DOI] [PubMed] [Google Scholar]
- Cai F, Frey JU, Sanna PP, Behnisch T (2010) Protein degradation by the proteasome is required for synaptic tagging and the heterosynaptic stabilization of hippocampal late-phase long-term potentiation. Neuroscience 169:1520–1526. 10.1016/j.neuroscience.2010.06.032 [DOI] [PubMed] [Google Scholar]
- Damsa C, Kosel M, Moussally J (2009) Current status of brain imaging in anxiety disorders. Curr Opin Psychiatry 22:96–110. 10.1097/YCO.0b013e328319bd10 [DOI] [PubMed] [Google Scholar]
- Descalzi G, Li XY, Chen T, Mercaldo V, Koga K, Zhuo M (2012) Rapid synaptic potentiation within the anterior cingulate cortex mediates trace fear learning. Mol Brain 5:6. 10.1186/1756-6606-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobie F, Craig AM (2007) A fight for neurotransmission: SCRAPPER trashes RIM. Cell 130:775–777. 10.1016/j.cell.2007.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. (2003) Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci 6:231–242. 10.1038/nn1013 [DOI] [PubMed] [Google Scholar]
- Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nägerl UV (2006) A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron 52:239–245. 10.1016/j.neuron.2006.08.015 [DOI] [PubMed] [Google Scholar]
- Fourcaudot E, Gambino F, Humeau Y, Casassus G, Shaban H, Poulain B, Lüthi A (2008) cAMP/PKA signaling and RIM1alpha mediate presynaptic LTP in the lateral amygdala. Proc Natl Acad Sci U S A 105:15130–15135. 10.1073/pnas.0806938105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ (2004) The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304:881–883. 10.1126/science.1094804 [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R (2004) The developmental origins of anxiety. Nat Rev Neurosci 5:545–552. 10.1038/nrn1429 [DOI] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB (2013) Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 14:488–501. 10.1038/nrn3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde AN, DiAntonio A (2002) Ubiquitin and the synapse. Nat Rev Neurosci 3:854–861. [DOI] [PubMed] [Google Scholar]
- Kandel ER. (2012) The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain 5:1–12. 10.1186/1756-6606-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova A, Mikhaylova M, Thomas U, Knöpfel T, Behnisch T (2006) Involvement of protein synthesis and degradation in long-term potentiation of Schaffer collateral CA1 synapses. J Neurosci 26:4949–4955. 10.1523/JNEUROSCI.4573-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET. (2015) The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci 16:5–16. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA (2008) Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci 9:813–825. 10.1038/nrn2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Wilding TJ, Li P, Zhuo M, Huettner JE (2001a) Presynaptic kainate receptors regulate spinal sensory transmission. J Neurosci 21:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Wang GD, Qiu CS, Huettner JE, Zhuo M (2001b) Direct presynaptic regulation of GABA/glycine release by kainate receptors in the dorsal horn: an ionotropic mechanism. Neuron 32:477–488. 10.1016/S0896-6273(01)00479-2 [DOI] [PubMed] [Google Scholar]
- Kim SS, Wang H, Li XY, Chen T, Mercaldo V, Descalzi G, Wu LJ, Zhuo M (2011) Neurabin in the anterior cingulate cortex regulates anxiety-like behavior in adult mice. Mol Brain 4:6. 10.1186/1756-6606-4-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Descalzi G, Chen T, Ko HG, Lu J, Li S, Son J, Kim T, Kwak C, Huganir RL, Zhao MG, Kaang BK, Collingridge GL, Zhuo M (2015a) Co-existence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 85:377–389. 10.1016/j.neuron.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Liu MG, Qiu S, Song Q, O'Den G, Chen T, Zhuo M (2015b) Impaired presynaptic long-term potentiation in the anterior cingulate cortex of Fmr1 knockout mice. J Neurosci 35:2033–2043. 10.1523/JNEUROSCI.2644-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Chang WC, Chang KB, Shyu BC (2007) Synaptic organization and input-specific short-term plasticity in anterior cingulate cortical neurons with intact thalamic inputs. Eur J Neurosci 25:2847–2861. 10.1111/j.1460-9568.2007.05485.x [DOI] [PubMed] [Google Scholar]
- Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M (2010) Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 330:1400–1404. 10.1126/science.1191792 [DOI] [PubMed] [Google Scholar]
- Lin A, Hou Q, Jarzylo L, Amato S, Gilbert J, Shang F, Man HY (2011) Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. J Neurochem 119:27–39. 10.1111/j.1471-4159.2011.07221.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Südhof TC, Linden DJ (2003) Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell 115:49–60. 10.1016/S0092-8674(03)00727-X [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S (2002) Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297:211–218. 10.1126/science.1071795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D (2005) Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci 6:863–876. 10.1038/nrn1786 [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Kinney GA, Liu YB, Billups D, Slater NT (1999) Glutamate transporters contribute to the time course of synaptic transmission in cerebellar granule cells. J Neurosci 19:9663–9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinetti GV, Schweizer FE (2010) Ubiquitination acutely regulates presynaptic neurotransmitter release in mammalian neurons. J Neurosci 30:3157–3166. 10.1523/JNEUROSCI.3712-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Hall BJ, Patrick GN (2010) Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. J Neurosci 30:16718–16729. 10.1523/JNEUROSCI.3686-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin RM, Tully K, Li Y, Cho JH, Higuchi M, Suhara T, Bolshakov VY (2010) Hierarchical order of coexisting pre- and postsynaptic forms of long-term potentiation at synapses in amygdala. Proc Natl Acad Sci U S A 107:19073–19078. 10.1073/pnas.1009803107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland HW, Li XY, Zhuo M (2012) Predicting aversive events and terminating fear in the mouse anterior cingulate cortex during trace fear conditioning. J Neurosci 32:1082–1095. 10.1523/JNEUROSCI.5566-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. (2004) The synaptic vesicle cycle. Annu Rev Neurosci 27:509–547. 10.1146/annurev.neuro.26.041002.131412 [DOI] [PubMed] [Google Scholar]
- Tai HC, Schuman EM (2008) Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci 9:826–838. [DOI] [PubMed] [Google Scholar]
- Takagi H, Setou M, Ito S, Yao I (2012) SCRAPPER regulates the thresholds of long-term potentiation/depression, the bidirectional synaptic plasticity in hippocampal CA3-CA1 synapses. Neural Plast 2012:352829. 10.1155/2012/352829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Kovalchuk Y, Attwell D (1995) Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. J Neurosci 15:5693–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Ko S, Ding HK, Qiu CS, Calejesan AA, Zhuo M (2005) Pavlovian fear memory induced by activation in the anterior cingulated cortex. Mol Pain 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S (1996) The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87:1327–1338. 10.1016/S0092-8674(00)81827-9 [DOI] [PubMed] [Google Scholar]
- Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, Zhuo M (2001) Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci 4:164–169. 10.1038/83993 [DOI] [PubMed] [Google Scholar]
- Widagdo J, Chai YJ, Ridder MC, Chau YQ, Johnson RC, Sah P, Huganir RL, Anggono V (2015) Activity-dependent ubiquitination of GluA1 and GluA2 regulates AMPA receptor intracellular sorting and degradation. Cell Rep pii:S2211-1247(15)00028-5. 10.1016/j.celrep.2015.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Hamid E, Shin W, Chiang HC (2014) Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol 76:301–331. 10.1146/annurev-physiol-021113-170305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M (2008) Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 28:7445–7453. 10.1523/JNEUROSCI.1812-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao I, Takagi H, Ageta H, Kahyo T, Sato S, Hatanaka K, Fukuda Y, Chiba T, Morone N, Yuasa S, Inokuchi K, Ohtsuka T, Macgregor GR, Tanaka K, Setou M (2007) SCRAPPER-dependent ubiquitination of active zone protein RIM1 regulates synaptic vesicle release. Cell 130:943–957. 10.1016/j.cell.2007.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao I, Takao K, Miyakawa T, Ito S, Setou M (2011) Synaptic E3 ligase SCRAPPER in contextual fear conditioning: extensive behavioral phenotyping of Scrapper heterozygote and overexpressing mutant mice. PLoS One 24:e17317. 10.1371/journal.pone.0017317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. (2015) Pain: converging on LTP. Nat Rev Neurosci 16:63. 10.1038/nrn3913 [DOI] [PubMed] [Google Scholar]
- Yi JJ, Ehlers MD (2005) Ubiquitin and protein turnover in synapse function. Neuron 47:629–632. 10.1016/j.neuron.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M (2005) Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47:859–872. 10.1016/j.neuron.2005.08.014 [DOI] [PubMed] [Google Scholar]
- Zhao MG, Ko SW, Wu LJ, Toyoda H, Xu H, Quan J, Li J, Jia Y, Ren M, Xu ZC, Zhuo M (2006) Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J Neurosci 26:8923–8930. 10.1523/JNEUROSCI.2103-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M. (2008) Cortical excitation and chronic pain. Trends Neurosci 31:199–207. 10.1016/j.tins.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Zhuo M. (2014) Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci 369:20130146. 10.1098/rstb.2013.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]


