Introduction
The amygdala is a limbic structure that regulates emotion-driven behaviors. Acute stress can modulate several amygdala-dependent functions, including fear conditioning and the expression of anxiety-like behavior. During acute stress, the hypothalamic–pituitary–adrenal (HPA) axis produces glucocorticoids, which interact with the noradrenergic system in the basolateral amygdala (BLA) to enhance fear memory storage (Roozendaal et al., 2006). This glucocorticoid stress response requires endocannabinoid signaling within BLA (Campolongo et al., 2009), but several questions regarding this signaling have remained unresolved. First, what molecular pathways link glucocorticoid binding to endocannabinoid release? Second, how does this stress response alter information processing within amygdala circuits and modulate functions such as anxiety-like behaviors or fear memory formation and expression?
In a recent issue of The Journal of Neuroscience, Di et al. (2016) identify a crucial piece of the molecular puzzle and provide convincing evidence showing that these molecular pathways are sufficient to produce an anxiety-like phenotype. The authors recorded from BLA principal cells (PCs) in slices and showed that bath application of glucocorticoids decreased the frequency of miniature IPSCs (mIPSCs). In addition, the authors electrically stimulated the external capsule to evoke IPSCs [evoked IPSCs (eIPSCs)] and showed that these responses did not decrease in amplitude upon glucocorticoid application. This surprising result argues against the straightforward interpretation that glucocorticoids decrease release probability at inhibitory synapses, since this would predict a reduction in both mIPSC frequency and eIPSC amplitude upon glucocorticoid application. Instead, the lack of correlated changes was interpreted as evidence that glucocorticoids may differentially modulate the two vesicle pools intended for spontaneous and evoked release.
Further experiments demonstrated that glucocorticoids suppress spontaneous inhibitory transmission by activating a G-protein-coupled, membrane-bound glucocorticoid receptor. This glucocorticoid receptor is located postsynaptically and triggers retrograde release of endocannabinoids (CBs) upon activation, suppressing presynaptic GABA release. This glucocorticoid–endocannabinoid signaling pathway bears a striking similarity to one already uncovered in the hypothalamus (Di et al., 2003), indicating that this may represent a core molecular pathway through which glucocorticoids can tune synaptic inhibition.
The authors demonstrated the behavioral relevance of this molecular pathway in two ways. First, exposure to acute stress caused the emergence of an anxiety-like phenotype, which was measured as a decrease in time spent at the center of an open field. This phenotype was blocked by intra-BLA infusion of either a CB1 receptor (CB1R) antagonist or a 2-arachidonoylglycerol (2-AG) synthesis inhibitor, indicating that acute stress acts via an endocannabinoid signaling pathway to produce this anxiety-like phenotype. Second, the glucocorticoid-mediated suppression of inhibition observed in slices was occluded by prior exposure to behavioral stress, suggesting that CB1 receptors had been saturated by endogenous cannabinoids during stress. Interestingly, CB1R blockade in unstressed animals produces anxiety-like behavior, implying that tonic CB1R activation under basal conditions has an anxiolytic effect. Together, these data indicate that the glucocorticoid signaling pathway elucidated here may be sufficient to modify certain amygdala-dependent behaviors.
How does this glucocorticoid-induced suppression of inhibition fit within a broader picture of BLA circuit function? A highly simplified BLA circuit is outlined below. BLA PCs receive excitatory input from cortical and thalamic afferents passing through the amygdalar external capsule. Inhibitory drive onto these BLA PCs comes from the following two major sources: local inhibitory interneurons provide feedforward inhibition, which is enhanced by noradrenergic input (Kaneko et al., 2008); and paracapsular intercalated cells in the external and internal capsules provide inhibitory input under dopaminergic modulation (Marowsky et al., 2005). With respect to the output of the circuit, a population of BLA principal cells project to the central amygdala (CeA) to decrease anxiety-related behaviors when activated (Tye et al., 2011).
Di et al. (2016) report that acute stress decreased the frequency of spontaneous GABA release, which might shape circuit activity. Spontaneous inhibition has been shown to suppress action potential generation and reduce the efficacy of coincident excitatory input (Carter and Regehr, 2002). A decrease in spontaneous inhibition may therefore result in either an increase in basal firing rate or more reliable responses to excitatory cortical input. If glucocorticoids increase activity selectively in CeA-projecting PCs, we would expect an anxiolytic effect (Tye et al., 2011), which is the opposite of the results reported by Di et al. (2016). It is therefore possible that PCs displaying glucocorticoid-induced activity changes do not directly project to CeA and instead cause anxiety-like phenotypes through other pathways. This highlights the fact that BLA influences behavior in a highly projection-specific manner, and that more work is required to link molecular modes of action with specific populations of BLA projection neurons.
Could glucocorticoids modulate evoked inhibitory transmission and not simply spontaneous transmission? Di et al. (2016) found no effect of glucocorticoids on evoked transmission, but they evoked IPSCs by electrically stimulating within the external capsule, which likely produces strong activation of inhibitory intercalated cells. In support of this, recent work in which cortical afferents to amygdala were optogenetically stimulated in the absence or presence of the external capsule showed that the external capsule provides a prominent inhibitory input onto PCs likely arising from intercalated cells (Morozov et al., 2011). Furthermore, the stimulation of cortical afferents in the external capsule would not produce excitatory synaptic currents in BLA interneurons due to the presence of glutamate antagonists DNQX and AP5, and therefore such stimulation would not produce feedforward inhibitory currents in BLA PCs. It is therefore possible that glucocorticoids reduce the probability of release from BLA interneurons onto BLA PCs, a mechanism that would decrease both spontaneous release frequency (detected by Di et al., 2016) and evoked event amplitude (undetected due to stimulating electrode placement) onto PCs.
Cholecystokinin (CCK) interneurons in BLA are a plausible target for presynaptic release probability modulations, because they express CB1 receptors and provide a distinct perisomatic inhibitory input onto PCs (Vereczki et al., 2016). During an acute stress response, the glucocorticoid cascade described by Di et al. (2016) could act on these CCK interneurons, increasing BLA PC activity by relieving cortically evoked feedforward inhibition and enhancing the PC drive onto downstream targets to cause anxiogenic effects. However, evidence indicates that a prominent class of PC projector neurons (CeA-projecting PC cells) in fact has an anxiolytic effect (Tye et al., 2011), implying again that greater circuit elucidation is required to fully link molecular pathways with behavior. Similar to the circuit hypothesis discussed here, recent work (Geddes et al., 2016) has shown that cortical input to dorsal raphe nucleus can be modulated in a switch-like manner due to endocannabinoid suppression of feedforward inhibition.
Vesicle pools for spontaneous and evoked synaptic transmission are thought to represent distinct entities (Fredj and Burrone, 2009), but it is unclear whether the release probability can be modulated independently for each pool. Di et al. (2016) reported that spontaneous, but not evoked, transmission was glucocorticoid sensitive, which was interpreted as a vesicle pool-specific modulation of release probability. This is an exciting and fundamentally novel concept and has not been observed for kainate, another neuromodulator. Mathew et al. (2008) used a styryl dye staining/destaining approach to show that kainate concomitantly enhances the release probability of both spontaneous and evoked vesicle pools in GABA terminals in layers II/III of cortex. Further work is needed to clarify whether spontaneous and evoked vesicle pools have related release probabilities or whether they can be modulated independently.
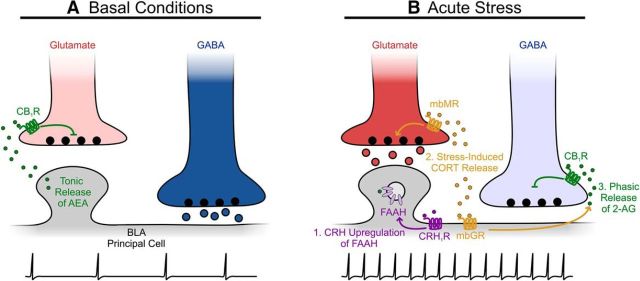
Together, these observations add to an emerging model of how acute stress can recruit endocannabinoid machinery to dynamically alter the excitation/inhibition balance within BLA (Hill et al., 2010; Fig. 1). Basal conditions are characterized by GABAergic inhibition of PCs, since glutamate release onto PCs is suppressed by tonic anandamide (AEA) release. Exposure to an acute stressor shifts the balance of the circuit toward excitation. First, the suppression of the excitatory drive onto PCs is relieved, as follows: corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus (PVN) binds to PCs to increase fatty acid amide hydrolase (FAAH) activity and decrease tonic AEA release (Gray et al., 2015). Second, glucocorticoids synthesized by the HPA axis enhance glutamate release onto PCs by binding to presynaptic membrane-bound mineralocorticoid receptors (mbMRs; Karst et al., 2010). Third, these glucocorticoids also suppress the inhibitory input to PCs by triggering the endocannabinoid signaling cascade uncovered by Di et al. (2016). Glucocorticoids may additionally disinhibit norepinephrine release through endocannabinoid actions (Campolongo et al., 2009), although more work is required to elucidate the behavioral and circuit-level implications of this disinhibition. Therefore, stress could lead to a constellation of synaptic alterations within BLA microcircuitry, shifting the excitation/inhibition balance of the network in a switch-like manner to influence behavior (Fig. 1).
Figure 1.
Glucocorticoids interact with the endocannabinoid system following acute stress exposure to shift excitation/inhibition balance in the BLA. A, Under basal conditions, tonic release of AEA from BLA principal neurons dampens glutamate release by binding to presynaptic CB1Rs, while GABA release is unrestrained. Thus, inhibition dominates the network and presumably suppresses principal neuron firing. B, Following exposure to acute stress, (1) CRH released from cells in PVN activates its receptor (CRH1R) on BLA principal neurons, thus upregulating FAAH activity and halting the tonic release of AEA; (2) glucocorticoids (CORT) produced by the HPA axis bind presynaptic mbMRs to enhance glutamate release, and, simultaneously, CORT binds glucocorticoid receptors (mbGR) on principal cells (3) to trigger the retrograde release of 2-AG, which activates presynaptic CB1Rs to selectively suppress GABA release. Under these conditions, excitation dominates the network and presumably increases the principal neuron firing rate.
Di et al. (2016) have elucidated a core molecular mechanism underlying the effects of the stress response on emotionally salient behavior. Clearly, a complex circuit in BLA shapes behavior, and stress-induced glucocorticoid release alters circuit function at several levels through mechanisms that are still not fully understood. Further work is now required to link expected activity changes in behaviorally defined pathways (e.g., anxiolytic CeA-projecting BLA PCs) with emerging molecular and synaptic details about circuit function. A deeper understanding of how networks in the brain are shaped by stress will help to provide new insights into conditions such as anxiety and post-traumatic stress disorder.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/preparing-manuscript#journalclub.
The laboratory of Dr. Jean-Claude Béïque is funded by the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health Research, the Heart and Stroke Foundation Canadian Partnership for Stroke Recovery, and the University of Ottawa Brain and Mind Research Institute. We thank Dr. Jean-Claude Béïque for providing a training environment that is simultaneously nurturing, exciting, and educational. We also thank Sean Geddes and Dr. Cary Soares for useful insights and comments.
The authors declare no competing financial interests.
References
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci U S A. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Regehr WG. Quantal events shape cerebellar interneuron firing. Nat Neurosci. 2002;5:1309–1318. doi: 10.1038/nn970. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Itoga CA, Fisher MO, Solomonow J, Roltsch EA, Gilpin NW, Tasker JG. Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J Neurosci. 2016;36:8461–8470. doi: 10.1523/JNEUROSCI.2279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12:751–758. doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes SD, Assadzada S, Lemelin D, Sokolovski A, Bergeron R, Haj-Dahmane S, Béïque JC. Target-specific modulation of the descending prefrontal cortex inputs to the dorsal raphe nucleus by cannabinoids. Proc Natl Acad Sci U S A. 2016;113:5429–5434. doi: 10.1073/pnas.1522754113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, McLaughlin RJ, Hassan KI, Kühne C, Wotjak CT, Deussing JM, Patel S, Hill MN. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 2015;35:3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J Neurosci. 2010;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Tamamaki N, Owada H, Kakizaki T, Kume N, Totsuka M, Yamamoto T, Yawo H, Yagi T, Obata K, Yanagawa Y. Noradrenergic excitation of a subpopulation of GABAergic cells in the basolateral amygdala via both activation of nonselective cationic conductance and suppression of resting K+ conductance: a study using glutamate decarboxylase 67-green fluorescent protein knock-in mice. Neuroscience. 2008;157:781–797. doi: 10.1016/j.neuroscience.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Erdmann G, Schütz G, Joëls M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci U S A. 2010;107:14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Mathew SS, Pozzo-Miller L, Hablitz JJ. Kainate modulates presynaptic GABA release from two vesicle pools. J Neurosci. 2008;28:725–731. doi: 10.1523/JNEUROSCI.3625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov A, Sukato D, Ito W. Selective suppression of plasticity in amygdala inputs from temporal association cortex by the external capsule. J Neurosci. 2011;31:339–345. doi: 10.1523/JNEUROSCI.5537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereczki VK, Veres JM, Müller K, Nagy GA, Rácz B, Barsy B, Hájos N. Synaptic organization of perisomatic GABAergic inputs onto the principal cells of the mouse basolateral amygdala. Front Neuroanat. 2016;10:20. doi: 10.3389/fnana.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]