Abstract
Fast cell-to-cell communication in the brain is achieved by action potential-dependent synaptic release of neurotransmitters. The fast kinetics of transmitter release are determined by transient Ca2+ elevations in presynaptic nerve terminals. Neuromodulators have previously been shown to regulate transmitter release by inhibiting presynaptic Ca2+ influx. Few studies to date have demonstrated the opposite, that is, neuromodulators directly driving presynaptic Ca2+ rises and increases in nerve terminal excitability. Here we use GCaMP Ca2+ imaging in brain slices from mice to address how nerve terminal Ca2+ is controlled in gonadotropin-releasing hormone (GnRH) neurons via action potentials and neuromodulators. Single spikes and bursts of action potentials evoked fast, voltage-gated Ca2+ channel-dependent Ca2+ elevations. In contrast, brief exposure to the neuropeptide kisspeptin-evoked long-lasting Ca2+ plateaus that persisted for tens of minutes. Neuropeptide-mediated Ca2+ elevations were independent of action potentials, requiring Ca2+ entry via voltage-gated Ca2+ channels and transient receptor potential channels in addition to release from intracellular store mechanisms. Together, these data reveal that neuromodulators can exert powerful and long-lasting regulation of nerve terminal Ca2+ independently from actions at the soma. Thus, GnRH nerve terminal function is controlled over disparate timescales via both classical spike-dependent and nonclassical neuropeptide-dependent mechanisms.
SIGNIFICANCE STATEMENT Nerve terminals are highly specialized regions of a neuron where neurotransmitters and neurohormones are released. Many neuroendocrine neurons release neurohormones in long-duration bursts of secretion. To understand how this is achieved, we have performed live Ca2+ imaging in the nerve terminals of gonadotropin-releasing hormone neurons. We find that bursts of action potentials and local neuropeptide signals are both capable of evoking large increases in nerve terminal Ca2+. Increases in Ca2+ driven by spike bursts last seconds; however, the increases in nerve terminal Ca2+ driven by neuropeptides can persist for tens of minutes. These findings reveal new mechanisms by which neuroendocrine nerve terminal Ca2+ can be controlled in the brain.
Keywords: calcium, GnRH, kisspeptin, median eminence, nerve terminal
Introduction
Nerve terminal boutons are the final point of regulation before the exocytosis of neurotransmitters and neuropeptides. Exocytosis of both small synaptic vesicles and large dense core vesicles (LDCVs) are highly dependent on Ca2+ concentration. Regulation of nerve terminal Ca2+ levels therefore represents a critical node for the control of neurotransmission. Previous studies have demonstrated that nerve terminal Ca2+ levels are highly dependent on the frequency and duration of invading action potentials (Jackson et al., 1991; Regehr et al., 1994). In addition, various neuromodulators can inhibit neurotransmitter release at the nerve terminal by inhibiting presynaptic Ca2+ influx (Kreitzer and Regehr, 2001; Kupferschmidt and Lovinger, 2015). Few studies to date have demonstrated the opposite, that is, neuromodulators directly driving presynaptic Ca2+ rises and increases in nerve terminal excitability that are independent of action potentials (Shakiryanova et al., 2011; Cheng and Yakel, 2014).
The nerve terminals of neuroendocrine neurons in the median eminence secrete hormones, which control anterior pituitary function and hence the endocrine axis. Remarkably little is known about how the excitability of these nerve terminals is controlled despite the fact that hormones secreted here control a wide variety of physiological functions. Gonadotropin-releasing hormone (GnRH) neurons release GnRH from their nerve terminals in the median eminence to control fertility (Herbison, 2015, 2016). The cell bodies of GnRH neurons reside in the basal forebrain and extend long projections to the median eminence. The release of GnRH from median eminence nerve terminals occurs in a pulsatile manner (Clarke and Cummins, 1982; Levine et al., 1982; Terasawa et al., 1988; Moenter et al., 1992), and this pattern of release is essential for normal reproductive function (Wildt et al., 1981). Evidence suggests that GnRH neuron projections to the median eminence are capable of supporting episodic secretion even when surgically disconnected from their soma (Blake and Sawyer, 1974; Krey et al., 1975; Soper and Weick, 1980; Meyer, 1987; Purnelle et al., 1997). This phenomenon may be in part explained by the fact that GnRH neuron projections to the median eminence function simultaneously as both a dendrite and axon, termed a dendron (Herde et al., 2013). This unique dendron projection allows the neuron to conduct action potentials and simultaneously integrate afferent information (Herde et al., 2013). This has led to the possibility that GnRH secretion from nerve terminals may be controlled via two semi-independent mechanisms. The first mechanism involves propagation of action potentials from the soma to the nerve terminals to drive GnRH secretion. The second involves direct neurotransmitter regulation of Ca2+ levels and excitability in the distal dendron and nerve terminals in the median eminence.
To understand how these two different mechanisms regulate neurosecretion, we have used genetically encoded Ca2+ sensors to image the distal GnRH neuron processes in and around the median eminence. Using this technique, we find that bursts of action potentials are efficient drivers for Ca2+ elevations in individual nerve terminals. However, these Ca2+ elevations quickly return to baseline levels within seconds of termination of the spike burst. To explore how neurotransmitters regulate nerve terminal function, we locally applied kisspeptin, a neuropeptide that potently stimulates GnRH secretion (Gottsch et al., 2004; Han et al., 2005). In contrast to bursts of action potentials, activation of kisspeptin receptors is capable of inducing Ca2+ plateaus in GnRH neuron nerve terminals that persist for tens of minutes. These long-lasting Ca2+ elevations do not require action potential firing but depend upon activation of autonomous G-protein-coupled receptors, voltage-gated Ca2+ channels, transient receptor potential C (TRPC) channels, and internal Ca2+ store mechanisms. We propose that this allows the distal projections of GnRH neurons to integrate inputs independently from the soma. These findings also suggest that, in addition to “fine-tuning” nerve terminal function, neuropeptides are capable of directly driving large and prolonged enhancements in nerve terminal Ca2+ and hence neural output.
Materials and Methods
Animals and stereotaxic injection of adeno-associated virus (AAV).
Mice were maintained under 12 h light/12 h dark lighting conditions, with food and water ad libitum. Male and female mice aged between 2 and 7 months were used for experiments. All experiments were approved by the University of Otago Animal Welfare and Ethics committee. The following strains of mice were used: GnRH-cre, JAX stock #021207 (Yoon et al., 2005); GnRH-cre/Rosa26-CAG-GCaMP3, JAX stock #014538 (Zariwala et al., 2012); GnRH-cre/GPR54−/− (Kirilov et al., 2013); and GnRH-GFP (Spergel et al., 1999). For experiments with GCaMP6s, GnRH-cre mice underwent prior stereotaxic surgery. Mice were first anesthetized with isoflurane and then placed in a stereotaxic frame. Then 1 μl of AAV9.CAG.Flex.GCaMP6s.WPRE.SV40 (1.74 × 1013 GC/ml; Penn Vector Core) was injected into the preoptic area over the course of 10 min. The injection needle was left in place for 5 min before and after injecting the virus. The coordinates for stereotaxic injections were as follows: midline, anteroposterior 0.10 cm, dorsoventral −0.43 cm from surface of brain. After recovery from surgery, animals were group housed until brain slices were prepared 3–5 weeks later.
Immunohistochemistry.
GnRH-cre/Rosa26-CAG-GCaMP3 or GnRH-cre mice injected with GCaMP6s AAV were deeply anesthetized with pentobarbital and perfused through the heart with 4% PFA in PBS. Brains were removed and postfixed in the same solution for 60 min. Thirty micrometer-thick coronal sections were cut on a freezing microtome and processed for GnRH and GFP immunohistochemistry using standard protocols as previously published (Herbison et al., 2008). The following antibodies were used: rabbit anti-GFP (Invitrogen, A-6455, 1:5000); guinea pig anti-GnRH (GA-2, gift from Greg Anderson, University of Otago, Dunedin, New Zealand, 1:10,000); donkey anti-rabbit fluorescein (Jackson ImmunoResearch Laboratories, 706-096-148, 1:200); and donkey anti-guinea pig TRITC (Jackson ImmunoResearch Laboratories, 706-025-148, 1:200).
Brain slice preparation for live imaging and electrophysiology.
Animals were killed by cervical dislocation and then decapitated. The brain was quickly removed and the optic tract peeled off. The dorsal surface of the brain was then glued to a vibratome cutting stage (VT1000s, Leica) and submerged in ice-cold cutting solution containing the following (in mm): 87 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 6 MgCl2, 25 d-glucose, 75 sucrose, bubbled with 95% O2/5% CO2. The vibratome blade was positioned to just touch the caudal extent of the hypothalamus and a single 500-μm-thick horizontal slice prepared (Constantin et al., 2012). The brain slice was then incubated for at least 1 h at 30°C in ACSF, which contained the following (in mm): 118 NaCl, 3 KCl, 11 d-glucose, 10 HEPES, 25 NaHCO3, 2.5 CaCl2, 1.2 MgCl2, bubbled with 95% O2/5% CO2.
Electrophysiology and confocal imaging.
Slices were placed on top of a mesh insert inside of a recording chamber. This allowed the brain slice to be continuously perfused with oxygenated ACSF on both surfaces. All experiments were performed at 25°C with a perfusion flow rate of 1–2 ml/min. GFP or GCaMP fluorescent neurons were visualized with an Olympus BX61WI confocal microscope. Loose seal on-cell recordings were performed with borosilicate glass pipettes filled with ACSF (resistance 3–6 mΩ). Recordings were performed in voltage clamp with the holding current kept at 0 pA. Extracellular electrical stimulation was delivered via tungsten bipolar electrodes (Microprobes, WE3ST30.2A10) controlled by a Grass S88X stimulus controller connected to a Grass biphasic current isolation unit. Electrodes were placed lateral to the rostral pole of the median eminence. Biphasic, constant current pulses were delivered at 0.1−0.5 mA with 200 μs pulse duration. Imaging was performed with an Olympus FV1000 confocal microscope fitted with a 40×, 0.8 NA objective lens. GCaMP was excited with a 488 nm Argon laser (Melles Griot). Emitted light was detected by a PMT after passing through a bandpass filter (505–605 nm). The confocal aperture was wide open during Ca2+ imaging experiments to collect maximum emitted fluorescence.
Drug application.
For the majority of experiments, drugs were dissolved in ACSF and locally puff-applied with a patch pipette (3–6 mΩ) at very low pressure (<1 psi). The tip of the puff pipette was positioned just above the surface of the slice and next to the nerve terminal being imaged. We chose to apply kisspeptin for 1 min periods based on recent studies (Han et al., 2015; Han and A.E.H., unpublished data) demonstrating that the optogenetic activation of arcuate nucleus kisspeptin neurons for 1 min is sufficient to trigger a pulse of luteinizing hormone secretion in vivo. To determine whether a GnRH nerve terminal was able to exhibit evoked Ca2+ elevations, KCl (20 mm) was locally puff-applied for 5–10 s. Only terminals that displayed fast Ca2+ rises in response to KCl were subsequently tested with kisspeptin puffs.
For the following experiments, drugs were not puff-applied but were instead bath-applied for a minimum of 15 min before locally puff applying kisspeptin: 2-aminoethoxydiphenyl borate (2-APB), CdCl2, and flufenamic acid (FFA). Cyclopiazonic acid (CPA, 30 μm) was bath-applied for a minimum of 60 min before locally applying kisspeptin. Previous work has shown that this duration and concentration of application are sufficient to inhibit the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase and deplete intracellular Ca2+ stores (Garaschuk et al., 1997; Meldolesi, 2001).
Data collection and analysis.
Electrophysiological recordings were collected with a Multiclamp 700B amplifier (Molecular Devices), using a low pass filter at 1 kHz and digitized with the Digidata 1440A (Molecular Devices) at 50 kHz. All electrophysiological data were analyzed with Clampex10 software (Molecular Devices).
Image acquisition was performed with Fluoview 1000 software. Frame scans (256 × 256 pixels) were performed on zoomed regions at ∼2 Hz frame rate with the lowest possible laser power. For the initial kisspeptin puff experiments with GCaMP6s, imaging was performed for 1000 frames (∼428 s). Kisspeptin was puff-applied starting at frame 200 (86 s since start of imaging) for 1 min. For long-duration imaging experiments (see Fig. 4B), 12 256 × 256 frames were imaged at ∼2 Hz, once every 2 min for up to 3 h. For long-duration imaging experiments shown in Figure 4C (left), 12 256 × 256 frames were imaged at ∼2 Hz, once every 15 min.
Figure 4.
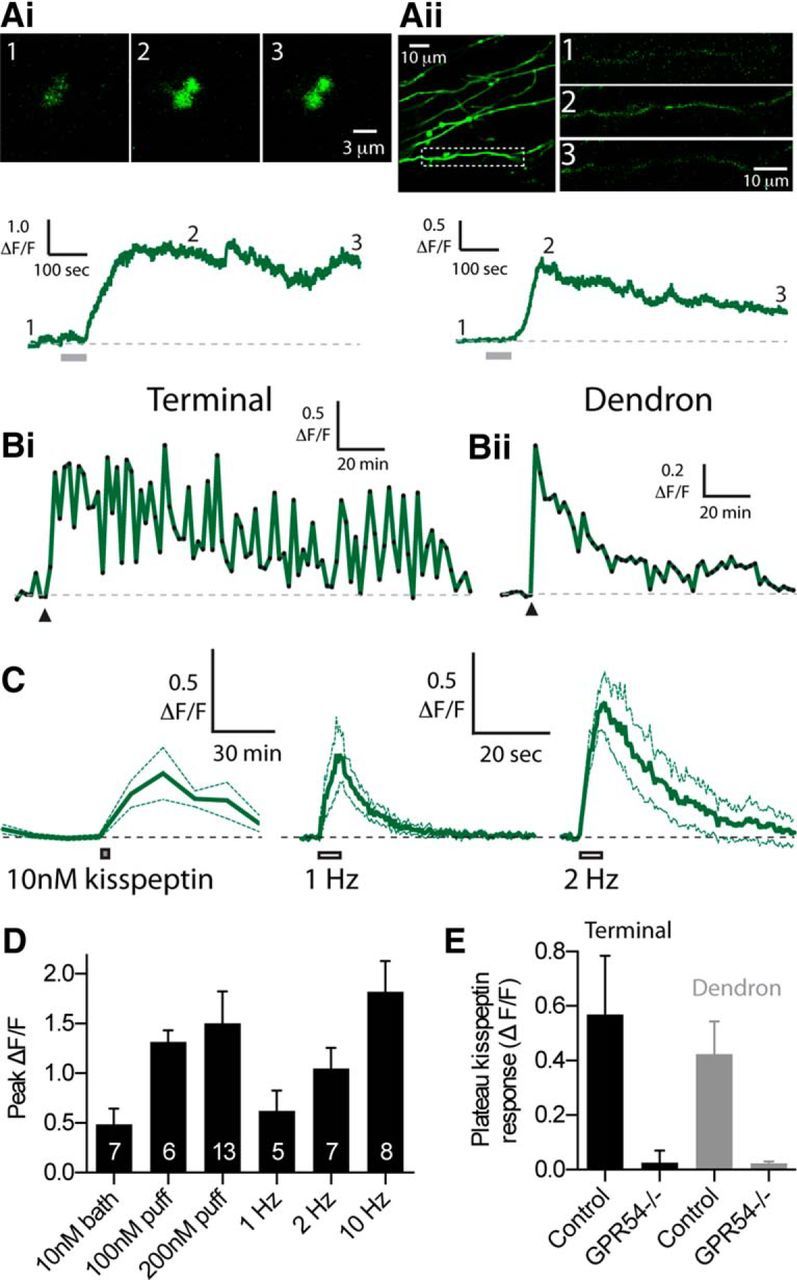
Kisspeptin induces long-duration Ca2+ plateaus in GnRH neuron dendrons and nerve terminals. Ai, Single frames showing the increase in GCaMP6s fluorescence in GnRH nerve terminals in response to a 1 min puff of 200 nm kisspeptin. Bottom, ΔF/F response. Gray bar under the trace represents duration of kisspeptin puff. Aii, Image of GCaMP6s-labeled GnRH neuron dendrons in the lateral zone of the median eminence. Region within the dashed box is expanded on the right (single frames). Bottom, ΔF/F response to a 1 min puff of 200 nm kisspeptin. Bi, Bii, Long-lasting ΔF/F response to a 1 min puff of 100 nm kisspeptin (at arrowhead) to either a GnRH neuron terminal or dendron. C, ΔF/F response to 5 min bath application of 10 nm kisspeptin (left, n = 7) compared with 5 s of electrical stimulation at 1 or 2 Hz (n = 5 and n = 7, respectively). Note the different time scales. D, Summary data showing the peak ΔF/F response evoked by different concentrations of kisspeptin or different frequencies of electrical stimulation. N values are noted inside the bars. E, Summary data comparing the magnitude of the 200 nm kisspeptin-evoked plateau response between nerve terminals and dendrons imaged in control and GnRH neuron-specific GPR54 knock-out mice (terminal control, n = 13; terminal knock-out, n = 9; dendron control, n = 10; dendron knock-out, n = 6).
Image analysis was performed with Fluoview 1000 software and ImageJ. Regions of interest were drawn around GnRH neuron elements before calculating ΔF/F. ΔF/F = (F − F0)/F0, where F is the fluorescence and F0 is the baseline fluorescence. In some imaging experiments that consisted of several minutes of continuous imaging, tissue drift was evident in the x-y axis. This drift was corrected with TurboReg (ImageJ) (Thévenaz et al., 1998) before calculating ΔF/F. Plateau ΔF/F responses after kisspeptin puffs were calculated as the average ΔF/F response during the last 60 s of the imaging experiment (total imaging time = 428 s, kisspeptin applied at 86 s into experiment).
All data are presented as mean ± SEM. N values refer to the number of individual nerve terminals imaged. For all experiments, a minimum animal number was 3. Statistical analyses were performed with nonparametric paired tests (Mann–Whitney) or repeated-measures tests (Kruskal–Wallis or Friedman test with a post hoc Dunn's Test). p < 0.05 was accepted as statistically significant.
Results
GCaMP3 labels GnRH neurons and reports spiking activity
Because of their small size, no studies to date have reported direct recordings from GnRH nerve terminals in the median eminence. This has hampered understanding of how secretion of GnRH from the median eminence is controlled. Because neurosecretion is driven by Ca2+ elevations, we set out to perform nerve terminal Ca2+ imaging in brain slices from adult male and female mice to understand how nerve terminal Ca2+ is controlled. To this end, we expressed the Ca2+ indicator GCaMP3 in GnRH neurons using a Cre-LoxP genetic approach (see Materials and Methods). This resulted in the specific expression of GCaMP3 in 94 ± 2% of GnRH neuron cell bodies (n = 4 mice; Fig. 1A). A similar, very high level of coexpression was also observed in GnRH dendron processes and nerve terminal boutons within the external zone of the median eminence (Fig. 1B).
Figure 1.
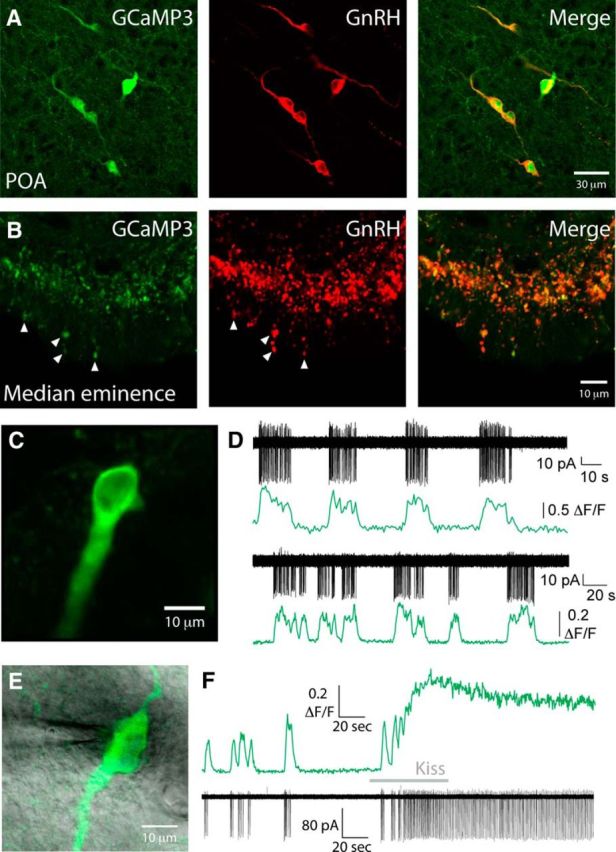
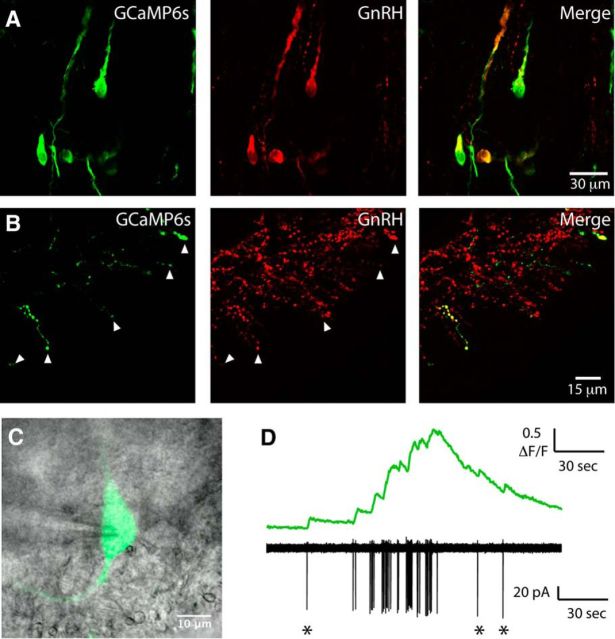
GCaMP3 expression is localized in GnRH neurons and reports spontaneous and evoked somatic spiking. Double-labeling immunohistochemistry was performed for GCaMP (green) and GnRH (red) in GnRH-GCaMP3 mice. Colocalization of GCaMP and GnRH was observed both at the level of cell bodies in the preoptic area (A) and in nerve terminals in the external zone of the median eminence (B). B, Arrows indicate several nerve terminals in the external layer of the median eminence that coexpress GCaMP and GnRH. C, Image of a GCaMP3 GnRH neuron from the anterior hypothalamic area of a live brain slice. D, Simultaneous electrical (black) and GCaMP3 fluorescence (green) traces from the soma of two representative GnRH neurons. Bottom traces, Cell shown in C. Traces illustrate that spontaneous bursts of spikes coincide with increases in GCaMP3 fluorescence. E, Image of a GCaMP3 GnRH neuron from the anterior hypothalamic area of a live brain slice with an on-cell recording electrode. F, Simultaneous GCaMP3 fluorescence (top, green) and on-cell electrical recording (bottom, black) from the soma of the GnRH neuron shown in E. A 1 min puff of kisspeptin (Kiss, 200 nm) onto the soma induces an increase in GCaMP3 fluorescence and spiking that persist for the duration of the recording.
To confirm that GCaMP3 could reliably report electrical activity in GnRH neurons, we performed simultaneous Ca2+ imaging and cell-attached electrical recordings from the soma of GCaMP3-GnRH neurons (n = 12; Fig. 1C–F). As reported previously, some GnRH neurons exhibit spontaneous burst firing both in vitro (Suter et al., 2000; Lee et al., 2010) and in vivo (Constantin et al., 2013). Spontaneous bursts of spiking corresponded with fast Ca2+ elevations that persisted for the duration of the burst and then returned to baseline upon burst termination. We next assessed the ability of GCaMP3 to report the activity of GnRH neurons in response to a strong activating stimulus. Kisspeptin is one of the most potent activators of GnRH neuron excitability (Han et al., 2005; Pielecka-Fortuna et al., 2008; Zhang et al., 2008; Constantin et al., 2013). Puff application of 200 nm kisspeptin to the soma of GnRH neurons induced consistent and robust elevations in GnRH neuron somatic Ca2+ and spiking that persisted for the duration of the recording (n = 8 of 8 cells tested from 4 mice; Fig. 1F). Together, these data show that GCaMP3 can report both spontaneous and evoked activity in the soma of GnRH neurons.
Characterization of spike-evoked Ca2+ transients in GnRH nerve terminals
Many studies have investigated the changes in GnRH neuron somatic Ca2+ in basal conditions and in response to various patterns of activity or neurotransmitters/neuromodulators (Terasawa et al., 1999; Jasoni et al., 2007; Constantin et al., 2009; Lee et al., 2010). However, no studies to date have investigated the changes in GnRH nerve terminal Ca2+ in response to electrical activity or neuromodulators.
To start to address how nerve terminal Ca2+ is controlled, we moved to imaging GnRH nerve terminal boutons in the external zone of the median eminence using parahorizontal brain slices (Fig. 2A) obtained from adult male and female mice. GnRH terminals often exhibited spontaneous Ca2+ transients that persisted even when imaging was performed in TTX (1 μm, data not shown). To characterize Ca2+ responses in GnRH nerve terminals, we electrically stimulated GnRH fibers lateral to the median eminence with a bipolar electrode (Fig. 2A). A single stimulus evoked a detectable GCaMP3 fluorescent transient in single GnRH neuron terminals (Fig. 2B–D), with increasing numbers of stimuli producing proportionally larger transients (peak ΔF/F for 1 spike = 0.15 ± 0.02; peak ΔF/F for 5 spikes = 0.42 ± 0.07, n = 12, p < 0.05; Friedman/Dunn's Test; Fig. 2D). As GnRH neurons can fire in bursts (Suter et al., 2000; Lee et al., 2010; Constantin et al., 2013), we also delivered short bursts of electrical stimulation. Ten Hz, 3 s bursts of stimulation evoked reliable, fast Ca2+ transients in GnRH boutons (Fig. 2E) that were abolished with local puff application of 1 μm TTX (inhibited to −3.0 ± 3.7% of control response, n = 9, p < 0.05, Friedman/Dunn's Test) confirming that these Ca2+ rises were completely action potential dependent (Fig. 2E,F). Stimulation-evoked Ca2+ transients were also significantly reduced with local puff application of the nonspecific voltage-gated Ca2+ channel (VGCC) antagonist CdCl2 (200 μm; inhibited to 17.9 ± 4.4% of control response, n = 14, p < 0.05, Friedman/Dunn's Test). To determine the VGCCs mediating nerve terminal Ca2+ transients, we locally puff-applied the L-, P/Q-, or N-type VGCC antagonists nifedipine (50 μm, n = 11), ω-agatoxin IVA (500 nm, n = 11), or ω-conotoxin GVIA (1 μm, n = 20), respectively. Only ω-conotoxin GVIA significantly reduced stimulation evoked Ca2+ transients (inhibited to 62.5 ± 8.4% of control response, n = 20, p < 0.05, Friedman/Dunn's Test; Fig. 2F). This is consistent with the involvement of N-type Ca2+ channels for GnRH secretion (Sahu et al., 1993). Puffs of ACSF alone were without effect (ΔF/F response = 104.1 ± 20.8% of control, n = 7, p > 0.05).
Figure 2.
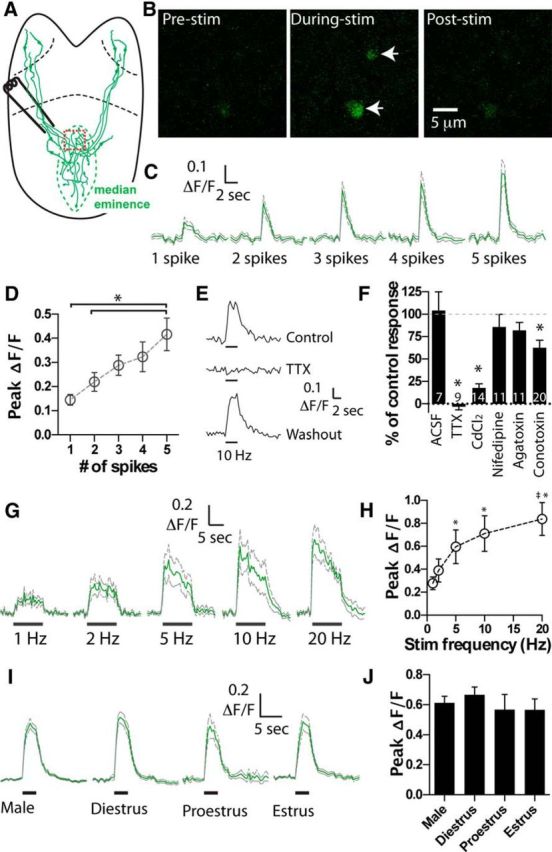
Spike-evoked Ca2+ elevations in GnRH nerve terminals. A, Brain slice preparation with location of stimulation electrode. Red box represents typical location for imaging of GnRH nerve terminals within median eminence. B, Single frames showing the increase in GCaMP3 fluorescence in two nerve terminals in response to 5 spikes at 10 Hz. C, Mean ± SEM ΔF/F nerve terminal responses to 1–5 action potentials delivered at 10 Hz (n = 12 terminals). Green represents mean. Gray represents ±SEM. D, The peak ΔF/F transient in GnRH nerve terminals shows a linear relationship to the number of spikes (n = 12). *The 5 spike response is significantly different from the 1 and 2 spike response. E, GCaMP3 fluorescence responses to bursts of spiking (10 Hz, 3 s) are reversibly blocked by puff application of TTX (n = 9). F, Summary data illustrating the degree of inhibition of nerve terminal GCaMP3 responses to various antagonists. *Significantly different from predrug application control. G, Mean ± SEM ΔF/F recorded from GCaMP3 terminals in response to 10 s trains of stimulation (1–20 Hz, n = 8). Peak ΔF/F response for the different stimulation frequencies is plotted in H. *Significantly different from the 1 Hz. ‡Significantly different from the 2 Hz. I, Mean ± SEM ΔF/F recorded from GCaMP3 terminals in response to 3 s trains of 10 Hz stimulation in males (n = 66) and females at different stages of the estrous cycle (diestrous, n = 33; proestrous, n = 12; estrous, n = 23). J, Summary data showing the peak ΔF/F response across the different groups.
Next, we investigated the Ca2+ dynamics of GnRH nerve terminals in response to different frequency bursts of electrical stimulation. Stimulation for 10 s at 1 Hz induced a small sustained increase in bouton GCaMP3 fluorescence (peak ΔF/F = 0.28 ± 0.06, n = 8; Fig. 2G,H). Ten second stimulation at 2, 5, 10, and 20 Hz produced larger increases in mean ΔF/F (Fig. 2G,H). Peak responses at 5, 10, and 20 Hz were significantly larger than the 1 Hz response (p < 0.05, n = 8; Fig. 2H; Friedman/Dunn's Test). While the ΔF/F response to 20 Hz stimulation was significantly greater than the 2 Hz response (p < 0.05, n = 8), it was not significantly greater than the 5 Hz or 10 Hz response (p > 0.05, n = 8). The same statistical relationship was found if mean ΔF/F during stimulation or the total integrated ΔF/F was analyzed (data not shown).
We then analyzed whether there were any sex- or estrus cycle-dependent differences in evoked Ca2+ responses. Calcium responses evoked by a 10 Hz, 3 s burst of spikes were not significantly different between nerve terminals from males (n = 66) and diestrous (n = 33), proestrous (n = 12), or estrous (n = 23) stage females. This lack of difference was true for both peak and mean ΔF/F measured during the 10 Hz stimulation burst (p > 0.05, Kruskal–Wallis/Dunn's Test; Fig. 2I,J).
Overall, these data show that GnRH nerve terminals exhibit fast, VGCC-dependent, transient increases in Ca2+ in response to trains of action potentials, similar to that seen in other CNS nerve terminals (Regehr et al., 1994).
Kisspeptin regulation of GnRH neuron Ca2+ levels
Kisspeptin is extremely potent at evoking spiking in GnRH neurons and driving GnRH secretion. As well as innervating the soma, kisspeptin neurons are known to send projections that form close appositions with GnRH fibers and nerve terminals in and around the median eminence (Uenoyama et al., 2011; Borsay et al., 2014; Yip et al., 2015). This suggests that kisspeptin neurons can directly communicate with GnRH neurons at their distal processes to control GnRH secretion from the median eminence. To investigate this possibility, we bath-applied kisspeptin (100 nm) and imaged GnRH nerve terminal GCaMP3 fluorescence. Surprisingly, only 1 nerve terminal of 11 responded with an increase in Ca2+ (from a total of 4 male and 4 female mice; data not shown).
We speculated that the lack of kisspeptin effect on GnRH-GCaMP3 nerve terminals may be due to the low affinity, low signal-to-noise, and/or low expression levels of GCaMP3 (Chen et al., 2013). To overcome this issue, we developed a new mouse model in which injections of a Cre-dependent GCaMP6s AAV were made into the preoptic area of GnRH-Cre mice resulting in the selective expression of the high-affinity, high-sensitivity Ca2+ sensor GCaMP6s in GnRH neurons (see Materials and Methods). As AAV injections only infected GnRH neuron cell bodies in the vicinity of the injection site, GCaMP6s was detected in ∼30% of all GnRH neuron cell bodies and their corresponding projections and nerve terminals in the median eminence (Fig. 3). Simultaneous on-cell recording and imaging confirmed that GCaMP6s could faithfully report single spike-evoked Ca2+ transients in GnRH neuron soma (Fig. 3D).
Figure 3.
GCaMP6s expression is localized in both GnRH neuron cell bodies and nerve terminals. Double-labeling immunohistochemistry was performed in sections taken from GnRH-cre mice injected with GCaMP6s AAV into the preoptic area. Green represents GCaMP6s. Red represents GnRH. Colocalization of GCaMP6s and GnRH was observed both at the level of cell bodies in the preoptic area (A) and in the median eminence (B). Arrowheads indicate nerve terminals in the external layer of the median eminence that coexpress GCaMP6s and GnRH. C, Image of a GnRH neuron from the medial preoptic area expressing GCaMP6s in an acute brain slice. The on-cell recording pipette is located on the left-hand side of the image. Fluorescence and contrast images are overlaid. D, Simultaneous recording of spontaneous GCaMP6s fluorescence (top) and electrical activity (bottom) from the neuron shown in C. *Single spikes.
Next, we moved to imaging GCaMP6s labeled GnRH nerve terminals in the external zone of the median eminence. Local 1 min puffs of kisspeptin (200 nm) were found to consistently evoke plateau-like elevations in GCaMP6s fluorescence in all GnRH nerve terminals tested (Fig. 4Ai). The plateau ΔF/F response to kisspeptin puffs was 0.57 ± 0.22 (n = 13). Puffs of kisspeptin onto dendrons lateral to the median eminence or in the anterior hypothalamic area also evoked similar Ca2+ responses (plateau ΔF/F = 0.42 ± 0.12, n = 10; Fig. 4Aii). In the majority of initial experiments, we noted that kisspeptin-evoked GCaMP6s fluorescence elevations did not return to baseline even after 6–12 min of imaging. This prompted us to perform imaging over a longer time window to determine the total duration of the kisspeptin-evoked response. In these experiments, local puffs of a lower concentration of kisspeptin (100 nm) onto GnRH nerve terminals evoked robust Ca2+ transients, which were variable in duration ranging between 16 and 178 min (mean = 62 ± 24 min, median = 43 min, n = 6; Fig. 4Bi). Similar long-duration responses were observed when 100 nm kisspeptin was puffed onto dendrons (mean duration = 65 ± 20 min, median = 68 min, n = 5; Fig. 4Bii).
The EC50 of kisspeptin is ∼4 nm when tested at the cell body (Pielecka-Fortuna et al., 2008; Zhang et al., 2008); therefore, we tested whether a lower concentration of kisspeptin would also be effective at inducing Ca2+ elevations in GnRH nerve terminals. Because the concentration of puff-applied drugs decreases greatly as it mixes with circulating ACSF, we bath-applied 10 nm kisspeptin for 5 min to enable a defined concentration of kisspeptin to be assessed; 10 nm kisspeptin-evoked a sustained Ca2+ elevation in all GnRH nerve terminal boutons tested that peaked at 0.48 ± 0.16 ΔF/F (n = 7; Fig. 4C). The duration of Ca2+ responses with bath-applied kisspeptin was variable, similar to that seen with the kisspeptin puff experiments. Specifically, kisspeptin-evoked Ca2+ responses did not fully return to baseline during the imaging period in 4 terminals (response >75 min) with the remaining 3 terminals showing responses that returned to baseline by 60 min after kisspeptin. In the same group of terminals, before the application of kisspeptin, we measured the response to 5 s of electrical stimulation at 1, 2, and 10 Hz. Peak ΔF/F Ca2+ responses evoked by different concentrations of kisspeptin (10 nm bath, 100 nm puff, and 200 nm puff) were similar to the Ca2+ responses evoked by 1–2 Hz bursts of action potentials. Specifically, 10 nm kisspeptin induced a peak Ca2+ elevation that was not statistically different from 1 or 2 Hz stimulation (peak 1 Hz ΔF/F = 0.62 ± 0.21, p > 0.05, n = 5; peak 2 Hz ΔF/F = 1.05 ± 0.21, p > 0.05, n = 7) but was significantly less than 10 Hz stimulation (peak 10 Hz ΔF/F = 1.82 ± 0.30, n = 8, p < 0.05; Fig. 4C,D). Indeed, across all groups shown in Figure 4D, the only statistically significant difference was found between 10 nm bath kisspeptin and 10 Hz electrical stimulation (Kruskal–Wallis/Dunn's Test). When all kisspeptin application experiments were compared between males and females, no differences were found in the kisspeptin responses (data not shown).
To establish whether the Ca2+ plateaus in response to kisspeptin required the kisspeptin receptor (GPR54, also referred to as KISS1R), we used genetically manipulated mice to selectively delete GPR54 from GnRH neurons (Kirilov et al., 2013) (see Materials and Methods). In these mice, Ca2+ responses to 200 nm kisspeptin puffs were absent from both GnRH terminals (GPR54−/− plateau ΔF/F = 0.03 ± 0.04, n = 9, p < 0.05 compared with control kisspeptin response) and dendrons (GPR54−/− plateau ΔF/F = 0.02 ± 0.01, n = 6, p < 0.05 compared with control kisspeptin response; Fig. 4E). GnRH nerve terminals in GPR54−/− mice were still capable of demonstrating Ca2+ elevations as electrical stimulation and puffs of KCl (20 mm) were both effective at evoking large Ca2+ transients in all terminals tested (data not shown).
Ca2+ entry pathways required for kisspeptin-evoked Ca2+ elevations in nerve terminals
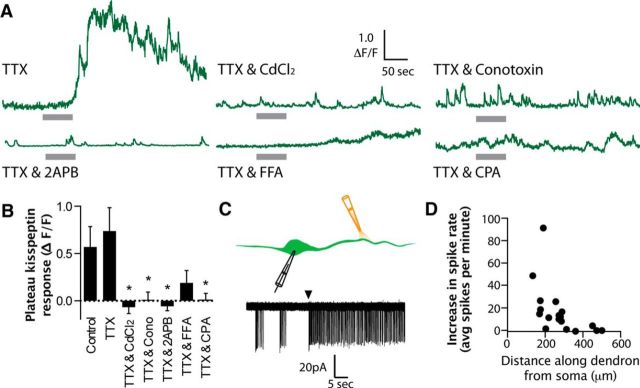
We next set out to determine the characteristics of kisspeptin-evoked Ca2+ elevations. Interestingly, 200 nm puffs of kisspeptin onto GnRH nerve terminals were equally effective at evoking Ca2+ rises in the presence and absence of TTX (plateau ΔF/F in control = 0.57 ± 0.22, n = 13 and TTX = 0.74 ± 0.25, n = 11, p > 0.05; Fig. 5A,B). We investigated this phenomenon further by performing on-cell electrical recordings from the soma of GnRH-GFP neurons and puffing 200 nm kisspeptin at different distances along the dendron projection toward the median eminence (Fig. 5C). Whereas puffs of kisspeptin close to the soma were very effective at increasing spiking, puffs at greater distances down the dendron were less effective or completely ineffective at increasing spike frequency recorded at the soma (Fig. 5D). These data further support the idea that, although kisspeptin may act at the soma and proximal dendron to increase spike discharge, the distal effects of kisspeptin are spike-independent.
Figure 5.
Kisspeptin-evoked Ca2+ plateaus require multiple Ca2+ pathways. A, Representative ΔF/F GCaMP6s traces showing the response to 1 min, 200 nm kisspeptin puff in GnRH neuron nerve terminals in the presence of various antagonists. Gray bar represents kisspeptin puff duration. B, Summary data showing the mean ± SEM plateau response across different drug conditions (plateau response taken as the average ΔF/F during the last 60 s of the trace). Control, n = 13; TTX, n = 11; CdCl2, n = 8; ω-conotoxin GVIA (Cono), n = 14; 2-APB, n = 9; FFA, n = 11; CPA, n = 11. *p < 0.05. C, Diagram and representative on-cell recording for experiments where kisspeptin was puffed at different distances along the GnRH neuron dendron as it projects toward the median eminence. Arrowhead indicates onset of kisspeptin puff. D, Graph plotting the average increase in spiking activity (measured in a 5 min bin before and after kisspeptin) versus the distance along the dendron where the puff was applied (n = 18 different cells).
Next, we interrogated the Ca2+ pathways involved for the nerve terminal kisspeptin response. Previous work has suggested that kisspeptin can activate VGCCs, Ca2+-permeable TRPC channels, and induce Ca2+ release from internal stores (Liu et al., 2008; Zhang et al., 2008; Glanowska and Moenter, 2015). As we demonstrated above, kisspeptin-evoked Ca2+ responses were unchanged in the presence of TTX. Therefore, for the remainder of experiments, TTX was included in the ACSF.
We first tested the involvement of VGCCs with the nonspecific antagonist CdCl2 (200 μm). In the presence of CdCl2, kisspeptin puffs were no longer able to evoke Ca2+ elevations in GnRH nerve terminal boutons (plateau ΔF/F = −0.07 ± 0.07, n = 8, p < 0.05 compared with the kisspeptin response in TTX alone; Fig. 5). Both our own (Fig. 2F) and other (Sahu et al., 1993) data suggest that N-type Ca2+ channels are present in GnRH nerve terminals. Therefore, we tested whether specific inhibition of N-type VGCCs with ω-conotoxin GVIA (1 μm) would modify the kisspeptin-evoked nerve terminal Ca2+ response. This revealed that the Ca2+ response to kisspeptin puffs was blocked in the presence of ω-conotoxin GVIA (plateau ΔF/F = 0.01 ± 0.08, n = 14, p < 0.05 compared with the kisspeptin response in TTX alone; Fig. 5). These data suggest that N-type VGCCs are essential for the kisspeptin-evoked Ca2+ responses.
We next determined the importance of TRPC channels as previous studies have shown that TRPC channels are activated in response to kisspeptin application at the cell body (Zhang et al., 2008, 2013). Two different TRPC antagonists were used as TRPC antagonists (Clapham et al., 2005) can also inhibit IP3 receptors (Maruyama et al., 1997) and voltage-gated ion channels (Guinamard et al., 2013). In the presence of 2-APB (100 μm), kisspeptin-evoked Ca2+ responses were blocked (plateau ΔF/F = −0.06 ± 0.05, n = 9, p < 0.05 compared with kisspeptin response in TTX alone). However, the second TRPC channel antagonist FFA (100 μm) exerted variable effects with 3 terminals demonstrating kisspepin-evoked Ca2+ plateaus, whereas the remaining 8 had no response to the kisspeptin puff. Because of this variability, overall the effect of kisspeptin in FFA was not significantly different from the control responses in TTX (plateau ΔF/F = 0.19 ± 0.13, n = 11, p > 0.05 compared with the kisspeptin response in TTX alone; Fig. 5).
Finally, we tested for the role of intracellular Ca2+ stores, as previous work has suggested that they are important for mediating kisspeptin-evoked GnRH secretion from nerve terminals (Glanowska and Moenter, 2015). To deplete intracellular Ca2+ stores, we preincubated slices for a minimum of 60 min with the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase pump inhibitor CPA (30 μm). We found that depletion of Ca2+ stores also blocked the kisspeptin-evoked nerve terminal Ca2+ elevation (plateau ΔF/F = 0.01 ± 0.07, n = 11, p < 0.05 compared with the kisspeptin response in TTX alone; Fig. 5).
Together, these data demonstrate that kisspeptin acts directly on GnRH nerve terminals to induce long-lasting elevations of intraterminal Ca2+. These long-lasting Ca2+ elevations require multiple Ca2+ entry and release mechanisms.
Discussion
We report here the first imaging of Ca2+ in individual GnRH nerve terminals. Using this technique, we find that bursts of action potentials are efficient drivers for Ca2+ elevations in GnRH nerve terminals, consistent with nerve terminals of other central neurons (Regehr et al., 1994). Remarkably, however, we demonstrate that local neuropeptide signals can induce Ca2+ elevations that persist for >1 h via direct activation of G-protein-coupled receptors. Importantly, these neuropeptide-driven Ca2+ elevations do not require action potential firing, thus allowing the distal projections of GnRH neurons to integrate inputs semiautonomously from the soma. An implication of these findings is that spiking recorded at the soma may not necessarily be an accurate indicator of secretion by GnRH neurons.
Synaptic nerve terminals use rapid changes in Ca2+ to release discrete quanta of neurotransmitter over microsecond timescales. We find that GnRH nerve terminals in the median eminence exhibit the same approximate behavior with action potentials resulting in fast Ca2+ elevations mediated by voltage-gated Ca2+ channels. GnRH neurons typically fire at 1–2 Hz in vivo (Constantin et al., 2013), and we show here that even this low firing rate induces a measurable elevation in nerve terminal Ca2+. However, recent research has demonstrated that GnRH neuron cell bodies or distal projections need to be activated at a minimum of 10 Hz for 2 min to evoke a pulse-like release of luteinizing hormone in vivo (Campos and Herbison, 2014). This suggests that large, prolonged increases in nerve terminal Ca2+ are required to evoke pulsatile GnRH secretion. While we show here that high-frequency burst discharges (5–20 Hz) evoke larger Ca2+ elevations in GnRH nerve terminals, both in vitro and in vivo experiments indicate that GnRH neuron soma rarely fire at these frequencies (Liu et al., 2008; Constantin et al., 2013). Instead, we speculate that the prolonged, high levels of nerve terminal Ca2+ likely required for pulsatile GnRH secretion may be achieved via nonclassical, spike-independent mechanisms at the terminal itself.
Previous studies have demonstrated that neuromodulators can directly elevate Ca2+ levels in the nerve terminals of other neurons in a transient and brief manner (Shakiryanova et al., 2011; Cheng and Yakel, 2014; Kakizawa et al., 2016). However, in stark contrast, we find here that a 1 min application of kisspeptin results in elevated GnRH nerve terminal Ca2+ levels that persist for tens of minutes and, in many cases, over an hour. The duration of these responses is remarkable but also quite unexpected. At their fastest, luteinizing hormone pulses can occur every 20–30 min in gonadectomized animals (Kinsey-Jones et al., 2008; Wakabayashi et al., 2010; Czieselsky et al., 2016), and it is therefore puzzling to find GnRH nerve terminal Ca2+ events that appear to last longer than the frequency of the secretion event itself. There are several possible explanations for this. One that we favor is the possibility that there may only be a transient secretion of GnRH in response to the kisspeptin-induced Ca2+ elevation due to depletion of docked and primed GnRH large dense core vesicles (LDCVs). It is possible that the initial peak Ca2+ rise in response to kisspeptin drives LDCV fusion and that the longer-lasting, lower-level plateau Ca2+ elevation is insufficient to stimulate additional release. This persistent, lower-level Ca2+ elevation could instead be important for the mobilization and priming of GnRH LDCVs in the terminal, which would be required for subsequent rounds of secretion. A similar scenario has been suggested for priming of LDCVs in adrenal chromaffin cells (Neher and Zucker, 1993; Burgoyne and Morgan, 1995; Robinson and Martin, 1998).
At present, it is technically not possible to measure GnRH secretion from a single GnRH nerve terminal. However, studies by others have clearly demonstrated that kisspeptin application to the median eminence region results in the secretion of GnRH. Although the temporal resolution has been limited, in vitro experiments using whole median eminence explants from several species have shown that 30–60 min applications of 10–1000 nm kisspeptin can increase GnRH release from these preparations (d'Anglemont de Tassigny et al., 2008; Smith et al., 2011; Uenoyama et al., 2011). A further in vitro study using fast-scan cyclic voltammetry reported that injection of 10 nm kisspeptin onto the median eminence region of brain slices increased GnRH signal from that region for ∼1 min (Glanowska and Moenter, 2015). Thus, it is highly likely that the 1 min puffs of kisspeptin administered here to the median eminence elevate GnRH secretion alongside the increase in Ca2+ observed within the GnRH neuron dendron and terminals. We note that the kisspeptin-evoked Ca2+ responses were absent in GnRH neuron-specific Kiss1 receptor-null mice. As KISS1R is present in all other neurons and cells in these mice, this demonstrates that kisspeptin acts directly on GnRH neuron projections within the median eminence to elevate Ca2+ levels.
At the GnRH neuron soma, many studies have now shown that KISS1R activation opens TRPC channels and inhibits potassium channels to generate long-lasting excitation (Piet et al., 2015). In contrast, the mechanisms through which kisspeptin activates GnRH secretion from nerve terminals are less well established (Glanowska and Moenter, 2015). Our present observations now show that the kisspeptin-induced elevation in Ca2+ concentrations within the GnRH nerve terminal is dependent upon at least two Ca2+ entry mechanisms and also involves release from internal stores. Interestingly, we find that both the N-type VGCC and TRPC channels are necessary for Ca2+ entry. The involvement of stores is somewhat reminiscent of the regulation of intracellular Ca2+ at the GnRH cell body itself where Ca2+-induced Ca2+ release from stores generates large Ca2+ transients in these cells (Jasoni et al., 2010; Lee et al., 2010). The coupling of KISS1R to possibly multiple different intracellular signaling cascades within the GnRH dendron and nerve terminal awaits further definition.
Finally, we note that the ability of neuropeptidergic inputs to directly regulate Ca2+ levels within GnRH neuron distal projections provides a scenario in which the neuron has two independent sites at which inputs can control output: one site close to the soma relying on classical action potential firing and the second site relying on action potential-independent modulation of nerve terminal Ca2+. The spike initiation site of GnRH neurons is located in the proximal dendron ∼100 μm from the cell body (Iremonger and Herbison, 2012) and allows the GnRH neuron to control secretion by regulating the number and pattern of action potentials that are conducted to the nerve terminal boutons located 2–4 mm away in the median eminence. As demonstrated here, local neuropeptide signals acting on GnRH neuronal elements in and around the median eminence are capable of inducing Ca2+ signals locally, yet these signals would not travel the extensive distance back to the spike initiation site to regulate spike firing. Therefore, at least three distinct modes of operation can be envisaged for the GnRH neuron: (1) somatic spiking-induced GnRH release; (2) GnRH release driven solely by local inputs to the distal dendron/nerve terminal; and (3) release driven by coincident distal inputs and spiking signals (Iremonger and Herbison, 2015).
Together, these data reveal a unique system of neural integration in GnRH neurons. Other neurons in the brain may use similar processing schemes to integrate information received at both proximal (soma/dendritic) and distal (axon terminal) sources. These findings also suggest that, in addition to “fine-tuning” nerve terminal function, neuropeptides are also capable of directly driving large and prolonged enhancements in nerve terminal Ca2+ and hence neural output.
Footnotes
This work was supported by Health Research Council of New Zealand Programme Grant to A.E.H. K.J.I. is currently a Sir Charles Hercus Health Research Fellow. We thank Ruth Empson and Jaideep Bains for comments on the manuscript.
The authors declare no competing financial interests.
References
- Blake CA, Sawyer CH (1974) Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology 94:730–736. 10.1210/endo-94-3-730 [DOI] [PubMed] [Google Scholar]
- Borsay BÁ, Skrapits K, Herczeg L, Ciofi P, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Hrabovszky E (2014) Hypophysiotropic gonadotropin-releasing hormone projections are exposed to dense plexuses of kisspeptin, neurokinin B and substance P immunoreactive fibers in the human: a study on tissues from postmenopausal women. Neuroendocrinology 100:141–152. 10.1159/000368362 [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A (1995) Ca2+ and secretory-vesicle dynamics. Trends Neurosci 18:191–196. 10.1016/0166-2236(95)93900-I [DOI] [PubMed] [Google Scholar]
- Campos P, Herbison AE (2014) Optogenetic activation of GnRH neurons reveals minimal requirements for pulsatile luteinizing hormone secretion. Proc Natl Acad Sci U S A 111:18387–18392. 10.1073/pnas.1415226112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499:295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yakel JL (2014) Presynaptic alpha7 nicotinic acetylcholine receptors enhance hippocampal mossy fiber glutamatergic transmission via PKA activation. J Neurosci 34:124–133. 10.1523/JNEUROSCI.2973-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G (2005) International Union of Pharmacology: XLIX. Nomenclature and structure–function relationships of transient receptor potential channels. Pharmacol Rev 57:427–450. 10.1124/pr.57.4.6 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT (1982) The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111:1737–1739. 10.1210/endo-111-5-1737 [DOI] [PubMed] [Google Scholar]
- Constantin S, Caligioni CS, Stojilkovic S, Wray S (2009) Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology 150:1400–1412. 10.1210/en.2008-0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin S, Piet R, Iremonger K, Hwa Yeo S, Clarkson J, Porteous R, Herbison AE (2012) GnRH neuron firing and response to GABA in vitro depend on acute brain slice thickness and orientation. Endocrinology 153:3758–3769. 10.1210/en.2012-1126 [DOI] [PubMed] [Google Scholar]
- Constantin S, Iremonger KJ, Herbison AE (2013) In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling. J Neurosci 33:9394–9401. 10.1523/JNEUROSCI.0533-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czieselsky K, Prescott M, Porteous R, Campos P, Clarkson J, Steyn FJ, Campbell RE, Herbison AE (2016) Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology 157:4794–4802. 10.1210/en.2016-1351 [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH (2008) Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149:3926–3932. 10.1210/en.2007-1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Yaari Y, Konnerth A (1997) Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J Physiol 502:13–30. 10.1111/j.1469-7793.1997.013bl.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanowska KM, Moenter SM (2015) Differential regulation of GnRH secretion in the preoptic area (POA) and the median eminence (ME) in male mice. Endocrinology 156:231–241. 10.1210/en.2014-1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA (2004) A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077. 10.1210/en.2004-0431 [DOI] [PubMed] [Google Scholar]
- Guinamard R, Simard C, Del Negro C (2013) Flufenamic acid as an ion channel modulator. Pharmacol Ther 138:272–284. 10.1016/j.pharmthera.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE (2005) Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356. 10.1523/JNEUROSCI.3328-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SY, McLennan T, Czieselsky K, Herbison AE (2015) Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci U S A 112:13109–13114. 10.1073/pnas.1512243112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. (2015) Physiology of the adult GnRH neuronal network. In Knobil and Neill's Physiology of Reproduction 4th Ed, Vol. 1 (Plant TM, Zelenik AJ, eds) pp. 399–467. Los Angeles: Academic Press. [Google Scholar]
- Herbison AE. (2016) Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol 12:452–466. 10.1038/nrendo.2016.70 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR (2008) Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149:597–604. 10.1210/en.2007-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herde MK, Iremonger KJ, Constantin S, Herbison AE (2013) GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci 33:12689–12697. 10.1523/JNEUROSCI.0579-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Herbison AE (2012) Initiation and propagation of action potentials in gonadotropin-releasing hormone neuron dendrites. J Neurosci 32:151–158. 10.1523/JNEUROSCI.3739-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Herbison AE (2015) Multitasking in gonadotropin-releasing hormone neuron dendrites. Neuroendocrinology 102:1–7. 10.1159/000368364 [DOI] [PubMed] [Google Scholar]
- Jackson MB, Konnerth A, Augustine GJ (1991) Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci U S A 88:380–384. 10.1073/pnas.88.2.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasoni CL, Todman MG, Strumia MM, Herbison AE (2007) Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. J Neurosci 27:860–867. 10.1523/JNEUROSCI.3579-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasoni CL, Romanò N, Constantin S, Lee K, Herbison AE (2010) Calcium dynamics in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 31:259–269. 10.1016/j.yfrne.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Kakizawa K, Watanabe M, Mutoh H, Okawa Y, Yamashita M, Yanagawa Y, Itoi K, Suda T, Oki Y, Fukuda A (2016) A novel GABA-mediated corticotropin-releasing hormone secretory mechanism in the median eminence. Sci Adv 2:e1501723. 10.1126/sciadv.1501723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey-Jones JS, Li XF, Luckman SM, O'Byrne KT (2008) Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology 149:1004–1008. 10.1210/en.2007-1505 [DOI] [PubMed] [Google Scholar]
- Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schütz G, Herbison AE (2013) Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun 4:2492. 10.1038/ncomms3492 [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG (2001) Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29:717–727. 10.1016/S0896-6273(01)00246-X [DOI] [PubMed] [Google Scholar]
- Krey LC, Butler WR, Knobil E (1975) Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey: I. Gonadotropin secretion. Endocrinology 96:1073–1087. 10.1210/endo-96-5-1073 [DOI] [PubMed] [Google Scholar]
- Kupferschmidt DA, Lovinger DM (2015) Inhibition of presynaptic calcium transients in cortical inputs to the dorsolateral striatum by metabotropic GABA(B) and mGlu2/3 receptors. J Physiol 593:2295–2310. 10.1113/JP270045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Duan W, Sneyd J, Herbison AE (2010) Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci 30:6214–6224. 10.1523/JNEUROSCI.6156-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JE, Pau KY, Ramirez VD, Jackson GL (1982) Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology 111:1449–1455. 10.1210/endo-111-5-1449 [DOI] [PubMed] [Google Scholar]
- Liu X, Lee K, Herbison AE (2008) Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149:4605–4614. 10.1210/en.2008-0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K (1997) 2-APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 122:498–505. 10.1093/oxfordjournals.jbchem.a021780 [DOI] [PubMed] [Google Scholar]
- Meldolesi J. (2001) Rapidly exchanging Ca2+ stores in neurons: molecular, structural and functional properties. Prog Neurobiol 65:309–338. 10.1016/S0301-0082(01)00004-1 [DOI] [PubMed] [Google Scholar]
- Meyer DC. (1987) In-vitro pulsatile luteinizing hormone-releasing hormone output is dependent on hypothalamic region and the stage of the estrous cycle. Biol Reprod 37:1207–1214. 10.1095/biolreprod37.5.1207 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RM, Midgley AR, Karsch FJ (1992) Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 130:503–510. 10.1210/endo.130.1.1727719 [DOI] [PubMed] [Google Scholar]
- Neher E, Zucker RS (1993) Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron 10:21–30. 10.1016/0896-6273(93)90238-M [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM (2008) Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986. 10.1210/en.2007-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piet R, de Croft S, Liu X, Herbison AE (2015) Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Front Neuroendocrinol 36:15–27. 10.1016/j.yfrne.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Purnelle G, Gérard A, Czajkowski V, Bourguignon JP (1997) Pulsatile secretion of gonadotropin-releasing hormone by rat hypothalamic explants without cell bodies of GnRH neurons. Neuroendocrinology 66:305–312. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Delaney KR, Tank DW (1994) The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci 14:523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Martin TF (1998) Docking and fusion in neurosecretion. Curr Opin Cell Biol 10:483–492. 10.1016/S0955-0674(98)80063-X [DOI] [PubMed] [Google Scholar]
- Sahu A, Crowley WR, Kalra SP, Kalra PS (1993) Role of multiple voltage-sensitive calcium channels in depolarization-induced release of neuropeptide Y and luteinizing hormone-releasing hormone from rat median eminence-arcuate nucleus. Mol Cell Neurosci 4:492–498. 10.1006/mcne.1993.1061 [DOI] [PubMed] [Google Scholar]
- Shakiryanova D, Zettel GM, Gu T, Hewes RS, Levitan ES (2011) Synaptic neuropeptide release induced by octopamine without Ca2+ entry into the nerve terminal. Proc Natl Acad Sci U S A 108:4477–4481. 10.1073/pnas.1017837108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ (2011) Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 152:1001–1012. 10.1210/en.2010-1225 [DOI] [PubMed] [Google Scholar]
- Soper BD, Weick RF (1980) Hypothalamic and extrahypothalamic mediation of pulsatile discharges of luteinizing hormone in the ovariectomized rat. Endocrinology 106:348–355. 10.1210/endo-106-1-348 [DOI] [PubMed] [Google Scholar]
- Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH (1999) GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Wuarin JP, Smith BN, Dudek FE, Moenter SM (2000) Whole-cell recordings from preoptic/hypothalamic slices reveal burst firing in gonadotropin-releasing hormone neurons identified with green fluorescent protein in transgenic mice. Endocrinology 141:3731–3736. 10.1210/endo.141.10.7690 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Krook C, Hei DL, Gearing M, Schultz NJ, Davis GA (1988) Norepinephrine is a possible neurotransmitter stimulating pulsatile release of luteinizing hormone-releasing hormone in the rhesus monkey. Endocrinology 123:1808–1816. 10.1210/endo-123-4-1808 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Schanhofer WK, Keen KL, Luchansky L (1999) Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19:5898–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenaz P, Ruttimann UE, Unser M (1998) A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7:27–41. 10.1109/83.650848 [DOI] [PubMed] [Google Scholar]
- Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, Ajiki K, Ichikawa M, Okamura H, Maeda KI, Tsukamura H (2011) Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol 23:863–870. 10.1111/j.1365-2826.2011.02199.x [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H (2010) Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132. 10.1523/JNEUROSCI.5848-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E (1981) Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385. 10.1210/endo-109-2-376 [DOI] [PubMed] [Google Scholar]
- Yip SH, Boehm U, Herbison AE, Campbell RE (2015) Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology 156:2582–2594. 10.1210/en.2015-1131 [DOI] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C (2005) Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123:669–682. 10.1016/j.cell.2005.08.039 [DOI] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen TW (2012) A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci 32:3131–3141. 10.1523/JNEUROSCI.4469-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK (2008) Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28:4423–4434. 10.1523/JNEUROSCI.5352-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ (2013) Kisspeptin activation of TRPC4 channels in female GnRH neurons requires PIP2 depletion and cSrc kinase activation. Endocrinology 154:2772–2783. 10.1210/en.2013-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]