Abstract
Objectives
We tried to clarify, by using representative national data in a real-world setting, whether single-pill combinations (SPCs) of antihypertensives actually improve medication adherence.
Design
A nationwide population-based study.
Setting
We used a 2.2% cohort (n=1 048 061) of the total population (n=46 605 433) that was randomly extracted by National Health Insurance of Korea from 2008 to 2013.
Participants
We included patients (n=116 677) who were prescribed with the same antihypertensive drugs for at least 1 year and divided them into groups of angiotensin II receptor blocker (ARB)-only, calcium channel blocker (CCB)-only, multiple-pill combinations (MPCs) and SPCs of ARB/CCB.
Primary outcome measures
Medication possession ratio (MPR), a frequently used indirect measurement method of medication adherence.
Results
Adjusted MPR was higher in combination therapy (89.7% in SPC, 87.2% in MPC) than monotherapy (81.6% in ARB, 79.7% in CCB), and MPR of SPC (89.7%, 95% CI 89.3 to 90.0) was higher than MPR of MPC (87.2%, 95% CI 86.7 to 87.7) (p<0.05). In subgroup analysis, adherence of SPC and MPC was 92.3% (95% CI 91.5 to 93.0) vs 88.1% (95% CI 87.1 to 89.0) in those aged 65–74 years and 89.3% (95% CI 88.0 to 90.7) vs 84.8% (95% CI 83.3 to 92.0) in those ≥75 years (p<0.05). According to total pill numbers, adherence of SPC and MPC was 90.9% (CI 89.8 to 92.0) vs 85.3% (95% CI 84.1 to 86.5) in seven to eight pills and 91.2% (95% CI 89.3 to 93.1) vs 82.5% (95% CI 80.6 to 84.4) in nine or more (p<0.05). The adherence difference between SPC and MPC started to increase at five to six pills and at age 50–64 years (p<0.05). When analysed according to elderly status, the adherence difference started to increase at three to four pills in the elderly (≥65 years) and at five to six in the non-elderly group (20–64 years) (p<0.05). These differences all widened further with increasing age and the total medications.
Conclusion
SPC regimens demonstrated higher adherence than MPC, and this tendency is more pronounced with increasing age and the total number of medications.
Keywords: hypertension, medication adherence, angiotensin li receptor blocker, calcium channel blocker, single pill combination
Strengths and limitations of this study.
The strength of this study is that we not only compared the adherence between combination and monotherapy of antihypertensive medications but also the adherence of single-pill combination (SPC) and multiple-pill combination regimens in a real-world setting by using National Health Insurance Service National Sample Cohort (NHIS-NSC), a representative large-scale health insurance claims data of Korea accounting for 2.2% of the total population.
Another strength of this study is that we analysed the differences in medication adherence of subjects who continued to take antihypertensive drugs for at least 1 year for the maximum of 6 observed years.
NHIS-NSC data do not provide detailed information regarding some specific factors that could affect the medication adherence, such as the patient’s education level, occupation, caregiver status, the family environment and healthcare provider factors.
We did not specify comorbidities according to severity and only adjusted with the average number of diagnoses of the subject during the observation period.
Introduction
Adherence to medication is an explanation of drug-taking behaviour and refers to taking drugs in compliance with the time, dose and frequency prescribed by the healthcare provider.1 The WHO defines medication non-adherence as a medically ill state because low medication adherence causes the illness to progress and lowers the overall health outcome.1 Non-adherence may lead to various clinical risks. In many studies, low adherence is associated with higher mortality and hospitalisation rates than higher adherence.2–4 Also, in terms of health economics, non-adherent patients use healthcare resources more than do adherent patients, and consequently, the burden of social illness increases because of the increase in additional medical expenses.5–7 Non-adherence is observed more frequently for chronic than acute diseases, especially for hypertension, for which non-adherence is reported in 50%–70% of the cases.1 7–9
Adherence to medication is determined by various aspects such as factors associated with the patient, condition, therapy, healthcare system, social/economic status and so on.1 5 7 10 Thus, to improve adherence, a strategic approach to the specific cause is needed. Regarding these factors, there were some previous studies showing a relationship between a lower number of medications taken by a patient and higher adherence in chronic diseases such as hypertension.11–15 This implies that selecting a single-pill combination (SPC) prescription could increase adherence compared with a multiple-pill combination (MPC) prescription.11–15 However, most of the previous research reported results obtained under certain centre conditions or were short-term studies of small samples, and systematic field surveys using real-world representative data were not common. Therefore, the aim of this study is to investigate the effect of SPC on adherence to antihypertensive medication in a real-world setting. In order to do this, we first checked the overall medication prescription status of patients with hypertension and investigated the relationship between multiple medication prescriptions, age and medication adherence to antihypertensive agents.
Methods
Data source
The data used in this study were obtained from the National Health Insurance Service National Sample Cohort (NHIS-NSC) of Korea. These data are a sample of 1 048 061 individuals, around 2.2% of the total population (n=46 605 433), and provide national health information according to sex, age and income. In addition, these cohort data are obtained through continuous observation every year and include qualification data (birth, death, sex, family relationship, address, property, income and insurance type), and medical service use data (billing statement, medical record, diagnosis record, prescription record and so on) (online supplementary figure 1).16
bmjopen-2019-029862supp001.pdf (256KB, pdf)
Study population
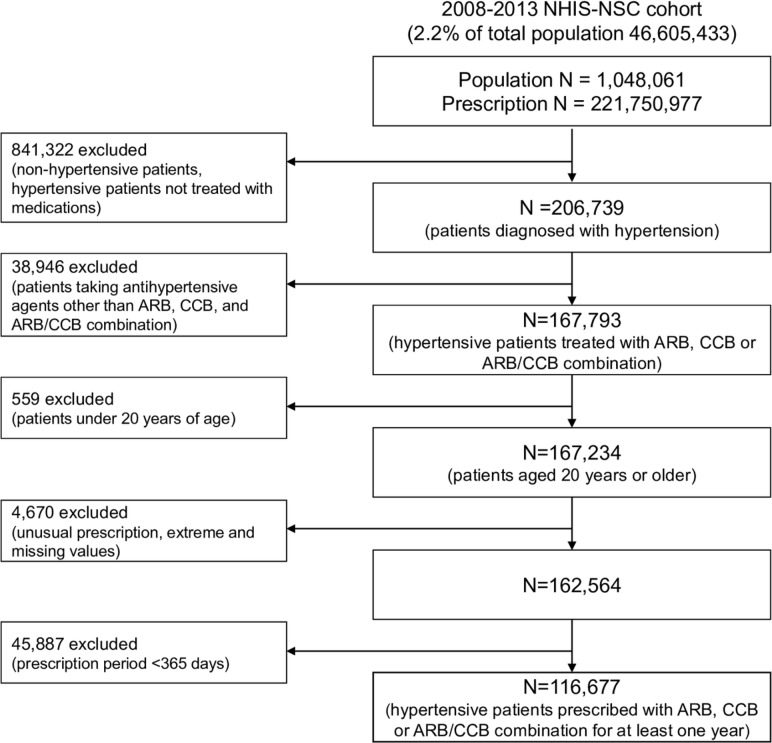
In total, 206 739 patients with hypertension taking antihypertensive medications were selected from the 2008 to 2013 NHIS-NSC (n=1 048 061, total outpatient prescriptions: 221 750 977 cases). Patients with hypertension were defined as all patients with the International Classification of Diseases Tenth Revision (ICD-10) codes that featured hypertension (I10, I11, I12, I13, I15). Our selection of antihypertensive agents was limited to dihydropyridine calcium channel blockers (CCBs) and angiotensin II receptor blockers (ARBs), the most commonly prescribed antihypertensive agents.17 18 This was to exclude the effects of adherence due to the class effect of antihypertensive medications. Therefore, all ARBs, CCBs and ARB/CCB compound drugs, as classified according to the Anatomical Therapeutic Chemical (ATC) classification system of drugs,19 that were sold domestically from 1 January 2008 to 31 December 2013 were included as antihypertensive medication. A total of 108 types of drugs were identified under the ATC system. Since the Korean release date of Exforge (amlodipine/valsartan combination), the first ARB/CCB compound drug, was 1 September 2007, the analysis period was set as starting from 2008. Of the 167 793 patients taking targeted antihypertensive agents (ARBs, CCBs and ARB/CCB compounds), only those aged ≥20 years were selected (n=167 234). To prevent statistical deviation caused by extreme values, the upper 0.01% values for a number of drugs and diagnoses, along with missing values were excluded. Most ARB, CCB and SPC of ARB/CCB are prescribed as once-a-day dosing. When a high-dose prescription is needed in Korea, most clinicians prescribe one high-dose tablet rather than two regular dose tablets, because of insurance coverage standards. Therefore, most antihypertensive agents are prescribed so that patients are directed to take a 0.5 or 1 tablet once a day. Thus, we excluded prescriptions that were not in the ‘0.5 or 1 tablet once a day’ form (n=162 564). In addition, only those who received antihypertensive medication for at least 1 year were selected to ensure a more objective and stable measurement of medication adherence. As a result, 116 677 patients were ultimately selected for the study (figure 1). Written informed consent was waived.
Figure 1.
Study population and data collection. ARB, angiotensin II receptor blockers; CCB, calcium channel blockers; MPC, multiple-pill combination; NHIS-NSC, National Health Insurance Service National Sample Cohort; SPC, single-pill combination.
Assessment of adherence
Medication adherence was calculated using the medication possession ratio (MPR), a frequently used indirect measurement method.5 7 20 MPR is calculated by dividing the total days supplied (excluding supplied days for the last clinic visit) by the number of days between the first and last refills.7
MPR=total days supplied/number of days between the first and last refills (prescription period)
The limitation of MPR is that adherence can be overestimated because the total days supplied is assumed to be the days the drug is actually used.20 21 Nevertheless, MPR was used in this study because it is considered the best method to evaluate the adherence of antihypertensive agents using retrospective data.21 Theoretically, MPR may exceed 100% if the patient visits prematurely before the drug is fully consumed. Thus, for the purposes of this study, MPR measuring over 100% was capped at 100%.
Factors related to adherence
Medication adherence is determined by the interactions of factors associated with the patient, condition, therapy, healthcare system, social/economic status and so on.1 5 7 10 In this study, factors associated with the patient (age, sex), condition (comorbidity), therapy (drug costs, number of concurrent drugs, prescription period), healthcare system (insurance coverage) and social/economic status (income, residence) were derived as confounding variables and used in the statistical analysis. Education, occupation, related symptoms, adverse effects of the treatment, family and caregiver status, and medical staff factors, which are known to affect adherence, were not included in the study, because they were not identifiable in the NHIS-NSC data (online supplementary figure 2). In this study, comorbidities were calculated as the mean number of the subjects’ diagnoses during the observation period. The number of drugs taken was calculated as the average number of medications taken by subjects during the observation period.
Statistical analysis
The study subjects were divided into four groups according to the type of antihypertensive drugs they were taking: the ARB-only group, CCB-only group, MPC group and SPC group. The average adherence of the four groups was examined. Each group was assigned according to the last drug taken by the subjects to categorise them without overlapping (online supplementary figure 3). The reason for dividing the group according to the last drug taken is that selecting the last period of hypertension treatment enables to attain relatively stabilised medication adherence than choosing an early period of hypertension treatment. Another reason is that if the group is divided according to the initial drug taken, the SPC group may not be selected at all. We compared the average adherence of the four groups before and after adjusting confounding factors using analysis of covariance (ANCOVA). A subgroup analysis, which compared the differences in adherence of each group according to age group (20–49 years, 50–64 years, 65–74 years and 75 years–) and number of medications, was conducted. We also compared the adherence difference between MPC and SPC therapies according to the combination of an old-age standard (65 years) and number of medications. Finally, a sensitivity analysis of age and the number of medications affecting differences in adherence was conducted. All analyses were conducted using STATA V.14.0 and p values <0.05 were regarded as statistically significant.
Patient and public involvement
There was no patient or public involvement in the development of this study.
Results
Baseline characteristics
Of the 116 677 subjects, 29 400 were in the ARB-only group, 58 401 in the CCB-only group, 10 458 in the MPC group and 18 418 in the SPC group. Among all subjects, 47.3% were men and 52.7% women. Most subjects were aged in their 60s, followed by those in their 50s, 70s and 40s. Subjects had an average of three to four diagnoses and were taking an average number of four medications (three to four drugs were the most common, followed by four to five) (table 1).
Table 1.
Baseline characteristics (n=116 677)
| ARB-only group N(%) or mean±SD |
CCB-only group N(%) or mean±SD |
MPC group N(%) or mean±SD |
SPC group N(%) or mean±SD |
P value | |
| Total | 29 400 (25.2%) | 58 401 (50.0%) | 10 458 (9.0%) | 18 418 (15.8%) | |
| Male (47.3%, n=55 210) | 13 834 | 25 499 | 5507 | 10 370 | <0.01 |
| Female (52.7%, n=61 467) | 15 566 | 32 902 | 4951 | 8048 | |
| Age (years) | 59.3±12.5 | 62.4±12.2 | 61.1±12.4 | 56.9±12.3 | <0.01 |
| 20–29 (0.6%) | 263 | 204 | 48 | 148 | |
| 30–39 (4.2%) | 1426 | 1695 | 417 | 1362 | |
| 40–49 (16.6%) | 5455 | 8003 | 1653 | 4283 | |
| 50–59 (26.4%) | 8259 | 14 621 | 2681 | 5212 | |
| 60–69 (27.7%) | 7761 | 17 177 | 2944 | 4475 | |
| 70–79 (19.0%) | 4997 | 12 604 | 2138 | 2412 | |
| ≥80 (5.5%) | 1239 | 4097 | 577 | 526 | |
| Income | <0.01 | ||||
| Low (33.8%) | 9396 | 20 277 | 3646 | 6063 | |
| Middle (25.6%) | 7304 | 15 081 | 2647 | 4868 | |
| High (40.6%) | 12 700 | 23 043 | 4165 | 7487 | |
| Residence | <0.01 | ||||
| Metropolitan (46.1%) | 13 711 | 26 482 | 4771 | 8874 | |
| City (44.1%) | 12 878 | 25 946 | 4670 | 7913 | |
| Rural (9.8%) | 2811 | 5973 | 1017 | 1631 | |
| Health insurance | <0.01 | ||||
| National health insurance (94.2%) | 27 679 | 55 113 | 9662 | 17 406 | |
| Medical aid (5.8%) | 1721 | 3288 | 796 | 1012 | |
| Average no. of diagnoses | 3.6±1.9 | 3.1±1.8 | 3.6±1.9 | 3.1±1.7 | <0.01 |
| Average no. of medications | 4.1±2.2 | 3.9±2.0 | 4.9±2.1 | 3.7±2.0 | <0.01 |
| Average cost of antihypertension drug (₩) | 651±185 | 413±141 | 982±316 | 824±196 | <0.01 |
| Prescription period (days) | 1,174±575 | 1,477±603 | 1,164±560 | 972±412 | <0.01 |
| Total days supplied (days) | 954±562 | 1,218±629 | 1,000±545 | 855±407 | <0.01 |
| Medication possession ratio (MPR) | 81.0±23.9 | 80.9±23.2 | 85.3±19.6 | 87.7±17.7 | <0.01 |
ARB, angiotensin II receptor blockers; CCB, calcium channel blockers; MPC, multiple-pill combination; SPC, single-pill combination.
Adherence comparison
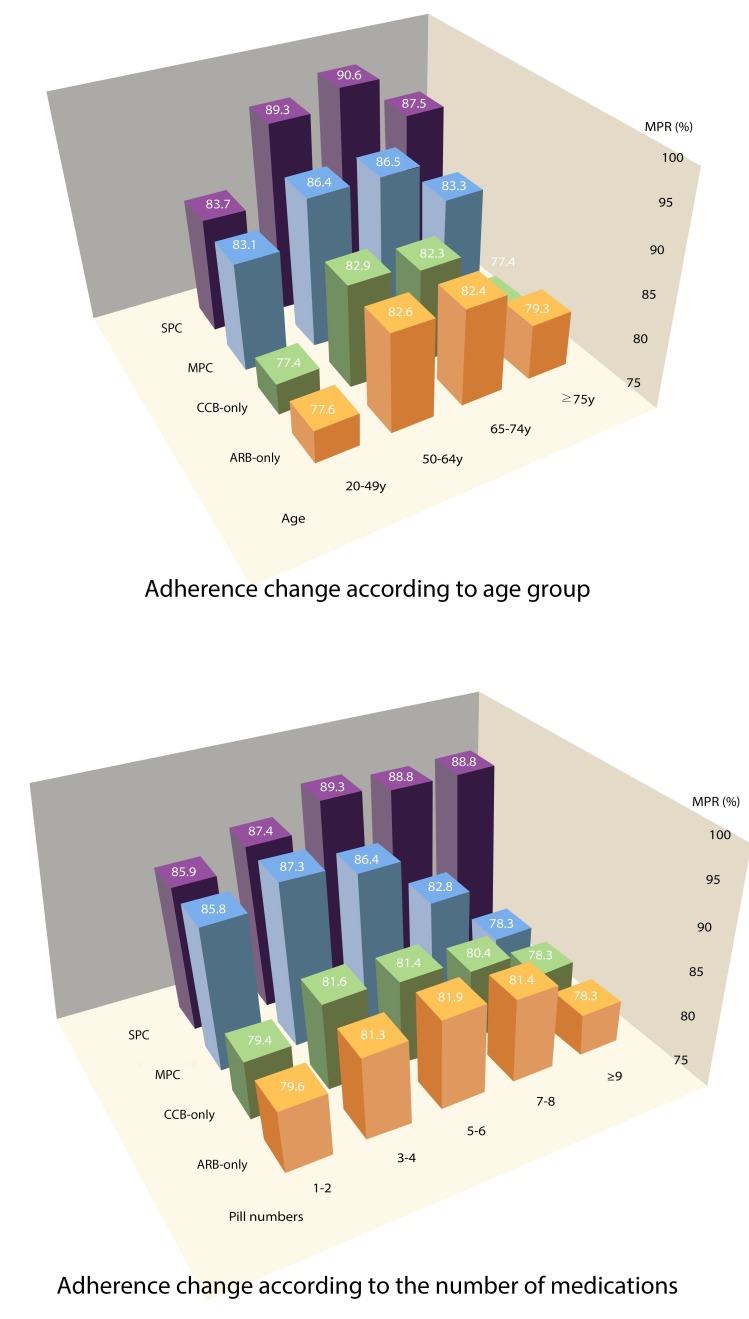
The crude mean (mean ±SD deviation, (SD)) of MPR for each group was 81.0%±23.9% in the ARB-only group, 80.9%±23.2% in the CCB-only group, 85.3%±19.6% in the MPC group and 87.7%±17.7% in the SPC group. The adjusted MPR was 81.6% (95% CI 81.3 to 81.9) in the ARB-only group, 79.7% (95% CI 79.5 to 79.9) in the CCB-only group, 87.2% (95% CI 86.7 to 87.7) in the MPC group and 89.7% (95% CI 89.3% to 90.0%) in the SPC group. Regardless of the adjustment, medication adherence was higher in the combination therapy than monotherapy groups, and adherence of the SPC group was higher than that of the MPC group when comparing combination therapies (p<0.05) (table 2). The adherence difference between the SPC and MPC groups was more significant as age and the number of drugs taken increased. The adherence difference between the two groups started to increase when the number of medications was at five to six and further widened when the number of drugs increased (p<0.05) (table 2). The adherence difference between the MPC and monotherapy groups began to decrease when the number of medications was at seven to eight and there was simply no difference between them when the number of total drugs taken was nine or more. However, the difference between the SPC and monotherapy groups remained high (table 2, figure 2).
Table 2.
Medication adherences according to age and numbers of medications
| ARB-only group (n=29 400) |
CCB-only group (n=58 401) |
MPC group (n=10 458) |
SPC group (n=18 418) |
p value* | MPR differences† | P value‡ | |||||
| Crude MPR mean |
Adjusted MPR mean§(95% CI) |
Crude MPR mean |
Adjusted MPR mean§(95% CI) |
Crude MPR mean |
Adjusted MPR mean§ (95% CI) |
Crude MPR mean |
Adjusted MPR mean§ (95% CI) |
||||
| 81.0 | 81.6 (81.3 to 81.9) | 80.9 | 79.7 (79.5 to 79.9) | 85.3 | 87.2 (86.7 to 87.7) | 87.7 | 89.7 (89.3 to 90.0) | <0.01 | 2.5 | <0.01 | |
| Age group, years (n=116 677) | |||||||||||
| 20–49 (n=24 957) | 77.6 | 77.9 (77.3 to 78.4) | 77.4 | 76.1 (75.5 to 76.7) | 83.1 | 84.9 (83.7 to 86.0) | 83.7 | 85.1 (84.4 to 85.8) | <0.01 | 0.2 | 0.20 |
| 50–64 (n=46 085) | 82.6 | 83.0 (82.6 to 83.4) | 82.9 | 81.9 (81.5 to 82.2) | 86.4 | 88.0 (87.2 to 88.8) | 89.3 | 90.8 (90.3 to 91.4) | <0.01 | 2.8 | <0.01 |
| 65–74 (n=30 652) | 82.4 | 83.0 (82.5 to 83.5) | 82.3 | 81.4 (81.0 to 81.8) | 86.5 | 88.1 (87.1 to 89.0) | 90.6 | 92.3 (91.5 to 93.0) | <0.01 | 4.2 | <0.01 |
| ≥75 (n=14 983) | 79.3 | 80.1 (79.3 to 81.0) | 77.4 | 76.6 (76.0 to 77.1) | 83.3 | 84.8 (83.3 to 86.3) | 87.5 | 89.3 (88.0 to 90.7) | <0.01 | 4.5 | <0.01 |
| Average no. of medications | |||||||||||
| 1–2 (n=19 523) | 79.6 | 80.3 (79.6 to 80.9) | 79.4 | 78.1 (77.4 to 78.7) | 85.8 | 87.6 (85.2 to 90.0) | 85.9 | 87.9 (87.0 to 88.9) | <0.01 | 0.3 | 0.68 |
| 3–4 (n=48 388) | 81.3 | 82.0 (81.6 to 82.5) | 81.6 | 80.6 (80.2 to 80.9) | 87.3 | 88.7 (87.9 to 89.4) | 87.4 | 89.2 (88.7 to 89.8) | <0.01 | 0.6 | 0.99 |
| 5–6 (n=30 105) | 81.9 | 82.3 (81.9 to 82.8) | 81.4 | 80.5 (80.1 to 80.9) | 86.4 | 87.5 (86.7 to 88.4) | 89.3 | 90.6 (89.9 to 91.3) | <0.01 | 3.1 | <0.01 |
| 7–9 (n=13 071) | 81.4 | 81.6 (80.9 to 82.3) | 80.4 | 78.9 (78.2 to 79.6) | 82.8 | 85.3 (84.1 to 86.5) | 88.8 | 90.9 (89.8 to 92.0) | <0.01 | 5.6 | <0.01 |
| ≥9 (n=5590) | 78.3 | 77.9 (76.8 to 79.0) | 78.3 | 76.3 (75.2 to 77.4) | 78.3 | 82.5 (80.6 to 84.4) | 88.8 | 91.2 (89.3 to 93.1) | <0.01 | 8.7 | <0.01 |
Analyses were performed using ANCOVA.
*P value of crude MPR mean.
†MPR differences=adjusted MPR of SPC group – adjusted MPR of MPC group.
‡P value of MPR differences.
§Adjusted for factors associated with the patient (age, sex), condition (comorbidity), therapy (drug costs, number of concurrent drugs, prescription period), the healthcare system (insurance coverage) and the social/economic status (income, residence).
ANCOVA, analysis of covariance; ARB, angiotensin II receptor blockers; CCB, calcium channel blockers; MPC, multiple-pill combination; MPR, medication possession ratio; SPC, single-pill combination
Figure 2.
Trends of medication adherences according to age group and the number of medications. ARB, angiotensin II receptor blockers; CCB, calcium channel blockers; MPC, multiple-pill combination; MPR, medication possession ratio; SPC, single-pill combination.
Subgroup analysis
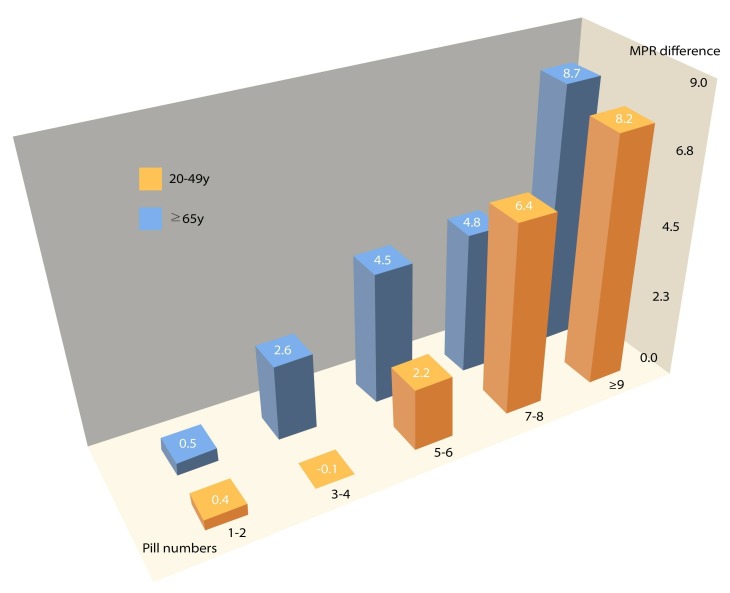
The number of medications and adherence were analysed by dividing subjects into elderly and non-elderly groups (cutoff age: 65 years). Regardless of the elderly status, the adherence difference between the SPC and MPC groups increased when the number of drugs increased. The adherence difference started to increase significantly when the number of drugs taken was at three to four in the elderly group (aged ≥65 years) and five to six in the non-elderly group (aged 20–64 years) (p<0.05) (figure 3). When a sensitivity analysis was conducted based on the number of drugs per detailed age group (20–49 years, 50–64 years, 65–74 years and ≥75 years), the same tendency emerged for overall medication adherence. The age groups 20–49 years and ≥75 years, which consisted of a relatively small number of samples, demonstrated a similar tendency, but the tendency was only marginally significant (table 3).
Figure 3.
Difference of medication adherences between MPC and SPC therapies according to combinations of pill numbers and age. The number of drugs for which the adherence difference begins to increase is three to four in the elderly group (≥65 year) and five to six in the non-elderly group (20–64 year) (p<0.05). MPC, multiple-pill combination; MPR, medication possession ratio; SPC, single-pill combination. *MPR difference=MPR of SPC group – MPR of MPC group.
Table 3.
Sensitivity analysis for medication adherences according to age and numbers of medications
| ARB-only group (n=29 400) |
CCB-only group (n=58 401) |
MPC group (n=10 458) |
SPC group (n=18 418) |
P value* | MPR differences† | P value‡ | |||||
| Crude MPR mean | Adjusted MPR mean§ (95% CI) | Crude MPR mean | Adjusted MPR mean§ (95% CI) | Crude MPR mean | Adjusted MPR mean§ (95% CI) | Crude MPR mean | Adjusted MPR mean§ (95% CI) | ||||
| 20–49 years (n=24 957) | |||||||||||
| 1–2 (n=6827) | 76.7 | 76.6 (75.6 to 77.6) | 75.8 | 74.7 (73.4 to 76.0) | 82.3 | 84.1 (80.2 to 88.0) | 83.4 | 84.8 (83.4 to 86.1) | <0.01 | 0.7 | 0.78 |
| 3–4 (n=11 768) | 78.2 | 78.5 (77.6 to 79.3) | 78.6 | 77.5 (76.8 to 78.3) | 85.6 | 86.7 (85.2 to 88.2) | 83.9 | 85.1 (84.1 to 86.1) | <0.01 | −1.6 | 0.01 |
| 5–6 (n=4595) | 78.1 | 78.6 (77.4 to 79.8) | 76.7 | 75.3 (74.0 to 76.6) | 82.2 | 83.9 (81.6 to 86.3) | 84.2 | 85.3 (83.5 to 87.0) | <0.01 | 1.3 | 0.70 |
| 7–9 (n=1360) | 79.4 | 79.3 (76.9 to 81.2) | 77.5 | 76.7 (74.1 to 79.4) | 76.7 | 78.6 (74.5 to 82.7) | 81.9 | 82.4 (79.0 to 85.8) | <0.01 | 3.8 | 0.13 |
| ≥9 (n=407) | 68.7 | 67.1 (62.6 to 71.6) | 72.4 | 68.0 (63.1 to 73.0) | 73.2 | 81.4 (74.0 to 88.8) | 86.5 | 89.8 (82.4 to 97.1) | <0.01 | 8.4 | 0.05 |
| 50–64 years (n=46 085) | |||||||||||
| 1–2 (n=7933) | 81.8 | 81.9 (81.0 to 82.9) | 81.4 | 81.0 (80.1 to 81.9) | 88.9 | 89.2 (85.6 to 92.9) | 88.5 | 89.3 (87.8 to 90.7) | <0.01 | 0.1 | 0.45 |
| 3–4 (n=20 396) | 82.5 | 83.0 (82.4 to 83.6) | 83.2 | 82.3 (81.9 to 82.8) | 88.3 | 89.5 (88.3 to 90.6) | 89.1 | 90.4 (89.6 to 91.2) | <0.01 | 0.9 | 0.72 |
| 5–6 (n=11 657) | 83.5 | 83.7 (83.0 to 84.4) | 83.8 | 83.2 (82.6 to 83.9) | 87.8 | 88.5 (87.3 to 89.8) | 90.3 | 91.1 (90.0 to 92.1) | <0.01 | 2.5 | <0.01 |
| 7–9 (n=4438) | 83.4 | 83.4 (82.2 to 84.5) | 82.5 | 80.4 (79.2 to 81.5) | 81.9 | 85.1 (83.1 to 87.1) | 89.9 | 92.3 (90.6 to 94.0) | <0.01 | 7.2 | <0.01 |
| ≥9 (n=1661) | 79.2 | 78.8 (76.9 to 80.6) | 81.5 | 78.7 (76.7 to 80.8) | 78.2 | 83.4 (80.3 to 86.4) | 89.6 | 91.3 (88.3 to 94.3) | <0.01 | 7.9 | <0.01 |
| 65–74 years (n=30 652) | |||||||||||
| 1–2 (n=3412) | 81.3 | 82.5 (80.6 to 84.4) | 81.1 | 80.3 (79.2 to 81.4) | 88.8 | 91.1 (85.5 to 96.8) | 88.9 | 91.0 (88.3 to 93.8) | <0.01 | −0.1 | 0.94 |
| 3–4 (n=11 308) | 83.5 | 83.9 (83.0 to 84.8) | 83.0 | 82.5 (81.9 to 83.1) | 88.7 | 89.2 (87.5 to 90.9) | 90.3 | 91.6 (90.3 to 92.9) | <0.01 | 2.4 | 0.03 |
| 5–6 (n=9267) | 83.1 | 83.1 (82.3 to 84.0) | 82.7 | 82.3 (81.6 to 83.0) | 88.0 | 88.3 (86.8 to 89.8) | 91.5 | 92.5 (91.2 to 93.8) | <0.01 | 4.2 | <0.01 |
| 7–9 (n=4562) | 81.3 | 81.7 (80.5 to 82.9) | 81.7 | 80.4 (79.3 to 81.5) | 85.4 | 87.4 (85.4 to 89.4) | 90.8 | 92.5 (90.7 to 94.4) | <0.01 | 5.2 | <0.01 |
| ≥9 (n=2103) | 79.8 | 79.3 (77.5 to 81.1) | 79.1 | 77.1 (75.4 to 81.1) | 78.6 | 83.1 (79.9 to 86.2) | 90.2 | 92.5 (89.5 to 95.6) | <0.01 | 9.5 | <0.01 |
| ≥75 years (n=14 983) | |||||||||||
| 1–2 (n=1351) | 80.6 | 81.0 (77.1 to 84.9) | 75.7 | 75.5 (73.8 to 77.3) | 82.4 | 83.1 (72.1 to 94.0) | 85.4 | 85.9 (80.3 to 91.5) | <0.01 | 2.9 | 0.69 |
| 3–4 (n=4916) | 79.2 | 80.4 (78.7 to 82.1) | 78.5 | 77.9 (77.0 to 78.9) | 85.2 | 85.6 (82.6 to 88.6) | 87.4 | 88.8 (86.3 to 91.2) | <0.01 | 3.2 | 0.42 |
| 5–6 (n=4586) | 79.8 | 80.5 (79.0 to 82.0) | 77.4 | 76.5 (75.4 to 77.6) | 83.4 | 85.2 (82.6 to 87.8) | 88.7 | 90.4 (87.9 to 92.8) | <0.01 | 5.2 | 0.01 |
| 7–9 (n=2711) | 79.3 | 79.6 (77.9 to 81.4) | 76.8 | 75.8 (74.3 to 77.2) | 83.1 | 85.3 (82.3 to 88.2) | 87.6 | 89.4 (86.4 to 92.3) | <0.01 | 4.1 | 0.18 |
| ≥9 (n=1419) | 77.8 | 77.4 (75.1 to 79.7) | 75.5 | 74.9 (72.8 to 77.0) | 80.2 | 81.3 (77.1 to 85.4) | 85.7 | 88.6 (84.2 to 93.0) | <0.01 | 7.4 | 0.06 |
Analyses were performed using ANCOVA.
*P value of crude MPR mean.
†MPR differences=adjusted MPR of SPC group – adjusted MPR of MPC group.
‡P value of MPR differences.
§Adjusted for factors associated with the patient (age, sex), condition (comorbidity), therapy (drug costs, number of concurrent drugs, prescription period), the healthcare system (insurance coverage) and the social/economic status (income, residence).
ANCOVA, analysis of covariance; ARB, angiotensin II receptor blockers; CCB, calcium channel blockers; MPC, multiple-pill combination; MPR, medication possession ratio; SPC, single-pill combination.
Discussion
First, among the 1 048 061 patients enrolled in the NHIS-NSC from 2008 to 2013, 206 739 were diagnosed with hypertension, a prevalence of 19.7%. This differs somewhat from the 23.7% prevalence of hypertension in Korea, as reported by the Korean Centers for Disease Control and Prevention in 2013.22 The reason for this difference seems to be that some people do not get medical treatment even when diagnosed with hypertension. In fact, according to the Korean National Health and Nutrition Examination Survey (KNHANES) in 2013, the hypertension unawareness rate in Korea is 38.5% and the untreated rate is 34.7%.22 Considering these values, the prevalence of hypertension in the sample of this study is similar to the prevalence in Korea. Thus, the data used in this study can be considered a representative sample reflecting the characteristics of the whole population without bias. Comparing these rates with other countries, the unawareness and untreated rates of hypertension in the USA during 2007–2010 were 18.9% and 26%, respectively.23 In England in 2006, the unawareness rate was 34.7% and untreated rate 48.7%.24 In Canada, the unawareness rate was 16.7% and untreated rate 20.1% in the period 2007–2009.24 These statistics indicate that the prevalence of hypertension identified in hospitals is slightly lower than the overall prevalence, suggesting the same tendency as found in this study.
In this study, the comparison of medication adherence of the four groups showed that adherence in combination therapy was higher than that in monotherapy. These results can be explained by applying the Health Belief Model.25 26 Those who think that the severity of their hypertension is higher (eg, by being prescribed combination therapy) are more likely to try to maintain adequate blood pressure by taking antihypertensive agents as prescribed.27 28 Schulz et al found that when prescribing antihypertensive agents such as ACE inhibitors, ARBs, beta blockers and CCBs with diuretics as SPC therapy, patients’ non-persistent risk was 8.4% lower and the possibility of non-adherence 19.4% lower than when prescribing these drugs as monotherapy without diuretics.29 Patel et al also reported that patients with SPC therapy including hydrochlorothiazide (HCTZ) demonstrated higher adherence than those using HCTZ monotherapy.30 Patel et al’s study did not include subjects’ baseline blood pressure information, but assumed that the monotherapy group was in the early stage of hypertension.30 In addition, Van Wijk et al reported that the group that had initiated hypertension treatment with combination therapy had higher drug persistence than the group that started with monotherapy. Furthermore, they assumed that the reason for the higher persistence for the combination therapy group was related to the severity of the disease.31 Another study by Hashmi et al reported that the average adherence of hypertensive patients was 79% when treated with monotherapy, 87% when treated with two drugs, and 90% when treated with three or more drugs.32 They also suggested that these results might be related to patients’ increased awareness, because of their hypertension severity. As such, patients treated with combination therapy may be more adherent, because they are more likely to take the medication with greater awareness than people treated with a single agent as their hypertension is more severe.
In this study, the medication adherence of the SPC group was found to be higher than that of the MPC group, consistent with the findings of previous research.11–15 A meta-analysis by Gupta et al, which compared antihypertensive medication adherence between SPC and MPC prescriptions, confirmed the significantly higher adherence of the SPC group than the MPC group in all three cohort studies and two trials (OR: 1.21; 95% CI 1.03 to 1.43).12 Sherrill et al also performed a meta-analysis of seven studies that compared adherence between two groups using MPR. All seven studies reported significantly higher adherence in the SPC than MPC group, regardless of the experience of antihypertensive agents.13
Furthermore, previous studies comparing medication adherence to SPC and MPC of ARB/CCB regimens, such as this study, indicated the same results.14 15 In a study using pharmacy claims data by Zeng et al, the proportion of good adherence in the ARB/CCB SPC group was 45.9%, higher than the 35.3% of the MPC group.14 However, their study had fewer subjects and shorter observation periods, and only included two types of ARB/CCB compound pills for the SPC group.14 A real-world study by Baser et al reported that the adherence of the ARB/CCB SPC group was higher than the MPC group (OR: 1.38; 95% CI 1.24 to 1.53).15 However, although Baser’s study was set in real world like this study, the sample size was small, including only 3259 subjects and short-term observation for 2 years. Regarding drug type, they included various types of ARB/CCB for the MPC group but limited the SPC group’s drug type to the valsartan/amlodipine compound.15 Compared with the two studies mentioned above, the current study may have confirmed the differences in adherence between SPC and MPC prescriptions by analysing long-term adherence for all ARBs, CCBs and ARB/CCB compounds available during the period of observation using a more systematic and representative large-scale data.
In addition, this study revealed that the higher the age, the greater the difference in adherence between the SPC and MPC groups (table 2, figure 2). According to Salas et al, cognitive impairment is a factor in decreasing adherence to antihypertensive medication in isolated patients.33 Moreover, according to Schwartz et al, the rate of drug use errors in patients aged >75 years was higher than those of patients <75 years.34 Presumably, it would be more difficult for the elderly to take both drugs accurately without omission when taking MPC medications as the frequency of decline in both physical and cognitive functions is higher in older age.33 35 In this regard, as the patient’s age increases, prescribing SPCs that simplifies the complexity of the medication regimen may be more beneficial in increasing adherence because for MPC prescriptions compliance is reduced even when only one of the prescribed drugs is omitted.
We also confirmed that the greater the number of drugs taken, the greater the difference in adherence between the SPC and MPC groups (table 2, figure 2). The reason for this tendency is that patients on MPC therapy need to take two drugs separately; the additional medication increases the complexity to a greater extent than when SPC medication is taken. Toh et al reported that a complex medication regimen such as multiple doses per day and multiple medications was significantly associated with higher non-compliance and readmissions.36 In addition, Pasina et al reported that for the elderly aged >65 years hospitalised in internal medicine wards, the greater the number of prescription drugs at discharge, the lower the medication adherence and understanding of the purpose of medication.37 Therefore, prescribing an SPC regimen would be one way to increase medication adherence, especially of patients taking a large number of medications.
Finally, comparing the adherence difference between the SPC and MPC groups according to both age and number of medications, there was a dose–response relationship tendency in which the more the number of drugs, the more prominent the difference regardless of age. However, this tendency started to be significant when a number of drugs taking was three or more in the elderly group (aged ≥65 years) and five or more in the non-elderly group (aged 20–64 years) (figure 3). Thus, the number of drugs affecting medication complexity showed a slight difference between the elderly and non-elderly group. The significant point of the number of medications, namely the significant point when the adherence difference between SPC and MPC becomes statistically significant, was slightly different between the detailed age groups, but the tendency remained the same (table 3). The reason for this difference is that it is more difficult for older patients to adapt to regimen complexity, because of impaired physical and cognitive functions mentioned above.33 35
Our study is meaningful for two reasons. First, we analysed the adherence of antihypertensive agents by using a sample of national cohort data that represent about 2.2% of the total population. Second, we analysed the differences in medication adherence using cohort subjects who continued to take antihypertensive medication for at least 1 year for the maximum of 6 observed years. Although previous research analysed medication adherence between the SPC and MPC of antihypertensive agents,11–15 they were either short-term studies or analysed in certain centres or under limited conditions. In addition, this study is meaningful, because it compared not only adherence with a combination therapy regimen type but also compared it with monotherapy. Furthermore, we investigated all of the prescriptions and the average number of associated diseases involved with the patients, which enabled us to more objectively adjust the factors associated with the therapy and the patients’ condition.
In contrast, because of limitations in data, this study did not reflect diverse socioeconomic factors such as the patients’ education level and occupation and did not include specific factors such as caregiver status, the family environment and healthcare provider factors. We also did not include antihypertensive agents other than ARBs and CCBs (eg, diuretics, beta blockers and so on) in the analysis. However, as the same class of drugs is homogeneous, we were able to focus on comparing the adherence between SPC and MPC by eliminating the effects on adherence of drug classes other than ARBs and CCBs.
Moreover, there is a weakness in the analysis regarding adjusting for patients’ comorbidities. This study did not specify comorbidities according to severity and only adjusted with the average number of diagnoses of the subject during the observation period. But in reality, some patients are diagnosed with many mild diseases, whereas others have few diagnoses but more severe diseases. Also, although new diseases can be additionally diagnosed at any point in the observation period, a new disease diagnosed at a certain point cannot be considered as having affected the medication adherence of the whole observation period. That is why we adjusted the comorbidities as the average number of diagnoses.
Finally, due to the inevitable limitation of real-world claims data, we could not compare the first-year adherence of each group even though the first year is usually an important phase for adherence in newly treated patients. When using real-world data such as the NHIS-NSC used here, it is practically impossible to divide the subjects into certain drug groups without implementing some operationalisation. This is due to the fact that medications prescribed to patients can be changed, added or even discontinued during the course of the observation period. Moreover, we concluded that categorising patients into four groups according to the last drug taken by subjects was the most ideal way as not many patients start with SPC as initial therapy unless their hypertension is severe. We also thought that comparing average adherence up to a maximum of 6 years was suitable, as the subjects in our study were not limited to newly treated patients.
In conclusion, those taking antihypertensive drugs as a combination therapy demonstrated higher adherence than those taking them as a monotherapy. Among the combination therapy patients, those on the SPC regimen demonstrated higher adherence than those taking the MPC prescription. This tendency was more pronounced with increasing age and the number of drugs taken. Therefore, if patients are older or taking numerous medications, prescribing antihypertensive agents as a SPC regimen may help improve medication adherence.
Supplementary Material
Footnotes
Contributors: SJK: conceived and designed the study, acquired and analysed the data, interpreted the study findings and drafted the manuscript. ODK: analysed the data and interpreted the study findings. S-WO, CML and BLC: critically reviewed the manuscript. H-CC: conceived and designed the study, supervised and directed the conduct of the study, interpreted the study findings and critically revised the manuscript; is the guarantor. All authors had full access to all of the data and the accuracy of the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no other meeting the criteria have been omitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: This study was approved by the institutional review board (IRB) at the Seoul National University Hospital (IRB No.E-15-5-079-673) and National Health Insurance review committee for research support (NHIS-2017-2-610). Written informed consent was waived
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are from the National Health Insurance service (NHIS). Interested researchers can request access to the data from NHIS. The detailed information for data access of NHIS could be obtained from the NHIS website (www.nhis.or.kr).
Patient consent for publication: Not required.
References
- 1. Sabaté E. Adherence to long-term therapies: evidence for action: World Health Organization, 2003. [PubMed] [Google Scholar]
- 2. Simpson SH, Eurich DT, Majumdar SR, et al. . A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 2006;333:15 10.1136/bmj.38875.675486.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shin S, Song H, Oh SK, et al. . Effect of antihypertensive medication adherence on hospitalization for cardiovascular disease and mortality in hypertensive patients. Hypertens Res 2013;36:1000–5. 10.1038/hr.2013.85 [DOI] [PubMed] [Google Scholar]
- 4. Hong JS, Kang HC. Relationship between oral antihyperglycemic medication adherence and hospitalization, mortality, and healthcare costs in adult ambulatory care patients with type 2 diabetes in South Korea. Med Care 2011;49:378–84. 10.1097/MLR.0b013e31820292d1 [DOI] [PubMed] [Google Scholar]
- 5. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028–35. 10.1161/CIRCULATIONAHA.108.768986 [DOI] [PubMed] [Google Scholar]
- 6. Marcum ZA, Sevick MA, Handler SM. Medication nonadherence: a diagnosable and treatable medical condition. JAMA 2013;309:2105–6. 10.1001/jama.2013.4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy 2014;7:35 10.2147/RMHP.S19801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 9. Park JH, Shin Y, Lee SY, et al. . Antihypertensive drug medication adherence and its affecting factors in South Korea. Int J Cardiol 2008;128:392–8. 10.1016/j.ijcard.2007.04.114 [DOI] [PubMed] [Google Scholar]
- 10. Tajouri TH, Driver SL, Holmes DR. ’Take as directed'--strategies to improve adherence to cardiac medication. Nat Rev Cardiol 2014;11:304–7. 10.1038/nrcardio.2013.208 [DOI] [PubMed] [Google Scholar]
- 11. Bangalore S, Kamalakkannan G, Parkar S, et al. . Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007;120:713–9. 10.1016/j.amjmed.2006.08.033 [DOI] [PubMed] [Google Scholar]
- 12. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension 2010;55:399–407. 10.1161/HYPERTENSIONAHA.109.139816 [DOI] [PubMed] [Google Scholar]
- 13. Sherrill B, Halpern M, Khan S, et al. . Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens 2011;13:898–909. 10.1111/j.1751-7176.2011.00550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeng F, Patel BV, Andrews L, et al. . Adherence and persistence of single-pill ARB/CCB combination therapy compared to multiple-pill ARB/CCB regimens. Curr Med Res Opin 2010;26:2877–87. 10.1185/03007995.2010.534129 [DOI] [PubMed] [Google Scholar]
- 15. Baser O, Andrews LM, Wang L, et al. . Comparison of real-world adherence, healthcare resource utilization and costs for newly initiated valsartan/amlodipine single-pill combination versus angiotensin receptor blocker/calcium channel blocker free-combination therapy. J Med Econ 2011;14:576–83. 10.3111/13696998.2011.596873 [DOI] [PubMed] [Google Scholar]
- 16. Lee J, Lee JS, Park SH, et al. . Cohort profile: the national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int J Epidemiol 2017;46:dyv319–e. 10.1093/ije/dyv319 [DOI] [PubMed] [Google Scholar]
- 17. Xu H, He Y, Xu L, et al. . Trends and patterns of five antihypertensive drug classes between 2007 and 2012 in China using hospital prescription data. Int J Clin Pharmacol Ther 2015;53:430–7. 10.5414/CP202243 [DOI] [PubMed] [Google Scholar]
- 18. Ishida T, Oh A, Hiroi S, et al. . Current use of antihypertensive drugs in Japanese patients with hypertension: Analysis by age group. Geriatr Gerontol Int 2018;18:899–906. 10.1111/ggi.13276 [DOI] [PubMed] [Google Scholar]
- 19. Organization WH. The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD). Oslo: WHO, 2006. [Google Scholar]
- 20. Andrade SE, Kahler KH, Frech F, et al. . Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 2006;15:565–74. 10.1002/pds.1230 [DOI] [PubMed] [Google Scholar]
- 21. Halpern MT, Khan ZM, Schmier JK, et al. . Recommendations for evaluating compliance and persistence with hypertension therapy using retrospective data. Hypertension 2006;47:1039–48. 10.1161/01.HYP.0000222373.59104.3d [DOI] [PubMed] [Google Scholar]
- 22. Statistics Korea. The 2013 report of the Korea National Health and Nutrition Examination Survey. http://knhanes.cdc.go.kr
- 23. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–50. 10.1001/jama.2010.650 [DOI] [PubMed] [Google Scholar]
- 24. Joffres M, Falaschetti E, Gillespie C, et al. . Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open 2013;3:e003423 10.1136/bmjopen-2013-003423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Educ Q 1988;15:175–83. 10.1177/109019818801500203 [DOI] [PubMed] [Google Scholar]
- 26. Rosenstock IM. Why people use health services. Milbank Q 2005;83:Online-only 10.1111/j.1468-0009.2005.00425.x [DOI] [Google Scholar]
- 27. Billups SJ, Malone DC, Carter BL. The relationship between drug therapy noncompliance and patient characteristics, health-related quality of life, and health care costs. Pharmacotherapy 2000;20:941–9. 10.1592/phco.20.11.941.35266 [DOI] [PubMed] [Google Scholar]
- 28. Shalansky SJ, Levy AR. Effect of number of medications on cardiovascular therapy adherence. Ann Pharmacother 2002;36:1532–9. 10.1345/aph.1C044 [DOI] [PubMed] [Google Scholar]
- 29. Schulz M, Krueger K, Schuessel K, et al. . Medication adherence and persistence according to different antihypertensive drug classes: A retrospective cohort study of 255,500 patients. Int J Cardiol 2016;220:668–76. 10.1016/j.ijcard.2016.06.263 [DOI] [PubMed] [Google Scholar]
- 30. Patel BV, Remigio-Baker RA, Thiebaud P, et al. . Improved persistence and adherence to diuretic fixed-dose combination therapy compared to diuretic monotherapy. BMC Fam Pract 2008;9:61 10.1186/1471-2296-9-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Wijk BL, Klungel OH, Heerdink ER, et al. . Rate and determinants of 10-year persistence with antihypertensive drugs. J Hypertens 2005;23:2101–7. 10.1097/01.hjh.0000187261.40190.2e [DOI] [PubMed] [Google Scholar]
- 32. Hashmi SK, Afridi MB, Abbas K, et al. . Factors associated with adherence to anti-hypertensive treatment in Pakistan. PLoS One 2007;2:e280 10.1371/journal.pone.0000280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salas M, In’t Veld BA, van der Linden PD, et al. . Impaired cognitive function and compliance with antihypertensive drugs in elderly: the Rotterdam Study. Clin Pharmacol Ther 2001;70:561–6. 10.1067/mcp.2001.119812 [DOI] [PubMed] [Google Scholar]
- 34. Schwartz D, Wang M, Zeitz L, et al. . Medication errors made by elderly, chronically ill patients. Am J Public Health Nations Health 1962;52:2018–29. 10.2105/AJPH.52.12.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turner A, Hochschild A, Burnett J, et al. . High prevalence of medication non-adherence in a sample of community-dwelling older adults with adult protective services-validated self-neglect. Drugs Aging 2012;29:741–9. 10.1007/s40266-012-0007-2 [DOI] [PubMed] [Google Scholar]
- 36. Toh MR, Teo V, Kwan YH, et al. . Association between number of doses per day, number of medications and patient’s non-compliance, and frequency of readmissions in a multi-ethnic Asian population. Prev Med Rep 2014;1:43–7. 10.1016/j.pmedr.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pasina L, Brucato AL, Falcone C, et al. . Medication non-adherence among elderly patients newly discharged and receiving polypharmacy. Drugs Aging 2014;31:283–9. 10.1007/s40266-014-0163-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-029862supp001.pdf (256KB, pdf)