Abstract
Both low birthweight (<2500g; LBW) and macrosomia (> 4000g) are considered adverse birth outcomes and are associated with later poor health conditions, yet the social determinants of macrosomia are understudied. In this study, we explore patterning of LBW, normal birthweight, and macrosomia by race/ethnicity and nativity. We examined data from all live births between 1999 and 2014 in New Jersey with a non-missing, plausible value of birthweight (n = 1,609,516). We compared the risk for LBW and macrosomia among non-Hispanic White, non-Hispanic Black, Hispanic, and non-Hispanic Asian mothers, and between the US- and native-born. For Hispanics and Asians, we also examined differences by country of origin. The racial/ethnic patterns for macrosomia mirrored those of LBW, suggesting that the factors underlying LBW shift birthweight distributions. For example, non-Hispanic White mothers had the lowest risk for LBW and the highest risk for macrosomia. Nativity patterns differed by subgroup, however, with unique risks for macrosomia among some origin groups, such as foreign-born Cubans.
The racial/ethnic and nativity patterns of macrosomia do not completely mirror those of LBW, suggesting some distinct social risk factors for macrosomia. Our findings raise questions about whether and how racial/ethnic and nativity patterning in both low and excess birthweight is retained in later conditions, such as childhood obesity.
Introduction
Research on the social determinants of health often considers birth outcomes a marker of health status, as birth outcomes are shaped by a complex web of maternal risk factors including health behaviors, exposures to chronic and acute stress, access to economic and social resources, and medical care (Kim and Saada, 2013). One of the most widely studied birth outcomes is birthweight, which is affected by conditions before and during pregnancy that bear on intrauterine growth as well as gestational age. Both low and excess birthweight are considered adverse birth outcomes, yet the social determinants of low birthweight (<2500g; LBW) have been studied considerably more often than the other extreme. In particular, researchers have long demonstrated significant variation by race/ethnicity and nativity in LBW (e.g., Acevedo-Garcia et al., 2005; James, 1993; Singh and Yu, 1996).
Previous research has shown that compared to non-Hispanic White women, the highest risk of LBW is observed for non-Hispanic Black women. Asian women have slightly elevated risk of LBW and Hispanic women exhibit similar risk to white women (Acevedo-Garcia et al., 2007; Borrell et al., 2016; James, 1993; Singh and Yu, 1996). Foreign-born status acts as a buffer against LBW within some, but not all, racial/ethnic groups. Foreign-born Hispanic and Black women generally have lower odds of LBW than their US-born counterparts, while foreign-born Asians have higher odds than US-born Asians (Acevedo-Garcia et al., 2005; David and Collins 1997). Some Hispanic subgroups, such as Puerto Ricans, have increased risk for LBW compared to others, such as Mexicans. Foreign-born status does not seem to confer the same protection to Puerto Ricans and Cubans that it does to Mexicans (Acevedo-Garcia et al., 2007). Among Asians, certain subgroups, such as Asian Indian, Filipino, Japanese, and Vietnamese women, have higher risk for LBW infants than non-Hispanic White women, while others, such as Chinese and Korean women, exhibit similar risk (Wartko et al., 2017).
These racial/ethnic and nativity patterns suggest that the risk factors associated with LBW, such as low maternal education, pre-pregnancy medical conditions (e.g., chronic hypertension), pregnancy medical complications (e.g., gestational hypertension), inadequate nutrition, neighborhood deprivation, poor access to health care, chronic stress, tobacco use, and environmental exposures (de Bernabe et al., 2004), are likely to be concentrated among certain racial/ethnic groups, such as Black and Puerto Rican mothers, and that US-born mothers are more likely on average to be exposed to such risk factors than foreign-born mothers. Chronic stress exposure (e.g., from chronic economic hardship and/or experiences of racial discrimination) is posited to be a particularly important pathway through which social risk factors influence birthweight (Braveman, 2011; Dominguez et al., 2008). Geronimus calls this phenomenon “weathering,” in which the health of African American women erodes as a physical consequence of social inequality (Geronimus, 1996).
The etiology of fetal growth is a complex combination of genetic factors, fetal hormones, uterine constraints, and maternal risk factors that vary in their influence over pregnancy (Dar and Gross, 2000; Langer, 2000). Early in gestation, genetic factors appear to be the primary driver of fetal growth, whereas external factors (e.g. uterine growth, maternal diet) are more important in later stages. The interplay between genetic and external factors is likely regulated via fetal hormones, such as insulin. While somewhat arbitrary (Chen et al., 1991; Paneth, 1995), the 2500 gram cut-off generally used for studying LBW implies that birthweights above this point are normal and pose little health risk to the mother or child. Yet excess birthweight, or macrosomia (birthweight over 4000 grams), is also a risk factor for infant morbidity and mortality. Macrosomia is associated with cesarean delivery, fetal injury, a higher risk of childhood overweight and obesity, postpartum hemorrhage, and low Agpar score (Chatfield, 2001; Henriksen, 2008). Very high birthweight (over 4500 grams) is associated with neonatal mortality, birth injury, and maternal morbidity (Zhang et al., 2008). Chronic or gestational diabetes may contribute to excessive fetal growth resulting from elevated insulin. Other maternal risk factors for macrosomia include multiparity, higher maternal weight/height, excessive maternal weight gain during pregnancy, maternal older age, smoking, and glycosuria (Gaudet et al., 2014; Lawlor et al., 2010; Zhang et al., 2008). There have been limited explorations into the social determinants of macrosomia, and we know particularly little about racial or nativity patterning.
In this paper, we explore patterning by race, ethnicity, and nativity across three categories of birthweight: LBW, normal birth weight, and macrosomia. This comprehensive view of birthweight offers insight into how the social inequalities that underlie racial/ethnic and nativity patterning in LBW may operate at the opposite end of the birthweight spectrum. On the one hand, the social inequalities that underlie LBW may affect the entire birthweight continuum, such that the distribution of birthweight among high-risk groups is shifted to the left. As a result, groups at high risk for LBW would have correspondingly low risk for macrosomia, and vice-versa. Alternatively, one of the central features of social determinants is that they create general vulnerability to disease rather than any specific disorder (Berkman and Kawachi, 2014); health risks that result from unhealthy social environments are associated with a constellation of poor health outcomes (Yen and Syme, 1999). Clinical risk factors that have been associated with macrosomia, such as chronic and gestational diabetes, are also highly prevalent among groups with high risk for LBW (CDC, 2011). It is thus alternatively possible that negative social risk factors keep certain groups from having babies in the normal weight range, and therefore some groups may have a high risk for both LBW and macrosomia.
The existing literature provides little insight into resolving these alternative scenarios. A limited number of advantageous social characteristics, such as higher levels of maternal education and being married at the time of birth, have been associated with macrosomia (Zhang et al., 2008). One Canadian study found mixed results by geography, however: in one province, higher SES mothers had higher odds for macrosomia while lower SES mothers had higher odds in another province (Dubois et al., 2007). The few existing studies that have examined race, ethnicity, or nativity suggest that foreign-born mothers in the US and Europe tend to have higher birthweights and higher odds of macrosomia than their native-born counterparts, as well as an increased risk for gestational diabetes, which is predictive of higher birth weight (Forna et al., 2003; Juarez and Revuelta-Eugercios, 2014; Restrepo-Mesa et al., 2015). Yet a study in Michigan observed lower odds of giving birth to a macrosomic infant among immigrant mothers; this study did not, however, include important controls in the multivariate models, such as race (El-Sayed and Galea, 2011). Another Australian study found non-indigenous women to have higher risk than indigenous women, suggesting concentration among the socially advantaged (Lahmann et al., 2009).
Some have suggested that the social determinants of gestational diabetes (GDM), a strong clinical predictor of macrosomia, may bear on the social patterning of birthweight (Ragnarsdottir and Conroy, 2010). In several US samples, Non-Hispanic White women displayed the lowest rates of GDM while Asian/Pacific Islander women had the highest (Hedderson et al., 2010; Nguyen et al., 2012; Tsai et al., 2013). Yet a US study of mothers in Hawaii found that among mothers with GDM, White women had the highest risk for macrosomia compared to Asian women (Tsai et al., 2013), suggesting that the social patterning of GDM may not always align with that of macrosomia.
To more comprehensively examine the social patterning of birthweight than has been done previously, this paper examines the racial/ethnic and nativity patterns of both low birthweight and macrosomia using population-level data from the State of New Jersey. To our knowledge, this is the first analysis to establish racial/ethnicity and nativity patterning for three birthweight categories simultaneously: low, normal, and macrosomic. By jointly considering the full range of birthweight outcomes, we explore whether mechanisms rooted in social inequality are specific to LBW or are also relevant to macrosomia. This work can also provide insight into understanding the origins of racial/ethnic and nativity patterning of later life conditions associated with poor birth outcomes, such as childhood obesity or chronic conditions (Barker, 1995). We acknowledge, however, that the etiologic pathway between birth outcomes and later health conditions may be highly dependent on gestational age, and thus also examine the robustness of our results to measurement of small for gestational age (SGA) and large for gestational age (LGA).
Methods
Sample
This was a cross-sectional study of all births between 1999 and 2014 in the State of New Jersey (n = 1,724,712). One of the six traditional US immigrant gateway states (Frey, 2006), New Jersey’s substantial racial/ethnic diversity and sizeable immigrant population enables analyses disaggregated by race/ethnicity, nativity, and origin country. The data came from state birth certificate records. We included live (n = 1,722,176), singleton (n = 1,648,042) births with a non-missing, plausible value of birthweight (n = 1,639,422), born to a mother belonging to one of the four major racial groups (White, Black, Asian, Hispanic, n= 1,609,516). We excluded cases with missing data on any of the covariates, resulting in a final analytic sample of 1,519,295 (n=1,149,835 for Hispanic subgroup analysis, n=905,004 for Asian subgroup analysis).
Variables
Outcome.
Birthweight was categorized as low birthweight (<2500 grams), normal birthweight (≥2500 and ≤4000 grams), and macrosomia (>4000 grams).
Exposure.
Our primary independent variables were maternal race/ethnicity and nativity. Maternal race/ethnicity was collected by self-report and categorized into non-Hispanic White (reference), non-Hispanic Black, Hispanic, and non-Hispanic Asian. For Hispanic and Asian mothers, we further identified country of origin if the number of mothers from that country in the dataset was larger than 10,000. Hispanic mothers were categorized as Mexican, Cuban, Puerto Rican, Central/South American, and other Hispanic. Given the demographic composition of New Jersey Hispanic immigrants, we suspect the “other Hispanic” category was primarily Dominican. Asian mothers were categorized as Chinese, Asian Indian, Korean, Filipina, and other Asian (combining Japanese and Vietnamese.) Mothers born in the contiguous 48 states, Alaska, or Hawaii were classified as US-born (reference); others were considered foreign-born.
Covariates.
We first controlled for demographic and socioeconomic characteristics that could confound racial patterns: maternal education [less than high school, high school diploma (reference), some college, bachelor’s degree or higher], participation in Medicaid during pregnancy versus not (reference), any employment in the year prior to birth versus no employment (reference), mother married at the time of birth versus not (reference), maternal age [less than 19 years, 20–24 years, 25–29 years (reference), 30–34 years, 35–39 years, 40+ years], gestational age in weeks (continuous), female infant sex versus male (reference), and parity [first (reference), second, third birth]. We also controlled for the presence of maternal health conditions associated in prior research with birthweight: chronic diabetes, gestational diabetes, pregnancy-induced hypertension, chronic hypertension, and pre-eclampsia or eclampsia. The reference group for each was the absence of the condition. In addition, we controlled for maternal health behaviors associated previously with birthweight: any prenatal smoking versus not (reference), pregnancy weight gain in pounds (continuous), and early/on time prenatal care (initiated within the first trimester of pregnancy) versus not (reference). Finally, we controlled for birth year and county in all models to account for unobserved time- or place-oriented factors associated with birthweight. Because omitting covariates can be beneficial when exploring social patterning across race-ethnic-nativity groups (Kaufman and Cooper, 2001), we also conducted unadjusted analyses, available in Appendix Table 1.
Analysis
We conducted a series of multinomial logistic regressions, with a three-category outcome: low birthweight, normal birthweight (reference), and macrosomia. We first modelled main effects for race/ethnicity and nativity. We then considered whether nativity differentials varied across racial/ethnic groups by including interaction terms between race/ethnicity and nativity. We interpreted a significant interaction term to mean that the comparison between foreign-born and US-born women for that particular racial/ethnic group was significantly different than the same comparison for the reference category (White women).We also conducted a joint test of interaction using an F-test with 3 degrees of freedom to assess whether all interaction coefficients were equal to zero. We then calculated predicted probabilities for low birthweight, normal birthweight, and macrosomia for each race/ethnicity and nativity combination, based on the interaction model with all covariate values held at their means. Finally, we conducted post-estimation Wald tests on the predicted probabilities to determine nativity differences in LBW and macrosomia within a racial/ethnic group and whether these differences were significantly different from zero. We then conducted similar analyses to examine differences among Hispanic and Asian mothers by country of origin and nativity status, relative to White mothers. We calculated relative risk ratios (with 95% confidence intervals, which corresponds to the conventional p<.05 cut-off for statistical significance) instead of odds ratios because the prevalence of LBW and macrosomia was low in our sample.
We conducted robustness checks considering small for gestational age (membership in the smallest decile of sex-specific, population-based birthweight-for-gestational-age curves) and large for gestational age (membership in the largest decile of sex-specific, population-based birthweight-for-gestational-age curves) as outcomes; our results were largely similar to the results for LBW and macrosomia (Appendix 2).
Results
Nearly 10% of Black mothers had low birthweight babies, compared to 4% of White mothers (Table 1). White mothers had the highest proportion of macrosomic infants (11.4%). A large majority of Asian and Hispanic mothers were foreign-born (92% and 70%, respectively), compared to 23% of Black and 12% of White mothers.
Table 1.
Descriptive Statistics of Analytic Variables from New Jersey EBC Records, 1999–2014
| Mean or Percent (%) | ||||
|---|---|---|---|---|
| Non-Hispanic White (n=803,294) | Non-Hispanic Black (n=251,558) | Asian (n=145,663) | Hispanic (n = 409,001) | |
| Low Birth Weight | 3.92% | 9.57% | 6.05% | 5.29% |
| Normal Birth Weight | 84.66% | 84.70% | 89.62% | 87.13% |
| Macrosomia | 11.41% | 5.71% | 4.31% | 7.57% |
| Mom foreign-born | 12.04% | 22.81% | 91.61% | 70.20% |
| Maternal education | ||||
| Less than high school | 4.29% | 16.32% | 2.71% | 33.61% |
| High school completion | 23.61% | 40.36% | 9.27% | 35.21% |
| Some college | 21.87% | 24.52% | 17.08% | 17.28% |
| Bachelor’s degree or higher | 50.23% | 18.80% | 70.94% | 13.90% |
| Mom participated in Medicaid during pregnancy | 11.75% | 46.09% | 7.46% | 46.69% |
| Mom employed in year prior to birth | 70.96% | 61.26% | 59.23% | 48.11% |
| Mom married | 83.94% | 32.12% | 95.10% | 42.57% |
| Maternal age | ||||
| Less than 19 | 2.54% | 13.56% | 0.56% | 10.93% |
| 20–24 | 11.45% | 26.23% | 6.33% | 26.57% |
| 25–29 | 24.12% | 24.70% | 30.72% | 27.45% |
| 30–37 | 36.27% | 20.64% | 40.43% | 21.46% |
| 35–39 | 20.97% | 11.60% | 18.34% | 10.93% |
| 40+ | 4.65% | 3.27% | 3.62% | 2.67% |
| Gestational age | 38.90 | 38.49 | 38.70 | 38.73 |
| Male infant | 51.31% | 50.99% | 51.30% | 51.02% |
| Parity | ||||
| 1− | 41.56% | 38.84% | 48.90% | 38.30% |
| 2− | 34.93% | 30.33% | 39.77% | 33.51% |
| 3+ | 23.41% | 30.70% | 11.28% | 28.14% |
| Birth year | 2006 | 2006 | 2007 | 2007 |
| Maternal medical risk factors | ||||
| Chronic (Type I or II) diabetes | 0.53% | 0.98% | 0.76% | 0.69% |
| Gestational diabetes | 3.87% | 4.12% | 9.57% | 5.08% |
| Pregnancy-induced hypertension | 2.84% | 3.93% | 1.85% | 2.32% |
| Chronic hypertension | 0.93% | 2.53% | 0.63% | 0.72% |
| Pre-eclampsia | 1.00% | 2.23% | 0.76% | 1.45% |
| Prenatal smoking | 9.45% | 10.70% | 0.97% | 4.18% |
| Weight gain during pregnancy (pounds) | 30.90 | 27.55 | 28.47 | 27.81 |
| Early/on-time initiation of prenatal care | 88.40% | 64.56% | 87.00% | 69.72% |
| Ethnicity | ||||
| Mexican (n=85,114) | 20.80% | |||
| Cuban (n=11,386) | 2.78% | |||
| Puerto Rican (n=101,804) | 24.87% | |||
| Central/South American (n=194,782) | 47.65% | |||
| Other Hispanic (n=15,915) | 3.89% | |||
| Chinese (n=21,032) | 14.47% | |||
| Asian Indian (n=78,100) | 53.46% | |||
| Korean (n=15,877) | 10.87% | |||
| Filipina (n = 21,781) | 14.99% | |||
| Other Asian (n = 8,873) | 6.20% | |||
Table 2 provides regression results for birthweight, with normal weight as the reference group. All relative risk ratios (RRR) are adjusted for the covariates identified above; unadjusted models are available in Appendix 1. Panel A models racial/ethnic and nativity differences among the full analytic sample of White, Black, Hispanic, and Asian mothers. Model 1 presents main effects for race/ethnicity and nativity. Compared to White mothers, Asian women had the highest relative risk for LBW (RRR=2.10, 95% CI=2.03, 2.19), followed by Black (RRR= 1.65, 95% CI=1.61, 1.70) and Hispanic women (RRR=1.12, 95% CI=1.09–1.16). Foreign-born women had lower relative risk of LBW than US-born mothers (RRR=0.91, 95% CI=0.89–0.93). Compared to White mothers, mothers of the three other racial/ethnic groups all had lower risk of macrosomia. Asian women had the lowest relative risk (RRR=0.40, 95% CI=0.39–0.42), followed by Black (RRR=0.63, 95% CI=0.61–0.64) and Hispanic women (RRR=0.83, 95% CI=0.81–0.84). Foreign-born status was not associated with any difference in macrosomia risk.
Table 2.
Multinomial Logistic Regression of LBW, Normal Birthweight (reference), and Macrosomia, by Race-Ethnicity, Nativity, and Country of Origin
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted RRR for LBW | Adjusted RRR for Macrosomia | Adjusted RRR for LBW | Adjusted RRR for Macrosomia | |||||
| Panel A. Race/Ethnic differences | ||||||||
| Maternal race/ethnicity | ||||||||
| Non-Hispanic White | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Non-Hispanic Black | 1.65 | (1.61, 1.70) | 0.63 | (0.61, 0.64) | 1.71 | (1.65–1.76) | 0.57 | (0.56–0.58) |
| Non-Hispanic Asian | 2.10 | (2.03, 2.19) | 0.40 | (0.39, 0.42) | 1.74 | (1.58–1.91) | 0.47 | (0.44–0.52) |
| Hispanic | 1.12 | (1.09, 1.16) | 0.83 | (0.81, 0.84) | 1.21 | (1.16–1.25) | 0.76 | (0.74–0.78) |
| Mom Foreign-born | 0.91 | (0.89, 0.93) | 0.99 | (0.97, 1.00) | 1.05 | (1.00–1.10) | 0.86 | (0.84–0.88) |
| Maternal race/ethnicity x Foreign-born | ||||||||
| Non-Hispanic Black x Foreign-born | 0.80 | (0.75–0.86) | 1.49 | (1.43–1.56) | ||||
| Non-Hispanic Asian x Foreign-born | 1.09 | (0.97–1.21) | 0.94 | (0.88–1.03) | ||||
| Hispanic x Foreign-born | 0.80 | (0.75–0.86) | 1.25 | (1.21–1.30) | ||||
| Joint test of interaction | p<.001 | p<.001 | ||||||
| Panel B. Hispanic subgroup differences | ||||||||
| Maternal ethnicity | ||||||||
| Non-Hispanic White | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Mexican | 0.91 | (0.86–0.97) | 0.87 | (0.84–0.90) | 1.00 | (0.86–1.17) | 0.83 | (0.74–0.92) |
| Cuban | 1.06 | (0.94–1.19) | 0.95 | (0.89–1.02) | 1.06 | (0.91–1.23) | 0.86 | (0.79–0.94) |
| Puerto Rican | 1.26 | (1.21–1.31) | 0.72 | (0.70–0.74) | 1.28 | (1.22–1.34) | 0.71 | (0.68–0.73) |
| Central/South American | 0.98 | (0.94–1.02) | 0.91 | (0.89–0.93) | 1.05 | (0.98–1.13) | 0.82 | (0.78–0.86) |
| Other Hispanic | 1.17 | (1.07, 1.28) | 0.87 | (0.81–0.93) | 1.08 | (0.90–1.30) | 0.71 | (0.62–0.81) |
| Mom Foreign-born | 1.00 | (0.97–1.04) | 0.91 | (0.89–0.93) | 1.06 | (1.01–1.11) | 0.86 | (0.84–0.89) |
| Maternal ethnicity x Foreign-born | ||||||||
| Mexican x Foreign-born | 0.85 | (0.72–1.00) | 1.11 | (1.00–1.24) | ||||
| Cuban x Foreign-born | 0.99 | (0.78–1.26) | 1.34 | (1.17–1.53) | ||||
| Puerto Rican x Foreign-born | 0.91 | (0.83–0.99) | 1.13 | (1.06–1.21) | ||||
| Central/South American x Foreign-born | 0.88 | (0.80–0.96) | 1.18 | (1.12–1.25) | ||||
| Other Hispanic x Foreign-born | 1.06 | (0.86–1.31) | 1.37 | (1.18–1.60) | ||||
| Joint test of interaction | p<.05 | p<.001 | ||||||
| Panel C. Asian subgroup differences | ||||||||
| Maternal ethnicity | ||||||||
| Non-Hispanic White | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Chinese | 1.11 | (1.01–1.23) | 0.56 | (0.53–0.60) | 1.29 | (1.03–1.61) | 0.50 | (0.43–0.59) |
| Asian Indian | 2.37 | (2.25–2.50) | 0.38 | (0.36–0.40) | 2.70 | (2.33–3.13) | 0.29 | (0.24–0.35) |
| Korean | 1.09 | (0.97–1.22) | 0.55 | (0.51–0.59) | 1.28 | (0.90–1.80) | 0.67 | (0.54–0.83) |
| Filipina | 1.45 | (1.34–1.56) | 0.55 | (0.51–0.59) | 1.26 | (1.05–1.52) | 0.59 | (0.51–0.69) |
| Other Asian | 1.49 | (1.31–1.69) | 0.47 | (0.42–0.52) | 1.24 | (.83–1.85) | 0.60 | (0.45–0.81) |
| Mom Foreign-born | 1.05 | (1.01–1.10) | 0.87 | (0.85–0.89) | 1.07 | (1.01–1.12) | 0.86 | (0.84–0.89) |
| Maternal ethnicity x Foreign-Born | ||||||||
| Chinese x Foreign-born | 0.83 | (0.65–1.06) | 1.13 | (0.95–1.36) | ||||
| Asian Indian x Foreign-born | 0.86 | (0.74–1.01) | 1.34 | (1.09–1.65) | ||||
| Korean x Foreign-born | 0.83 | (0.58–1.20) | 0.80 | (0.64–1.00) | ||||
| Filipina x Foreign-born | 1.16 | (0.95–1.43) | 0.91 | (0.77–1.07) | ||||
| Other Asian x Foreign-born | 1.21 | (0.79–1.85) | 0.75 | (0.54–1.03) | ||||
| Joint test of interaction | NS | p<.05 | ||||||
Note: Models controlled for maternal education, Medicaid participation, employed in year prior to birth, married, maternal age, gestational age, male infant, parity, chronic diabetes, gestational diabetes, pregnancy-induced hypertension, chronic hypertension, pre-eclampsia, prenatal smoking, weight gain, and early prenatal care initiation. “NS” indicates not statistically significant at the p < .05 level or better.
Model 2, Panel A incorporates interactions between race/ethnicity and nativity. The main effect coefficients for racial/ethnic categories refer to the US-born. US-born Asian mothers had the highest relative risk of LBW compared to US-born White mothers (RRR=1.74, 95% CI=1.58–1.91), followed by Blacks (RRR=1.71, 95% CI=1.65–1.76) and Hispanics (RRR=1.21, 95% CI=1.16–1.25). For macrosomia, Asian US-born women had the lowest relative risk for macrosomia compared to US-born Whites (RRR=0.47, 95% CI=0.44–0.52), followed by Black women (RRR=0.57, 95% CI=0.56=0.58); US-born Hispanics were the closest to US-born Whites (RRR=0.76, 95% CI=0.74–0.78). The joint test of interaction was statistically significant (p<.001) for LBW and macrosomia. For both LBW and macrosomia, the interaction terms for Black and Hispanic mothers were significantly different from 1.0, indicating that nativity differentials for these groups were significantly different than for White women.
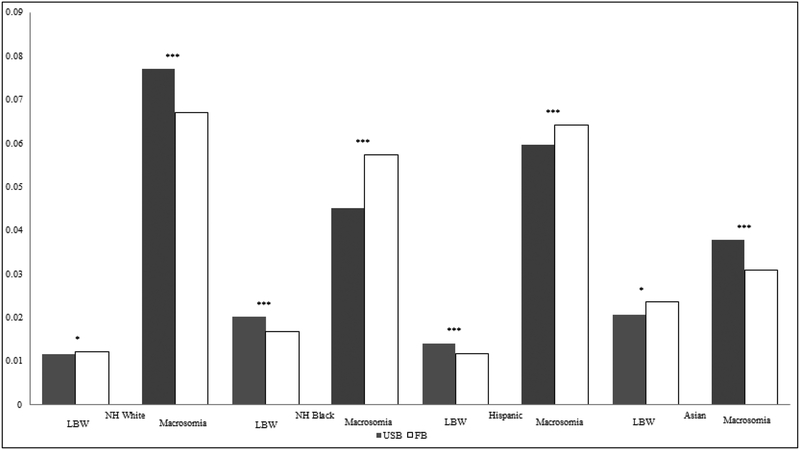
Figure 1 provides predicted probabilities based on the Model 2 interaction results, and indicates (with asterisks) significant nativity differentials within each racial/ethnic group. For both Asians and Whites, foreign-born women had a higher predicted probability for LBW compared to their US-born counterparts. Foreign-born Black and Hispanic women had lower predicted probability for LBW compared to their US-born counterparts. For macrosomia, foreign-born White and Asian women had lower predicted probabilities for macrosomia compared to their US-born counterparts, while foreign-born Black and Hispanic women had higher probabilities.
Figure 1.
Predicted Probabilities of LBW and Macrosomia, by racial/ethnic group and nativity
Predicted probabilities are calculated from Model 2 in Table 2, Panel A. Significance markers represent Wald tests comparing US-born (USB) and foreign-born (FB) women within each racial/ethnic group, *** p<.001, **p<.01, *p<.05
Panel B in Table 2 provides origin country subgroup comparisons for Hispanic women relative to non-Hispanic White women. In Model 1, compared to White women, Mexicans were the only subgroup with significantly lower relative risk for LBW (RRR=0.91, 95% CI=0.86–0.97). Puerto Rican and other Hispanic women had significantly higher relative risk (RRR=1.26, 95% CI=1.21–1.31, RRR=1.17, 95% CI 1.07–1.28). Cuban and Central/South American women were not significantly different in their LBW risk from White women. There was no significant difference in LBW risk by nativity. For macrosomia, all subgroups except for Cubans had a significantly lower relative risk for macrosomia compared to White women. Foreign-born women had significantly lower relative risk of macrosomia than US-born women (RRR=0.91, 95% CI=0.89–0.93).
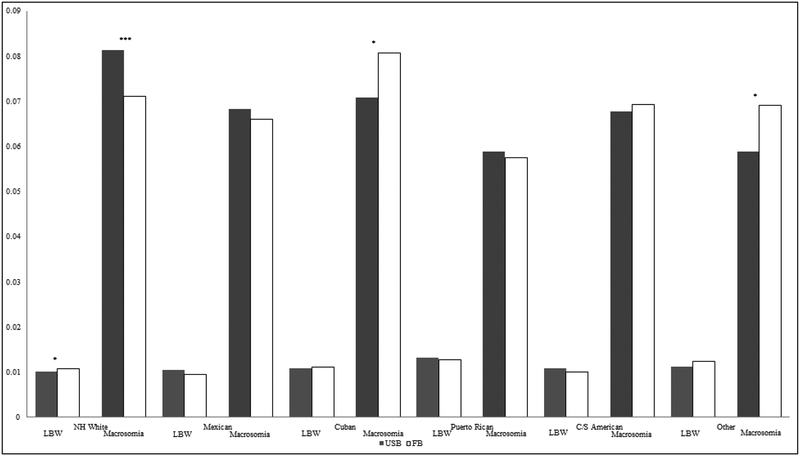
Model 2 includes the interactions between nativity and Hispanic subgroups. For LBW, the joint test of significance for the interactions was statistically significant (p < .05), as were the interaction terms for Puerto Ricans and Central/South Americans. For macrosomia, all interaction terms were significant, except for Mexicans. Figure 2 graphs the predicted probabilities of LBW and macrosomia based on Panel B, Model 2. While White mothers exhibited significant nativity differences, there were few significant nativity differences in either LBW or macrosomia for any of the Hispanic subgroups. The only exceptions were Cubans and Other Hispanics; foreign-born women in these groups had significantly higher risk for macrosomia than their US-born counterparts. The predicted probability of macrosomia for foreign-born Cubans was nearly equivalent to that of US-born Whites.
Figure 2.
Predicted Probabilities of LBW and Macrosomia, by Hispanic sub-group and nativity
Predicted probabilities are calculated from Model 2 in Table 2, Panel B. Significance markers represent Wald tests comparing US-born (USB) and foreign-born (FB) women within each racial/ethnic group, *** p<.001, **p<.01, *p<.05
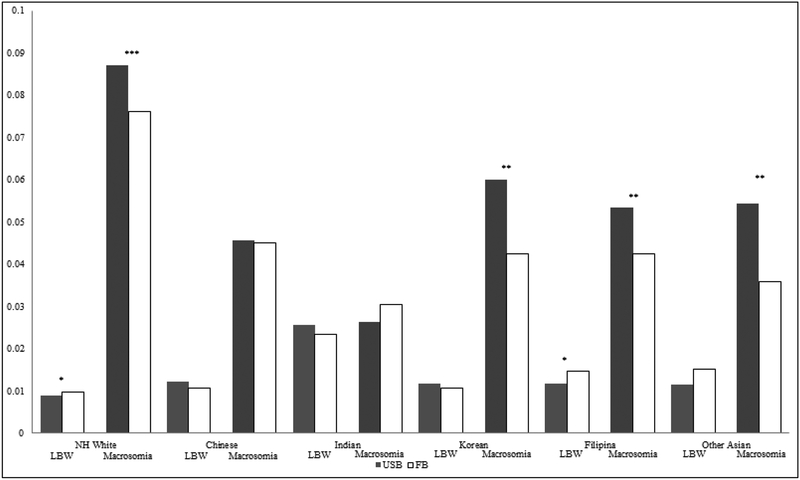
Table 2, Panel C provides the origin country subgroup comparisons for Asian women relative to non-Hispanic White women. In Model 1, all Asian subgroups except Koreans had significantly higher relative risk of LBW compared to White mothers. Foreign-born mothers also had significantly higher risk relative to US-born mothers (RRR=1.05 95% CI=1.01–1.10). For macrosomia, all groups had significantly lower relative risk than White mothers. Foreign-born mothers had significantly lower risk than the US-born (RRR=0.87, 95% CI=0.85–0.89). In Model 2, no interaction terms were significant for LBW. For macrosomia, the interaction with nativity was statistically significant for Asian Indians. The joint test of interaction was significant at the p < .05 level. Figure 3 shows few nativity differences in LBW, with the exception of Filipina mothers, who exhibit an immigrant disadvantage. Foreign-born Korean, Filipina, and other Asian mothers had lower risk for macrosomia relative to their US-born counterparts.
Figure 3.
Predicted Probabilities of LBW and Macrosomia, by Asian sub-group and nativity
Predicted probabilities are calculated from Model 2 in Table 2, Panel C. Significance markers represent Wald tests comparing US-Born and FB women within each racial/ethnic group, *** p<.001, **p<.01, *p<.05
Discussion
To our knowledge, this is the first paper to jointly consider the racial/ethnic and nativity patterning of LBW, normal birth weight, and macrosomia. We found that racial and nativity groups with a low risk for LBW had a higher risk for macrosomia and vice versa. The complementary racial/ethnic and nativity patterning between LBW and macrosomia suggests that instead of elevated risk for both adverse birth outcomes, the social factors associated with LBW simultaneously reduce the risk for macrosomia by shifting the entire birthweight distribution to the left.
Our trends for LBW largely confirmed racial/ethnic patterns observed in previous research; with controls for key covariates, non-Hispanic Black and Asian women had substantially higher risk of LBW compared to non-Hispanic White women, with the risk for Hispanic women much closer to that of Whites (Acevedo-Garcia et al., 2005; James, 1993). The racial/ethnic patterns for macrosomia were nearly perfectly reversed from those of LBW. Whites exhibited the highest risk for macrosomia, followed in descending order by Hispanic, Black, and Asian women. We know of only one other study that has examined racial patterning across a range of birthweights (Alexander et al., 1999). This study similarly found Whites to have higher birthweight percentile values compared to Blacks and Hispanics. Our study built on this work by including Asian women, incorporating subgroup comparisons within the Hispanic and Asian pan-ethnic categories, and considering nativity.
The nativity patterns of LBW and macrosomia were similarly reversed. Among Whites and Asians, immigrants had higher risk of LBW and lower risk for macrosomia compared to their US-born counterparts. Foreign-born Black and Hispanic mothers had lower risk for LBW and higher risk for macrosomia.
The complementarity between LBW and macrosomia was maintained among individual Asian subgroups. All but one Asian subgroup had higher risk of LBW compared to Whites and all subgroups had lower risk for macrosomia. Foreign-born status was particularly protective against macrosomia for Korean, Filipina, and “other” Asian mothers. These complementary patterns were observed among two Hispanic subgroups, as well: Puerto Ricans and other Hispanics; overall, the higher relative risk of LBW observed for Hispanics appears to have been driven by these two subgroups. Mexican mothers represented a notable exception to the dominant pattern. Mexican mothers, regardless of nativity, had birth weights more concentrated in the healthy weight range than White mothers. Thus, instead of either of the two proposed scenarios—complementary patterning or a risk for both LBW and macrosomia—Mexican mothers were protected from both low and excess birthweight.
Finally, the aggregated sample of foreign-born Hispanic women exhibited a higher probability for macrosomia and a lower probability for LBW compared to their US-born counterparts. Exploring these associations within Hispanic subgroups showed this nativity disparity in macrosomia was driven by Cubans and other Hispanics. Interestingly, however, foreign Cubans and Other Hispanic mothers did not have corresponding lower LBW risk. Taken together, these findings suggest unique risks for macrosomia for these subgroups.
Our study contained some limitations. While the New Jersey birth certificate data are diverse with respect to race/ethnicity and nativity, it may not be generalizable to other states. However, the consistency of our LBW findings with published national trends suggests that our data are not anomalous. Further, we were not able to consider detailed migration information, such as duration in the United States or generational status. However birth records files do not typically contain this level of detail. The birth records also did not include pre-pregnancy weight, an important predictor of birthweight and a necessary input to distinguish recommended weight gain from raw weight gain. We were also unable to distinguish very low and very high birthweight from the standard LBW and macrosomia cut-offs due to small cell sizes in subgroup analyses. Finally, some measures, such as race, nativity, and educational status, were self-reported, which could have introduced misreporting bias. In contrast, other measures like birthweight and clinical factors were not self-reported, which is a strength of the data.
Conclusion
Our results raise questions about the racial/ethnic patterning of later associated outcomes, such as childhood obesity. While our results indicated that non-Hispanic White women have the highest risk for macrosomia, their children do not display correspondingly high levels of childhood obesity (Anderson and Whitaker, 2009). Conversely, Hispanic children have the highest prevalence of childhood obesity (Singh et al., 2009), yet we found that Mexican women (who constitute the largest proportion of Hispanics in the U.S.) had a high likelihood of normal birthweight babies. We encourage future work that explores the pathways between birth outcomes, early childhood environments, and later outcomes such as childhood obesity more fully. This work could also consider different etiologies to birthweight that include gestational age and pre-pregnancy weight, and consider a wider range of birth outcomes, such as very low and very high birthweight.
Highlights.
We explore racial/ethnic and nativity patterning across three birthweight categories.
Groups at lowest LBW risk generally have the highest risk for macrosomia.
Some groups have uniquely high risk for macrosomia (e.g., foreign-born Cubans).
Appendix Table 1. Multinomial Logistic Regression of LBW, Normal Birthweight (reference), and Macrosomia, by Race-Ethnicity, Nativity, and Country of Origin, Unadjusted
| Model 1 | Model 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LBW | Macrosomia | LBW | Macrosomia | |||||||||
| RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | |||||
| Maternal race/ethnicity | ||||||||||||
| Non-Hispanic White | ||||||||||||
| Non-Hispanic Black | 2.51 | 2.46 | 2.55 | 0.50 | 0.49 | 0.51 | 2.59 | 2.54 | 2.64 | 0.44 | 0.43 | 0.45 |
| Non-Hispanic Asian | 1.82 | 1.77 | 1.87 | 0.35 | 0.34 | 0.36 | 1.57 | 1.53 | 1.61 | 0.58 | 0.57 | 0.60 |
| Hispanic | 1.53 | 1.50 | 1.57 | 0.63 | 0.62 | 0.64 | 1.48 | 1.37 | 1.59 | 0.40 | 0.37 | 0.44 |
| Mom Foreign-born | 0.76 | 0.74 | 0.77 | 1.03 | 1.01 | 1.04 | 0.86 | 0.83 | 0.89 | 0.87 | 0.85 | 0.88 |
| Maternal race/ethnicity x Foreign-born | ||||||||||||
| Non-Hispanic Black x Foreign-born | 0.79 | 0.75 | 0.83 | 1.74 | 1.66 | 1.81 | ||||||
| Non-Hispanic Asian x Foreign-born | 0.87 | 0.83 | 0.91 | 1.30 | 1.26 | 1.35 | ||||||
| Hispanic x Foreign-born | 1.13 | 1.04 | 1.23 | 0.99 | 0.90 | 1.08 | ||||||
| Maternal ethnicity | ||||||||||||
| Non-Hispanic White | ||||||||||||
| Mexican | 1.33 | 1.29 | 1.37 | 0.68 | 0.66 | 0.70 | 1.38 | 1.24 | 1.54 | 0.59 | 0.53 | 0.65 |
| Cuban | 1.09 | 1.01 | 1.17 | 0.91 | 0.85 | 0.96 | 1.15 | 1.02 | 1.28 | 0.74 | 0.68 | 0.81 |
| Puerto Rican | 1.63 | 1.59 | 1.67 | 0.65 | 0.64 | 0.67 | 1.75 | 1.69 | 1.80 | 0.55 | 0.54 | 0.57 |
| Central/South American | 1.22 | 1.19 | 1.25 | 0.81 | 0.79 | 0.82 | 1.20 | 1.14 | 1.27 | 0.63 | 0.60 | 0.65 |
| Other Hispanic | 1.37 | 1.30 | 1.45 | 0.73 | 0.69 | 0.77 | 1.82 | 1.62 | 2.05 | 0.54 | 0.47 | 0.61 |
| Mom Foreign-born | 0.99 | 0.97 | 1.01 | 0.92 | 0.90 | 0.93 | 0.86 | 0.83 | 0.89 | 0.87 | 0.85 | 0.88 |
| Maternal ethnicity x Foreign-born | ||||||||||||
| Mexican x Foreign-born | 0.90 | 0.80 | 1.02 | 1.14 | 1.03 | 1.26 | ||||||
| Cuban x Foreign-born | 1.20 | 1.00 | 1.44 | 1.46 | 1.28 | 1.66 | ||||||
| Puerto Rican x Foreign-born | 1.17 | 1.09 | 1.25 | 1.16 | 1.09 | 1.23 | ||||||
| Central/South American x Foreign-born | 1.02 | 0.96 | 1.10 | 1.30 | 1.23 | 1.37 | ||||||
| Other Hispanic x Foreign-born | 1.42 | 1.23 | 1.63 | 1.38 | 1.19 | 1.59 | ||||||
| Maternal Ethnicity | ||||||||||||
| Non-Hispanic White | ||||||||||||
| Chinese | 1.16 | 1.10 | 1.23 | 0.58 | 0.55 | 0.61 | 1.04 | 0.87 | 1.24 | 0.43 | 0.37 | 0.51 |
| Asian Indian | 2.50 | 2.43 | 2.58 | 0.39 | 0.38 | 0.41 | 1.99 | 1.77 | 2.24 | 0.24 | 0.19 | 0.29 |
| Korean | 1.22 | 1.15 | 1.30 | 0.54 | 0.51 | 0.58 | 0.96 | 0.74 | 1.25 | 0.58 | 0.47 | 0.71 |
| Filipina | 1.62 | 1.55 | 1.70 | 0.61 | 0.58 | 0.65 | 1.52 | 1.32 | 1.74 | 0.49 | 0.42 | 0.56 |
| Other Asian | 1.96 | 1.83 | 2.09 | 0.47 | 0.43 | 0.52 | 1.38 | 1.03 | 1.87 | 0.51 | 0.38 | 0.68 |
| Mom Foreign-born | 1.03 | 1.00 | 1.05 | 0.85 | 0.84 | 0.87 | 0.86 | 0.83 | 0.89 | 0.87 | 0.85 | 0.88 |
| Maternal Race/Ethnicity x Foreign-Born | ||||||||||||
| Chinese x Foreign-born | 0.90 | 0.73 | 1.09 | 1.32 | 1.11 | 1.58 | ||||||
| Asian Indian x Foreign-born | 0.98 | 0.87 | 1.11 | 1.37 | 1.12 | 1.67 | ||||||
| Korean x Foreign-born | 0.99 | 0.75 | 1.31 | 0.85 | 0.68 | 1.06 | ||||||
| Filipina x Foreign-born | 1.27 | 1.09 | 1.47 | 0.98 | 0.83 | 1.14 | ||||||
| Other Asian x Foreign-born | 1.02 | 0.74 | 1.40 | 0.77 | 0.56 | 1.05 | ||||||
Appendix Table 2. Multinomial Logistic Regression of small for gestational age (SGA), Normal Birthweight (reference), and large for gestational age (LGA), by Race-Ethnicity, Nativity, and Country of Origin LGA and SGA
| Model 1 | Model 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | |||||
| Maternal race/ethnicity | ||||||||||||
| Non-Hispanic White | ||||||||||||
| Non-Hispanic Black | 1.69 | 1.66 | 1.72 | 0.67 | 0.65 | 0.68 | 1.75 | 1.72 | 1.79 | 0.61 | 0.60 | 0.63 |
| Non-Hispanic Asian | 2.29 | 2.24 | 2.34 | 0.44 | 0.43 | 0.45 | 1.99 | 1.87 | 2.11 | 0.51 | 0.47 | 0.55 |
| Hispanic | 1.17 | 1.15 | 1.19 | 0.85 | 0.83 | 0.86 | 1.26 | 1.23 | 1.30 | 0.78 | 0.76 | 0.80 |
| Mom foreign-born | 0.93 | 0.91 | 0.94 | 0.98 | 0.96 | 0.99 | 1.08 | 1.05 | 1.11 | 0.86 | 0.84 | 0.87 |
| Maternal Race/Ethnicity x Foreign-Born | ||||||||||||
| non-Hispanic Black x Foreign-born | 0.79 | 0.76 | 0.83 | 1.45 | 1.39 | 1.51 | ||||||
| non-Hispanic Asian x Foreign-born | 1.03 | 0.96 | 1.10 | 0.96 | 0.89 | 1.05 | ||||||
| Hispanic x Foreign-born | 0.79 | 0.76 | 0.82 | 1.25 | 1.21 | 1.29 | ||||||
| Maternal Ethnicity (ref = nH White) | ||||||||||||
| Mexican | 1.01 | 0.97 | 1.04 | 0.90 | 0.87 | 0.93 | 1.11 | 1.01 | 1.21 | 0.87 | 0.79 | 0.95 |
| Cuban | 1.02 | 0.95 | 1.11 | 0.94 | 0.88 | 1.00 | 1.07 | 0.97 | 1.18 | 0.86 | 0.80 | 0.93 |
| Puerto Rican | 1.32 | 1.29 | 1.35 | 0.76 | 0.74 | 0.78 | 1.34 | 1.30 | 1.38 | 0.74 | 0.72 | 0.76 |
| Central/South American | 1.02 | 1.00 | 1.05 | 0.93 | 0.91 | 0.95 | 1.13 | 1.08 | 1.18 | 0.82 | 0.79 | 0.86 |
| Other Latina | 1.11 | 1.05 | 1.18 | 0.89 | 0.84 | 0.94 | 1.10 | 0.98 | 1.24 | 0.74 | 0.66 | 0.84 |
| Mom foreign-born | 1.02 | 1.00 | 1.04 | 0.91 | 0.89 | 0.93 | 1.08 | 1.05 | 1.11 | 0.86 | 0.84 | 0.88 |
| Maternal Race/Ethnicity x Foreign-Born | ||||||||||||
| Mexican x Foreign-born | 0.86 | 0.78 | 0.95 | 1.09 | 0.99 | 1.21 | ||||||
| Cuban x Foreign-born | 0.86 | 0.73 | 1.00 | 1.28 | 1.13 | 1.45 | ||||||
| Puerto Rican x Foreign-born | 0.92 | 0.87 | 0.98 | 1.15 | 1.09 | 1.22 | ||||||
| Central/South American x Foreign-born | 0.85 | 0.80 | 0.90 | 1.21 | 1.15 | 1.27 | ||||||
| Other Latina x Foreign-born | 0.96 | 0.84 | 1.11 | 1.34 | 1.17 | 1.53 | ||||||
| Maternal Ethnicity (ref = nH White) | ||||||||||||
| Chinese | 1.23 | 1.16 | 1.30 | 0.57 | 0.54 | 0.61 | 1.49 | 1.30 | 1.69 | 0.53 | 0.46 | 0.61 |
| Asian Indian | 2.50 | 2.42 | 2.58 | 0.43 | 0.41 | 0.45 | 2.84 | 2.59 | 3.10 | 0.32 | 0.26 | 0.38 |
| Korean | 1.22 | 1.15 | 1.30 | 0.57 | 0.53 | 0.61 | 1.24 | 1.02 | 1.52 | 0.68 | 0.56 | 0.82 |
| Filipina | 1.64 | 1.56 | 1.72 | 0.59 | 0.56 | 0.63 | 1.63 | 1.45 | 1.83 | 0.61 | 0.53 | 0.69 |
| Other Asian | 1.82 | 1.70 | 1.95 | 0.52 | 0.47 | 0.57 | 1.47 | 1.16 | 1.86 | 0.69 | 0.53 | 0.89 |
| Mom foreign-born | 1.07 | 1.04 | 1.10 | 0.86 | 0.84 | 0.88 | 1.09 | 1.06 | 1.12 | 0.86 | 0.84 | 0.88 |
| Maternal Race/Ethnicity x Foreign-Born | ||||||||||||
| Chinese x Foreign-born | 0.79 | 0.68 | 0.91 | 1.10 | 0.94 | 1.29 | ||||||
| Asian Indian x Foreign-born | 0.86 | 0.78 | 0.95 | 1.38 | 1.15 | 1.66 | ||||||
| Korean x Foreign-born | 0.96 | 0.78 | 1.19 | 0.83 | 0.67 | 1.02 | ||||||
| Filipina x Foreign-born | 0.99 | 0.87 | 1.12 | 0.97 | 0.84 | 1.12 | ||||||
| Other Asian x Foreign-born | 1.24 | 0.97 | 1.59 | 0.73 | 0.55 | 0.97 | ||||||
Note: Models controlled for maternal education, Medicaid participation, employed in year prior to birth, married, maternal age, gestational age, male infant, parity, chronic diabetes, gestational diabetes, pregnancy-induced hypertension, chronic hypertension, pre-eclampsia, prenatal smoking, weight gain, and early prenatal care initiation. “NS” indicates not statistically significant at the p < .05 level or better.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Annie Ro, Email: annie.ro@uci.edu, Program in Public Health, University of California, Irvine, 653 E. Peltason Drive, AIRB 2036, Irvine, CA 92617.
Rachel E. Goldberg, Department of Sociology, University of California, Irvine.
Jennifer B. Kane, Department of Sociology, University of California, Irvine.
References
- Acevedo-Garcia D, Soobader M-J, Berkman LF, 2007. Low birthweight among US Hispanic/Latino subgroups: The effect of maternal foreign-born status and education. Social Science & Medicine 65:2503–16. [DOI] [PubMed] [Google Scholar]
- Acevedo-Garcia D, Soobader MJ, Berkman LF, 2005. The differential effect of foreign-born status on low birth weight by race/ethnicity and education. Pediatrics 115:E20–E30. [DOI] [PubMed] [Google Scholar]
- Alexander GR, Kogan MD, Himes JH, 1999. 1994–1996 U.S. Singleton Birth Weight Percentiles for Gestational Age by Race, Hispanic Origin, and Gender. Maternal and Child Health Journal 3:225–31. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Whitaker RC, 2009. Prevalence of obesity among US preschool children in different racial and ethnic groups. Arch Pediatr Adolesc Med 163:344–8. [DOI] [PubMed] [Google Scholar]
- Barker DJ, 1995. Fetal origins of coronary heart disease. BMJ 311:171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, Kawachi I, 2014. A Historial Framework for Social Epidemiology, in: Berkman LF, Kawachi I, Glymour M (Eds.), Social Epidemiology. Oxford Unviersity Press, New York, New York. [Google Scholar]
- Borrell LN, Rodriguez-Alvarez E, Savitz DA, Baquero MC, 2016. Parental Race/Ethnicity and Adverse Birth Outcomes in New York City: 2000–2010. American Journal of Public Health 106:1491–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P, 2011. Black–white disparities in birth outcomes: is racism-related stress a missing piece of the puzzle?, in: Lemelle AA, Reed W, Taylor S (Eds.), Handbook of African American Health Social and Behavioral Interventions. Spring, New York, NY, pp. 155–63. [Google Scholar]
- CDC, 2011. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. National Center for Chronic Disease Prevention and Health Promotion, Atlanta, GA. [Google Scholar]
- Chatfield J, 2001. ACOG Issues Guidelines on Fetal Macrsomia. American Family Physician 64:169–70. [PubMed] [Google Scholar]
- Chen R, Wax Y, Lusky A, Toppelberg G, Barell V, 1991. A criterion for a standardized definition of low birthweight. Int J Epidemiol 20:180–6. [DOI] [PubMed] [Google Scholar]
- Dar P, Gross SJ, 2000. Macrosomia: A genetic perspective. Clin Obstet Gynecol 43:298–308. [DOI] [PubMed] [Google Scholar]
- David RJ, Collins JWJ, 1997. Differing Birth Weight among Infants of U.S.-Born Blacks, African-Born Blacks, and U.S.-Born Whites. New England Journal of Medicine 337:1209–14. [DOI] [PubMed] [Google Scholar]
- de Bernabe JV, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martinez D, Dominguez-Rojas V, 2004. Risk factors for low birth weight: a review. Eur J Obstet Gyn R B 116:3–15. [DOI] [PubMed] [Google Scholar]
- Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, Sandman CA, 2008. Racial differences in birth outcomes: The role of general, pregnancy, and racism stress. Health Psychol 27:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois L, Girard M, Tatone-Tokuda F, 2007. Determinants of high birth weight by geographic region in Canada. Chronic Diseases in Canada 28:63–70. [PubMed] [Google Scholar]
- El-Sayed AM, Galea S, 2011. Maternal immigrant status and high birth weight: implications for childhood obesity. Ethn Dis 21:47–51. [PubMed] [Google Scholar]
- Forna F, Jamieson DJ, Sanders D, Lindsay MK, 2003. Pregnancy outcomes in foreign-born and US-born women. Int J Gynaecol Obstet 83:257–65. [DOI] [PubMed] [Google Scholar]
- Frey WH, 2006. Immigration Goes Nationwide: Recent dispersal has made immigrants and new minorities more visible. The Brookings Institution, Washington, DC. [Google Scholar]
- Gaudet L, Ferraro ZM, Wen SW, Walker M, 2014. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. Biomed Res Int 2014:640291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, 1996. Black/white differences in the relationship of maternal age to birthweight: A population-based test of the weathering hypothesis. Social Science & Medicine 42:589–97. [DOI] [PubMed] [Google Scholar]
- Hedderson MM, Darbinian JA, Ferrara A, 2010. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol 24:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T, 2008. The macrosomic fetus: a challenge in current obstetrics. Acta Obstet Gyn Scan 87:134–45. [DOI] [PubMed] [Google Scholar]
- James SA, 1993. Racial and ethnic differences in infant mortality and low birth weight A psychosocial critique. Annals of Epidemiology 3:130–36. [DOI] [PubMed] [Google Scholar]
- Juarez SP, Revuelta-Eugercios BA, 2014. Too heavy, too late: investigating perinatal health outcomes in immigrants residing in Spain. A cross-sectional study (2009–2011). J Epidemiol Community Health 68:863–8. [DOI] [PubMed] [Google Scholar]
- Kaufman JS, Cooper RS, 2001. Commentary: Considerations for use of racial/ethnic classification in etiologic research. Am J Epidemiol 154:291–98. [DOI] [PubMed] [Google Scholar]
- Kim D, Saada A, 2013. The Social Determinants of Infant Mortality and Birth Outcomes in Western Developed Nations: A Cross-Country Systematic Review. International Journal of Environmental Research and Public Health 10:2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahmann PH, Wills R-A, Coory M, 2009. Trends in birth size and macrosomia in Queensland, Australia, from 1988 to 2005. Paediatric and Perinatal Epidemiology 23:533–41. [DOI] [PubMed] [Google Scholar]
- Langer O, 2000. Fetal macrosomia: Etiologic factors. Clin Obstet Gynecol 43:283–97. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Fraser A, Lindsay RS, Ness A, Dabelea D, Catalano P, Davey Smith G, Sattar N, Nelson SM, 2010. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 53:89–97. [DOI] [PubMed] [Google Scholar]
- Nguyen BT, Cheng YW, Snowden JM, Esakoff TF, Frias AE, Caughey AB, 2012. The effect of race/ethnicity on adverse perinatal outcomes among patients with gestational diabetes mellitus. American Journal of Obstetrics and Gynecology 207:322.e1–22.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneth NS, 1995. The problem of low birth weight. Future Child 5:19–34. [PubMed] [Google Scholar]
- Ragnarsdottir LH, Conroy S, 2010. Development of Macrosomia Resulting From Gestational Diabetes Mellitus: Physiology and Social Determinants of Health. Advances in Neonatal Care 10:7–12. [DOI] [PubMed] [Google Scholar]
- Restrepo-Mesa SL, Estrada-Restrepo A, Gonzalez-Zapata LI, Agudelo-Suarez AA, 2015. Newborn birth weights and related factors of native and immigrant residents of Spain. J Immigr Minor Health 17:339–48. [DOI] [PubMed] [Google Scholar]
- Singh GK, Kogan MD, Yu SM, 2009. Disparities in Obesity and Overweight Prevalence Among US Immigrant Children and Adolescents by Generational Status. Journal of Community Health 34:271–81. [DOI] [PubMed] [Google Scholar]
- Singh GK, Yu SM, 1996. Adverse pregnancy outcomes: differences between US- and foreign-born women in major US racial and ethnic groups. American Journal of Public Health 86:837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PJ, Roberson E, Dye T, 2013. Gestational diabetes and macrosomia by race/ethnicity in Hawaii. BMC Res Notes 6:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartko PD, Wong EY, Enquobahrie DA, 2017. Maternal Birthplace is Associated with Low Birth Weight Within Racial/Ethnic Groups. Matern Child Health J 21:1358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen IH, Syme SL, 1999. The Social Environment and Health: A Discussion of the Epidemiologic Literature. Annual Review of Public Health 20:287–308. [DOI] [PubMed] [Google Scholar]
- Zhang X, Decker A, Platt RW, Kramer MS, 2008. How big is too big? The perinatal consequences of fetal macrosomia. Am J Obstet Gynecol 198:517 e1–6. [DOI] [PubMed] [Google Scholar]