Abstract
Objective.
To explore the association between findings on advanced, but available, magnetic resonance imaging (MRI) sequences of the cervical spinal cord and muscular system, in tandem with biomechanical measures of maximum volitional plantar flexion torques as a proxy for a mild incomplete spinal cord injury.
Design.
Observational case series.
Setting.
University research laboratory.
Subjects.
Three patients with chronic whiplash and one patient with history of whiplash injury but no current symptoms.
Methods.
We measured lower extremity muscle fat, morphological changes in descending spinal cord pathways with advanced MRI applications and maximal activation of the plantar flexors.
Results.
Larger magnitudes of lower extremity muscle fat corresponded to altered spinal cord anatomy and reductions in the ability to maximally activate plantar flexor torques in the three subjects with chronic whiplash. Such findings were not present in the recovered participant.
Conclusions.
The potential value of MRI to quantify neuromuscular degeneration in chronic whiplash is recognized. Larger scaled prospective studies are warranted before stronger conclusions can be drawn.
Keywords: MRI, Whiplash, Neurology, Neck (Pain), Spinal Cord
Introduction
Whiplash-associated disorders (WADs) from motor vehicle collisions (MVCs) afflict over 4-million Americans annually, reducing quality of life and accounting for an estimated $100 billion in indirect medical costs [1]. Up to 50% will fail to fully recover [2], presenting with complex physical and psychological signs/symptoms, suggesting whiplash is not only a medical issue but also a condition that is influenced by external noninjury-related factors [3]. We report three chronic cases whose clinical presentation bears striking similarities to the known pathophysiology of incomplete spinal cord injury, including anatomical evidence of spinal tract damage, muscle fat infiltration, and reduced volitional generation of plantar flexion torques. It is likely that similar cases are under-recognized and these, considering current treatments have not substantially influenced functional recovery [4,5], could be crucial for exploring and developing more informed and effective management options.
Three patients with chronic whiplash were evaluated in a research laboratory because of persistent pain-related disability of varying durations. Consistent across the three patients was the lack of available findings for structural cervical spine pathology from imaging studies (magnetic resonance imaging [MRI], computed tomography [CT], radiography). The purpose of this proof-of-concept study was to explore the association between findings on advanced, but available, MRI sequences of the cervical spinal cord and muscular system, in tandem with biomechanical measures of maximum volitional plantar flexion torques as a proxy for an initial injury involving the cervical spinal cord. One patient who nominated full recovery at 3-months post whiplash event was included for comparison.
Patient 1
A 42-year-old man with 3 years of persistent pain-related disability in addition to cognitive impairments, insomnia, psychological distress (depression, post-traumatic stress disorder), social withdrawal, labile temperament, and poor endurance in the neck and lower extremity muscles reports his head was turned left when struck from behind while stopped at a red light waiting to make a right hand turn. The bullet “or striking” vehicle was traveling approximately 25 miles/h. His medical history was notable for previous low back pain with complete symptom resolution following L5/S1 lumbar fusion in 2001. It is noteworthy that a previous spinal surgery has been implicated as a prognostic factor for delayed recovery in whiplash [3]. The patient had no prior history of mental or systemic illnesses. He has returned to full-time employment as a computer software consultant. Numerous imaging studies (radiography, MRI, CT) were unremarkable for salient pathology. Ongoing treatment included over 70 visits of physical therapy and chiropractic combined, eight Botulinum Toxin injections (BOTOX® [onabotulinumtoxinA], Allergan, Inc. Irvine, CA, USA) to the neck muscles, and two intraarticular cervical facet injections, all of which provided short-term palliative relief. Brief assessment of reflexes indicated hyperexcibility of bilateral patellar and triceps surae responses, with one to two beats of clonus during the latter assessments. The patient did not demonstrate positive Babinski responses. Current medications: tigabine 4 mg PO QID, alprazolam 0.25 mg PO BID, hydrocodone 5/325 mg PO BID, and ibuprofen 200 mg PO QID.
Patient 2
A 41-year-old female with 4 years of persistent pain-related disability, widespread hyperalgesia, difficulty concentrating, insomnia, psychological distress, fatigue, and general muscle weakness in the neck and bilateral lower extremities was struck from behind while traveling at ~35 miles/h with straight head position on impact from the bullet vehicle traveling ~75 miles/h. Her medical history was notable for depression but no physical ailments requiring medical or rehabilitative treatment. She was unable to work as a registered nurse. Clinical presentation included positive Babinski reflexes and elevated patellar tendon-tap responses bilaterally. Current medications: hydrocodone 5/325 mg PO PRN for pain, pregabalin 50 mg PO TID, metaxalone 800 mg PO BID, and ibuprofen 800 mg PO PRN.
Patient 3
A 27-year-old female with 3.5 months of pain-related disability, widespread hyperalgesia, weakness, and fatigue of the neck and bilateral lower extremities, difficulty concentrating, insomnia, and signs of psychological distress was struck from behind while stopped at a traffic light and reports her head was turned to the right at time of impact. The bullet vehicle was traveling ~15 miles/h. Her medical history was notable for depression but no physical ailments requiring medical or rehabilitative treatment. She was working part-time as a professional musician. Treatment continued to include physical therapy (20+ visits to date). Clinical presentation included positive bilateral Babinski responses, with normal tendon-tap responses in the lower extremities. Current medications: tizanidine 4 mg PO BID, diazepam 5 mg PO BID, and ibuprofen 600 mg PO BID.
Patient 4
A 28-year-old female self-nominated full recovery at 3-months post MVC, where she was struck from behind while stopped at a traffic light and reports her head was straight at the time of impact. The bullet vehicle was traveling ~10 miles/h. Her medical history was unremarkable and she was working full-time as a university research lab assistant. The patient demonstrated no altered reflex excitability upon clinical assessment, and she was taking ibuprofen 600 mg PO TID.
Methods
Each patient underwent the following lab based protocols and pain-related disability was measured using percentage scores on the neck disability index (NDI). The NDI is a 10-item questionnaire that enjoys considerable support as a valid and reliable measure of neck-related disability. Higher scores equate to more pain-related disability[6].
3D Fat–Water Separation MRI
A number of MRI techniques, based on the different precessional frequencies of fat and water protons, can be used to quantify muscle fat. One such technique, a three-dimensional (3D) multi-echo gradient echo acquisition fat/water separation, can be used to rapidly collect the data required for the quantitative analysis of fat and water [7]. Accordingly, a 3D two point Dixon fat–water separation technique, which has demonstrated accuracy for fat quantification when compared with the gold standard, muscle biopsy [8], was performed for the neck extensor muscles (C3–C7) and the bilateral lower extremity muscles for all subjects on a 3 T Siemens magnet (Siemens, Erlangen, Germany). For the cervical spine, a standard 12-channel head coil and 4-channel neck coil were used as receiver coils to improve signal to noise. The axial FLASH dual echo, gradient echo sequence had duration of 4.23 minutes with an in-plane resolution of 0.49 mm and thickness of 3 mm. A single slab was placed over the cervical spine. The same sequence was used in the lower extremities but required 5.13 minutes to cover the increased field of view. Both the left and right lower extremities were acquired in a single acquisition using a 16-channel body array surface coil. Image reconstruction calculated the in-phase, opposed-phase, fat-only, and water only images. Muscle fat data were combined for the right and left neck extensors as well as the bilateral plantar and dorsiflexors.
Magnetization Transfer Imaging of the Cervical Spinal Cord
Characterizing the demyelination/degeneration of ascending and descending white matter spinal pathways in patients with cervical SCI is crucial for assessing prognosis of functional rehabilitation [9]. Magnetization transfer (MT) imaging provides a more sensitive and specific marker of white matter pathology in the cervical cord when compared with traditional T1- and T2-weighted signals [9]. Furthermore, MT measures of the spinal pathways in SCI have shown to be predictive of motor and sensory disability levels, suggesting a noninvasive MRI measure of cord degeneration and determination of impairment is possible [9]. Accordingly, MT MRI was used to quantify the myelin content in the cervical spinal cord white matter. We employed the imaging method of multiple-echo data image combination, which samples the magnetization at several echoes and combines them to improve the signal to noise without increasing scan time or increasing specific absorption rate. The MT scan was4.06 minutes with an in-plane resolution of 0.25 mm, a slice thickness of 4 mm, and 11 slices oriented parallel to the disk planes of C4–C6. The MT pulse was applied1.5 kHz off-resonance with a 540° flip angle and 10 millisecond long duration to saturate the bound water pool. The non-MT scan was identical except that the MT saturation pulse was turned off and run as a separate acquisition but co-registered with the MT scan. The MT ratio (MTR) was calculated on a voxel-by-voxel basis.
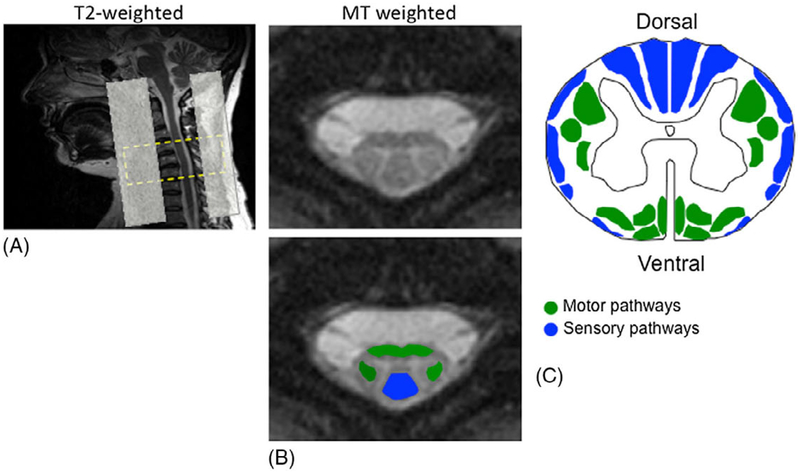
Regions of interest (ROIs) at the C5 vertebral level were placed in spinal white matter containing the ventromedial and the dorsolateral descending motor pathways, and the dorsal columns (Figure 1).
Figure 1.
(A) T2-weighted sagittal localizer demonstrating typical patient positioning and the slice prescription for MT weighted acquisition, covering C4–C6 vertebral levels. Saturation bands were set ventrally and dorsally to limit aliasing and ghosting artifacts. (B) Anatomically defined ROIs on the MT-weighted image (for MTR) were selected over the ventromedial and dorsolateral (green) descending motor pathways and the dorsal columns (blue) of the cervical spinal cord. (C) Motor and sensory pathways.
Central Activation Ratios of the Plantar Flexors
Volitional torque generation and central activation of the plantar flexors were determined by having each subject perform three baseline maximum volitional-effort (MVE) isometric contractions of 3–8 seconds duration of the plantar flexors in an isokinetic dynamometer (Biodex Rehabilitation System v3, Biodex Medical Systems, Shirley, NY, USA) attached to a six degree of freedom load cell (ATI, Apex, NC, USA) while receiving identical standardized verbal encouragement under constant environmental conditions. To assess any central activation failure during each baseline MVE, a brief train of electrical stimulation (10 pulses, 600 microsecond duration, 100 Hz, 135 V; Grass S48, external isolation; Grass Technologies, West Warwick, RI, USA) was delivered through 8 × 13 cm stimulation electrodes placed over the plantar flexor muscle surface. Stimulation was triggered manually when torques appeared to reach a plateau during maximal efforts. The electrically elicited torque superimposed on the maximum volitional torque was used to estimate voluntary plantar flexor activation by: mean volitional torque (100 millisecond epoch prior to stimulation)/peak torque with stimulation.
The result was termed the central activation ratio (CAR) with a range of 0.0–1.0 (normal >0.85–0.90).
We certify that all applicable institutional regulations concerning the ethical use of human volunteers were followed during the course of this investigation.
Results
In the three patient cases, duration of symptoms was 36, 48, and 3.5 months with NDI scores of 62%, 68%, and 48%, indicating severe disability. Table 1 displays demographic details for the three subjects and the overall MRI and plantar flexor output measures. The magnitude of neck muscle fatty infiltrates was consistent with previous reports [10], but we remain unaware of any available comparison data for plantar flexor muscle fat in whiplash or iSCI. Accordingly, one subject, that nominated full recovery at 3 months post whiplash exposure from a MVC, was included to provide a visual and quantitative comparison of lower extremity muscle fat infiltration, MTR data, and plantar flexor CARs (Figure 2A,B and Table 1). All three chronic participants demonstrated lowered MTRs in selected regions of the spinal cord that were consistent with previous reports from known SCI [9], suggestive of reduced myelin in spinal regions corresponding to the location of sensorimotor pathways. CARs ranged from 58–62% in the three subjects compared with 100% in the recovered subject. In all three chronic cases we found that the expression of lower leg muscle fat infiltration corresponded to altered cervical spinal cord pathway integrity and reductions in the ability to maximally generate plantar flexion torques and muscle fatigue.
Table 1.
Results of the lab based protocols
| Subject Age (Gender) |
Muscle Fat (%) | Mean (sd) MTR* | Plantar Flexor CAR† |
Neck Disability Index % Score (Time)§ |
Head Rotation/ Position at Impact¶ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neck | Legs‡ | Spinal Cord C5 | |||||||||
| Right | Left | Total | Ventral | Right | Left | Dorsal | |||||
| Severe 42 (M) | 30 | 20.9 | 12.0 | 16.5 | 38.93 (1.90) | 25.95 (0.71) | 47.18 (1.01) | 27.02 (2.80) | 0.62 | 62 (36 months) | Left |
| Severe 41 (F) | 29 | 15.2 | 15.8 | 14.6 | 32.42 (1.30) | 42.84 (0.75) | 39.98 (1.17) | 28.44 (0.74) | 0.58 | 68 (48 months) | Straight |
| Severe 27 (F) | 31 | 15.2 | 14.5 | 15.9 | 31.67 (2.63) | 40.64 (0.41) | 21.98 (1.19) | 29.98 (0.64) | 0.62 | 48 (3.5 months) | Right |
| Recovered 28 (F) | 10.5 | 7.4 | 7.2 | 7.6 | 38.79 (1.33) | 45.38 (3.51) | 42.55 (0.88) | 43.65 (0.99) | 1.00 | 4 (3 months) | Straight |
Reductions relative to one another suggest degeneration in specific regions of the cervical spinal cord.
Lower CAR values suggest reductions in volitional torque production.
Total muscle fat in legs = right and left values of plantar- and dorsiflexors combined.
Elapsed time since MVC.
Subjective report from the patient involved in rear-end impact.
MTR = magnetization transfer ratio; CAR = central activation ratio; MVC = motor vehicle collision.
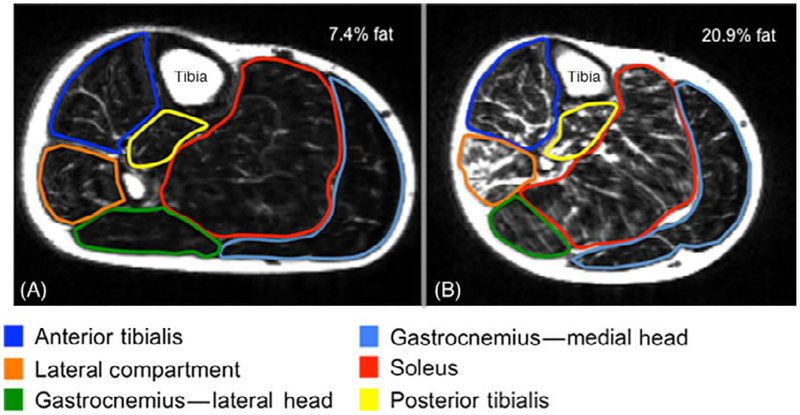
Figure 2.
MRI (fat-only image) of the right plantar/dorsiflexors in (A) recovered subject and (B) severe whiplash subject. Note the increased signal throughout the plantar/dorsiflexors in the severe whiplash subject(B) suggestive of fatty infiltrates. This is not observed in the recovered whiplash subject (A).
NB. The data for the recovered subject (A) (at 3 months post-injury event) was presented solely for visual observation of and comparison with the lower extremity muscles in this chronic subject (B).
Discussion
These findings provide preliminary evidence to suggest that the expression of neck and lower extremity muscle fatty infiltrates and reduced central activation in this small sample of patients with chronic WAD could very-well be the result of an initial mild injury involving the spinal cord, as suggested from the cord MTRs. While the preliminary observations are intriguing, larger-scale, prospective investigations over an extended duration and across a sample of participants with varying levels of functional disability are necessary to draw confident conclusions.
Characterizing the integrity of white matter spinal pathways in SCI is crucial for assessing prognosis with functional rehabilitation [9]. MTR measures of the spinal cord have shown to be predictive of motor and sensory disability levels following a SCI, suggesting a non-invasive MRI determination of impairment is possible [9]. Significant abnormalities from cord and brain MTR have also been reported for other common conditions, e.g., SCI [11], Parkinson’s disease [12,13], multiple sclerosis [14], stroke[15], and Alzheimer’s disease [16].
As the attempt to evidence structural pathology in whiplash by conventional MRI is usually inconclusive [17], a non-invasive MTR measure could prove valuable for identifying region-specific neuronal injury in spinal cord pathways and for determining the clinical course in high-risk patients. The three chronic subjects demonstrated MTRs of the regional cervical cord that suggest a loss in spinal cord motor pathways. This theory is further supported by the concordant findings of muscle fatty infiltration of the neck and lower leg muscles and markedly reduced plantar flexor CARs in the three patients with chronic WAD.
The origins of muscle fatty infiltration are complex and it would be premature to ascribe the results seen here to purely iSCI. The utility of our model describing the expression of muscle fatty infiltrates and central activation deficits secondary to a mild injury of the cord could be improved by concurrently testing the influence of decreased patient activity levels [18]. It has been documented that decreased activity after a MVC increases the risk of chronic WAD [19], and that depriving previously fit individuals of activity may lead to fatigue, mood swings, and higher levels of muscle fat [20]. However, inactivity (or disuse) would not fully explain the findings of dramatic reductions in CARs observed in our three chronic cases(0.58–0.62), as the average reported CARs of aging and less active knee extensors range from 0.87 to 0.95 [21]. While inactivity may play a role, it does not fully explain the findings reported herein. It is also prudent to consider the potential influence of a neuroinflammatory response [22] that could affect the functioning of the peripheral and central nervous system [23] as well the structure (and possibly functional strength) of the skeletal muscle system[24].
Measures of reduced volitional maximum plantar flexor torques, as determined with electrically elicited twitch interpolation, suggest signs of disrupted descending neural commands (e.g., central activation deficits) [25,26] and are observed in our three patients with severe WAD. The low CAR values in our participants (0.58–0.62) are consistent with previous results for quadriceps in those with known SCI [27]. While all three patients with severe WAD were prescribed medications, which depress central excitability and could alter CARs, the magnitude of deficits in central activation are in stark contrast to data for able-bodied individuals, and unlikely due to medication use[27]. Further, the magnitude of changes in central activation are unlikely due to disuse or reduced function, as indicated in other orthopedic populations (CARs:0.80–0.90) [28].
Accumulating evidence suggests that chronic whiplash is not only a medical condition but also one that is heavily influenced by external, noninjury-related factors [3]. A recent retrospective review of over 5,500 patients with whiplash indicated that individuals with chronic WAD-related disability were more likely to (1) be female; (2) present clinically with lower limb pain or “nonorganic” signs; (3) have returned to work; (4) retained a lawyer; and/or (5) have undergone a previous spinal surgery unrelated to the whiplash (all or some of these factors are similar to our three patient cases). The authors concluded that many medical and noninjury factors influence outcomes [3]. While we do not dispute this position, it is our contention that combining available measures to quantify muscle degeneration, altered spinal cord integrity, volitional muscle activation, and key psychosocial factors (e.g., coping, expectations, anxiety, and depression) is crucial for advancing our mechanistic understanding of why some, but not others, develop chronic WAD-related disability.
Conclusions
These observational data provide foundation for future research focusing on available imaging applications to quantify losses in neural substrates within specific spinal cord pathways following traumatic spinal injuries. This work sets focus on quantifying deficits in motor output in a larger population of patients with varying levels of pain-related disability following a whiplash injury. Such data could provide a more comprehensive picture to support the hypothesis that a mild injury involving the cervical spinal cord, in tandem with associated psychosocial factors, predicts the clinical course of whiplash in high-risk patients. The implications for exploring and developing more informed treatments such as specific exercise, pharmacological and psychological control of pain, and emerging physical rehabilitation interventions consistent with current evidenced-based treatment of patients with incomplete SCI are clear.
Acknowledgments
We thank Mark Hoggarth, Ryan Schmid, and Hyosub Kim for their assistance in collecting data for this proof-of-concept study.
Footnotes
Conflict of Interest: Elliott and Parrish have ownership and investment interests in a medical consultation startup, Pain ID, LLC. James Elliott is supported by a NIH KL2 grant-KL2 RR025740.
This study was initiated, conducted, and completed at Northwestern University, Feinberg School of Medicine—Department of Physical Therapy and Human Movement Sciences.
References
- 1.Naumann RB, Dellinger AM, Zaloshnja E, Lawrence B, Miller TR. Incidence and total lifetime costs of motor vehicle-related fatal and nonfatal injury by road user type, United States. Traffic Inj Prev 2010;11:353–60. [DOI] [PubMed] [Google Scholar]
- 2.Carroll LJ, Holm LW, Hogg-Johnson S, et al. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): Results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33:S83–92. [DOI] [PubMed] [Google Scholar]
- 3.Dufton JA, Bruni SG, Kopec JA, Cassidy JD, Quon J. Delayed recovery in patients with whiplash-associated disorders. Injury 2012;43:1141–7. [DOI] [PubMed] [Google Scholar]
- 4.Jull G, Kenardy J, Hendrikz J, Cohen M, Sterling M. Management of acute whiplash: A randomized controlled trial of multidisciplinary stratified treatments. Pain 2013; 154:1798–806. [DOI] [PubMed] [Google Scholar]
- 5.Lamb SE, Gates S, Williams MA, et al. Emergency department treatments and physiotherapy for acute whiplash: A pragmatic, two-step, randomised controlled trial. Lancet 2013; 381:546–56. [DOI] [PubMed] [Google Scholar]
- 6.Vernon H, Mior S. The neck disability index: A study of reliability and validity. J Manipulative Physiol Ther 1991; 14:409–15. [PubMed] [Google Scholar]
- 7.Elliott JM, Walton DM, Rademaker A, Parrish TB. Quantification of cervical spine muscle fat: A comparison between T1-weighted and multi-echo gradient echo imaging using a variable projection algorithm (VARPRO). BMC Med Imaging 2013; 13:30. doi: 10.1186/1471-2342-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith AC, Parrish TB, Abbott R, et al. Muscle-fat magnetic resonance imaging: 1.5 Tesla and 3.0 Tesla vs histology. Muscle Nerve 2014; 50:170–6. doi: 10.1002/mus.24255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen-Adad J, El Mendili MM, Lehericy S, et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 2011;55:1024–33. [DOI] [PubMed] [Google Scholar]
- 10.Elliott J, Pedler A, Kenardy J, et al. The temporal development of fatty infiltrates in the neck muscles following whiplash injury: An association with pain and posttraumatic stress. PLoS ONE 2011;6: e21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SA, Pekar JJ, van Zijl PC. Advanced MRI strategies for assessing spinal cord injury. Handb Clin Neurol 2012;109:85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckert T, Sailer M, Kaufmann J, et al. Differentiation of idiopathic Parkinson’s disease, multiple system atrophy, progressive supranuclear palsy, and healthy controls using magnetization transfer imaging. Neuroimage 2004;21:229–35. [DOI] [PubMed] [Google Scholar]
- 13.Tambasco N, Pelliccioli GP, Chiarini P, et al. Magnetization transfer changes of grey and white matter in Parkinson’s disease. Neuroradiology 2003;45:224–30. [DOI] [PubMed] [Google Scholar]
- 14.Dehmeshki J, Chard DT, Leary SM, et al. The normal appearing grey matter in primary progressive multiple sclerosis: A magnetisation transfer imaging study. J Neurol 2003;250:67–74. [DOI] [PubMed] [Google Scholar]
- 15.Homayoon N, Ropele S, Hofer E, et al. Microstructural tissue damage in normal appearing brain tissue accumulates with Framingham Stroke Risk Profile Score: Magnetization transfer imaging results of the Austrian Stroke Prevention Study. Clin Neurol Neurosurg 2013;115:1317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanyu H, Shimizu S, Tanaka Y, et al. Differences in magnetization transfer ratios of the hippocampus between dementia with Lewy bodies and Alzheimer’s disease. Neurosci Lett 2005;380:166–9. [DOI] [PubMed] [Google Scholar]
- 17.Sterling M, McLean SA, Sullivan MJ, et al. Potential processes involved in the initiation and maintenance of whiplash-associated disorders: Discussion paper 3. Spine (Phila Pa 1976) 2011;36:S322–9. [DOI] [PubMed] [Google Scholar]
- 18.McLean SA, Clauw DJ, Abelson JL, Liberzon I. The development of persistent pain and psychological morbidity after motor vehicle collision: Integrating the potential role of stress response systems into a biopsychosocial model. Psychosom Med 2005;67: 783–90. [DOI] [PubMed] [Google Scholar]
- 19.Borchgrevink GE, Kaasa A, McDonagh D, et al. Acute treatment of whiplash neck sprain injuries. A randomized trial of treatment during the first 14 days after a car accident. Spine 1998;23:25–31. [DOI] [PubMed] [Google Scholar]
- 20.Manini TM, Clark BC, Nalls MA, et al. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 2007;85:377–84. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida Y, Marcus RL, Lastayo PC. Intramuscular adipose tissue and central activation in older adults. Muscle Nerve 2012;46:813–6. [DOI] [PubMed] [Google Scholar]
- 22.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 2009;10:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuve O, Zettl U. Neuroinflammation of the central and peripheral nervous system: An update. Clin Exp Immunol 2014;175:333–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterling M, Elliott JM, Cabot PJ. The course of serum inflammatory biomarkers following whiplash injury and their relationship to sensory and muscle measures: A longitudinal cohort study. PLoS ONE 2013;8: e77903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulow PM, Norregaard J, Mehlsen J, Danneskiold-Samsoe B. The twitch interpolation technique for study of fatigue of human quadriceps muscle. J Neurosci Methods 1995;62:103–9. [DOI] [PubMed] [Google Scholar]
- 26.Lin KH, Chen YC, Luh JJ, Wang CH, Chang YJ. H-reflex, muscle voluntary activation level, and fatigue index of flexor carpi radialis in individuals with incomplete cervical cord injury. Neurorehabil Neural Repair 2012;26:68–75. [DOI] [PubMed] [Google Scholar]
- 27.Hornby TG, Lewek MD, Thompson CK, Heitz R. Repeated maximal volitional effort contractions in human spinal cord injury: Initial torque increases and reduced fatigue. Neurorehabil Neural Repair 2009;23: 928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtney CA, Lewek MD, Witte PO, Chmell SJ, Hornby TG. Heightened flexor withdrawal responses in subjects with knee osteoarthritis. J Pain 2009; 10:1242–9. [DOI] [PubMed] [Google Scholar]