Abstract
Background
The Philippines is ranked 3rd globally for tuberculosis incidence (554/100,000 population). The tuberculosis ward at San Lazaro Hospital, Manila receives 1,800–2,000 admissions of acutely unwell patients per year with high mortality. Objectives of this prospective cohort study were to quantify the association of under-nutrition (primary) and diabetes (secondary) with inpatient mortality occurring between 3–28 days of hospital admission in patients with suspected or previously diagnosed TB.
Methods and results
We enrolled 360 adults (≥18 years); 348 were eligible for the primary analysis (alive on day 3). Clinical, laboratory, anthropometric and enhanced tuberculosis diagnostic data were collected at admission with telephone tracing for mortality up to 6 months post-discharge. In the primary analysis population (mean age 45 years, SD = 15.0 years, 70% male), 58 (16.7%) deaths occurred between day 3–28 of admission; 70 (20.1%) between day 3 and discharge and documented total post-day 3 mortality including follow-up was 96 (27.6%). In those in whom it could be assessed, body mass index (BMI) ranged from 11.2–30.6 kg/m2 and 141/303 (46.5%) had moderate/severe undernutrition (BMI<17 kg/m2). A sex-specific cut-off for mid-upper arm circumference predictive of BMI<17 kg/m2 was associated with inpatient Day 3–28 mortality in males (AOR = 5.04, 95% CI: 1.50–16.86; p = 0.009; p = 0.032 for interaction by sex). The inability to stand for weight/height for BMI assessment was also associated with mortality (AOR = 5.59; 95% CI 2.25–13.89; p<0.001) as was severe compared to normal/mild anaemia (AOR = 9.67; 95% CI 2.48–37.76; p<0.001). No TB specific variables were associated with Day 3–28 mortality, nor was diabetes (HbA1c ≥6.5% or diabetes treatment). Similar effects were observed when the same multivariable model was applied to confirmed TB patients only and to the outcome of all post-day 3 in-patient mortality.
Conclusion
This research supports the use of mid-upper arm circumference for triaging acutely unwell patients and the design and testing of nutrition-based interventions to improve patient outcomes.
Introduction
Tuberculosis (TB) is the leading cause of death from a single infectious agent [1]. Malnutrition, in this case “under-nutrition” is both a risk factor for and complication of active TB disease [2–4]. Clinical wasting and under-nutrition are common clinical findings in patients infected with Mycobacterium tuberculosis (MTB) and are associated with mortality and adverse outcomes [5–7]. A recent systematic review [8] identified under-nutrition as a consistently demonstrable risk factor for death in TB patients on treatment, both within 2 months of treatment initiation as well as late deaths after completion of treatment.
The contribution of under-nutrition to poorer TB treatment outcomes may be mediated through effects on immunity, or altered treatment pharmacokinetics such as increased drug toxicity [9] or decreased drug absorption [10–12]. Both humoral and cell mediated immune responses active in TB disease are negatively affected by under-nutrition, particularly protein energy under-nutrition [13,14]. Immune responses may be important not only against the TB organisms but also against co-infecting organisms [15], which have been associated with increased mortality. In acutely unwell patients, under-nutrition may also increase the risk of inpatient death due to increased risk of severe metabolic and electrolyte disturbances [16–18]. Type 2 Diabetes Mellitus (T2DM) is another known risk factor for active TB [19]. T2DM, especially when poorly controlled, may increase the risk of death and relapse during TB disease [20] whilst TB may also negatively affect glycaemic control [21].
Body Mass Index (BMI; kg/m2) is a widely accepted measure to detect under- and over-nutrition, reflecting relative thinness or fatness, but may be difficult to assess in acutely unwell patients, whilst weight is affected by hydration and fluid balance, even when these are not clinically detectable and can affect BMI classification [22]. Mid-upper arm circumference (MUAC) is simple, less affected by fluid balance and is commonly used in young children [22,23] but there are no accepted cut-offs for adults and little data to support such for more severe levels of under-nutrition, which has limited its use [24]. Studies specifically investigating suitable anthropometric measurements in acutely unwell patients with TB are lacking.
The Philippines has a population of around 100 million and is a high burden country for both TB and multidrug resistant TB (MDR-TB), with a recent estimated TB incidence of 554/100,000 population, placing it third in the world for TB incidence [1]. In 2013, the national prevalence of undernutrition (BMI<18.5kg/m2) for adults 20 years and older was 9.4% for males and 10.5% for females [25]. The national prevalence of T2DM in 2013 ranged from 4.1–12.8% depending on the criteria used [25].
There is a lack of quality evidence to inform a comprehensive nutritional management strategy for malnourished TB patients [26,27]. The aim of this prospective cohort study was to quantify the association of under-nutrition with inpatient mortality within 28 days of hospital admission of Filipino patients with suspected or previously diagnosed TB. The specific primary objective was to assess the effect of moderate or severe under-nutrition measured as BMI<17 kg/m2). A secondary objective was to investigate the impact of diabetes on in-patient mortality.
Materials and methods
Study design and setting
This was a prospective cohort study in San Lazaro Hospital (SLH), a single large urban public hospital and tertiary level infectious diseases referral center serving a poor population in Metro Manila in the Philippines with high admission rates to its TB ward [15,28]. Participants were enrolled from July 25th, 2016 –May 3rd, 2017 and followed up until death or the patient leaving the ward alive (including discharges, transfers or absconding from the ward). The last study participant was discharged on the 8th June 2017. Telephone tracing was conducted at one, two and six months post-discharge to determine vital status and TB treatment status.
Study participants
Participants were enrolled sequentially from patients with suspected or previously diagnosed TB admitted to the TB ward at SLH. Study nurses checked the ward admissions register twice daily (on weekdays) and approached newly admitted patients for consent to participate within 24 hours of admission. The only exclusion criteria were those aged less than 18 years or if too unwell to give written consent or participate (e.g. unconscious, incoherent, severe shortness of breath or life threatening presentations).
Primary outcome
The primary outcome was defined as all-cause in-patient mortality between day three and day 28 (D3 and D28) after admission, inclusive (alive or died). Patients transferred or discharged out of the TB ward prior to D28 or still alive on D28 were included as alive.
Data collection and clinical procedures
At admission, study questionnaires included participant demographics, previous TB symptoms, past medical history, recent food intake and history of weight loss. Research nurses completed anthropometric measurements within 24 hours of admission as previously described [29]. In brief, these included weight (to the nearest 0.1kg; Seca 803 Clara Digitial Personal Non-Medical Scale), height (to nearest 0.5cm; Seca 216 Mechanical Stadiometer), mid-upper arm circumference (MUAC; to the nearest 0.5cm; Seca 201 measuring tape), four-site skinfold measurements (mm units; Harpenden calipers model 68875, Country Technology) and handgrip strength (kg; Jamar Hydraulic Hand Dynamometer Lafayette Instruments, USA). BMI was calculated as kg/m2. Our primary exposure was moderate/severe undernutrition defined as BMI <17 kg/m2, based upon the WHO cutoff for moderate thinness [30,31]. The MUAC measurement from the non-dominant hand was used in analyses. Moderate/severe under-nutrition using MUAC was defined as ≤18.5 cm for females and ≤20.5 cm for males, following an investigation of the performance of MUAC to predict BMI<17 kg/m2 in the same study population [29]. Body fat percentage was calculated using the Durnin and Wormersley equation [32]. Grip strength was analyzed as the maximum recorded value for either hand.
Routine laboratory and clinical data obtained by the hospital was extracted from patient charts. HIV screening (where additional consent was provided) was conducted using standard hospital procedures and Standard Diagnostics Bioline HIV-1/2 Ag/Ab Combo Rapid Test kits. A previous HIV diagnosis or reactive HIV screening was classified as HIV positive. Individuals with a non-reactive screening test were classified as HIV negative. Individuals not agreeing to undergo testing were classified as HIV status unknown. Sputum collection pots and explanations on expectoration were provided to all participants at enrollment and research nurses assisted patients to expectorate and collect samples in the early morning. Sputa samples were assessed using direct sputum smear microscopy using the Ziehl Neelsen stain (AFB; Acid Fast Bacilli smear) and GeneXpert MTB/RIF (Cepheid) testing on all samples. Digital photos of admission chest x-rays (as available) were uploaded to the study database and were assessed by two independent, trained study physicians for results consistent with pulmonary TB and a severity score generated [33]. Venous blood was collected for study-specific assessments of glycated haemoglobin (HbA1c) and C-reactive protein (CRP) (Alere Afinion AS100 point of care analyzer) and additional electrolytes; phosphate, magnesium and calcium, assessed at an external quality assured laboratory (Hi-Precision Diagnostics, Manila). Standard electrolytes, liver and kidney function tests were conducted as per hospital protocols. Critical levels of electrolytes were defined according to published criteria [34] as a corrected calcium <1.5 or >3.4 mmol/L, magnesium <0.3 or >3.3 mmol/L, a phosphate level <0.3 or >1.6 mmol/L, potassium <2.5 or >7 mmol/L, and sodium <120 or >160 mmol/L. Serum calcium was corrected for albumin using the formula “Corrected calcium = (uncorrected Calcium [mmol/L] + (0.02*albumin [g/L]) if albumin <40.0 g/L” [35]. Where albumin was missing, the average value was used for the purpose of calcium correction. T2DM was defined as HbA1c ≥6.5% or on current diabetes medication. Haemoglobin values at admission were used to generate anaemia classifications using standard WHO cutoffs to define mild, moderate and severe [36]. Systemic Inflammatory Response Syndrome (SIRS) was defined as presence of two or more of the following; body temperature >38.3° or <36.0°C, heart rate > 90 beats per minute, respiratory rate > 20 breaths per minute, and a total white blood cell count <4 or >12 x 109 [37].
A hospital diagnosis of TB was defined as the final diagnosis of the treating clinician extracted from the clinical notes. Study-defined TB included i) any patient with AFB positive sputum smear or MTB detected by GeneXpert, except individuals with high grade positive AFB and no MTB detected by GeneXpert (deemed to be non-TB mycobacterium); ii) receiving anti-TB treatment prior to admission, regardless of bacteriological confirmation; iii) a new chest X-ray indicative of TB, with reported cough of duration ≥2 weeks.
Sample size
We pre-specified a sample size of 348 patients who survive the first 48 hours after admission (alive on D3) to detect an increase from 15% to 30% mortality between exposure groups, with 5% significance and at least 90% power if the proportion of patients exposed (BMI<17.0 kg/m2) was between 30–60% [28].
Statistical analysis
All data were collected on electronic questionnaires using Open Data Kit (opendatakit.org) software on Android tablets, uploaded to a secure server at the end of every day [38]. Data were analyzed with Stata version 14 (College Station TX; StataCorp LP). The primary analysis population comprised patients still alive on D3. Data summaries were calculated for those that did not survive beyond D3 and for patients who survived beyond D3, with and without BMI values, and combined. Characteristics of patients at the time of admission were summarized as per data type. Univariable associations with in-patient mortality were analysed using logistic regression. MUAC measurement was expected to be more complete than BMI, given that MUAC can be measured in patients that cannot be weighed or have height measured. In an exploratory analysis, the predicted probability of death within D3-D28 was visualized by plotting predicted values of MUAC from unadjusted fractional polynomial logistic regression models, by sex.
The primary analysis approach was to build a multivariable model to demonstrate the effect of under-nutrition (the primary exposure of interest), adjusting for confounders and effect modifiers of the effect of under-nutrition and independent risk factors of in-patient mortality. Under-nutrition was analysed based on two definitions; i) using a categorical variable based on BMI (<17.0, ≥17.0 and missing, due to the numbers of missing data due to patient incapacity) and ii) a binary variable based on MUAC<18.5 cm in females and<20.5 cm in male patients. To build a final multivariable model, age was included a priori, and then the important nutrition and anthropometric variables were identified. Inclusion of each additional variable in turn was evaluated by comparing adjusted models with and without the new variable using a likelihood ratio test (LRT), with retention in the adjusted model if the LRT p-value ≤ 0.1. After adjustment for age and important anthropometric variables, variables associated with in-patient mortality (LRT p ≤ 0.1 in univariable analysis) and variables demonstrating a confounding effect on the association between under-nutrition and mortality were investigated in turn with the same retention criterion. Interactions in the final model were also tested and retained based on LRT results.
The final multivariable model was re-fitted in two subsets; study-defined TB patients and bacteriologically confirmed TB patients. After adjustment for the same fixed terms additional clinical factors of interest relevant only to these subgroups were investigated for associations with in-patient mortality.
Ethical statement
The study protocol and informed consent forms received approval by the Institutional Review Boards of Nagasaki University (NEKKEN) and San Lazaro Hospital. Research nurses obtained informed written consent, and all research nurses completed Good Clinical Practice certification prior to patient enrollment.
Results and discussion
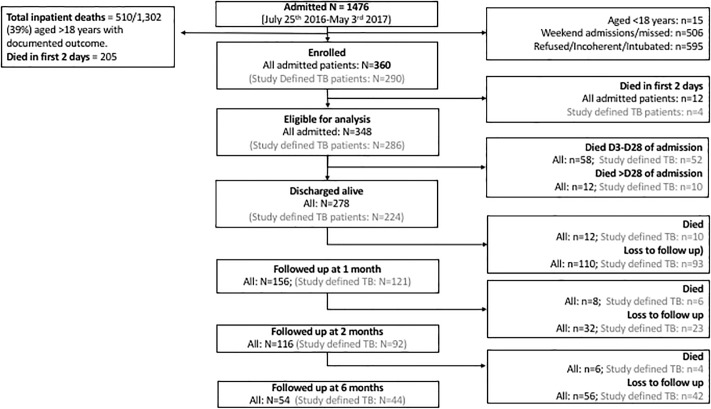
We enrolled 360 individuals from a total of 1476 admissions with known or suspected TB (Fig 1). The main reasons for exclusion were weekend (Friday night to Saturday) or holiday admissions (34.3%), or the patient being too unwell to participate, give consent or refused (40.3%).
Fig 1. Study flow diagram.
Study participants
Patient characteristics and missing data summaries are presented in Table 1 and S1 Table. In the primary analysis population, 70% were male with a mean age of 45 years (SD 15). Within this group, BMI could not be assessed for 45 patients (12.9%) due to incapacity to stand. Of the 303/348 patients in the primary analysis population with BMI assessed, BMI ranged from 11.2–30.6; 13.5% had moderate undernutrition (BMI 16.0–16.9 kg/m2) and 33% severe undernutrition (BMI <16 kg/m2). According to our study-defined, sex-specific MUAC cut-offs, 53% of 348 patients had moderate or severe undernutrition. In patients in whom BMI could not be assessed, MUAC was significantly lower than in those with BMI available (p = 0.0001) with more severe anaemia (p = 0.003 test for trend) and a greater probability of one or more critically low electrolytes (p<0.0001). Thus, exclusion of more unwell patients with missing BMI would introduce selection bias.
Table 1. Characteristics of study participants.
| Died <D3 | Alive after D3 of admission | ||||
|---|---|---|---|---|---|
| Characteristic | N = 12 | All (N = 348) | With BMI (N = 303) | BMI missing (N = 45) | |
| Age (years) | mean (SD) | 47.8 (18.0) | 45.3 (15.0) | 45.5 (14.8) | 44.0 (16.5) |
| Age (category) | 18–40 | 5 (41.7) | 139 (39.9) | 118 (38.9) | 21 (46.7) |
| 41–65 | 4 (33.3) | 177 (50.9) | 157 (51.8) | 20 (44.4) | |
| >65 | 3 (25.0) | 32 (9.2) | 28 (9.2) | 4 (8.9) | |
| Sex | Female | 3 (25.0) | 106 (30.5) | 92 (30.4) | 14 (31.1) |
| Male | 9 (75.0) | 242 (69.5) | 211 (69.6) | 31 (68.9) | |
| Highest Education Achieved | Primary | 6 (50.0) | 117 (33.6) | 99 (32.7) | 18 (40.0) |
| Secondary | 4 (33.3) | 169 (48.6) | 148 (48.8) | 21 (46.7) | |
| Tertiary | 2 (16.7) | 48 (13.8) | 43 (14.2) | 5 (11.1) | |
| Vocational | 14 (4.0) | 13 (4.3) | 1 (2.2) | ||
| Occupation | Not Working | 5 (41.7) | 152 (44.1) | 127 (42.3) | 25 (55.6) |
| Service and Sales | 3 (25.0) | 107 (31.0) | 97 (32.3) | 10 (22.2) | |
| Trades and Manual Labour | 3 (25.0) | 63 (18.3) | 54 (18.0) | 9 (20.0) | |
| Office Work | 1 (8.3) | 23 (6.7) | 22 (7.3) | 1 (2.2) | |
| BMI (kg/m2) | Mean (SD) | 14.1 (3.1) [N = 5] | - | 17.9 (3.7) | - |
| BMI (category; kg/m2) | > = 17kg/m2 | - | - | 162 (53.5) | |
| <17kg/m2 | - | - | 141 (46.5) | ||
| MUAC (cm) | Mean (SD) | 18.1 (3.3) | 20.0 (3.6) | 20.3 (3.5) | 18.0 (3.7)a |
| Any food intake in the past 24 hours | No | 14 (6.1) | 11 (5.2) | 3 (17.6) | |
| Yes | 4 (100.0) | 214 (93.9) | 200 (94.8) | 14 (82.4) | |
| Food intake over past month | No decrease | 99 (43.4) | 93 (44.1) | 6 (35.3) | |
| Moderate decrease | 3 (75.0) | 112 (49.1) | 104 (49.3) | 8 (47.1) | |
| Severe decrease | 1 (25.0) | 17 (7.5) | 14 (6.6) | 3 (17.6) | |
| Diabetic | Non-diabetic (HbA1c<6.5%) | 8 (72.7) | 283 (82.3) | 248 (82.4) | 35 (81.4) |
| Diabetic (HbA1c> = 6.5% or on treatment) | 3 (27.3) | 61 (17.7) | 53 (17.6) | 8 (18.6) | |
| HbA1c category | Normal HbA1c (<6.5%) | 8 (72.7) | 291 (84.6) | 254 (84.4) | 37 (86.0) |
| Mild/Mod HbA1c (> = 6.5%-7.9%) | 2 (18.2) | 24 (7.0) | 22 (7.3) | 2 (4.7) | |
| Severe HbA1c (> = 8%) | 1 (9.1) | 29 (8.4) | 25 (8.3) | 4 (9.3) | |
| Anaemia Status | Non-Anaemic | 4 (36.4) | 97 (28.8) | 90 (30.7) | 7 (15.9) |
| Mild Anaemia (Hgb 11.0–12.9/11.0–11.9 g/dL [M/F]) | 4 (36.4) | 94 (27.9) | 84 (28.7) | 10 (22.7) | |
| Moderate Anaemia (Hgb 8.0–10.9 g/dL) | 1 (9.1) | 108 (32.0) | 91 (31.1) | 17 (38.6) | |
| Severe Anaemia (Hgb <8 g/dL) | 2 (18.2) | 38 (11.3) | 28 (9.6) | 10 (22.7)b | |
| HIV Status | Negative | 1 (8.3) | 116 (33.3) | 112 (37.0) | 4 (8.9) |
| Positive | 22 (6.3) | 17 (5.6) | 5 (11.1) | ||
| Not known (refused) | 11 (91.7) | 210 (60.3) | 174 (57.4) | 36 (80.0) | |
| Study Defined TB | Not TB | 5 (41.7) | 47 (13.5) | 40 (13.2) | 7 (15.6) |
| TB | 4 (33.3) | 286 (82.2) | 252 (83.2) | 34 (75.6) | |
| Unclassifiable | 3 (25.0) | 15 (4.3) | 11 (3.6) | 4 (8.9) | |
| Hospital Diagnosed TB | No | 34 (10.1) | 31 (10.7) | 3 (6.7) | |
| Yes | 12 (100.0) | 301 (89.9) | 259 (89.3) | 42 (93.3) | |
| MDR TB | No | 4 (100.0) | 277 (89.9) | 249 (90.5) | 28 (84.8) |
| Yes (Xpert RIF resistance or on MDR-regimen) | 31 (10.1) | 26 (9.5) | 5 (15.2) | ||
| Direct sputum smear microscopy | Acid-fast bacilli negative | 4 (33.3) | 201 (57.8) | 186 (61.4) | 15 (33.3) |
| Acid-fast bacilli positive | 99 (28.4) | 84 (27.7) | 15 (33.3) | ||
| Missing | 8 (66.7) | 48 (13.8) | 33 (10.9) | 15 (33.3) | |
| Albumin (g/L) | Mean (SD) | 22.7 (5.8) | 23.5 (6.8) | 24.2 (6.5) | 20.1 (7.2) |
| Hypoalbuminemia (<20 g/L) | No | 4 (66.7) | 119 (67.6) | 106 (73.6) | 13 (40.6) |
| Yes | 2 (33.3) | 57 (32.4) | 38 (26.4) | 19 (59.4) | |
| C-reactive protein (mmol/L) | Median (IQR) | 11.9 (6.5–14.7) | 6.9 (2.3–12.2) | 6.5 (2.2–11.5) | 10.7 (5.2–14.6) |
| C-reactive protein category | <0.5 mmol/L | 19 (6.9) | 19 (7.9) | ||
| 0.5–16 mmol/L | 7 (77.8) | 220 (80.3) | 194 (80.2) | 26 (81.2) | |
| >16.0 mmol/L | 2 (22.2) | 35 (12.8) | 29 (12.0) | 6 (18.8) | |
| Serum Creatinine (umol/L) | Median (IQR) | 69.2 (37.3–86.0) | 73.1 (57.1–95.4) | 73.1 (57.5–93.5) | 73.2 (56.6–108.1) |
| Serum AST (IU/L) | Median (IQR) | 51.0 (33–216) | 27.0 (18–47) | 27.0 (18–47) | 27.0 (18–45) |
| Serum ALT (IU/L) | Median (IQR) | 32.0 (22–53) | 27.0 (17–41) | 28.0 (17–42) | 21.5 (15–31) |
| Critical AST, ALT, creatinine or blood urea nitrogenc | No | 3 (50.0) | 174 (90.2) | 151 (91.0) | 23 (85.2) |
| Yes | 3 (50.0) | 19 (9.8) | 15 (9.0) | 4 (14.8) | |
| Serum Potassium (mmol/L) | Mean (SD) | 4.5 (0.9) | 4.1 (0.7) | 4.2 (0.7) | 3.8 (0.8) |
| Serum Sodium (mmol/L) | Mean (SD) | 140.6 (4.8) | 138.4 (6.0) | 138.9 (5.6) | 134.8 (7.9) |
| Serum Chloride (mmol/L) | Mean (SD) | 101.8 (6.1) | 100.6 (6.9) | 101.3 (6.7) | 96.9 (7.0) |
| Serum Phosphate (mmol/L) | Mean (SD) | 1.6 (0.8) | 1.3 (0.5) | 1.3 (0.5) | 1.3 (0.4) |
| Serum Magnesium (mmol/L) | Mean (SD) | 0.8 (0.2) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) |
| Serum corrected Calcium (mmol/L) | Mean (SD) | 2.4 (0.3) | 2.4 (0.2) | 2.4 (0.2) | 2.4 (0.2) |
| Any Critical Electrolyted | No | 5 (71.4) | 214 (80.1) | 198 (82.2) | 16 (61.5) |
| Yes | 2 (28.6) | 53 (19.9) | 43 (17.8) | 10 (38.5)e | |
| Systemic Inflammatory Response Syndrome (SIRS)f | No | 1 (8.3) | 69 (19.8) | 60 (19.8) | 9 (20.0) |
| Yes | 11 (91.7) | 279 (80.2) | 243 (80.2) | 36 (80.0) | |
BMI, Body Mass Index; MUAC, Mid-Upper Arm Circumference.
a MUAC was significantly lower in the group in whom BMI could not be assessed compared to those with BMI available (t-test, p = 0.0002)
b More severe anaemia was observed in the group without BMI compared to those with BMI data (chi2 test for trend, p = 0.003)
c Critical levels defined as 5 x upper limit of normal according to San Lazaro Hospital laboratory reference ranges
e A greater probability of one or more critically low electrolytes was observed in the group without BMI compared to those with BMI data (chi2 test p<0.0001)
f SIRS defined as body temperature >38.3° or <36.0°C, heart rate > 90 beats per minute, respiratory rate > 20 breaths per minute, and a total white blood cell count <4 or >12 x 109
Within the primary analysis population, 17.7% (61/344) had newly or previously diagnosed diabetes. The proportion with study defined TB was 82% (286/348), with 53% of those being (151/286) bacteriologically confirmed and 48% (135/282) classified as new cases, 33% as relapse (94/282) and 19% (53/282) as treatment after loss to follow-up or previous treatment outcome unknown. The prevalence of MDR-TB (defined as RIF resistance mutation identified by GeneXpert or already on an MDR treatment regimen) was 10.1% of 308 patients. The proportion of patients who were HIV positive was 6.3% whilst those who were HIV negative was 33%. The remaining had an unknown status due to refusal for HIV screening.
Inpatient mortality
Inpatient mortality between D3-D28 was 16.7% (58/348; 95% CI 13.1–21.0%). A further 12 deaths occurred during admissions (>28 days), resulting in total inpatient mortality post-D3 was 20.1% (70/348, 95% CI 15.9–24.3%) and 22.8% (82/360, 95% CI 18.4–27.1%) in all enrolled patients (Fig 1).
Risk factors for inpatient mortality (D3-D28)
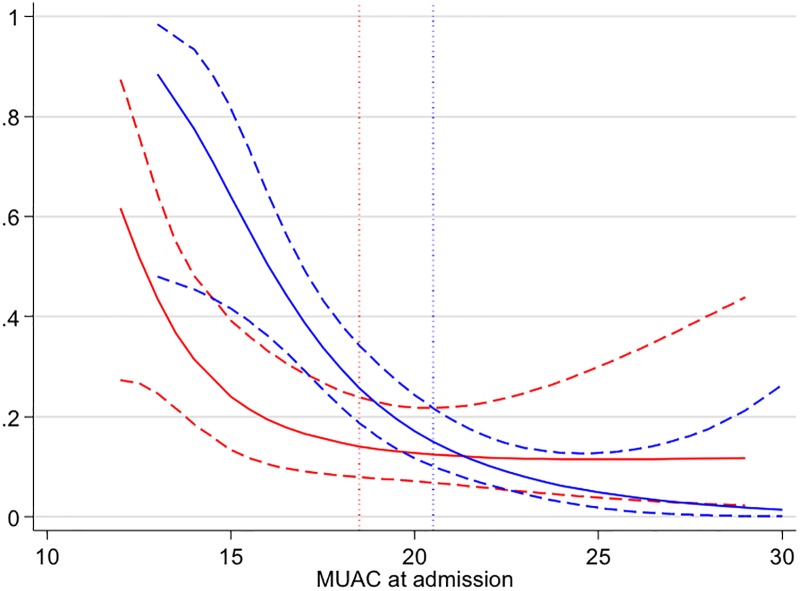
In univariable analyses, increased odds of in-patient mortality (D3 to D28) were seen for moderate or severe undernutrition assessed by MUAC (p<0.001), MUAC as a continuous variable (Fig 2), missing BMI status compared to BMI > = 17.0 kg/m2, lower handgrip strength, lower percentage body fat, both HIV positive status and missing HIV status compared to HIV negative status, and moderate and severe anaemia compared to non-anaemic patients (Table 2). When limited to those with BMI data available, BMI<17 kg/m2 was not associated with in-patient mortality (p = 0.45), whilst MUAC diagnosed moderate/severe under-nutrition was associated, with the effect size increasing in the BMI adjusted model (S2 Table) There was some weak evidence of an association with age (p = 0.070) and for increased risk in those with hospital diagnosed TB (p = 0.051). Higher serum phosphate levels were strongly associated with increased odds of mortality (p = 0.002). Hyperphosphataemia (phosphate >1.6 mmol/L; 38/303) compared to normal or hypophosphataemia (latter defined as phosphate <0.8 mmol/L; 1/303) was associated with increased odds of death (OR = 3.48, 95% CI 1.60–7.60, p = 0.002). Presence of critical electrolyte levels were significantly associated with increased odds of death (p<0.001). A hospital diagnosis of community-acquired pneumonia (CAP) extracted from the discharge/final diagnosis in the clinical notes was also associated with increased odds of mortality (p<0.001). MUAC diagnosed moderate/severe undernutrition was non-significantly associated with increased odds of CAP (OR = 1.44, 95% CI 0.92–2.27; p = 0.112). The presence of systemic inflammatory response syndrome (SIRS) or its individual criteria of tachycardia, tachypnoeia, fever or abnormal white cell count were not associated with mortality. There was no evidence of associations with mortality for level of education or measures of socioeconomic status.
Fig 2. Predicted probability of death by MUAC at admission from unadjusted fractional polynomial logistic regression.
Red = females, blue = males, dotted lines are sex specific MUAC cut-off values for under-nutrition.
Table 2. Univariable associations with D3-D28 mortality (primary analysis population, N = 348).
| Characteristic | N | Deaths (%) | OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Age (years) | linear effect | 348 | 0.98 (0.96–1.00) | 0.070 | |
| Sex | Female | 106 | 18 (17.0) | 1 | 0.917 |
| Male | 242 | 40 (16.5) | 0.97 (0.53–1.78) | ||
| BMI Category | > = 17kg/m2 | 162 | 16 (9.9) | 1 | <0.001 |
| <17kg/m2 | 141 | 18 (12.8) | 1.34 (0.65–2.73) | ||
| Not assessable | 45 | 24 (53.3) | 10.43 (4.78–22.76) | ||
| MUAC Category | Normal/mild undernutrition | 164 | 13 (7.9) | 1 | <0.001 |
| Moderate/severe undernutrition | 184 | 45 (24.5) | 3.76 (1.95–7.27) | ||
| Grip strength (kg) | linear effect | 346 | 0.91 (0.88–0.94) | <0.001 | |
| % Body Fat | linear effect | 335 | 0.94 (0.90–0.98) | 0.001 | |
| Diabetic | Non-diabetic (HbA1c<6.5%) | 283 | 48 (17.0) | 1 | 0.450 |
| Diabetic (HbA1c> = 6.5% or on treatment) | 61 | 8 (13.1) | 0.74 (0.33–1.65) | ||
| HbA1c category | Normal HbA1c (<6.5%) | 291 | 48 (16.5) | 1 | 0.426 |
| Mild/Mod HbA1c (> = 6.5%-7.9%) | 24 | 2 (8.3) | 0.46 (0.10–2.02) | ||
| Severe HbA1c (> = 8%) | 29 | 6 (20.7) | 1.32 (0.51–3.42) | ||
| Anaemia Status | Non-Anaemic | 97 | 7 (7.2) | 1 | 0.001 |
| Mild Anaemia (Hgb 11.0–12.9/11.0–11.9 g/dL [M/F]) | 94 | 15 (16.0) | 2.44 (0.95–6.29) | ||
| Moderate Anaemia (Hgb 8.0–10.9 g/dL) | 108 | 21 (19.4) | 3.10 (1.26–7.67) | ||
| Severe Anaemia (Hgb <8 g/dL) | 38 | 14 (36.8) | 7.50 (2.72–20.65) | ||
| HIV Status | Negative | 116 | 6 (5.2) | 1 | <0.001 |
| Positive | 22 | 6 (27.3) | 6.88 (1.98–23.93) | ||
| Unknown | 210 | 46 (21.9) | 5.14 (2.12–12.45) | ||
| Hospital Diagnosed TB | No | 34 | 2 (5.9) | 1 | 0.051 |
| Yes | 301 | 53 (17.6) | 3.42 (0.79–14.71) | ||
| Study Diagnosed TB | No | 62 | 6 (9.7) | 1 | 0.085 |
| Yes | 286 | 52 (18.2) | 2.07 (0.85–5.07) | ||
| Bacteriologically confirmed TB | No | 167 | 19 (11.4) | 1 | 0.072 |
| Yes | 151 | 28 (18.5) | 1.77 (0.94–3.33) | ||
| MDR TB | No | 277 | 39 (14.1) | 1 | 0.761 |
| Yes (Xpert Rif resist or on MDR-regimen) | 31 | 5 (16.1) | 1.17 (0.43–3.24) | ||
| Albumin (g/L) | linear effect | 176 | 0.86 (0.80–0.92) | <0.001 | |
| Hypoalbuminemia (<20g/L) | No | 119 | 10 (8.4) | 1 | <0.001 |
| Yes | 57 | 24 (42.1) | 7.93 (3.44–18.26) | ||
| C-reactive protein (mmol/L) | linear effect | 274 | 1.07 (1.01–1.14) | 0.031 | |
| C-reactive protein category | <0.5 | 19 | 1 (5.3) | 1 | 0.374 |
| 0.5–16 | 220 | 34 (15.5) | 3.29 (0.43–25.47) | ||
| >16.0 | 35 | 6 (17.1) | 3.72 (0.41–33.52) | ||
| Critical AST, ALT, creatinine, or blood urea nitrogena | No | 174 | 30 (17.2) | 1 | 0.056 |
| Yes | 19 | 7 (36.8) | 2.80 (1.02–7.70) | ||
| Serum Potassium (mmol/L) | linear effect | 307 | 0.69 (0.46–1.05) | 0.086 | |
| Serum Sodium (mmol/L) | linear effect | 305 | 0.94 (0.90–0.99) | 0.013 | |
| Serum Chloride (mmol/L) | linear effect | 305 | 0.98 (0.93–1.03) | 0.408 | |
| Serum Phosphate (mmol/L) | linear effect | 303 | 2.60 (1.42–4.76) | 0.002 | |
| Serum Magnesium (mmol/L) | linear effect | 303 | 5.93 (0.48–72.84) | 0.163 | |
| Serum corrected Calcium (mmol/L) | linear effect | 303 | 0.72 (0.15–3.32) | 0.70 | |
| Any Critical Electrolyteb | No | 214 | 22 (10.3) | 1 | 0.196 |
| Yes | 53 | 16 (30.2) | 3.77 (1.81–7.86) | <0.001 | |
| Clinical diagnosis CAP | No | 114 | 3 (2.6) | 1 | |
| Yes | 221 | 52 (23.5) | 11.38 (3.47–37.36) | <0.001 |
After adjustment for age and moderate/severe undernutrition diagnosed by MUAC, odds of death in patients with BMI<17.0 kg/m2 were not increased compared to those with BMI≥17.0 kg/m2 (OR = 0.38, 95% CI 0.15–0.98), but were substantially increased for patients with missing BMI compared to BMI ≥ 17.0 kg/m2 (OR = 2.94, 95% CI 1.07–8.13). It was considered plausible that missing BMI value was a proxy indicator of very poor prognosis based on missing BMI status occurring in immobile and incapacitated patients. A proxy variable for immobility was created based on missing versus non-missing BMI value that was associated with mortality after adjustment for age and under-nutrition based on MUAC (p<0.001).
Increased handgrip strength was associated with decreased odds of mortality after adjustment for age, MUAC and immobility (p<0.001), with the effect of reducing, but not eliminating the effect estimate of MUAC. However handgrip strength was not retained due to MUAC having a much greater potential for practical implementation in this setting. Percentage body fat was not associated with mortality after adjustment for age, MUAC and immobility (p = 0.464).
The final model included age, immobility, anaemia (none or mild, moderate or severe), plus an interaction between sex and MUAC diagnosed moderate or severe undernutrition (LRT p = 0.0316 for interaction) and HIV status (negative, positive or unknown), and clinical diagnosis of CAP (Table 3). Under-nutrition was associated with a large increase in the odds of death in male patients (OR = 5.04, 95% CI 1.50–16.86, p = 0.009). Female patients were more likely to be severely anaemic (20% vs 7%), which was associated with increased odds of death (OR = 9.67 95% CI 2.48–37.76, p<0.001) compared to non-anaemic or mildly anaemic patients. Positive HIV status or missing HIV status (due to lack of consent, often in older patients) and final clinical diagnosis of CAP also had independently increased odds of mortality (Table 3). Due to reduced numbers of observations, hyperphosphataemia was not included in main multivariable analysis, but when included in the same model as reported above (N = 281) was independently associated with increased odds of mortality (OR = 3.74; 95% CI 1.20–11.62; p = 0.023) whilst not appreciably changing the effect estimates of the other independent exposure variables (S3 Table).
Table 3. Multivariable analysis of D3-D28 mortality in all admitted patients (N = 325).
| Characteristic | Value | Adj OR (95% CI) | p-value |
|---|---|---|---|
| Under-nutrition status in women | Normal/Mild | Ref | |
| Moderate/Severe undernutrition | 0.81 (0.24–2.77) | 0.737 | |
| Under-nutrition status in men | Normal/Mild | Ref | |
| Moderate/Severe undernutrition | 5.04 (1.50–16.86) | 0.009 | |
| Sex in normal/mild undernutrition | Female | Ref | |
| Male | 0.38 (0.09–1.58) | 0.182 | |
| Sex in moderate/severe undernutrition | Female | Ref | |
| Male | 2.65 (0.63–11.10) | 0.182 | |
| HIV status | Negative | Ref | |
| Positive | 6.86 (1.31–35.77) | 0.022 | |
| Unknown status | 5.46 (1.77–16.84) | 0.003 | |
| Anemia status | Normal/Mild (Hgb > = 11 g/dL) | Ref | |
| Moderate (Hgb 8–10.9 g/dL) | 2.58 (0.89–7.45) | 0.080 | |
| Severe (Hgb <8 g/dL) | 9.67 (2.48–37.76) | <0.001 | |
| Immobility | Mobile | Ref | |
| Immobile | 5.59 (2.25–13.89) | <0.001 | |
| Clinical diagnosis CAP | No | Ref | |
| Yes | 19.23 (4.55–81.20) | <0.001 | |
| Age (years) | 0.97 (0.94–0.99) | 0.030 |
CAP, Community Acquired Pneumonia
P-values are Wald test p-values. LRT p-value for the interaction term is 0.0316. Overall LRT p-values are: age p = 0.0274; immobility p = 0.002; anaemia p = 0.035; HIV status p = 0.0032; CAP p = <0.0001. Moderate/Severe undernutrition as assessed by MUAC cut-offs of 18.5cm in women and 20.5 cm in men. Immobility assessed as too immobile to get out of bed for measurement of height or weight.
Diabetes and risk of inpatient mortality
In the univariable analyses, neither diabetes nor level of HbA1C % was associated with inpatient mortality (p = 0.450, p = 0.426; Table 2).
Subset analyses
When added into the same multivariable model as above, there was no evidence of an association with D3-D28 inpatient death for TB specific factors including: if on anti-tuberculosis treatment (ATT) on admission; new (GeneXpert identified RIF resistance mutation) or existing diagnosis of MDR-TB, basis of TB diagnosis, chest x-ray severity score or presence of cavitation. There was also no association with diabetes or HbA1c levels. Only MTB load from sputum microscopy (semi-quantitative grade) was non-significantly associated. The final multivariable models for study defined TB (N = 283) and bacteriologically confirmed TB cases (N = 151) are shown in Table 4. Evidence of the effect modification of MUAC defined undernutrition by sex remained in the study defined TB subset (LRT p-value for interaction = 0.041) with moderate/severe undernutrition in male TB patients associated with increased odds of mortality but with low precision due to data scarcity (OR = 7.38; 95% CI 1.54–35.38). The interaction term could not be included in the bacteriologically defined sub-set due to reduced observations. Effect estimates for exposure variables included in the final models were similar to those in the main analysis but with reduced precision.
Table 4. Multivariable analysis of risk of mortality (D3-D28) in Study-defined TB cases (N = 261) [excluding 3 EXPTB] and Bacteriologically confirmed (N = 140).
| Study Defined TB patients (n = 261) | Bacteriologically confirmed TB (n = 140) | ||||
|---|---|---|---|---|---|
| Characteristic | Adj OR (95% CI) | p-value | Adj OR (95% CI) | p-value | |
| Under-nutrition status in women | Normal/Mild | Ref | - | ||
| Moderate/Severe undernutrition | 0.90 (0.21–3.77) | 0.896 | - | ||
| Under-nutrition status in men | Normal/Mild | Ref | - | ||
| Moderate/Severe undernutrition | 7.38 (1.54–35.38) | 0.012 | - | ||
| Under-nutrition in men & womena | Normal/Mild | - | Ref | ||
| Moderate/Severe undernutrition | - | 13.21 (1.59–109.61) | 0.023 | ||
| Sex in non-malnourished | Female | Ref | - | ||
| Male | 0.29 (0.05–1.75) | 0.177 | - | ||
| Sex in malnourished | Female | Ref | - | ||
| Male | 2.35 (0.79–6.98) | 0.124 | - | ||
| Sex | Female | - | Ref | ||
| Male | - | 1.08 (0.31–3.74) | 0.901 | ||
| HIV status | Negative | Ref | Ref | ||
| Positive | 9.66 (1.54–60.51) | 0.015 | 7.34 (0.78–68.66) | 0.081 | |
| Missing | 5.04 (1.49–17.02) | 0.009 | 8.09 (1.63–40.23) | 0.011 | |
| Anemia status | Normal/Mild | Ref | Ref | ||
| Moderate | 2.93 (0.90–9.56) | 0.074 | 2.11 (0.23–19.30) | 0.508 | |
| Severe | 8.74 (1.84–41.44) | 0.006 | 2.19 (0.17–28.96) | 0.553 | |
| Mobility | Mobile | Ref | Ref | ||
| Immobile | 5.30 (1.84–41.44) | 0.001 | 11.92 (2.99–47.42) | <0.001 | |
| Clinical CAP diagnosisb | No | Ref | - | ||
| Yes | 16.16 (3.59–72.79) | <0.001 | - | ||
| Age (years) | Linear effect | 0.98 (0.95–1.00) | 0.095 | 0.95 (0.91–0.99) | 0.033 |
CAP, Community Acquired Pneumonia
P-values in are Wald test p-values. For the Study Defined TB population LRT p-value for the interaction term is 0.0414. Overall LRT p-values are: age p = 0.0923; immobility p = 0.0010; anaemia p = 0.018; HIV status p = 0.0060; CAP p = <0.0001. Moderate/Severe undernutrition as assessed by MUAC cut-offs of 18.5cm in women and 20.5 cm in men. Immobility assessed as too immobile to get out of bed for measurement of height or weight.
a The MUAC under-nutrition sex interaction could not be modelled in the BC subpopulation due to data sparsity.
b Clinical CAP diagnosis perfectly predicted mortality in the BC subpopulation.
Mortality post-D28 and post-discharge
There were 12 further inpatient deaths in those that survived until D3. Of the 278 patients discharged alive, attrition was high (Fig 1). Based on known deaths, the minimum mortality by one month after discharge was 4.3% (12/278) in those discharged alive and 23.6% (82/348) in patients alive on D3. By two months, it was 7.2% of those discharged alive and 25.8% in patients alive on D3. By six months the minimum mortality was 9.4% of those discharged alive and 27.6% in patients alive on D3.
Under-nutrition and all inpatient mortality and total mortality post-D3
Using the same model as above, the effect of MUAC defined moderate or severe undernutrition was also observed for all inpatient mortality after D3 (N = 70 deaths: OR = 4.17 95% CI 1.35–12.91) and to inpatient mortality after D3 and post-discharge mortality combined (N = 96 deaths: OR = 3.35; 95% CI 1.53–7.30) with the effect limited to men in both outcomes and the interaction terms being statistically significant (LRT p-values = 0.0462 and 0.0402).
The primary objective of this prospective cohort study was to compare inpatient mortality of patients with and without evidence of moderate or severe under-nutrition who were admitted to the SLH TB ward. This study demonstrates that the use of sex-specific MUAC cut-offs previously shown in this population to have a high positive predictive value for BMI<17 kg/m2 are strongly associated with risk of inpatient death within the first month of admission and excluding early deaths, when nutritional interventions would be unlikely to have an impact [29]. This observation was robust to variations in outcome definition, and patient subsets (all admissions, study defined or bacteriologically confirmed TB). Furthermore, when limited to the subset of those with BMI available, MUAC was associated with risk of death, independent of BMI whilst BMI<17 was not associated. This is very similar to previous observations in emergency admission patients in the UK [40]. Thus MUAC represents a logistically simpler measurement and better predicts risk of death. Moderate evidence suggested a greater effect of under-nutrition on risk of death in males than females. A simple assessment of a patients ability to stand was also strongly and independently associated with risk of death, of a similar effect size as MUAC defined moderate/severe under-nutrition and HIV status. Although body fat percentage and grip strength were also associated with risk of death, MUAC assessment is much simpler and captured more of the variation in outcome. BMI<17 kg/m2 has been previously shown in admissions to this ward to be associated with increased risk of inpatient death within 2 weeks in TB-PCR positive admissions (RR 1.95; 95%CI 1.03–3.69), but due to missing data was not reported in multivariable models and no other nutritional assessments were undertaken [15].
We assessed a panel of electrolytes to investigate if these might underlie an effect of acute under-nutrition on inpatient mortality through mechanisms such as dehydration or refeeding syndrome [41]. Similar to previous observations, hyponatraemia was associated with mortality [17]. Contrary to expectations, hyperphosphataemia was associated with mortality, but the aetiology and role of hyperphosphataemia in mortality is unclear.
TB disease and associated mortality are often reportedly higher in males, with epidemiological drivers, delay in seeking treatment, and attrition from treatment programs posited as potential reasons [42]. Increased time-to-treatment is expected to contribute to nutritional deterioration and is often proposed as the underlying reason for observations of low nutritional status being associated with adverse outcomes [6]. However, in this study, there was no evidence of difference in undernutrition by sex in all admissions or in the TB sub-groups. It is possible that sex differences in immune responses extend to TB and is more robust to the effects of under-nutrition in women than men [43].
WHO guidance recommends nutritional assessments for all individuals with active TB, but weights and heights are not measured routinely on admission to the ward and follow-up assessments to track weight gain/loss are not done [26]. In a clinical setting with a high turnover of acutely unwell patients, a simple and quick anthropometric measurement of nutritional state is essential to the diagnosis and management of undernutrition. This is important as despite showing a high prevalence of moderate and severe under-nutrition on the ward it was not recognized or managed by clinical staff. The reasons include limitations in resources (personnel, equipment, interventions, and knowledge), lack of standardized guidelines, the widespread belief that nutritional interventions may be associated with an increased risk of adverse events and that treatment of TB is sufficient for nutritional recovery. Whilst the financial costs of hospital prescribed nutritional support, unlike ATT and basic inpatient costs, have to be borne by the patient. In poor populations with already marginal food intakes and inpatients dependent on standard hospital-provided diets, TB treatment alone is unlikely to be sufficient for nutritional recovery. This is supported by evidence from India in which 23% of patients who completed treatment still had BMI<16 kg/m2 from that at diagnosis.5 However, surprisingly, there is a very limited evidence base to support the efficacy of interventions to diagnose and treat under-nutrition in TB patients [26,27].
Diabetes was considerably more common in this patient population than in the general population[44]. The lack of association for diabetes or level of HbA1c, indicating the degree of glycaemic dysregulation on risk of death was surprising. However, diabetes was associated with higher BMI (mostly within the normal range) and it is possible that any effects of the diabetes is superseded by the better nutritional status and the advantage that may confer.
A limitation of this study was the unknown HIV status for >50% of participants due to a high testing refusal rate, more common in older patients (100% in > 65 years). However, as older age was not associated with increased risk of death, this does not appear to explain the observation of increased risk of death in those with unknown status. Our study population compared with all admissions to this ward had lower mortality, especially very early, before D3 mortality, but the association between under-nutrition and inpatient death is likely to hold true for all those that survive D3.
Conclusions
This study establishes the association between under-nutrition assessed using MUAC with risk of inpatient death in this acutely unwell adult population. In this study, there was no apparent effect of diabetes. Further research is required in different populations to optimize MUAC cut-offs to predict risk of death and develop suitable nutritional interventions in active TB disease.
Supporting information
(DOCX)
Moderate/Severe under-nutrition as assessed by MUAC cut-offs of 18.5cm in women and 20.5 cm in men.
(DOCX)
P-values are Wald test p-values. LRT p-value for the interaction term is 0.0257. Overall LRT p-values are: age p = 0.033; immobility p = 0.0087; anaemia p = 0.0149; HIV status p = 0.0053; CAP p = <0.0001; hyperphosphataemia p = 0.024. Moderate/Severe undernutrition as assessed by MUAC cut-offs of 18.5cm in women and 20.5 cm in men. Immobility assessed as too immobile to get out of bed for measurement of height or weight.
(DOCX)
Acknowledgments
We are grateful to the patients, families, San Lazaro Hospital staff and research nurses, Marjen Nicole Quema, Alan Kevin Q. Llantada and Reby Marie T. Garcia for their contributions in making this work possible.
Data Availability
The anonymised dataset and data dictionary are available at https://doi.org/10.6084/m9.figshare.8003318.
Funding Statement
This research was supported by funding from Nagasaki University to Professor Sharon E Cox. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Global Tuberculosis Report. Geneva; 2017. [Google Scholar]
- 2.Paton NI, Ng Y-M. Body composition studies in patients with wasting associated with tuberculosis. Nutrition. 2006;22: 245–51. 10.1016/j.nut.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 3.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: Evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8: 286–298. [PubMed] [Google Scholar]
- 4.Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39: 149–155. 10.1093/ije/dyp308 [DOI] [PubMed] [Google Scholar]
- 5.Bhargava A, Chatterjee M, Jain Y, Chatterjee B, Kataria A, Bhargava M, et al. Nutritional Status of Adult Patients with Pulmonary Tuberculosis in Rural Central India and Its Association with Mortality. PLoS One. 2013;8: 1–11. 10.1371/journal.pone.0077979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zachariah R, Spielmann MP, Harries a. D, Salaniponi FML. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002;96: 291–294. 10.1016/s0035-9203(02)90103-3 [DOI] [PubMed] [Google Scholar]
- 7.Lai HH, Lai YJ, Yen YF. Association of body mass index with timing of death during tuberculosis treatment. PLoS One. 2017;12: 1–12. 10.1371/journal.pone.0170104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15: 871–885. 10.5588/ijtld.10.0352 [DOI] [PubMed] [Google Scholar]
- 9.Satyaraddi A, Velpandian T, Sharma SK, Vishnubhatla S, Sharma A, Sirohiwal A, et al. Correlation of plasma anti-tuberculosis drug levels with subsequent development of hepatotoxicity. Int J Tuberc Lung Dis. 2014;18: 188–95, i–iii. 10.5588/ijtld.13.0128 [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran G, Hemanth Kumar AK, Bhavani PK, Poorana Gangadevi N, Sekar L, Vijayasekaran D, et al. Age, nutritional status and INH acetylator status affect pharmacokinetics of anti-tuberculosis drugs in children. Int J Tuberc Lung Dis. 2013;17: 800–806. 10.5588/ijtld.12.0628 [DOI] [PubMed] [Google Scholar]
- 11.Polasa K, Murthy K, Krishnaswamy K. Rifampicin kinetics in undernutrition. Br J Clin Pharmacol. 1984;17: 481–484. 10.1111/j.1365-2125.1984.tb02377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeremiah K, Denti P, Chigutsa E, Faurholt-Jepsen D, PrayGod G, Range N, et al. Nutritional supplementation increases rifampin exposure among tuberculosis patients coinfected with HIV. Antimicrob Agents Chemother. 2014;58: 3468–3474. 10.1128/AAC.02307-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrasekaran P, Saravanan N, Bethunaickan R, Tripathy S. Malnutrition: Modulator of immune responses in tuberculosis. Front Immunol. 2017;8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaible UE, Kaufmann SHE. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med. 2007;4: 0806–0812. 10.1371/journal.pmed.0040115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimazaki T, Taniguchi T, Saludar NRD, Gustilo LM, Kato T, Furumoto A, et al. Bacterial co-infection and early mortality among pulmonary tuberculosis patients in Manila, The Philippines. Int J Tuberc Lung Dis. 2018;22: 65–72. 10.5588/ijtld.17.0389 [DOI] [PubMed] [Google Scholar]
- 16.Zietse R, Zoutendijk R, Hoorn EJ. Fluid, electrolyte and acid-base disorders associated with antibiotic therapy. Nat Rev Nephrol. 2009;5: 193–202. 10.1038/nrneph.2009.17 [DOI] [PubMed] [Google Scholar]
- 17.Sharma SK, Mohan A, Banga A, Saha PK, Guntupalli KK. Predictors of development and outcome in patients with acute respiratory distress syndrome due to tuberculosis. Int J Tuberc Lung Dis. 2006;10: 429–435. [PubMed] [Google Scholar]
- 18.Hill AR, Uribarri J, Mann J, Berl T. Altered water metabolism in tuberculosis: Role of vasopressin. Am J Med. 1990;88: 357–364. 10.1016/0002-9343(90)90489-Z [DOI] [PubMed] [Google Scholar]
- 19.Hayashi S, Chandramohan D. Risk of active tuberculosis among people with diabetes mellitus: systematic review and meta-analysis. Trop Med Int Heal. 2018;23: 1058–1070. 10.1111/tmi.13133 [DOI] [PubMed] [Google Scholar]
- 20.Riza AL, Pearson F, Ugarte-Gil C, Alisjahbana B, Van de Vijver S, Panduru NM, et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol. Elsevier Ltd; 2014;2: 740–753. 10.1016/S2213-8587(14)70110-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang CY, Bai KJ, Lin HH, Chien ST, Lee JJ, Enarson DA, et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS One. 2015;10: 1–15. 10.1371/journal.pone.0121698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benitez Brito N, Suarez Llanos JP, Fuentes Ferrer M, Oliva Garcia JG, Delgado Brito I, Pereyra-Garcia Castro F, et al. Relationship between mid-upper arm circumference and body mass index in inpatients. PLoS One. 2016;11: 1–10. 10.1371/journal.pone.0160480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madden AM, Smith S. Body composition and morphological assessment of nutritional status in adults: A review of anthropometric variables. J Hum Nutr Diet. 2016;29: 7–25. 10.1111/jhn.12278 [DOI] [PubMed] [Google Scholar]
- 24.Tang AM, Chung M, Dong K, Wanke C, Bahwere P, Bose K, et al. Determining a Global Mid-Upper Arm Circumference Cutoff to Assess Underweight in Adults (Men and Nonpregnant Women) Recommended Citation Determining a Global Mid-Upper Arm Circumference Cutoff to Assess Underweight in Adults (Men and Nonpregnant Women) i [Internet]. Washington, DC; 2017. www.fantaproject.org
- 25.Food and Nutrition Research Institute. 8th National Nutrition Survey. Manila; 2013.
- 26.World Health Organization. Nutritional care and support for patient with tuberculosis. Geneva; 2013. [PubMed] [Google Scholar]
- 27.Sinclair D, Abba K, Grobler L, Sudarsanam TD. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimazaki T, Marte SD, Saludar NRD, Dimaano EM, Salva EP, Ariyoshi K, et al. Risk factors for death among hospitalised tuberculosis patients in poor urban areas in Manila, The Philippines. Int J Tuberc Lung Dis. 2013;17: 1420–1426. 10.5588/ijtld.12.0848 [DOI] [PubMed] [Google Scholar]
- 29.White L V., Lee N, Marin FP, Saludar NR, Edwards T, Cox SE. Performance of alternative measures to body mass index in the assessment of moderate and severe under-nutrition among acutely unwell patients hospitalized in a TB ward in the Philippines: A cross-sectional study. Cheungpasitporn W, editor. PLoS One. 2019;14: e0215968 10.1371/journal.pone.0215968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organization technical report series. 1995. pp. 1–452. [PubMed]
- 31.James WP, Ferro-Luzzi A, Waterlow JC. Definition of chronic energy deficiency in adults. Report of a working party of the International Dietary Energy Consultative Group. Eur J Clin Nutr. European Journal of Clinical Nutrition; 1988;42: 969–981. [PubMed] [Google Scholar]
- 32.Harrison G, Buskirk E, Carter J. Skinfold thickness and measurement technique In: Lohman T, Roche A, Martorell R, editors. Anthropometric Standardization Reference Manual: Human Kinetics. 1988. pp. 55–70. [Google Scholar]
- 33.Ralph AP, Ardian M, Wiguna A, Maguire GP, Becker NG, Drogumuller G, et al. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. 2010; 863–870. 10.1136/thx.2010.136242 [DOI] [PubMed] [Google Scholar]
- 34.Stanford Health Care. Laboratory Critical/Panic Value List [Internet].
- 35.Jain A, Bhayana S, Vlasschaert M, House A. A formula to predict corrected calcium in haemodialysis patients. Nephrol Dial Transplant. 2008;23: 2884–2888. 10.1093/ndt/gfn186 [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva; 2011. [Google Scholar]
- 37.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101: 1644–1655. 10.1378/chest.101.6.1644 [DOI] [PubMed] [Google Scholar]
- 38.Brunette W, Sundt M, Dell N, Chaudhri R, Breit N, Borriello G. Open data kit 2.0. Proc 14th Work Mob Comput Syst Appl—HotMobile ‘13. 2013; 1–6.
- 39.Ronco C, Bellomo R, Kellum J. Critical Care Nephrology. 2nd ed Elsevier Health Sciences; 2008. [Google Scholar]
- 40.Powell-Tuck J, Hennessy EM. A comparison of mid upper arm circumference, body mass index and weight loss as indices of undernutrition in acutely hospitalized patients. Clin Nutr. 2003;22: 307–312. 10.1016/S0261-5614(03)00009-8 [DOI] [PubMed] [Google Scholar]
- 41.Mehanna H, Nankivell PC, Moledina J, Travis J. Refeeding syndrome—awareness, prevention and management. Head Neck Oncol. 2009;1: 4 10.1186/1758-3284-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton KC, Macpherson P, Houben RMGJ, White G, Corbett EL. Sex Differences in Tuberculosis Burden and Notifications in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. 2016;21: 1–23. 10.1371/journal.pmed.1002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nhamoyebonde S, Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. 2014;209: S100–6. 10.1093/infdis/jiu147 [DOI] [PubMed] [Google Scholar]
- 44.Jimeno CA, Kho SA, Bien, Matawaran J, Duante CA, Jasul G V, et al. Prevalence of Diabetes Mellitus and Pre-Diabetes in the Philippines: A Sub-study of the 7 th National Nutrition and Health Survey (2008). 2008;53: 1–8. Available: https://www.pcp.org.ph/files/PJIM%20Vol53%20No2/Prevalence_of_Diabetes_Mellitus_and_Pre-Diabetes_in_the_Philippines_A_Sub-study_of_the_7th_National_Nutrition_and_Health_Survey_2008.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Moderate/Severe under-nutrition as assessed by MUAC cut-offs of 18.5cm in women and 20.5 cm in men.
(DOCX)
P-values are Wald test p-values. LRT p-value for the interaction term is 0.0257. Overall LRT p-values are: age p = 0.033; immobility p = 0.0087; anaemia p = 0.0149; HIV status p = 0.0053; CAP p = <0.0001; hyperphosphataemia p = 0.024. Moderate/Severe undernutrition as assessed by MUAC cut-offs of 18.5cm in women and 20.5 cm in men. Immobility assessed as too immobile to get out of bed for measurement of height or weight.
(DOCX)
Data Availability Statement
The anonymised dataset and data dictionary are available at https://doi.org/10.6084/m9.figshare.8003318.