Abstract
Background
Although landmark studies in the 1990s demonstrated that adolescents and young adults (AYAs, ages 15–39 years) with cancer had lower survival improvement compared to other ages, therapeutic advances warrant reappraisal of those observations. We utilized more recent data to study site-specific AYA survival trends and disparities and gain a more contemporary understanding of this problem.
Methods
Using California Cancer Registry data from 1988 to 2014, we calculated 1) 5-year overall survival improvement for AYAs compared to other age groups; 2) hazard ratios (HRs) of death for AYAs comparing 2001–2014 with 1988–2000 stratified by site, stage, sex, age group, race and ethnicity, and socioeconomic status (SES); and 3) site-specific adjusted HRs (aHRs) for AYA risk groups and interaction analyses by time period.
Results
For all cancers combined, AYAs demonstrated survival improvement that exceeded all other age groups, largely due to reduced mortality in human immunodeficiency virus and acquired immunodeficiency syndrome-related cancers. The strongest predictor of death was cancer stage (aHR = 6.32 for distant vs localized, 95% confidence interval [CI] = 6.20 to 6.45). The aHR of death was statistically significantly higher for blacks (1.46, 95% CI = 1.42 to 1.50), Asian and Pacific Islanders (1.12, 95% CI = 1.09 to 1.15), and Latino whites (1.06, 95% CI = 1.04 to 1.08) compared to non-Latino whites, and was statistically significantly higher for low SES compared to high (1.31, 95% CI = 1.29 to 1.34). Survival disparities by stage, race and ethnicity, and SES worsened over time.
Conclusions
For AYAs in aggregate, the historical cancer survival improvement gap has been closed. However, the growing survival disparities in AYA subsets reported here, including advanced stage disease, racial and ethnic minorities, and low SES, highlight new priorities in need of increased attention, including inequities in cancer care and delivery within this vulnerable population.
Although advances in cancer treatment have resulted in improved survival, outcome differences exist among subgroups, indicating that multiple factors (ie, stage, sex, age, race and ethnicity, and socioeconomic status [SES]) determine cancer survival (1–3). In the 2006 landmark report, Closing the Gap, the National Cancer Institute (NCI) Progress Review Group reported that from 1977 to 1997, adolescents and young adults (AYAs, ages 15–39 years) in the Surveillance, Epidemiology, and End Results (SEER) Program had an alarming lack of improvement in cancer survival compared with other age groups (4).
With Closing the Gap serving as a call to action, diverse efforts were undertaken in subsequent years to improve outcomes for AYAs with cancer, including characterization of survival and toxicity disparities (5); elucidation of tumor biology (6,7); introduction of more effective therapy (8); improvement of AYA participation in clinical trials (9); establishment of an AYA Oncology Discipline Committee in the Children’s Oncology Group (COG) and some adult cooperative oncology groups in the United States (5,9); and emergence of the discipline of AYA oncology (10). However, potential improvements may not have extended to all AYA subgroups: recent data published by our group at the Los Angeles Cancer Surveillance Program documented inferior survival for AYAs who had advanced stage disease and were male, black, or of low SES (11). With 1 million new cases of AYA cancer diagnosed worldwide each year with variable race and ethnicity, SES, and available resources, a better understanding of factors that affect cancer survival in this vulnerable population is needed (12).
Thus, in light of the availability of more recent data reflecting at least some of these important developments, we undertook this study to provide a current, comprehensive, and fully transparent description of AYA site-specific cancer survival. Leveraging California’s rich racial, ethnic, and socioeconomic diversity, we used California Cancer Registry (CCR) data from 1988 to 2014 to evaluate cancer survival trends (changes in survival over time) for AYAs compared with other age groups, including by site and subgroup, and delineate sociodemographic subgroups of AYAs at risk for survival disparities. We hypothesized that AYA cancer survival has improved for certain cancer sites (eg, Kaposi sarcoma [KS], leukemia, lymphoma) and subgroups (eg, non-Latino whites [NLW], higher SES), but not for others. We also postulated that survival disparities persist for at-risk AYA subgroups (eg, blacks, lower SES).
Methods
Cancer Cases
The state of California requires that all cancer diagnoses among California residents be reported to the CCR. We obtained data on all AYA patients diagnosed in California with first primary invasive cancer, benign cancers in the brain and central nervous system (CNS), and bladder cancer in situ (defined as not invading the basement membrane of the mucosa of the bladder wall) from January 1, 1988, through December 31, 2014 (most recent data completion year) and reported to the CCR by August 1, 2017.
AYA Cancer Site Recode
For AYAs, cancer cases were subdivided by site [as defined by the AYA Site Recode and World Health Organization 2008 definition (13,14)]. Twenty-two of the most common AYA cancer sites were included plus “other,” comprising all other invasive cancer sites and bladder cancer in situ, for a total of 23 sites. For additional comparisons to the AYA age group, case counts and survival data from 1988 to 2014 were provided for all invasive cancers combined (including bladder cancer in situ) for younger children (0–14 years of age) and older adults (≥40 years of age).
Stage, Sex, Age, Race, Ethnicity, and SES
For each cancer case, stage at diagnosis (localized, regional, distant, unknown); sex (male, female, other or unknown); age at diagnosis (divided into 5-year intervals); race and ethnicity (NLW, black, Latino white [LW], Asian and Pacific Islander [API], and other, reported to the CCR by hospitals and physician offices as documented in medical records); and SES quintile (high, mid-high, middle, mid-low, low) were obtained. The CCR provides area-based SES estimates based on census block group level using information collected by the Census Bureau, which is ranked by quintiles from low (SES = 1) to high (SES = 5) (15,16). The 1990 census-based SES was used for cancer cases diagnosed during 1988–1995, the 2000 census-based SES for cases during 1996–2005, and the American Community Survey 2006–2010-based SES for cases during 2006–2014.
Time Periods
The period 1988–2014 was divided into two intervals (1988–2000 and 2001–2014) to allow for observation of trends over time. This demarcation was selected as a chronological midpoint in our follow-up period that roughly corresponds to emergence of an era with growing emphasis on AYA disparities by the NCI, the COG, and certain adult cooperative oncology groups in the United States, as well as advocacy organizations (4,5,9,17).
Survival Analyses
We calculated 5-year observed survival by time period and quantified the change between the two time periods. For deceased patients, survival time was measured in days from the date of diagnosis to the date of death from any cause. Mortality as an endpoint is ascertained by the registry through a combination of methods including information sharing from the reporting hospitals, record linkage with vital statistics, Social Security Administration, driver license information, and credit records. Patients alive at the end of the study period (December 31, 2014) or patients lost to follow-up before the end of the study period were censored at the end of study date or date of last known previous contact while alive, respectively, through the methods listed above. Five-year observed survival is defined as the proportion of patients alive 5 years after diagnosis.
Absolute differences in sex-specific 5-year survival between 1988–2000 and 2001–2014 were calculated for all invasive cancers combined for all ages, including bladder cancer in situ and excluding benign brain and CNS (not reportable until 2001), for cases at 5-year age intervals, with the objective of gaining a broad understanding of how AYA survival in aggregate is changing compared to younger and older patients. Absolute survival differences were chosen to minimize the amount of statistical transformation needed to quantify improvements. We then performed this comparison excluding KS (a human immunodeficiency virus [HIV] or acquired immunodeficiency syndrome [AIDS]-related cancer). To verify that survival changes over time were statistically significant, ratios and confidence limits of 5-year survival between the two time periods were calculated (Supplementary Table 2, available online).
Further analyses were performed among subsets of AYAs only. Site-specific 5-year survival for AYAs was calculated using the nonparametric Kaplan-Meier survival function. To quantify higher and lower risk factors for death, hazard ratios (HRs), consisting of a ratio of mortality between two groups, were calculated (HR >1.0 corresponded to a higher risk of death and HR <1.0 to a lower risk of death compared to the reference group). Crude hazard ratios for death between the two time periods were calculated for each site and further stratified by stage, sex, age, race and ethnicity, and SES (excluding benign brain and CNS as not reportable until 2001). Site-specific crude hazard ratios and 95% confidence intervals (CIs) by time period were then calculated within each subgroup, and then adjusted for stage, sex, age, race and ethnicity, and SES. Survival analyses and curves were constructed for each site stratified by time period. Survival differences between time periods were evaluated using the log-rank test.
Multivariable Cox proportional adjusted hazard ratios (aHRs) for death for the entire 1988–2014 time period were calculated for each site by risk factors for stage, sex, age, race and ethnicity, and SES (including benign brain and CNS from 2001 to 2014) and adjusted for all other risk factors listed. Older and younger AYAs were defined as 25–34 years old and 15–24 years old, respectively, for bone and soft tissue sarcoma (STS), acute lymphocytic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), ovarian germ cell, and testis (ie, sites with higher frequencies in younger AYAs to compare two similarly sized age groups). For all remaining sites, older and younger AYAs were defined as 35–39 years old and 15–34 years old, respectively, because the frequency of cases in these sites was more equivalent in the two age groups. To test for statistical differences in adjusted hazard ratios over time, adjusted hazard ratios by site and subgroup were further stratified by time period (1988–2000 and 2001–2014), and interaction analyses by time period were performed. All statistical analyses were carried out using SAS Version 9.4 (Cary, NC). All P values were two-sided (P < .05).
Results
For 1988–2000 and 2001–2014, a total of 107 747 and 117 746 first cancers were diagnosed among AYA California residents, respectively (Table 1). In the later time period, a higher proportion of cases had localized disease and were female, LW, younger, and of lower SES. Site-specific subgroup case counts and distribution details are shown in Supplementary Table 1 (available online). A cancer site list with case counts for “other” site category is provided in Supplementary Table 2 (available online).
Table 1.
Distribution of all adolescent and young adult cancers by time period, tumor, and patient characteristics, California, 1988–2014
| Characteristic | Distribution, % |
|
|---|---|---|
| 1988–2000 | 2001–2014 | |
| (n = 107 747) | (n = 117 746) | |
| Cancer site | ||
| Bone and soft tissue sarcoma | 2.4 | 2.7 |
| Brain and CNS: Benign | — | 3.9 |
| Brain and CNS: Invasive | 4.2 | 4.1 |
| Breast | 14.1 | 13.8 |
| Cervix | 6.2 | 4.5 |
| Colorectal | 3.7 | 4.8 |
| Kaposi sarcoma | 8.5 | 1.0 |
| Kidney | 1.1 | 2.1 |
| Leukemia: ALL | 1.3 | 1.8 |
| Leukemia: AML | 1.7 | 1.9 |
| Leukemia: CML | 0.9 | 0.9 |
| Lip, oral cavity, and pharynx | 1.6 | 1.6 |
| Lung | 1.8 | 1.3 |
| Lymphoma: Hodgkin | 5.0 | 5.1 |
| Lymphoma: Non-Hodgkin | 7.5 | 5.8 |
| Melanoma | 10.6 | 9.1 |
| Ovary: Carcinoma | 1.6 | 1.4 |
| Ovary: Germ cell | 0.5 | 0.6 |
| Stomach | 1.1 | 1.1 |
| Testis | 7.5 | 8.5 |
| Thyroid | 7.8 | 12.4 |
| Uterus | 1.1 | 1.8 |
| Other* | 9.8 | 9.7 |
| Stage | ||
| Localized | 46.5 | 51.6 |
| Regional | 21.6 | 25.4 |
| Distant | 21.1 | 18.8 |
| In situ | 0.2 | 0.5 |
| Unknown | 10.7 | 3.7 |
| Sex | ||
| Male | 46.9 | 40.7 |
| Female | 53.1 | 59.3 |
| Other or unknown | 0.0 | 0.0 |
| Age group, y | ||
| 15–19 | 5.1 | 7.1 |
| 20–24 | 8.5 | 11.2 |
| 25–29 | 16.6 | 16.8 |
| 30–34 | 28.5 | 25.8 |
| 35–39 | 41.2 | 39.1 |
| Race and ethnicity | ||
| Asian and Pacific Islander | 7.3 | 9.8 |
| Black | 6.9 | 5.7 |
| Latino white | 23.1 | 33.5 |
| Non-Latino white | 59.5 | 45.9 |
| Other or unknown | 3.2 | 5.1 |
| Socioeconomic status | ||
| High | 21.3 | 20.3 |
| Mid-high | 22.7 | 21.7 |
| Middle | 20.9 | 20.4 |
| Mid-low | 18.5 | 19.4 |
| Low | 16.6 | 18.2 |
Other indicates all noncategorized invasive cancers and benign intracranial tumors. See Supplementary Table 2 (available online) for cancer sites included in other. Of the other cancers, 2% and 5.5% are in situ stage in 1988–2000 and 2001–2014, respectively. ALL = acute lymphocytic leukemia; AML = acute myeloid leukemia; CML = chronic myeloid leukemia; CNS = central nervous system.
Overall AYA Survival Trends
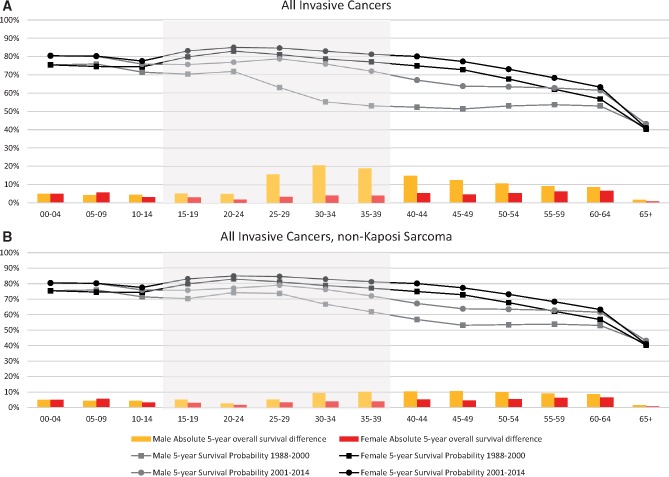
To gain an overall perspective of survival trends, we first compared 5-year survival improvements for AYAs to other ages. For all cancers between 1988–2000 and 2001–2014, we found that survival improvement was at least as large among AYAs as in younger children and older adults. For all cancers combined including KS, AYA males demonstrated the largest survival improvement (Figure 1A). Among 30- to 34-year-olds, 5-year survival increased by 20.6% in males but only 4.2% in females, and among 35- to 39-year-olds by 18.9% in males and 4.2% in females. Among males of all ages, survival improvement was greatest for AYAs. By way of contrast, when KS was excluded, survival also increased for AYA males, but not as dramatically (9.5% and 10.3% for the 30- to 34-years-olds and 35- to 39-year-olds, respectively Figure 1B). Excluding KS, between 1988–2000 and 2001–2014, AYAs demonstrated survival improvement that was larger than younger children, but similar to older adults. Five-year survival rate ratios for each 5-year age interval are presented in Supplementary Table 3 (available online).
Figure 1.
Absolute observed 5-year survival (lines) and survival difference (bars) of all invasive cancer by sex and age group at 5-year intervals, California, 1988–2000 to 2001–2014. A) All invasive cancers. B) All invasive cancers excluding Kaposi sarcoma. Absolute 5-year survival difference calculated by subtracting 1988–2000 5-year survival from 2001–2014 5-year survival. Invasive cancer is all cancers with invasive behavior code (excluding brain and central nervous system benign behavior code) and including bladder in situ. Shaded area indicates ages 15–39 years. All differences in survival between the two time periods (bars) meet statistical significance (see Supplementary Table 3, available online).
Site- and Demographic Subgroup-Specific AYA Survival Trends
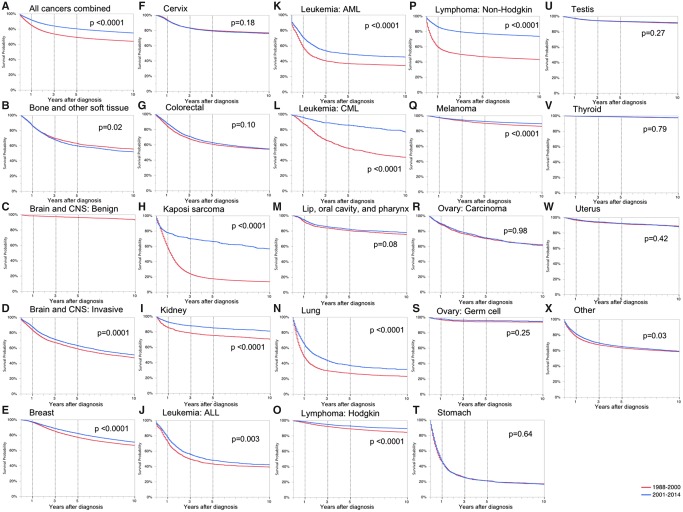
We next examined changes in site- and subgroup-specific AYA survival over time. For each cancer site, Table 2 displays the overall crude and adjusted hazard ratios for death comparing 2001–2014 with 1988–2000. For all cancer sites combined, the overall adjusted hazard ratio (0.70, 95% CI = 0.69 to 0.71) indicated a lower risk for death in the later time period. Survival improved for 15 of 22 cancer sites. There were no statistically significant differences in survival between the time periods for bone and STS, ovarian carcinoma, ovarian germ cell tumor, stomach cancer, testicular cancer, thyroid cancer, and uterine cancer. Sites showing the greatest improvement in survival were KS (aHR = 0.26, 95% CI = 0.24 to 0.29), chronic myeloid leukemia (CML; aHR = 0.33, 95% CI = 0.28 to 0.39), and non-Hodgkin lymphoma (NHL; aHR = 0.38, 95% CI = 0.36 to 0.41) (Table 2, Figure 2). There were no cancer sites that had statistically significant worse survival after adjusting for cancer and demographic factors.
Table 2.
Crude hazard ratio (HR) for death in adolescent and young adult California residents during 2001–2014 compared with 1988–2000
| Site | Overall: Crude | Overall: Adjusted | Stage |
Sex |
Age group, y |
Race and ethnicity |
Socioeconomic status |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Localized | Regional | Distant | Unknown | Male | Female | 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | Asian and Pacific Islander | Black | Latino white | Non-Latino white | Other or unknown | High | Mid-high | Middle | Mid-low | Low | |||
| Total | 0.65* | 0.70* | 0.66* | 0.74* | 0.70* | 0.58* | 0.55* | 0.84* | 0.83* | 0.84* | 0.63* | 0.58* | 0.66* | 0.75* | 0.66* | 0.69* | 0.57* | 0.99 | 0.58* | 0.59* | 0.64* | 0.69* | 0.72* |
| Bone and soft tissue sarcoma | 1.10† | 1.03 | 1.11 | 1.04 | 0.99 | 0.90 | 1.09 | 1.11 | 0.91 | 1.20 | 1.27† | 1.08 | 1.10 | 1.27 | 1.39† | 1.02 | 1.02 | 3.43† | 1.05 | 1.00 | 1.25† | 1.12 | 1.06 |
| Brain and CNS: Invasive | 0.89* | 0.93* | 0.89* | 1.00 | 0.99 | 0.88 | 0.90* | 0.88* | 0.92 | 0.90 | 0.94 | 0.86* | 0.93 | 0.94 | 0.86 | 0.94 | 0.83* | 1.34 | 0.93 | 0.90* | 0.87 | 0.87* | 0.86* |
| Breast | 0.84* | 0.77* | 0.83* | 0.72* | 0.75* | 1.16 | 0.63 | 0.84* | 1.80 | 0.77 | 0.74* | 0.79* | 0.88* | 0.86* | 0.92 | 0.81* | 0.82* | 1.09 | 0.78* | 0.80* | 0.83* | 0.88* | 0.91 |
| Cervix | 1.06 | 0.91* | 0.93 | 0.89* | 0.91 | 0.60* | — | 1.06 | 2.39 | 1.72† | 1.07 | 1.02 | 1.02 | 0.82 | 1.17 | 1.01 | 1.08 | 2.62† | 1.10 | 0.93 | 1.05 | 1.05 | 1.16† |
| Colorectal | 0.95 | 0.86* | 0.88 | 0.90 | 0.82* | 0.80 | 0.93 | 0.98 | 0.79 | 0.94 | 1.11 | 0.93 | 0.92 | 1.07 | 0.93 | 1.01 | 0.88* | 1.38 | 0.86* | 0.98 | 0.85* | 0.95 | 1.07 |
| Kaposi sarcoma | 0.30* | 0.26* | 0.22* | 0.37* | 0.34* | 0.36* | 0.29* | 0.75 | 2.07 | 0.49* | 0.29* | 0.32* | 0.28* | 0.22* | 0.40* | 0.33* | 0.24* | 0.39* | 0.17* | 0.28* | 0.31* | 0.29* | 0.42* |
| Kidney | 0.61* | 0.81* | 0.82 | 1.08 | 0.83 | 0.60 | 0.64* | 0.55* | 0.47 | 0.76 | 0.91 | 0.63* | 0.55* | 1.21 | 0.81 | 0.63* | 0.49* | 1.24 | 0.53* | 0.51* | 0.56* | 0.81 | 0.59* |
| Leukemia: ALL | 0.87* | 0.84* | — | — | 0.87* | — | 0.84* | 0.92 | 0.70* | 0.94 | 1.10 | 0.98 | 0.89 | 0.81 | 0.82 | 0.85* | 0.79* | 2.07 | 0.83 | 0.98 | 0.86 | 0.88 | 0.76* |
| Leukemia: AML | 0.71* | 0.71* | — | — | 0.71* | — | 0.75* | 0.68* | 0.64* | 0.80* | 0.78* | 0.65* | 0.71* | 0.63* | 0.70* | 0.76* | 0.69* | 0.71 | 0.61* | 0.63* | 0.65* | 0.78* | 0.84* |
| Leukemia: CML | 0.33* | 0.33* | — | — | 0.33* | — | 0.38* | 0.23* | 0.26* | 0.24* | 0.34* | 0.39* | 0.34* | 0.22* | 0.51* | 0.35* | 0.31* | 0.33 | 0.30* | 0.40* | 0.26* | 0.28* | 0.41* |
| Lip, oral cavity, and pharynx | 0.88 | 0.86* | 0.89 | 0.79* | 0.79 | 0.75 | 0.91 | 0.88 | 2.21 | 2.03 | 1.06 | 0.85 | 0.81* | 0.82 | 0.46* | 1.01 | 0.99 | 0.82 | 0.93 | 0.77 | 1.01 | 0.97 | 0.70* |
| Lung | 0.71* | 0.70* | 0.59* | 0.45* | 0.69* | 0.80 | 0.71* | 0.73* | 1.00 | 0.75 | 0.81 | 0.70* | 0.78* | 0.97 | 0.62* | 0.66* | 0.68* | 0.90 | 0.69* | 0.70* | 0.63* | 0.79* | 0.76* |
| Lymphoma: Hodgkin | 0.69* | 0.68* | 0.70 | 0.74* | 0.68* | 0.42* | 0.65* | 0.78* | 0.71* | 0.81 | 0.64* | 0.54* | 0.87 | 0.99 | 0.71* | 0.71* | 0.60* | 1.13 | 0.72* | 0.62* | 0.65* | 0.85 | 0.59* |
| Lymphoma: Non-Hodgkin | 0.35* | 0.38* | 0.20* | 0.38* | 0.43* | 0.39* | 0.32* | 0.57* | 0.53* | 0.46* | 0.36* | 0.31* | 0.36* | 0.47* | 0.41* | 0.41* | 0.29* | 0.39* | 0.27* | 0.26* | 0.33* | 0.39* | 0.48* |
| Melanoma | 0.78* | 0.75* | 0.63* | 0.65* | 1.00 | 1.00 | 0.79* | 0.80* | 0.90* | 0.74* | 0.77* | 0.85* | 0.74* | 0.61 | 0.44 | 0.85 | 0.77* | 0.36 | 0.77* | 0.71* | 0.83* | 0.74* | 0.90 |
| Ovary: Carcinoma | 1.00 | 1.11 | 1.16 | 0.96 | 1.19† | 0.74 | — | 1.00 | 1.14 | 1.55 | 0.78 | 0.95 | 1.05 | 0.89 | 1.10 | 1.00 | 0.95 | 1.31 | 0.98 | 0.91 | 1.00 | 1.21 | 0.85 |
| Ovary: Germ cell | 0.75 | 0.85 | 0.81 | 0.15 | 0.93 | — | — | 0.75 | 0.70 | 0.42 | 0.98 | 0.53 | 2.10 | 0.15 | 1.00 | 0.90 | 0.86 | — | 0.56 | 0.78 | 1.11 | 0.50 | 0.82 |
| Stomach | 0.98 | 0.92 | 0.96 | 0.88 | 0.92 | 0.85 | 1.00 | 0.96 | 1.36 | 0.91 | 1.06 | 0.94 | 0.97 | 1.04 | 0.89 | 1.03 | 0.81* | 0.84 | 0.92 | 0.94 | 0.88 | 1.08 | 1.02 |
| Testis | 0.94 | 0.93 | 0.87 | 0.95 | 1.03 | 0.94 | 0.94 | — | 0.83 | 1.06 | 0.92 | 0.82 | 0.97 | 1.04 | 1.31 | 0.94 | 0.74* | 1.16 | 1.01 | 0.77 | 0.98 | 0.81* | 1.00 |
| Thyroid | 0.97 | 1.00 | 1.01 | 0.93 | 1.48 | 1.29 | 1.05 | 0.95 | 0.67 | 1.43 | 0.77 | 1.13 | 0.91 | 1.42 | 0.70 | 0.86 | 1.04 | 0.31 | 0.84 | 1.01 | 0.96 | 0.94 | 1.06 |
| Uterus | 1.10 | 1.06 | 1.09 | 0.90 | 0.93 | 2.29 | — | 1.10 | — | 4.75 | 1.15 | 1.04 | 1.10 | 1.37 | 1.08 | 1.07 | 1.04 | 5.67† | 0.89 | 1.18 | 1.22 | 0.87 | 1.35 |
| Other‡ | 0.95* | 0.94* | 1.23† | 0.97 | 0.86* | 0.98 | 0.94* | 0.99 | 1.06 | 1.09 | 1.09 | 0.94 | 0.90* | 0.80* | 0.92 | 0.94 | 0.96 | 1.58† | 0.90* | 0.98 | 0.96 | 0.99 | 0.91* |
Indicates a statistically significant lower risk for death in 2001–2014 compared to 1988–2000. Risk group 2001–2014, reference group 1988–2000. Adjusted overall hazard ratio = hazard ratio for time period adjusted for stage, sex, age, race and ethnicity, and socioeconomic status. ALL = acute lymphocytic leukemia; AML = acute myeloid leukemia; CML = chronic myeloid leukemia; CNS = central nervous system.
Indicates a statistically significant higher risk for death in 2001–2014 compared with 1988–2000.
No superscript indicates no statistically significant difference in mortality in 2001–2014 compared to 1988–2000.
Other indicates all noncategorized invasive cancers and benign intracranial tumors. See Supplementary Table 2 (available online) for cancer sites included in other.
Figure 2.
Kaplan-Meier survival curves by time period for all cancers by site, adolescent and young adult, California, 1988–2000 and 2001–2014. Log-rank two-sided P values are reported. A) All cancers combined; B) bone and other soft tissue; C) brain and CNS: benign; D) brain and CNS: invasive; E) breast; F) cervix; G) colorectal; H) Kaposi sarcoma; I) kidney; J) leukemia: ALL; K) leukemia: AML; L) leukemia: CML; M) lip, oral cavity, and pharynx; N) lung; O) lymphoma: Hodgkin; P) lymphoma: non-Hodgkin; Q) melanoma; R) ovary: carcinoma; S) ovary: germ cell; T) stomach; U) testis; V) thyroid; W) uterus; X) other. Other indicates all noncategorized invasive cancers and benign intracranial tumors. See Supplementary Table 2 (available online) for cancer sites included in other. ALL = acute lymphocytic leukemia; AML = acute myeloid leukemia; AYA = adolescent and young adult; CML = chronic myeloid leukemia; CNS = central nervous system.
Table 2 also summarizes the subgroup-specific crude hazard ratios for death. Subgroups that showed worsening trends in survival between the two time periods included black AYAs with bone and STS (HR = 1.39, 95% CI = 1.07 to 1.80), 20- to 24-year-old women with cervical cancer (HR = 1.72, 95% CI = 1.15 to 3.15), and AYAs of low SES with cervical cancer (HR = 1.16, 95% CI = 1.02 to 1.33). The survival improvements in KS and NHL were more evident in those from higher SES groups compared to the lowest SES group. In addition, survival improvements in invasive brain and CNS cancer, colorectal cancer, melanoma, stomach cancer, and testicular cancer were statistically significant among NLW patients; however, no statistically significant improvements were seen among other racial or ethnic groups (Table 2).
AYA Survival Disparities and Trends
We then evaluated whether tumor and host characteristics were associated with mortality for each cancer site. Table 3 shows the site-specific adjusted hazard ratios for death over the entire 1988–2014 time period for each at-risk subgroup compared to each indicated reference group. Interaction analyses between the two time periods (1988–2000 and 2001–2014) are represented by superscripts to indicate if adjusted hazard ratios increased or decreased between the two time periods (Table 3; Supplementary Table 4, available online).
Table 3.
Adjusted hazard ratios for death by site among AYAs by selected patient and tumor characteristics, California, 1988–2014
| Risk group | Distant | Male | Older AYAs* | Asian and Pacific Islander | Black | Latino white | Low and mid-low SES |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Reference group | Localized | Female | Younger AYAs | Non-Latino white | Non-Latino white | Non-Latino white | High and mid-high SES |
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Total | 6.32† (6.20 to 6.45) | 1.47‡ (1.44 to 1.49) | 1.42‡ (1.40 to 1.44) | 1.12† (1.09 to 1.15) | 1.46† (1.42 to 1.50) | 1.06† (1.04 to 1.08) | 1.31† (1.29 to 1.34) |
| Bone and soft tissue sarcoma | 7.19 § (6.48 to 7.98) | 1.14 § (1.06 to 1.24) | 1.14 § (1.05 to 1.24) | 1.26 § (1.09 to 1.46) | 1.39 § (1.20 to 1.60) | 1.23 § (1.11 to 1.35) | 1.10 § (1.00 to 1.21) |
| Brain and CNS: Benign | — | 1.13 (0.86 to 1.48) | 1.07 (0.81 to 1.40) | 0.87 (0.52 to 1.46) | 0.75 (0.40 to 1.37) | 1.20 (0.88 to 1.64) | 2.13 (1.50 to 3.05) |
| Brain and CNS: Invasive | 2.00 § (1.69 to 2.36) | 1.29 § (1.22 to 1.37) | 1.38 § (1.30 to 1.47) | 1.22 § (1.10 to 1.36) | 1.09 § (0.95 to 1.24) | 1.05† (0.98 to 1.13) | 1.20 § (1.12 to 1.29) |
| Breast | 11.85† (11.07 to 12.68) | 1.00 § (0.65 to 1.54) | 0.93 § (0.89 to 0.97) | 0.90 § (0.84 to 0.96) | 1.46 § (1.37 to 1.56) | 0.99 § (0.94 to 1.04) | 1.36† (1.29 to 1.42) |
| Cervix | 18.99† (16.99 to 21.22) | — | 1.10 § (1.03 to 1.19) | 1.13 § (0.96 to 1.32) | 1.66 § (1.46 to 1.89) | 1.01 § (0.92 to 1.10) | 1.31 § (1.19 to 1.44) |
| Colorectal | 13.37† (12.06 to 14.82) | 1.34 § (1.26 to 1.43) | 0.99 § (0.93 to 1.06) | 1.00† (0.90 to 1.10) | 1.14 § (1.02 to 1.27) | 0.93 § (0.86 to 1.00) | 1.39† (1.29 to 1.51) |
| Kaposi sarcoma | 1.78 § (1.67 to 1.90) | 1.31‡ (1.05 to 1.64) | 0.99 § (0.94 to 1.03) | 0.91 § (0.77 to 1.08) | 1.08† (1.01 to 1.17) | 0.90 § (0.85 to 0.95) | 1.07† (1.02 to 1.13) |
| Kidney | 33.90† (28.42 to 40.44) | 1.29 § (1.11 to 1.50) | 1.00 § (0.87 to 1.15) | 1.14 § (0.86 to 1.51) | 1.49† (1.21 to 1.84) | 0.95 § (0.80 to 1.13) | 1.26 § (1.07 to 1.50) |
| Leukemia: ALL | — | 1.10 § (1.00 to 1.21) | 1.83† (1.67 to 2.01) | 1.15 § (0.95 to 1.40) | 1.69 § (1.36 to 2.11) | 1.29 § (1.14 to 1.45) | 1.26 § (1.12 to 1.43) |
| Leukemia: AML | — | 1.17 § (1.08 to 1.27) | 1.17 § (1.07 to 1.28) | 0.98 § (0.86 to 1.12) | 1.20 § (1.02 to 1.42) | 0.98 § (0.88 to 1.08) | 1.14† (1.03 to 1.27) |
| Leukemia: CML | — | 1.20† (1.03 to 1.39) | 1.00 § (0.86 to 1.16) | 0.81 § (0.63 to 1.03) | 1.27 § (0.99 to 1.63) | 0.97 § (0.81 to 1.15) | 1.54 § (1.29 to 1.83) |
| Lip, oral cavity, and pharynx | 6.23 § (5.09 to 7.63) | 1.47 § (1.28 to 1.69) | 1.47‡ (1.28 to 1.68) | 1.10 § (0.88 to 1.37) | 1.41‡ (1.13 to 1.76) | 0.87 § (0.72 to 1.04) | 1.37 § (1.17 to 1.60) |
| Lung | 13.07† (10.96 to 15.58) | 1.18 † (1.09 to 1.28) | 1.23 § (1.13 to 1.35) | 0.82 § (0.73 to 0.92) | 0.98 § (0.87 to 1.12) | 0.82 § (0.73 to 0.91) | 1.27 § (1.16 to 1.40) |
| Lymphoma: Hodgkin | 2.12† (1.81 to 2.48) | 1.44 § (1.31 to 1.59) | 1.46 § (1.32 to 1.62) | 1.12† (0.89 to 1.40) | 1.51 § (1.29 to 1.77) | 1.15 § (1.02 to 1.30) | 1.51 § (1.34 to 1.69) |
| Lymphoma: Non-Hodgkin | 1.70† (1.60 to 1.81) | 2.28‡ (2.14 to 2.42) | 1.18 § (1.12 to 1.24) | 0.69† (0.62 to 0.77) | 1.12† (1.03 to 1.22) | 1.01† (0.95 to 1.07) | 1.29† (1.21 to 1.37) |
| Melanoma | 31.42† (28.22 to 34.97) | 1.71 § (1.59 to 1.84) | 1.20 § (1.11 to 1.29) | 1.78 § (1.34 to 2.38) | 1.21‡ (0.80 to 1.83) | 1.01 § (0.90 to 1.15) | 1.63 § (1.49 to 1.77) |
| Ovary: Carcinoma | 8.13 § (6.82 to 9.69) | — | 1.14 § (1.02 to 1.28) | 1.21 § (1.02 to 1.44) | 1.33 § (1.04 to 1.70) | 1.10 § (0.95 to 1.28) | 1.14 § (0.99 to 1.31) |
| Ovary: Germ cell | 5.09 § (3.03 to 8.54) | — | 1.92 § (1.19 to 3.09) | 0.77 § (0.33 to 1.79) | 1.56 § (0.70 to 3.48) | 1.10 § (0.62 to 1.97) | 1.72 § (0.93 to 3.18) |
| Stomach | 11.10 § (8.83 to 13.95) | 1.08 § (0.99 to 1.18) | 0.98 § (0.90 to 1.07) | 0.96 § (0.83 to 1.10) | 1.22 § (1.00 to 1.48) | 1.04† (0.92 to 1.18) | 1.06 § (0.94 to 1.19) |
| Testis | 7.76† (6.96 to 8.65) | — | 1.26‡ (1.12 to 1.40) | 1.49 § (1.15 to 1.93) | 1.54† (1.18 to 2.02) | 1.28† (1.15 to 1.42) | 1.54 § (1.37 to 1.72) |
| Thyroid | 5.02† (4.00 to 6.31) | 2.46 § (2.10 to 2.87) | 1.75 § (1.50 to 2.04) | 0.82 § (0.64 to 1.07) | 1.03 § (0.69 to 1.52) | 0.95 § (0.79 to 1.14) | 1.87 § (1.56 to 2.24) |
| Uterus | 20.70† (16.02 to 26.74) | — | 1.33 § (1.08 to 1.64) | 1.16 § (0.87 to 1.55) | 1.61 § (1.06 to 2.42) | 0.89 § (0.69 to 1.14) | 1.22 § (0.96 to 1.56) |
| Other‖ | 5.96 § (5.59 to 6.35) | 1.29 § (1.24 to 1.35) | 1.31‡ (1.26 to 1.37) | 1.25‡ (1.18 to 1.33) | 1.21 § (1.12 to 1.30) | 1.12 § (1.06 to 1.19) | 1.32 § (1.25 to 1.39) |
Older to younger AYAs included 25–34 to 15–24 years for bone and soft tissue sarcoma, ALL, AML, Hodgkin lymphoma, ovarian germ cell, and testis (sites with higher frequencies in younger AYAs). Older to younger AYAs included 35–39 to 15–34 years for remaining sites (sites with higher frequencies in older AYAs). Adjusted hazard ratio signifies adjustment for all other factors (stage, sex, age, race and ethnicity, socioeconomic status [SES]) for time period 1988–2014. aHR = adjusted hazard ratio; ALL = acute lymphocytic leukemia; AML = acute myeloid leukemia; AYA = adolescent and young adult; CI = confidence interval; CML = chronic myeloid leukemia; CNS = central nervous system.
Indicates that when testing for interaction by time period, there is a statistically significant increase in adjusted hazard ratio from 1988–2000 to 2001–2014.
Indicates that when testing for interaction by time period, there is a statistically significant decrease in adjusted hazard ratio from 1988–2000 to 2001–2014.
§indicates that when testing for interaction by time period, no statistically significant change in adjusted hazard ratio from 1988–2000 to 2001–2014. No superscript indicates there was no testing for interaction by time period, as for benign brain and CNS, as it was not reportable until 2001. See Supplementary Table 4 (available online) for details.
Other indicates all noncategorized invasive cancers and benign intracranial tumors. See Supplementary Table 2 (available online) for cancer sites included in other.
Stage of disease remained the strongest predictor of mortality among AYAs (aHR distant to localized for all cancers = 6.32, 95% CI = 6.20 to 6.45) for every applicable cancer site. Further, adjusted hazard ratios for distant to localized disease increased over time for most cancer sites. For all cancers from 1988 to 2014, males had an elevated adjusted hazard ratio of death compared to females (1.47, 95% CI = 1.44 to 1.49); however, this disparity decreased over the two time periods. By age, older AYAs had an increased adjusted hazard ratio compared to younger AYAs (1.42, 95% CI = 1.40 to 1.44); this disparity also decreased over time. For race and ethnicity, compared to NLWs, blacks had the highest adjusted hazard ratio for all cancers combined (1.46, 95% CI = 1.42 to 1.50), a disparity present in almost every cancer site, followed by APIs (1.12, 95% CI = 1.09 to 1.15) and LWs (1.06, 95% CI = 1.04 to 1.08). For every race and ethnicity compared to NLWs, survival disparities for all cancers combined worsened over time. For all cancers, AYA patients of lower SES had an increased adjusted hazard ratio of death compared to higher SES (1.31, 95% CI = 1.29 to 1.34); and like the racial and ethnic disparities, this risk increased over time.
Discussion
With landmark reports from the 1990s highlighting lower survival improvement for AYAs relative to other ages, the overall objective of this study was to provide a more current, enriched understanding of AYA cancer survival trends and disparities. Using California population-based data from 1988 to 2014, our study shows that, after excluding KS, AYAs have experienced similar survival improvements to both younger and older patients. This improvement held true for multiple AYA cancers and was particularly striking for HIV and AIDS-related cancers following the introduction of combined antiretroviral therapy in 1996. At the same time, our study clearly indicates that progress has not been uniformly shared among all AYA subsets, notably those with certain types of cancer and with advanced stage disease, as well as among racial and ethnic minorities and lower SES groups. These results are important because they highlight that, although there has been gratifying progress resulting from nearly three decades of well-deserved emphasis on AYA cancer resulting in closure of the AYA survival improvement gap, challenges involving cancer biology and therapeutics, treatment delivery, and health-care equity remain.
Recent articles may appear somewhat contradictory in their conclusions about the current status of the AYA survival. For example, SEER-17 data from 2000 to 2009 showed AYA survival to be equivalent or better to younger children and older adults in virtually all cancer sites, except for female breast cancer and leukemia (7). However, another contemporary report observed lower survival improvements for certain cancers among AYAs as compared to younger and older adults (18). These seemingly conflicting observations can be reconciled by a recent article from our group evaluating AYA survival trends using SEER-wide data from 1973 to 2009, which found that after excluding HIV and AIDS-related cancers and the HIV and AIDS epidemic time period, AYAs with cancer in aggregate had the highest survival compared to other age groups and a consistently improving 5-year relative survival trend over time; however, there was slightly lower survival improvement than in other ages because of a higher baseline survival for AYAs rather than an absolute failure to improve (19). In addition to highlighting the important distinction between measuring survival improvements and survival itself, in that report (19), we substantiated the initial hypothesis (4,20) that the negative survival improvement gap observed in AYAs from 1977 to 1997 was essentially driven by the rise and height of HIV and AIDs-related cancers in AYA males. Similarly, the dramatic survival improvement we found among AYAs in this study from 1988 to 2014, especially among AYA males, was the result of the baseline survival corresponding to the height of the HIV and AIDS epidemic and the large survival improvement resulting from its fall.
This otherwise optimistic picture of recent AYA trends is also tempered by our data in showing that survival improvement has not been shared equally by all AYAs, differentiated by site, stage, and sociodemographic characteristics. A few cancer sites have demonstrated dramatic survival improvement over the two time periods [KS and NHL, resulting from therapeutic advances for HIV (21) and targeted anti-CD20 immunotherapy (22), and CML, from the introduction of tyrosine kinase inhibitors (23)]. In contrast, certain cancer sites have not shown any survival improvement. Whereas survival for certain sites is excellent and likely nearing asymptotic levels (thyroid, testis, ovarian germ cell cancers), other sites (bone and STS, ALL, AML, lung cancer, stomach cancer) have considerable room for improvement.
For each cancer site, the strongest predictor of survival was stage. Of concern, this stage-related survival gap appears to be worsening over time. These findings suggest that research specifically focused on elucidating the biology of advanced-stage solid tumors and inherently systemic cancers, such as leukemias and lymphomas, has potential for substantial impact on AYA survival.
In addition, we found that host factors contribute to the likelihood of AYA cancer survival. Sex- and age-specific survival disparities, although present, are improving over time. In contrast, survival disparities among some racial and ethnic minorities and those of low SES have stagnated or worsened. These trends hold true even in sites with a favorable prognosis (testicular cancer, HL) and with marked survival gains (KS, NHL). Racial-, ethnic-, and SES-related survival disparities among AYAs have been described in many common AYA cancer types, such as HL, NHL, testicular, thyroid, and breast cancer (24–28). Troubling racial and ethnic survival trends over time have also been reported for black patients with breast and other cancers, and for Latino children with ALL (1–3,24–31). Although survival differences by race, ethnicity, and SES could be influenced by differences in disease or host biology, treatment response, adherence, or issues related to cultural competency of health services, our study raises an overarching concern about systemic inequality in care access and delivery for AYAs, a population already known to be at risk for lacking adequate health insurance (32).
Our study offers several strengths including the large, robust, and diverse CCR dataset, allowing for site-specific survival adjusted for demographic factors as well as racial-, ethnic-, and socioeconomic-specific analyses; incorporation of differential survival over two time periods permitting demonstration of a trajectory of survival disparities; and the ability to have reliable 5-year vital status data in both time-period groups. However, there are also potential limitations inherent to registry-based research. These include possible misclassification of stage, race, or ethnicity provided by the reporting site; misclassification of SES, a block-level measure based on census data using the address provided from medical records; and potential for misclassification of vital status. Despite these concerns, SEER registries, including the CCR data used in this study, capture high-quality data, adhere to strict quality control standards, and are used as benchmarks for registry-based data worldwide.
Even though the historical gap in AYA survival improvement has been closed with the advent of effective treatment for HIV and AIDS-related cancers and increased focus on AYA cancer, AYAs remain a vulnerable population with unique age-related challenges. Future directions include in-depth analyses of site- and subgroup-specific survival trends to explain continued disparities and lack of survival improvement and to determine effective interventions for improving outcomes in certain cancer sites, in late stage disease, and among sociodemographically disadvantaged subgroups.
Funding
Diana J. Moke was supported by grant T32 CA09659 from the National Institutes of Health. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s SEER Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Notes
Affiliations of authors: Children’s Center for Cancer and Blood Diseases, Children’s Hospital Los Angeles, Los Angeles, CA (DJM, DRF); Los Angeles Cancer Surveillance Program, Department of Preventive Medicine, Keck School of Medicine, University of Southern California (USC), Los Angeles, CA (KT, ASH, AH, LL, DD); USC Norris Comprehensive Cancer Center, Los Angeles, CA (AH, DRF, DD).
There are no conflicts of interests to disclose.
Diana J. Moke contributed to the overall concept of the study, literature search, table and figure design, and data interpretation; wrote the first draft of the paper; and approved the final manuscript. Kaiya Tsai contributed to the data analysis, data interpretation, figures, and tables and approved the final manuscript. David R. Freyer contributed to the overall concept of the study, literature search, and data interpretation and approved the final manuscript. Ann S. Hamilton contributed to the overall concept of the study, figures, tables, data analysis, and data interpretation and approved the final manuscript. Amie Hwang contributed to the figures, tables, data analysis, and data interpretation and approved the final manuscript. Lihua Liu contributed to the figures, tables, data analysis, and data interpretation and approved the final manuscript. Dennis Deapen contributed to the overall concept of the study, figures, tables, and data interpretation and approved the final manuscript.
David R. Freyer and Dennis Deapen are co-senior authors.
Supplementary Material
References
- 1. Aizer AA, Wilhite TJ, Chen MH, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120(10):1532–1539. [DOI] [PubMed] [Google Scholar]
- 2. Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99(18):1384–1394. [DOI] [PubMed] [Google Scholar]
- 3. Kish JK, Yu M, Percy-Laurry A, Altekruse SF.. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. J Natl Cancer Inst Monogr. 2014;2014(49):236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adolescent and Young Adult Progress Review Group. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, LIVESTRONG Young Adult Alliance; 2006.
- 5. Freyer DR, Felgenhauer J, Perentesis J, on behalf of the COG Adolescent and Young Adult Oncology Discipline Committee. Children’s Oncology Group’s 2013 blueprint for research: adolescent and young adult oncology. Pediatr Blood Cancer. 2013;60(6):1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tricoli JV, Seibel NL, Blair DG, Albritton K, Hayes-Lattin B.. Unique characteristics of adolescent and young adult acute lymphoblastic leukemia, breast cancer, and colon cancer. J Natl Cancer Inst. 2011;103(8):628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis DR, Seibel NL, Smith AW, Stedman MR.. Adolescent and young adult cancer survival. J Natl Cancer Inst Monographs. 2014;2014(49):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Orellana-Noia VM, Douvas MG.. Recent developments in adolescent and young adult (AYA) acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2018;13(2):100–108. [DOI] [PubMed] [Google Scholar]
- 9. Weiss AR, Hayes-Lattin B, Kutny MA, Stock W, Stegenga K, Freyer DR.. Inclusion of adolescents and young adults in cancer clinical trials. Semin Oncol Nurs. 2015;31(3):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaw PH, Reed DR, Yeager N, Zebrack B, Castellino SM, Bleyer A.. Adolescent and young adult (AYA) oncology in the United States: a specialty in its late adolescence. J Pediatr Hematol Oncol. 2015;37(3):161–169. [DOI] [PubMed] [Google Scholar]
- 11. Liu L HA, Moke D, Tsai KY, Wojcik KY, Cockburn M, Deapen D, eds. Cancer in Los Angeles County: Survival Among Adolescents and Young Adults 1988-2014. Los Angeles, CA: Los Angeles Cancer Surveillance Program, University of Southern California; 2017. [Google Scholar]
- 12. Barr RD. Adolescents, young adults, and cancer–the international challenge. Cancer. 2011;117(S10):2245–2249. [DOI] [PubMed] [Google Scholar]
- 13. Barr RD, Holowaty EJ, Birch JM.. Classification schemes for tumors diagnosed in adolescents and young adults. Cancer. 2006;106(7):1425–1430. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute Surveillance, Epidemiology, and End Results Program. AYA Site Recode ICD-O-3/WHO 2008. http://www.seer.cancer.gov/ayarecode/aya-who2008.html. Accessed June 22, 2017.
- 15.US Census Bureau. https://www.census.gov/en.html. Last revised March 8, 2013. Accessed January 10, 2018.
- 16.US Census Bureau. American Community Survey. https://www.census.gov/programs-surveys/acs/. Accessed January 10, 2018.
- 17. Smith AW, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: an update on progress and recommendations for the future. Cancer. 2016;122(7):988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009–1016. [DOI] [PubMed] [Google Scholar]
- 19. Liu L, Moke D, Tsai K, et al. A reappraisal of sex-specific cancer survival trends among adolescents and young adults in the United States. J Natl Cancer Inst. 2019;111(5):djy140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bleyer A, O’Leary M, Barr R, Ries LAG, eds. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000 Bethesda, MD: National Cancer Institute; 2006.
- 21. International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92(22):1823–1830. [DOI] [PubMed] [Google Scholar]
- 22. Dotan E, Aggarwal C, Smith MR.. Impact of rituximab (Rituxan) on the treatment of B-cell non-Hodgkin’s lymphoma. PT. 2010;35(3):148–157. [PMC free article] [PubMed] [Google Scholar]
- 23. Kantarjian HM, Cortes JE, O'Brien S, et al. Imatinib mesylate therapy in newly diagnosed patients with Philadelphia chromosome-positive chronic myelogenous leukemia: high incidence of early complete and major cytogenetic responses. Blood. 2003;101(1):97–100. [DOI] [PubMed] [Google Scholar]
- 24. Keegan TH, DeRouen MC, Parsons HM, et al. Impact of treatment and insurance on socioeconomic disparities in survival after adolescent and young adult Hodgkin lymphoma: a population-based study. Cancer Epidemiol Biomarkers Prev. 2016;25(2):264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kent EE, Breen N, Lewis DR, de Moor JS, Smith AW, Seibel NL.. US trends in survival disparities among adolescents and young adults with non-Hodgkin lymphoma. Cancer Causes Control. 2015;26(8):1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Derouen MC, Gomez SL, Press DJ, Tao L, Kurian AW, Keegan TH.. A population-based observational study of first-course treatment and survival for adolescent and young adult females with breast cancer. J Adolesc Young Adult Oncol. 2013;2(3):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keegan TH, Grogan RH, Parsons HM, et al. Sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults in California. Thyroid. 2015;25(6):635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeRouen MC, Mujahid M, Srinivas S, Keegan TH.. Disparities in adolescent and young adult survival after testicular cancer vary by histologic subtype: a population-based study in California 1988-2010. J Adolesc Young Adult Oncol. 2016;5(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L, Bhatia S, Gomez SL, Yasui Y.. Differential inequality trends over time in survival among U.S. children with acute lymphoblastic leukemia by race and ethnicity, age at diagnosis, and sex. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Caggiano V. Racial disparities in breast cancer mortality among the regions of California. J Clin Oncol. 2015;33(suppl 28):106. [Google Scholar]
- 31. Kent EE, Sender LS, Largent JA, Anton-Culver H.. Leukemia survival in children, adolescents, and young adults: influence of socioeconomic status and other demographic factors. Cancer Causes Control. 2009;20(8):1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adams SH, Newacheck PW, Park MJ, Brindis CD, Irwin CE.. Health insurance across vulnerable ages: patterns and disparities from adolescence to the early 30s. Pediatrics. 2007;119(5):e1033–1039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.