Abstract
Background
This study aimed to evaluate the prognostic significance of plasma fibrinogen, serum albumin, the mean platelet volume (MPV), and the neutrophil-to-lymphocyte ratio (NLR) in patients with laryngeal squamous cell carcinoma (LSCC) who underwent surgical resection.
Material/Methods
A retrospective study included 110 patients with LSCC who underwent surgical resection between January 2008 to June 2015. Clinicopathologic and demographic data were recorded. Preoperative levels of plasma fibrinogen, serum albumin, MPV, and NLR were measured, and all patients underwent postoperative follow-up. The Kaplan-Meier method was used to determine the impact of these factors on overall survival (OS) and disease-free survival (DFS).
Results
Preoperative hyperfibrinogenemia was significantly correlated with clinical stage, T stage, and tumor location in patients with LSCC (P<0.05). Serum albumin, MPV, and NLR were significantly correlated with the clinical stage and the T stage (P<0.05). The OS and DFS were significantly reduced in patients with hyperfibrinogenemia compared with patients with plasma fibrinogen <4 g/dL (P<0.05). Serum albumin of 35 g/L was not significantly correlated with OS (P>0.05). Patients with an MPV <9.5 fL had a significantly longer OS compared with patients with an MPV ≥9.5 fL (P=0.026). The DFS of patients with an NLR <2.22 was significantly longer than for those with an NLR ≥2.22.
Conclusions
Preoperative hyperfibrinogenemia, increased MPV and NLR were associated with reduced prognosis in patients with LSCC.
MeSH Keywords: Laryngeal Neoplasms, Nutrition Assessment, Preoperative Period
Background
Laryngeal squamous cell carcinoma (LSCC) is the most common type of laryngeal cancer, accounting for 95–98% cases [1,2]. Early diagnosis and treatment of LSCC with surgery or radiotherapy can achieve favorable results, but the treatment of advanced-stage LSCC has a poor prognosis, and the 5-year survival rate is low [3–5]. Therefore, early diagnosis and early treatment are essential for improved prognosis of patients with LSCC.
The primary risk factors for LSCC include male gender, smoking, drinking, and infection with human papillomavirus (HPV) [6–9]. The tumor grade and stage of LSCC are considered to be independent prognostic factors for prognosis, and other prognostic factors include the primary site, tumor size, lymph node metastasis, and the presence of distant metastases. Recently, several studies have been undertaken to identify preoperative prognostic factors several types of cancer, including peripheral blood-derived inflammatory factors that include the neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), C-reactive protein (CRP) levels, the platelet-to-lymphocyte ratio (PLR), the prognostic nutritional index (PNI), serum albumin concentration, total lymphocyte count, fibrinogen levels, red cell distribution width (RDW), and mean platelet volume (MPV) [10–20].
This retrospective study aimed to evaluate the prognostic significance of plasma fibrinogen, serum albumin, MPV, and NLR in patients with LSCC who underwent surgical resection at a single center.
Material and Methods
Patients
A retrospective study included 110 patients with laryngeal squamous cell carcinoma (LSCC) who underwent surgical resection at Guangdong Provincial Peoples’ Hospital between January 2008 and June 2015. Surgical procedures included CO2 laser surgery (35 cases, 31.82%), partial laryngectomy (51 cases, 46.36%), and total laryngectomy (24 cases, 21.82%). The clinical stage of laryngeal carcinoma was determined according to the 8th American Joint Committee on Cancer (AJCC) TNM staging system. The study inclusion criteria were histologically-confirmed LSCC, no previous history of surgery, radiotherapy, or chemotherapy before hospital admission, no history of other malignancy, no distant metastases, complete clinical, radiologic, laboratory, and follow-up data, no hematological disorders, no autoimmune disease or treatment with glucocorticoids. Patients underwent 3-monthly postoperative follow-up and their survival status, disease progression, and time of death were recorded. This study was approved by the Ethics Committee of Guangdong General Hospital and Guangdong Academy of Medical Sciences. All clinical procedures were performed in accordance with the Declaration of Helsinki. All patients signed informed consent to be included in this study.
Biochemical analysis
Blood samples of all patients were collected before breakfast at two weeks before surgery. Biochemical analysis, including tests for plasma fibrinogen, serum albumin, the mean platelet volume (MPV), and the neutrophil-to-lymphocyte ratio (NLR), was conducted using clinical laboratory equipment, according to the manufacturers’ instructions. Briefly, the levels of fibrinogen were measured by a CA-7000 automatic coagulation analyzer (Sysmex Corporation, Kobe, Japan). Serum albumin was measured using the bromocresol green (BCG) dye method. MPV was measured from blood drawn into EDTA-treated tubes using a Beckman-Coulter analyzer (Sysmex Corporation, Tokyo, Japan). Neutrophils and lymphocytes were measured with an automatic nephelometer (Sysmex Corporation, Tokyo, Japan) and the NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count.
Statistical analysis
Statistical analysis of the study data was performed using SPSS version 19.0 (IBM, Chicago, IL, USA). Categorical variables were expressed as counts and percentages and were compared using a chi-squared (χ2) test or Fisher’s exact test. Univariate and multivariate analysis were performed to determine survival differences using Cox proportional hazards models and were expressed as the hazard ratio (HR) and the 95% confidence interval (CI). The optimal cutoff score for preoperative NLR was defined by receiver operating characteristic (ROC) curve analysis. The cutoff value was the point closest to both maximum sensitivity and specificity. The variables that were shown to be associated with overall survival (OS) and disease-free survival (DFS) by univariate analysis underwent multivariate analysis with the Cox proportional-hazards model. Survival curves were calculated using the Kaplan-Meier method and compared using the log-rank test. P<0.05 was considered as statistically significant.
Results
Demographic data
A total of 110 patients, including seven women (6.36%) and 103 men (93.64%) with laryngeal squamous cell carcinoma (LSCC), were eligible for this study. The median age of the recruited patients was 61.5 years (range, 43–85 years). The tumor site was glottic in 72 (65.45%) patients, supraglottic in 34 (30.91%), and subglottic in four (3.64%) patients. There were 53 (48.18%) patients with stage I–II LSCC and 57 (51.82%) patients with stage III–IV LSCC (Table 1).
Table 1.
The clinicopathological characteristics of the patients with laryngeal squamous cell carcinoma (LSCC) treated with surgical resection and included in the study.
| Characteristic | Values n (%) |
|---|---|
| Age# (yrs) * | 61.5 ± 10.38 (range, 43–85 yrs) |
| Gender (Male: Female) | 103 (93.64%): 7 (6.36%) |
| Tumor location (glottic: supraglottic: subglottic) | 72 (65.45%): 34 (30.91%): 4 (3.64%) |
| T stage (T1–T2: T3–T4) | 58 (52.73%): 52 (47.27%) |
| N stage (N0: N1–N3) | 83 (75.45%): 27 (24.55%) |
| TNM stage (I–II: III–IV) | 53 (48.18%): 57 (51.82%) |
| Histological grade (highly/moderately/poorly) | 33 (30.00%): 56 (50.91%): 21 (19.19%) |
| OS, months* | 44 (range, 4–72) |
| DFS, months* | 43 (range, 4–69) |
| Fibrinogen, g/L (≥4: <4) | 43 (39.09%): 67 (60.91%) |
| Albumin, g/L (≥35: <35) | 76 (69.09%): 34 (30.91%) |
| MPV, fL (≥9.5: <9.5) | 80 (72.73%): 30 (27.27%) |
| ALC# | 2.10 ± 0.81 |
| AMC# | 0.75 ± 0.89 |
| ANC# | 4.34 ± 1.93 |
T – tumor; OS – overall survival; DFS – disease-free survival; ALC – absolute lymphocyte count; AMC – absolute monocyte count; ANC – absolute neutrophil count; MPV – mean platelet volume.
Values are expressed as the median (range);
Values are expressed as the mean ± standard deviation (SD).
TNM staging: clinical stage of laryngeal cancer was determined according to the American Joint Committee on Cancer (AJCC) TNM stage (7th edition, 2010).
Selection of the cutoff values for fibrinogen, albumin, mean platelet volume (MPV), and the neutrophil-to-lymphocyte ratio (NLR)
The normal ranges at our institution are: plasma fibrinogen, 1.90–4.00 g/L; serum albumin 35.0–55.0 g/L; and mean platelet volume (MPV), 9.5–13 fL. For adults, a serum albumin level <3.5 g/dL represents hypoalbuminemia [21,22]]. MPV <9.5 fL was defined as the cutoff value for a low MPV, according to previously reported findings [20]. The cutofff value of the NLR was determined by the receiver operating characteristic (ROC) curve analysis. According to the ROC curve, the optimal cutoff value of preoperative NLR was 2.22. The area under the ROC curves (AUC) was 0.692 with a 95% confidence interval (CI) of between 0.515–0.713 (Figure 1). There were 43 patients (39.09%) with hyperfibrinogenemia (≥4 g/L), 34 patients (30.91%) with hypoalbuminemia (<35 g/L), and the MPV of 30 fL (27.27%) patients was <9.5 fL (Table 1).
Figure 1.
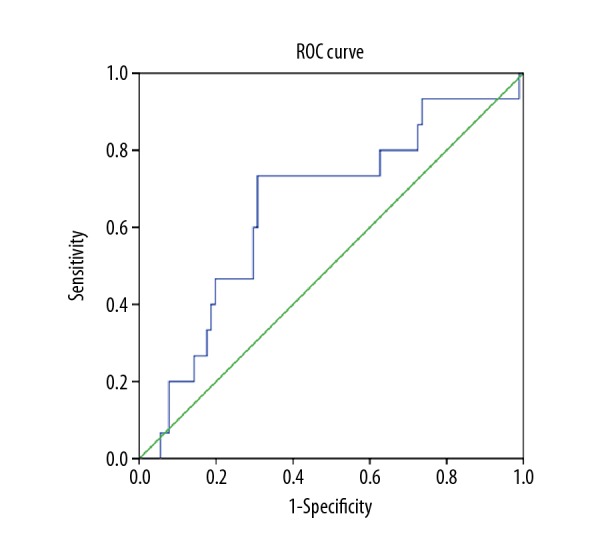
The optimal cutoff score of the preoperative neutrophil-to-lymphocyte ratio (NLR) was defined by the receiver operating characteristic (ROC) curve analysis. The area under the ROC curves (AUC) was 0.692 with a 95% confidence interval (CI) of 0.515–0.713.
Correlations between plasma fibrinogen, serum albumin, MPV, and NLR with the clinicopathological findings in patients with LSCC
The patients were divided into high-level and low-level groups according to the above cutoff values and correlations were calculated for patient clinicopathological features (Table 2). Hyperfibrinogenemia was significantly correlated with the clinical stage, T stage, and tumor location in patients with LSCC (P<0.05). Serum albumin, MPV, and the NLR were significantly correlated with the clinical stage and T stage (Table 2) (P<0.05). Also, levels of albumin and the NLR were significantly correlated with plasma levels of fibrinogen (Table 3).
Table 2.
Correlation between preoperative plasma fibrinogen, serum albumin, mean platelet volume (MPV), and the neutrophil-to-lymphocyte ratio (NLR) with clinicopathological features of the patients with laryngeal squamous cell carcinoma (LSCC) treated with surgical resection.
| n | Fibrinogen (g/L) | Albumin (g/L) | MPV | NLR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | >4 | P-value | Normal | <35 | P-value | Normal | <9 | P-value | >2.22 | ≤2.22 | P-value | ||
| Age (years) | |||||||||||||
| <60 | 44 | 31 | 13 | 0.094 | 34 | 10 | 0.158 | 31 | 12 | 0.804 | 31 | 13 | 0.094 |
| ≥60 | 66 | 36 | 30 | 42 | 24 | 49 | 17 | 36 | 30 | ||||
| Gender | |||||||||||||
| Male | 103 | 64 | 39 | 0.429 | 72 | 31 | 0.674 | 74 | 28 | 0.749 | 64 | 39 | 0.429 |
| Female | 7 | 3 | 4 | 4 | 3 | 6 | 1 | 3 | 4 | ||||
| Clinical stage | |||||||||||||
| I–II | 53 | 42 | 11 | <0.01 | 43 | 10 | 0.008 | 43 | 9 | 0.036 | 39 | 14 | 0.009 |
| III–IV | 57 | 25 | 32 | 33 | 24 | 37 | 20 | 28 | 29 | ||||
| T | |||||||||||||
| T1–T2 | 58 | 46 | 12 | <0.01 | 48 | 10 | 0.001 | 47 | 10 | 0.025 | 42 | 15 | 0.009 |
| T3–T4 | 52 | 21 | 31 | 28 | 24 | 33 | 19 | 25 | 27 | ||||
| N | |||||||||||||
| N0 | 83 | 53 | 30 | 0.267 | 58 | 25 | 0.754 | 50 | 22 | 0.273 | 53 | 30 | 0.267 |
| N1–N3 | 27 | 14 | 13 | 18 | 9 | 22 | 5 | 14 | 13 | ||||
| Tumor location | |||||||||||||
| Glottic | 72 | 50 | 22 | 0.029 | 53 | 19 | 0.339 | 57 | 14 | 0.082 | 44 | 28 | 0.311 |
| Supraglottic | 34 | 16 | 18 | 21 | 13 | 21 | 13 | 22 | 12 | ||||
| Subglottic | 4 | 1 | 3 | 2 | 2 | 2 | 2 | 1 | 3 | ||||
| Histological grade | |||||||||||||
| 1 | 33 | 16 | 17 | 0.615 | 22 | 11 | 0.700 | 23 | 10 | 0.943 | 22 | 11 | 0.441 |
| 2 | 56 | 41 | 15 | 39 | 17 | 44 | 12 | 33 | 23 | ||||
| 3 | 21 | 10 | 11 | 15 | 6 | 13 | 7 | 12 | 9 | ||||
| Smoking history | |||||||||||||
| No (0) | 74 | 44 | 30 | 0.655 | 52 (0) | 22 | 0.701 | 56 | 18 | 0.320 | 45 | 29 | |
| Yes (1) | 36 | 23 | 13 | 24 | 12 | 24 | 12 | 25 | 11 | ||||
| Alcohol | |||||||||||||
| No (0) | 91 | 55 | 36 | 0.825 | 64 | 12 | 0.538 | 68 | 12 | 0.303 | 59 | 32 | 0.889 |
| Yes (1) | 19 | 12 | 7 | 12 | 7 | 12 | 7 | 12 | 7 | ||||
Table 3.
Correlation between preoperative plasma fibrinogen, serum albumin, and the neutrophil-to-lymphocyte ratio (NLR) in patients with laryngeal squamous cell carcinoma (LSCC) treated with surgical resection.
| n | Albumin (g/L) | NLR | |||||
|---|---|---|---|---|---|---|---|
| Normal | <35 | P-value | >2.22 | ≤2.22 | P-value | ||
| Fibrinogen | |||||||
| Normal | 67 | 56 | 11 | <0.01 | 15 | 52 | <0.01 |
| >4 | 43 | 20 | 23 | 24 | 19 | ||
| Albumin (g/L) | |||||||
| Normal | 76 | – | – | 26 | 50 | 0.683 | |
| <35 | 34 | – | – | 13 | 21 | ||
The prognostic significance of hyperfibrinogenemia, MPV, and NLR on overall survival (OS) and disease-free survival (DFS)
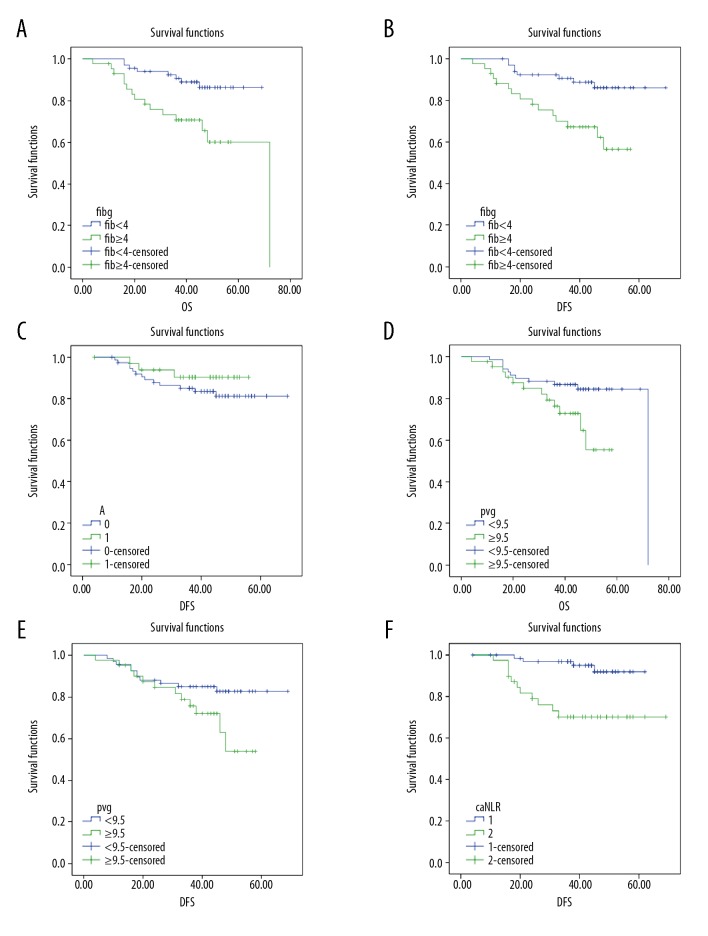
The median overall survival (OS) for all patients was 44 months (range, 4–72 months), and the median disease-free survival (DFS) was 43 months (range, 4–69 months). There were 87 patients (79.09%) who were alive at the end of the follow-up period, and 11 patients (10.00%) developed tumor recurrence. The prognostic significance of the four indicators was further determined by Kaplan–Meier analysis. As shown in Figure 2A and 2B, the OS and DFS of patients with hyperfibrinogenemia (fibrinogen ≥4 g/L) were significantly shorter compared with the patients with fibrinogen <4 g/L (P=0.02 and P=0.005, respectively). For serum albumin, the level of 35 g/L could not distinguish between patients with LSCC with an increased OS from those with reduced OS (P= 0.317) (Figure 2C). The OS of patients with LSCC and an MPV <9.5 fL was significantly longer compared with patients with MPV ≥0.5 fL (P=0.026). The DFS of patients with a MPV <9.5 fL compared with patients with MPV ≥9.5 fl showed no significant difference (P=0.059) (Figure 2D, 2E). The DFS of patients in the NLR <2.22 group was significantly longer compared with patients in the NLR ≥2.22 group (Figure 2F). Also, multivariate analysis with the Cox proportional hazards model showed that preoperative levels of fibrinogen and MPV were independent markers for poor prognosis in patients with LSCC (Table 4).
Figure 2.
(A–F) Kaplan-Meier survival curves for the overall survival (OS) and disease-free survival (DFS) of patients with laryngeal squamous cell carcinoma (LSCC) with the four indicators of plasma fibrinogen, serum albumin, mean platelet volume (MPV), and the neutrophil-to-lymphocyte ratio (NLR)
Table 4.
Multivariate analysis of overall survival (OS) in patients with laryngeal squamous cell carcinoma (LSCC) treated with surgical resection and preoperative plasma fibrinogen, mean platelet volume (MPV), and the neutrophil-to-lymphocyte ratio (NLR).
| Variables | Regression coefficient | P-value | HR 95% CI |
|---|---|---|---|
| Fibrinogen | −1.068 | 0.017 | 0.344 (0.143–0.827) |
| MPV | −0.918 | 0.033 | 0.399 (0.171–0.9310) |
| NLR | 0.878 | 0.059 | 2.405 (0.968–5.974) |
HR – hazard ratio; CI – confidence interval; MPV – mean platelet volume; NLR – neutrophil-to-lymphocyte ratio.
Discussion
Morbidity and mortality from advanced laryngeal squamous cell carcinoma (LSCC) are high due to the high rate of metastasis and local recurrence. Surgery and radiation therapy are first-line treatments that result in similar overall survival (OS) and disease-free survival (DFS). Combined modality therapy is generally recommended for approximately 60% of patients with head and neck cancers, according to the National Comprehensive Cancer Network (NCCN) [23]. The present study selected surgery as an independent treatment to analyze the prognostic factors associated with outcome following surgery for LSCC.
In the present study, preoperative plasma fibrinogen was a significant prognostic factor for both DFS and OS. In tumor progression, levels of inflammatory cytokines have been shown to be increased [24–26]. Fibrinogen is a pro-inflammatory protein, which involved in the formation of extracellular matrix and has important roles in tumor initiation and progression [27–29]. It is possible that high plasma fibrinogen levels in LSCC are secondary to the increased systemic inflammatory response due to tumor progression. Serum albumin has been used as a surrogate marker to reflect malnutrition associated with malignancy, and a previously published study showed that the prognostic nutritional index (PNI), which was calculated as serum albumin + 5×total lymphocyte count, could predict survival in patients with LSCC after curative laryngectomy [17]. However, in the present study, serum albumin levels of <35 g/L did not distinguish between the patients with LSCC with a long OS from those with a short OS (P=0.317). This finding might be explained in two ways. First, the study sample size was relatively small and only 110 patients were enrolled. Second, total lymphocyte count was not evaluated when serum albumin was studied. Therefore, further studies are still needed to evaluate the prognosis value of measuring preoperative serum albumin levels in patients with LSCC.
The neutrophil-to-lymphocyte ratio (NLR), as an indicator of inflammatory and immune status, has previously been shown to be associated with improved patient survival with many types of cancers, including liver cancer, stomach cancer, esophageal cancer, soft tissue sarcoma, and breast cancer [30,31]. In the present study, receiver operating characteristic (ROC) curve analysis was used to identify the optimal cutoff value of the NLR as 2.22, which was different from previously reported values. The cutoff value of the NLR for patients with breast cancer was 2.06 with an AUC of 0.56 (95% CI, 0.43–0.69) in a previous study [32]. In patients with LSCC, Fu et al. established the cutoff value of the NLR as 2.59 with an AUC of 0.61 (95% CI, 0.55–0.66) [33]. The cutoff value of the NLR in another previous study was identified as 2.17 with an AUC of 0.614 (95% CI, 0.515–0.713) [34]. In the present study, the AUC was 0.692 (95% CI, 0.515–0.713), which was higher than that reported in previous studies. Further analysis suggested that the patients with an increased NLR ≥2.22 had a significantly shorter DFS and OS when compared with patients with a low NLR <2.22. Although a different cutoff value of the NLR was identified in the present study, the general conclusion regarding the prognostic value of the preoperative NLR was consistent with previous studies.
In this study, evaluation of the prognostic role of the preoperative mean platelet volume (MPV) for patients with LSCC showed that the OS of patients with LSCC with MPV <9.5 fL was significantly increased when compared with patients with MPV ≥9.5 fL. However, the DFS of patients with MPV <9.5 fL compared with patients with MPV ≥9.5 fL showed no significant difference. MPV is a platelet volume index that is usually used as a marker of platelet activation [35]. Altered MPV levels have been reported in patients with several types of cancers, but its prognostic value remains controversial as low MPVs have been associated with poor prognosis in non-small cell lung cancer (NSCLC) [30,36], bladder cancer, and renal cell carcinoma [37,38], but in patients with colorectal cancer, increased MPV was associated with poor prognosis [39]. This was the first study to investigate the prognostic value of MPV in patients with LSCC and the findings supported that increased MPV was associated with poor prognosis.
The findings from the present study showed some relationships between peripheral blood hematological and serological parameters, patient demographics, clinicopathologic characteristics, and patient prognosis in LSCC. There have been previous reports that high preoperative plasma levels of fibrinogen correlated with poor patient survival in esophageal squamous cell carcinoma [40,41] and advanced hypopharyngeal squamous cell carcinoma [42]. Pretreatment measurement of the NLR and the platelet-to-lymphocyte ratio (PLR) have been shown to be a useful complement to TNM staging in the prognostic assessment of patients with nasopharyngeal carcinoma (NPC) [43]. Also, a recent study showed that the NLR was significantly associated with the progression of LSCC and with DFS and cancer-specific survival (CSS), which supports the view that hematological parameters could be considered independent prognostic parameters for patients with LSCC [44]. The findings of the present study also showed that hyperfibrinogenemia, increased MPV and an increased NLR were associated with poor prognosis for patients with LSCC, while an association between serum albumin levels and patient survival was not detected.
The parameters investigated in the present study, including plasma fibrinogen, serum albumin, MPV, and NLR have been previously reported to have independent predictive value for patient prognosis in several types of cancer. However, previous studies have usually investigated one factor and few studies have been conducted to compare these factors, especially in LSC. However, this study had several limitations. First, this was a single-center retrospective study with a relatively small sample size with only 110 patients with LSCC. Second, all patients were Chinese and further studies should be conducted that include other ethnic groups. Also, it is difficult to clearly identify the relationship between peripheral hematological parameters and outcome following surgical resection for malignancy from a retrospective study. Therefore, further large-scale prospective and multicenter studies are needed to determine the prognostic role of the factors studied before any recommendations can be made for their clinical use.
Conclusions
The findings from this study showed that preoperative hyperfibrinogenemia, an increased mean platelet volume (MPV), and an increased neutrophil-to-lymphocyte ratio (NLR) were associated with reduced overall survival (OS) and disease-free survival (DFS) in patients with laryngeal squamous cell carcinoma (LSCC) who underwent surgical resection. There was no detected prognostic value for serum albumin levels. This was a small retrospective study conducted at a single center and further large-scale multicenter studies are needed to validate these findings in patients with LSCC.
Footnotes
Source of support: This study was funded by the Medical Scientific Research Foundation of Guangdong Province (A2018294), the National Natural Science Foundation of China (81573000), the Talent Introduction Fund of Guangdong Provincial Peoples’ Hospital (Y012018142), and Guangzhou Science and Technology Project in China (201607010389)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Shim YS. Recent advances in management of laryngeal cancer. Cancer Res Treat. 2004;36(1):13–18. doi: 10.4143/crt.2004.36.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yilmaz M, Karatas OF, Yuceturk B, et al. Alpha-B-crystallin expression in human laryngeal squamous cell carcinoma tissues. Head Neck. 2015;37(9):1344–48. doi: 10.1002/hed.23746. [DOI] [PubMed] [Google Scholar]
- 5.Megwalu UC, Sikora AG. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(9):855–60. doi: 10.1001/jamaoto.2014.1671. [DOI] [PubMed] [Google Scholar]
- 6.Maasland DH, van den Brandt PA, Kremer B, et al. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: Results from the Netherlands Cohort Study. BMC Cancer. 2014;14:187. doi: 10.1186/1471-2407-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman ND, Abnet CC, Leitzmann MF, et al. Prospective investigation of the cigarette smoking-head and neck cancer association by sex. Cancer. 2007;110(7):1593–601. doi: 10.1002/cncr.22957. [DOI] [PubMed] [Google Scholar]
- 8.Zuo JJ, Tao ZZ, Chen C, et al. Characteristics of cigarette smoking without alcohol consumption and laryngeal cancer: Overall and time-risk relation. A meta-analysis of observational studies. Eur Arch Otorhinolaryngol. 2017;274(3):1617–31. doi: 10.1007/s00405-016-4390-x. [DOI] [PubMed] [Google Scholar]
- 9.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–31. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 10.Son HJ, Park JW, Chang HJ, et al. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol. 2013;20(9):2908–13. doi: 10.1245/s10434-013-2968-8. [DOI] [PubMed] [Google Scholar]
- 11.Kawai K, Kitayama J, Tsuno NH, et al. Hyperfibrinogenemia after preoperative chemoradiotherapy predicts poor response and poor prognosis in rectal cancer. Int J Colorectal Dis. 2011;26(1):45–51. doi: 10.1007/s00384-010-1054-y. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita H, Kitayama J, Taguri M, Nagawa H. Effect of preoperative hyperfibrinogenemia on recurrence of colorectal cancer without a systemic inflammatory response. World J Surg. 2009;33(6):1298–305. doi: 10.1007/s00268-009-9992-7. [DOI] [PubMed] [Google Scholar]
- 13.Shu YJ, Weng H, Bao RF, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: A retrospective and in vitro study. BMC Cancer. 2014;14:566. doi: 10.1186/1471-2407-14-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki T, Shimada H, Nanami T, et al. Hyperfibrinogenemia is associated with inflammatory mediators and poor prognosis in patients with gastric cancer. Surg Today. 2016;46(12):1394–401. doi: 10.1007/s00595-016-1339-z. [DOI] [PubMed] [Google Scholar]
- 15.Feng Z, Wen H, Bi R, et al. Thrombocytosis and hyperfibrinogenemia are predictive factors of clinical outcomes in high-grade serous ovarian cancer patients. BMC Cancer. 2016;16:43. doi: 10.1186/s12885-016-2070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazici P, Demir U, Bozkurt E, et al. The role of red cell distribution width in the prognosis of patients with gastric cancer. Cancer Biomark. 2017;18(1):19–25. doi: 10.3233/CBM-160668. [DOI] [PubMed] [Google Scholar]
- 17.Fu Y, Chen SW, Chen SQ, et al. A preoperative nutritional index for predicting cancer-specific and overall survival in chinese patients with laryngeal cancer: a retrospective study. Medicine (Baltimore) 2016;95(11):e2962. doi: 10.1097/MD.0000000000002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Chen S, Zheng C, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17(1):285. doi: 10.1186/s12885-017-3220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu ST, Zhou Z, Cai Q, et al. Prognostic value of the C-reactive protein/albumin ratio in patients with laryngeal squamous cell carcinoma. Onco Targets Ther. 2017;10:879–84. doi: 10.2147/OTT.S128391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seles M, Posch F, Pichler GP, et al. Blood platelet volume represents a novel prognostic factor in patients with nonmetastatic renal cell carcinoma and improves the predictive ability of established prognostic scores. J Urol. 2017;198(6):1247–52. doi: 10.1016/j.juro.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 21.Di Fiore F, Lecleire S, Pop D, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol. 2007;102(11):2557–63. doi: 10.1111/j.1572-0241.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishizuka M, Nagata H, Takagi K, et al. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246(6):1047–51. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]
- 23.Adelstein D, Gillison ML, Pfister DG, et al. NCCN guidelines insights: Head and neck cancers, version 2.2017. J Natl Compr Canc Netw. 2017;15(6):761–70. doi: 10.6004/jnccn.2017.0101. [DOI] [PubMed] [Google Scholar]
- 24.Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002;3(1):27–34. doi: 10.1016/s1470-2045(01)00619-2. [DOI] [PubMed] [Google Scholar]
- 25.Musolino C, Allegra A, Pioggia G, Gangemi S. Immature myeloid-derived suppressor cells: A bridge between inflammation and cancer (Review) Oncol Rep. 2017;37(2):671–83. doi: 10.3892/or.2016.5291. [DOI] [PubMed] [Google Scholar]
- 26.Shrihari TG. Dual role of inflammatory mediators in cancer. Ecancermedicalscience. 2017;11:721. doi: 10.3332/ecancer.2017.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann NY Acad Sci. 2001;936:406–25. [PubMed] [Google Scholar]
- 28.Sahni A, Khorana AA, Baggs RB, et al. FGF-2 binding to fibrin(ogen) is required for augmented angiogenesis. Blood. 2006;107(1):126–31. doi: 10.1182/blood-2005-06-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahni A, Simpson-Haidaris PJ, Sahni SK, et al. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2) J Thromb Haemost. 2008;6(1):176–83. doi: 10.1111/j.1538-7836.2007.02808.x. [DOI] [PubMed] [Google Scholar]
- 30.Kang MH, Go SI, Song HN, et al. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer. 2014;111(3):452–60. doi: 10.1038/bjc.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu SJ, Shen SL, Li SQ, et al. Prognostic value of preoperative peripheral neutrophil-to-lymphocyte ratio in patients with HBV-associated hepatocellular carcinoma after radical hepatectomy. Med Oncol. 2013;30(4):721. doi: 10.1007/s12032-013-0721-6. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi H, Kawanaka H, Fukuyama S, et al. Comparison of the prognostic values of preoperative inflammation-based parameters in patients with breast cancer. PLoS One. 2017;12(5):e0177137. doi: 10.1371/journal.pone.0177137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu Y, Liu W, OuYang D, et al. Preoperative neutrophil-to-lymphocyte ratio predicts long-term survival in patients undergoing total laryngectomy with advanced laryngeal squamous cell carcinoma: A single-center retrospective study. Medicine (Baltimore) 2016;95(6):e2689. doi: 10.1097/MD.0000000000002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu XP, Qiu QH, Chen LS, et al. Preoperative neutrophil-to-lymphocyte ratio is an independent prognostic marker in patients with laryngeal squamous cell carcinoma. BMC Cancer. 2015;15:743. doi: 10.1186/s12885-015-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson CB, Jakubowski JA. The pathophysiology and clinical relevance of platelet heterogeneity. Blood. 1988;72(1):1–8. [PubMed] [Google Scholar]
- 36.Kumagai S, Tokuno J, Ueda Y, et al. Prognostic significance of preoperative mean platelet volume in resected non-small-cell lung cancer. Mol Clin Oncol. 2015;3(1):197–201. doi: 10.3892/mco.2014.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Cui MM, Xu Y, et al. Decreased mean platelet volume predicts poor prognosis in invasive bladder cancer. Oncotarget. 2017;8(40):68115–22. doi: 10.18632/oncotarget.19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun ZY, Zhang X, Liu YS, et al. Lower mean platelet volume predicts poor prognosis in renal cell carcinoma. Sci Rep. 2017;7(1):6700. doi: 10.1038/s41598-017-07168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li N, Yu Z, Zhang X, et al. Elevated mean platelet volume predicts poor prognosis in colorectal cancer. Sci Rep. 2017;7(1):10261. doi: 10.1038/s41598-017-11053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S, Yan H, Du J, et al. Prognostic significance of pre-resection albumin/fibrinogen ratio in patients with non-small cell lung cancer: A propensity score matching analysis. Clinica Chimica Acta. 2018;482:203–8. doi: 10.1016/j.cca.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Zhang D, Zhou X, Bao W, et al. Plasma fibrinogen levels are correlated with postoperative distant metastasis and prognosis in esophageal squamous cell carcinoma. Oncotarget. 2015;6(35):38410. doi: 10.18632/oncotarget.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuwahara T, Takahashi H, Sano D, et al. Fibrinogen and neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hypopharyngeal squamous cell carcinoma. Anticancer Res. 2018;38(9):5321–30. doi: 10.21873/anticanres.12859. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y, Qu S, Pan X, Huang S, Zhu X. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in intensity modulated radiation therapy for nasopharyngeal carcinoma. Oncotarget. 2018;9(11):9992–10004. doi: 10.18632/oncotarget.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsueh C, Tao L, Zhang M, et al. The prognostic value of preoperative neutrophils, platelets, lymphocytes, monocytes and calculated ratios in patients with laryngeal squamous cell cancer. Oncotarget. 2017;8(36):60514–27. doi: 10.18632/oncotarget.16234. [DOI] [PMC free article] [PubMed] [Google Scholar]