Abstract
Background
Short‐acting insulin analogue use for people with diabetes is still controversial, as reflected in many scientific debates.
Objectives
To assess the effects of short‐acting insulin analogues versus regular human insulin in adults with type 1 diabetes.
Search methods
We carried out the electronic searches through Ovid simultaneously searching the following databases: Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (1946 to 14 April 2015), EMBASE (1988 to 2015, week 15), the Cochrane Central Register of Controlled Trials (CENTRAL; March 2015), ClinicalTrials.gov and the European (EU) Clinical Trials register (both March 2015).
Selection criteria
We included all randomised controlled trials with an intervention duration of at least 24 weeks that compared short‐acting insulin analogues with regular human insulins in the treatment of adults with type 1 diabetes who were not pregnant.
Data collection and analysis
Two review authors independently extracted data and assessed trials for risk of bias, and resolved differences by consensus. We graded overall study quality using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) instrument. We used random‐effects models for the main analyses and presented the results as odds ratios (OR) with 95% confidence intervals (CI) for dichotomous outcomes.
Main results
We identified nine trials that fulfilled the inclusion criteria including 2693 participants. The duration of interventions ranged from 24 to 52 weeks with a mean of about 37 weeks. The participants showed some diversity, mainly with regard to diabetes duration and inclusion/exclusion criteria. The majority of the trials were carried out in the 1990s and participants were recruited from Europe, North America, Africa and Asia. None of the trials was carried out in a blinded manner so that the risk of performance bias, especially for subjective outcomes such as hypoglycaemia, was present in all of the trials. Furthermore, several trials showed inconsistencies in the reporting of methods and results.
The mean difference (MD) in glycosylated haemoglobin A1c (HbA1c) was ‐0.15% (95% CI ‐0.2% to ‐0.1%; P value < 0.00001; 2608 participants; 9 trials; low quality evidence) in favour of insulin analogues. The comparison of the risk of severe hypoglycaemia between the two treatment groups showed an OR of 0.89 (95% CI 0.71 to 1.12; P value = 0.31; 2459 participants; 7 trials; very low quality evidence). For overall hypoglycaemia, also taking into account mild forms of hypoglycaemia, the data were generally of low quality, but also did not indicate substantial group differences. Regarding nocturnal severe hypoglycaemic episodes, two trials reported statistically significant effects in favour of the insulin analogue, insulin aspart. However, due to inconsistent reporting in publications and trial reports, the validity of the result remains questionable.
We also found no clear evidence for a substantial effect of insulin analogues on health‐related quality of life. However, there were few results only based on subgroups of the trial populations. None of the trials reported substantial effects regarding weight gain or any other adverse events. No trial was designed to investigate possible long‐term effects (such as all‐cause mortality, diabetic complications), in particular in people with diabetes related complications.
Authors' conclusions
Our analysis suggests only a minor benefit of short‐acting insulin analogues on blood glucose control in people with type 1 diabetes. To make conclusions about the effect of short acting insulin analogues on long‐term patient‐relevant outcomes, long‐term efficacy and safety data are needed.
Plain language summary
Short‐acting insulin analogues versus regular human insulin for type 1 diabetes mellitus
Review question
Are short‐acting insulin analogues more useful than regular human insulin for adults with type 1 diabetes?
Background
Diabetes is a condition that causes a person's blood sugar (glucose) level to become too high. Insulin is a hormone that is released by the pancreas (a small organ behind the stomach); it controls the blood levels of glucose. In type 1 diabetes, the pancreas does not produce any insulin so the person has to inject insulin to control their glucose levels and keep well. Short‐acting insulin analogues (such as insulin lispro, insulin aspart and insulin glulisine) act more quickly than regular human insulin. They can be injected immediately before meals and lead to lower blood sugar levels after food intake.
Study characteristics
We found nine randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) comparing the insulin analogues, insulin lispro and insulin aspart, to regular human insulin delivered to 2693 participants. The people in the included studies were monitored (called follow‐up) for between 24 and 52 weeks.
This evidence is up‐to‐date as of 15 April 2015.
Key results
According to our analysis, short‐acting insulin analogues were slightly better than regular human insulin regarding long‐term glycaemic control (where blood glucose is at controlled levels) and showed similar episodes of low blood sugar (called hypoglycaemia), especially with regard to severe (night‐time) hypoglycaemia. We found no information on late diabetes complications such as problems with the eyes, kidneys or feet. The studies did not report costs and they were too short to investigate death from any cause reliably. We also found no clear evidence for a marked effect of insulin analogues on the health‐related quality of life (which is physical, mental, emotional and social health).
Quality of the evidence
The quality of the included studies was low or very low, mainly because none of the studies was carried out in a blinded way (where healthcare professionals and participants do not know which treatment they received) so that risk of bias, especially for outcomes such as hypoglycaemic episodes, was present in all of the studies. Furthermore, several studies showed inconsistencies in the reporting of methods and results.
Summary of findings
Summary of findings for the main comparison. Short‐acting insulin analogues compared with regular human insulin for adults with type 1 diabetes mellitus.
| Short‐acting insulin analogues compared with regular human insulin for adults with type 1 diabetes mellitus | ||||||
| Patient: adults with type 1 diabetes mellitus Settings: outpatients Intervention: short‐acting insulin analogues Comparison: regular human insulin | ||||||
| Outcomes | Regular human insulin | Short‐acting insulin analogues | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
All‐cause mortality Follow‐up: 24‐52 weeks |
See comment | See comment | See comment | See comment | See comment | Mortality was not a primary outcome in any of the included trials. Overall, there was only 1 death in 6 trials, that reported on deaths as an adverse event |
| Macrovascular complications | See comment | See comment | See comment | See comment | See comment | Not reported |
| Microvascular complications | See comment | See comment | See comment | See comment | See comment | Not reported |
|
Severe hypoglycaemic episodes
(heterogeneous definitions of severe hypoglycaemia) Follow‐up: 24‐52 weeks |
166 per 1000 | 150 per 1000 (124 to 182) | OR 0.89 (0.71 to 1.12) | 2459 (7) | ⊕⊝⊝⊝ very lowa | ‐ |
|
Health‐related quality of life Follow‐up: 24‐52 weeks |
See comment | See comment | See comment | See comment | See comment | Health‐related quality of life was either only assessed in subpopulations of 3 trials or insufficiently reported. Overall, there was no clear evidence for a substantial effect of short‐acting insulin analogues on this outcome |
|
HbA1c at end of follow‐up [%] Follow‐up: 24‐52 weeks |
The mean HbA1c ranged across control groups from 6.3% to 9.3% | The mean HbA1c in the intervention groups was 0.15% lower (0.2 lower to 0.1 lower) | ‐ | 2608 (9) | ⊕⊕⊝⊝ lowb | ‐ |
| Costs | See comment | See comment | See comment | See comment | See comment | Not reported |
| CI: confidence interval; HbA1c: glycosylated haemoglobin A1c; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
*Assumed risk was derived from the event rates in the comparator groups
aDowngraded by three levels because of high risk for performance bias, pooling of different outcome definitions and participant populations and wide confidence intervals being compatible with both beneficial and harmful effects bDowngraded by two levels because of inconsistencies in reporting of the results and indirectness (HbA1c as a surrogate outcome measure)
Background
Description of the condition
Type 1 diabetes mellitus is a metabolic disorder caused by a cellular‐mediated autoimmune destruction of pancreatic β cells. The resulting deficiency in insulin secretion in turn leads to chronic hyperglycaemia (i.e. elevated levels of plasma glucose). To date, there is no cure and treatment consists of life‐long insulin replacement to control blood sugar levels. Long‐term complications of diabetes mellitus include retinopathy, nephropathy, neuropathy and increased risk of cardiovascular disease.
Description of the intervention
Blood sugar control through insulin therapy is the main priority of therapy for people with type 1 diabetes. Since the Diabetes Control and Complications Trial (DCCT 1993), intensive insulin therapy using a basal‐bolus regimen has become the standard. In this regimen, a bolus of insulin is injected before every meal whereas a longer‐acting insulin type is injected once or twice a day. The bolus insulin can either be regular human insulin (RHI) or a short‐acting insulin analogue; and the basal insulin can either be neutral protamine Hagedorn (NPH) insulin or a long‐acting insulin analogue. Alternatively, people can use insulin pumps, in which short‐acting insulin can be injected as a bolus or continuously in very small amounts, so that no special long‐acting insulin component is necessary.
In contrast to human endogenous insulin, insulin analogues have a modified molecular structure resulting in different pharmacokinetic profiles. When RHI is injected subcutaneously, the plasma insulin concentration peaks about two to four hours after injection, unlike the much earlier plasma insulin peak in people without diabetes after meal ingestion. This low rise to peak insulin concentration makes it difficult to mimic physiological temporal insulin profiles and is likely to account for much of the observed hyperglycaemia following meals in people with diabetes (Zinman 1989). Furthermore, since the action of RHI last for about six to eight hours with a peak at about two to four hours, people run the risk of experiencing late post‐absorptive hypoglycaemic episodes (Brunelle 1998; DeWitt 2003; Vignati 1997). The delay in the absorption of subcutaneously administered regular insulin is due to the fact that in this preparation, insulin tends to associate in 'clusters' of six molecules (hexamers), and time is needed after injection for these clusters to dissociate to single molecules that can be used by the body (Mosekilde 1989). Short‐acting insulin analogues with less tendency toward self association are therefore absorbed more quickly, achieving peak plasma concentrations about twice as high and within approximately half the time compared to regular insulin (Howey 1994; Torlone 1994).
Currently there are three different short‐acting insulin analogues available: insulin aspart, insulin glulisine and insulin lispro. Compared to RHI, insulin aspart has aspartic acid instead of proline at position 28 of the B‐region; in glulisine, the amino acid asparagine is replaced by lysine at position 3 and lysine with glutamic acid at position 29 of the B‐chain; and in insulin lispro, proline at position 28 and lysine at position 29 of the B‐region are interchanged.
Adverse effects of the intervention
The key risk associated with any insulin therapy is the occurrence of hypoglycaemic episodes. Insulin analogues have been promoted as lowering the risk of hypoglycaemia because their faster pharmacokinetic profile might help avoid hypoglycaemic episodes in post‐meal periods. However, the evidence needs to be carefully evaluated also considering different patient subgroups and methodological challenges associated with the assessment of hypoglycaemia in clinical trials. For example, Singh 2009 point out that several trials on insulin analogues have excluded participants with a history of severe hypoglycaemia. Open‐label designs combined with measurements of hypoglycaemia solely relying on participants' reports may bring about results at high risk for bias.
Another potential adverse effect of insulin analogues is weight gain. In general, improvement in glycaemic control through insulin therapy is frequently associated with weight gain, which in turn can have negative consequences on blood pressure and lipid profiles (Russell‐Jones 2007).
Finally, the structural homology of insulin analogues to insulin‐like growth‐factor I (IGF‐I) has caused concern regarding the progression of diabetic late complications and potential mitogenic (induction of cell division) effects, especially with long‐term use of insulin analogues. IGF‐I may affect the progression of retinopathy (Grant 1993; King 1985), and certain modified insulin analogues have shown a carcinogenic effect in the mammary glands in female rats (Jørgensen 1992), or mitogenic potency in osteosarcoma cells (Kurtzhals 2000).
Overall, only limited data on the long‐term safety of insulin analogues are currently available, mainly because of short follow‐up periods and because people with clinically relevant complications are often excluded from clinical studies.
How the intervention might work
Due to their faster pharmacokinetics, insulin analogues could lead to lower glucose levels after meals (Heinemann 1996; Howey 1994), and potentially also improve overall glycaemic control. It has been proposed that lower post‐prandial glucose may be associated with a lower risk of cardiovascular complications in diabetes (Haffner 1998).
Furthermore, insulin analogues might have additional beneficial effects on people's health‐related quality of life by requiring less restrictive mealtime planning. For people treated with RHI, insulin should be administered at least 30 minutes before meals. However, this recommendation is often not followed because of its inconvenience (Overmann 1999). Short‐acting insulin analogues, in contrast, can be injected directly before meals or even after meals without a deterioration of prandial glycaemic control (Brunner 2000; Schernthaner 1998).
Why it is important to do this review
Insulin analogues have been heavily promoted by the pharmaceutical industry. Based on their pharmacokinetic profile we might expect short‐acting insulin analogues to improve the insulin therapy of people with diabetes mellitus. The evidence collected in previous reviews and meta‐analyses showed at best only modest benefits on glycaemic control and the frequency of hypoglycaemic episodes compared to therapy with RHI (Garg 2010; Gough 2007; Singh 2009; WHO 2011). While some reviews find a stronger reduction in glycosylated haemoglobin A1c (HbA1c) with rapid‐acting insulin analogues compared to RHI, the effects were smaller than published minimal clinically relevant differences. Furthermore, potential adverse effects of treatment with these insulin analogues have not been ruled out sufficiently and there is a lack of evidence regarding the effects on long‐term clinical outcomes (Singh 2009; WHO 2011).
Based on the results of cost‐effectiveness analyses (Cameron 2009; Holden 2011), the heavy use of insulin analogues promoted through aggressive marketing of the pharmaceutical industry has become a matter of political debate (Frick 2008; Gale 2011; Holleman 2007a; Sawicki 2011). This issue is of particular importance for low‐ to middle‐income countries, where people still die due to the lack of affordable insulin (Cohen 2011; Gale 2011).
Considering this background, the availability of up‐to‐date evidence is highly relevant. The aim of this work is to systematically review the clinical efficacy and safety of the short‐acting insulin analogues aspart, glulisine and lispro in the treatment of people with type 1 diabetes mellitus with a particular focus on long‐term clinical outcomes. In contrast to the previous review (Siebenhofer 2006), this update is therefore restricted to only include studies with a follow‐up duration of at least 24 weeks.
Objectives
To assess the effects of short‐acting insulin analogues versus regular human insulin in adults with type 1 diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCT; blinded and open, parallel and cross‐over design) with a treatment duration of 24 weeks or more, designed to compare people with diabetes who were treated with the currently 'on the market' available short‐acting insulin analogues lispro, aspart or glulisine versus RHI in the review, regardless of dose or schedule, if insulin was injected subcutaneously via syringe, pen or pump. Concerning vascular mortality and morbidity, studies with a follow‐up of several years would be needed. For the assessment of metabolic control, studies with a shorter duration can be useful, if the blood glucose lowering effect of the investigated treatments can be assessed with sufficient confidence and compared to patient relevant outcomes (e.g. avoidance of hypoglycaemic events). Thus, we considered trials with a minimum duration of 24 weeks for inclusion in this review. This also concurs with the requirement of the European Medicines Agency for confirmatory studies in the treatment of diabetes mellitus (EMA 2002).
Types of participants
Adults (aged 18 years and older) with type 1 diabetes mellitus who were not pregnant.
Diagnostic criteria (diabetes mellitus)
To be consistent with changes in classification and diagnostic criteria of diabetes mellitus through the years, the diagnosis should have been established using the standard criteria valid at the time of the beginning of the trial (e.g. ADA 1999; ADA 2008; WHO 1998). Ideally, diagnostic criteria should have been described. If necessary, we used trial authors' definition of diabetes mellitus. We planned to subject diagnostic criteria to a sensitivity analysis.
Types of interventions
We considered all participants with diabetes receiving a short‐acting insulin analogue treatment (intervention group) in comparison to people receiving treatment with RHI (control group), whether the short‐acting insulin treatment was used with or without other long‐acting or intermediate‐acting insulin, as long as any additional treatment was given equally to both groups.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Macrovascular and microvascular complications.
Severe hypoglycaemic episodes.
Secondary outcomes
Glycaemic control (glycosylated haemoglobin A1c (HbA1c)).
Adverse events.
Health‐related quality of life.
Costs.
Method and timing of outcome measurement
All‐cause mortality measured after a time interval of less than 12 months (short‐term) or more than 12 months (long‐term).
Macrovascular complications: non‐fatal and fatal myocardial infarction and stroke measured after a time interval of less than 12 months (short‐term) or more than 12 months (long‐term).
Microvascular complications: manifestation and progression of retinopathy, nephropathy and neuropathy, and end‐stage renal disease measured after a time interval of less than 12 months (short‐term) or more than 12 months (long‐term).
Severe hypoglycaemic episodes: number of participants with at least one severe hypoglycaemic episode, measured after a time interval of less than 12 months (short‐term) or more than 12 months (long‐term).
Glycaemic control: HbA1c measured after a time interval of less than 12 months (short‐term) or more than 12 months (long‐term).
Adverse events: number of overall, severe and non‐severe hypoglycaemic episodes; number of participants who experienced at least one episode of ketoacidosis, weight gain and other adverse events measured after a time interval of less than 12 months (short‐term) or more than 12 months (long‐term).
Health‐related quality of life assessment, measured by a validated instrument, such as the Diabetes Treatment Satisfaction Questionnaire (Bradley 1990), after a time interval of less than 12 months (short‐term) or more than 12 months (long‐term).
Costs measured after a time interval of less than 12 months (short‐term) or more than 12 months (long‐term).
'Summary of findings' table
We presented a 'Summary of findings' table reporting the following outcomes listed according to priority.
All‐cause mortality.
Macrovascular complications.
Microvascular complications.
Severe hypoglycaemic episodes.
Health‐related quality of life
HbA1c.
Costs.
Search methods for identification of studies
Electronic searches
We carried out the electronic search through Ovid, simultaneously searching the following databases.
The Cochrane Central Register of Controlled Trials (CENTRAL) (March 2015).
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) (1946 to 14 April 2015).
EMBASE (1988 to 2015, week 15).
We used highly sensitive search filters to identify RCTs and applied various search terms for short‐acting insulin analogues and diabetes mellitus (for details see Appendix 1). For ongoing trials, we searched ClinicalTrials.gov (www.clinicaltrials.gov) and the European (EU) Clinical Trials register (www.clinicaltrialsregister.eu).
We included trials published in any language.
Searching other resources
In addition to the electronic search, we reviewed references from original articles and reviews.
For the original review, we also screened abstracts of major diabetology meetings (European Association for the Study of Diabetes, American Diabetes Association) ongoing from 1992 and articles of diabetes journals (Diabetologia, Diabetic Medicine, Diabetes Care, Diabetes) to December 2003. With the help of the International Register of Clinical Trials registers at (www.trialscentral.org) and the register of Current Science at (www.controlled‐trials.com), we looked for ongoing trials.
We directed inquiries to the three main pharmaceutical companies producing short‐acting insulin analogues (Aventis, Eli Lilly and Novo Nordisk). We contacted experts and approval agencies (the European Agency for the Evaluation of Medicinal Products (EMEA), the US Food and Drug Administration (FDA), the Medicines Control Agency (MCA) and the Therapeutic Goods Administration (TGA)).
For economic analyses, we contacted the Pharmaceutical Evaluation Section of the Pharmaceutical Benefits Branch of the Commonwealth Department of Health and Aged Care of Australia.
We reviewed the bibliography of standard textbooks (Diabetes Annual, 12. Elsevier Science B.V. (Marshall 1999); Praxis der Insulintherapie (Berger 2001), and Evidence‐based Diabetes Care (Gerstein 2001)).
Data collection and analysis
Selection of studies
Two review authors (BF or MS, KH or TS) independently scanned the abstract, title or both sections of every record retrieved to determine the trials to be assessed further. A third review author (AS) resolved any differences in opinion. If resolution of disagreements had not been possible, we planned to add the article to those 'awaiting classification' and contact trial authors for clarification. We present a PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow‐diagram of study selection (Liberati 2009).
Data extraction and management
For trials that fulfilled inclusion criteria, two review authors (BF, MS) independently abstracted relevant population and intervention characteristics using standard data extraction forms with any disagreements to be resolved by discussion, or, if required, by a third review author (AS) (for details see Characteristics of included studies table; Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10).
1. Overview of study populations.
| Characteristic | Intervention(s) and comparator(s) | Sample sizea | Screened/eligible (n) | Randomised (n) | Safety (n) | ITT (n) | Finishing study (n) | Randomised finishing study (%) | Follow‐upb |
|
Ferguson 2001 cross‐over trial |
I: insulin lispro | ‐ | 40/39 | 39 | 35 | 33c | 34 | 87 | 24 weeks |
| C: regular human insulin | |||||||||
| total: | 39 | 35 | 33 | 34 | 87 | ||||
| Home 2000 | I: insulin aspart | ‐ | 1237/1110 | 708 | 707 | 698 | 676 | 96 | 6 months |
| C: regular human insulin | 362 | 358 | 349 | 335 | 94 | ||||
| total: | 1070 | 1065 | 1047 | 1011 | 94 | ||||
| Iwamoto 2001 | I: insulin aspart | ‐ | 146 | 145d | 143 | 143 | 136 | 94 | 24 weeks |
| C: regular human insulin | 65 | 64e | 62 | 62 | 60 | 94 | |||
| total: | 209 | 205 | 205 | 196 | 94 | ||||
|
Provenzano 2001 cross‐over trial |
I: insulin lispro | ‐ | 12 | 12 | 12 | 12 | 12 | 100 | 6 monthsf |
| C: regular human insulin | |||||||||
| total: | 12 | 12 | 12 | 12 | 100 | ||||
| Raskin 2000 | I: insulin aspart | ‐ | 884 | 597 | 596 | 596 | 552 | 93 | 6 monthsh |
| C: regular human insulin | 287 | 286 | 286 | 263 | 92 | ||||
| total: | 884g | 882 | 882 | 815 | |||||
| Recasens 2003 | I: insulin lispro | ‐ | 45 | 22 | 22 | 22 | ‐ | ‐i | 12 months |
| C: regular human insulin | 23 | 23 | 23 | ‐ | ‐i | ||||
| total: | 45 | 45 | 45 | ‐ | ‐ | ||||
| Z011 2007 | I: insulin lispro | ‐ | ‐ | 81 | 81 | 81 | 74j | 91 | 12 months |
| C: regular human insulin | 86 | 86 | 86 | 79j | 92 | ||||
| total: | 167 | 167 | 167 | 153 | 92 | ||||
| Z013 2007 | I: insulin lispro | ‐ | 81 | 81 | 81 | 75j | 93 | 12 months | |
| C: regular human insulin | 88 | 88 | 88 | 83j | 94 | ||||
| total: | 169 | 169 | 169 | 158 | 93 | ||||
| Z015 2007 | I: insulin lispro | ‐ | 50 | 50 | 50 | 45j | 90 | 12 months | |
| C: regular human insulin | 48 | 48 | 48 | 43j | 90 | ||||
| total: | 98 | 98 | 98 | 88 | 90 | ||||
| Grand total | All interventions | 1735 | |||||||
| All comparators | 1009 | ||||||||
| All interventions and comparators | 2744k | ||||||||
aAccording to power calculation in study publication or report bDuration of intervention or follow‐up (or both) under randomised conditions until end of study cOne participant who completed the trial was not analysed because of inconsistencies between the home glucose monitoring diary, HbA1c results and the content of the glucose meter memory.dTwo participants not exposed to treatment eOne participant not exposed to treatment, one person removed because of protocol violation fThree months on Mediterranean diet and three months on normal diet gAccording to original study report, 884 participants were randomised, but only 882 received the treatment hParticipants were treated with insulin aspart or regular human insulin for six months, but could continue their assigned treatment in a six‐months extension of the study iIt was not explicitly stated, but based on the presentation of the results, we assume that all participants finished the study jBased on number of drop‐outs reported in IQWIG 2007 kParticipants of cross‐over trials were counted both in interventions and comparator groups
"‐" denotes not reported
C: comparator; HbA1c: glycosylated haemoglobin A1c; I: intervention; ITT: intention‐to‐treat
We sent an email request to authors of included trials to enquire whether they were willing to answer questions regarding their trials. Appendix 11 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the authors of the article, if required.
Dealing with duplicate publications and companion papers
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximised yield of information by collating all available data. In case of doubt, we prioritised the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (BF, MS or AS) assessed each trial independently. We planned to resolve possible disagreements by consensus, or with consultation of a third party. In cases of disagreement, we consulted the other review authors and made a judgement on consensus.
We assessed risk of bias using the Cochrane 'Risk of bias' tool (Higgins 2011a; Higgins 2011b). We used the following bias criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias), separated for blinding of participants and personnel and blinding of outcome assessment.
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We assessed outcome reporting bias by integrating the results of 'Examination of outcome reporting bias' (Appendix 6), 'Matrix of study endpoints (publications)' (Appendix 5), and section 'Outcomes (outcomes reported in abstract of publication)' of the 'Characteristics of included studies' table. This analysis formed the basis for the judgement of selective reporting (reporting bias).
We judged risk of bias criteria as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented a 'Risk of bias' graph and a 'Risk of bias summary' figure.
We assessed the impact of individual bias domains on trial results at endpoint and trial levels.
For performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessors) and attrition bias (incomplete outcome data), we intended to evaluate risk of bias separately for subjective and objective outcomes (Hróbjartsson 2013). We considered the implications of missing outcome data from individual participants.
We defined the following endpoints as subjective outcomes.
Hypoglycaemic episodes.
Adverse events other than hypoglycaemic episodes.
Health‐related quality of life.
Diabetic complications.
We defined the following outcomes as objective outcomes.
All‐cause mortality.
Glycosylated HbA1c.
Costs.
Measures of treatment effect
We expressed dichotomous data as odds ratios (ORs) or risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MD) with 95% CIs.
Unit of analysis issues
We took into account the level at which the randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome.
Dealing with missing data
We obtained relevant missing data from authors, if feasible, and evaluated important numerical data such as screened, eligible, randomised participants as well as intention‐to‐treat (ITT), as‐treated and per‐protocol populations. We investigated attrition rates, for example drop‐outs, losses to follow‐up and withdrawals, and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Where standard deviations for outcomes were not reported, we imputed these values by assuming the standard deviation of the missing outcome to be the mean of the standard deviations from those studies where this information was reported. We investigated the impact of imputation on meta‐analyses by means of sensitivity analysis.
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we did not report trial results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1, in view of the low power of this test. We examined heterogeneity using the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011a).
Had we found heterogeneity, we would have attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
We expected the following characteristics to introduce clinical heterogeneity.
Sex.
Age.
Duration of disease.
Duration of follow‐up.
Hypoglycaemia unawareness.
Assessment of reporting biases
Had we included 10 studies or more for a particular outcome, we planned to use funnel plots to assess small‐study effects. Due to several explanations for funnel plot asymmetry, we interpreted results carefully (Sterne 2011).
Data synthesis
Unless there was good evidence for homogeneous effects across studies, we primarily summarised low‐risk of bias data by means of a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual study (Riley 2011). In addition, we performed statistical analyses according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We calculated MDs for the percentage of HbA1c and used a random‐effects model for the meta‐analysis. We tried to incorporate the two different study designs used, cross‐over and parallel trials, into the meta‐analysis (Curtin 2002; Elbourne 2002). We only included cross‐overs trials in meta‐analyses if we considered the risk of carry‐over effects low. For continuous outcomes, CIs taking into account the cross‐over nature of trials could be calculated if the publication provided the MD plus the standard deviation, standard error, CI or P value of a paired analysis. If there was no measure of within‐person variance provided, we approximated the correlation between treatment outcomes using the lowest observed correlation among the other studies. For binary data, we calculated ORs and CIs for cross‐over trials using the technique by Becker and Balagtas (Becker 1993; Stedman 2009). We pooled data using the generic invariance method. We assessed the robustness of the results by repeating the analysis using unpaired analyses and a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses for participants with type 1 diabetes in order to explore effect size differences as follows.
Different interventions.
Duration of intervention.
Different types of insulin analogues (insulin lispro versus insulin aspart versus insulin glulisine).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by restricting analysis to the following.
Published studies.
Taking into account risk of bias, as specified in the Assessment of risk of bias in included studies section.
Very long or large studies to establish how much they dominated the results.
Trials using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other) and country.
We also wanted to test the robustness of the results by repeating the analysis using different measures of effect size (RR, OR, etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of trials, see Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The electronic search using the search strategies described yielded 3043 trials after duplicates were removed. We found one additional trial by handsearching the references of other review articles. For further details, see the flow chart in Figure 1.
1.
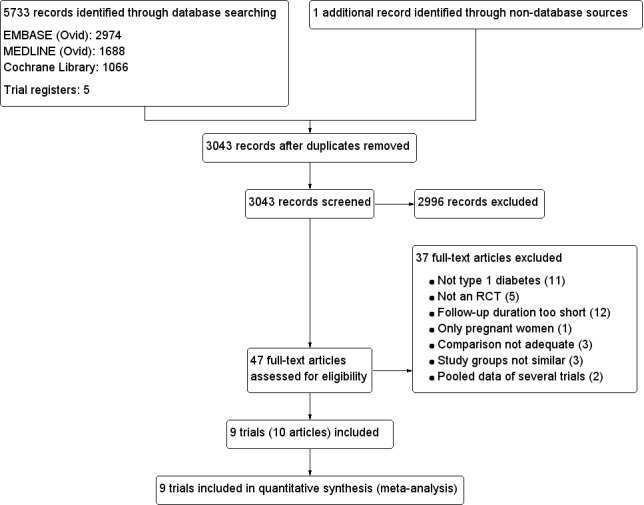
Study flow diagram.
After investigation of these 3043 records, we excluded 2996 articles according to the inclusion and exclusion criteria (Criteria for considering studies for this review). For the remaining 47 records, we obtained the full‐text, which resulted in the exclusion of another 37 articles.
Included studies
We found nine RCTs, described in 10 articles, to be potentially appropriate for inclusion in the meta‐analysis. A detailed description of the characteristics of included studies is in the Characteristics of included studies table; Appendix 3 and Appendix 4. The following is a succinct overview.
Source of data
The results of all of the trials were at least partially published in scientific journals between 1996 and 2006. For six of the trials, we relied on additional information based on the original trial reports, which were published in a report by the Institute for Quality and Efficiency in Health Care (IQWIG 2007). Therefore, we cited this report as an additional source for these six trials. The publications by Anderson 1997 and Garg 1996 were based on the combined data of two (Anderson 1997) and three (Garg 1996) different trials. Just from the publications alone, it does not become clear that the data of different trials were combined. However, for the report by IQWIG 2007, the original trial reports were available and therefore, we continued to treat these trials separately in this review using the same study names (Z011, Z013 and Z015) as in IQWIG 2007. We also contacted all trial authors to request missing data or clarify issues regarding the methodology of the trial. However, only one of the trial authors replied (see Appendix 11).
Comparisons
Six trials compared the insulin analogue lispro with RHI (Ferguson 2001; Provenzano 2001; Recasens 2003; Z011 2007; Z013 2007; Z015 2007), the other three studies used the insulin analogue Aspart (Home 2000; Iwamoto 2001; Raskin 2000). None of the included trials used Glulisine.
Overview of study populations
Overall, 2693 people with type 1 diabetes participated in the nine included trials; 1735 participants were randomised to the treatment group receiving a short‐acting insulin analogue, 1009 participants were randomised to the control group receiving RHI and 51 participants were in both treatment arms in the two cross‐over trials (Ferguson 2001; Provenzano 2001). Altogether, 94% of randomised participants finished the trial in the intervention groups and 92% of randomised participants finished the trials in the control groups.
The individual sample size ranged from 12 to 1070 participants across trials.
Study design and setting
All included trials were RCTs. Seven trials used a parallel design and two trials were cross‐over studies (Ferguson 2001; Provenzano 2001). All trials were open‐label with no blinding of either participants or investigators. Four trials provided no information regarding the years in which the studies were carried out (Ferguson 2001; Iwamoto 2001; Provenzano 2001; Recasens 2003). The other five trials were performed between 1992 and 1997. Overall, six of the trials were carried out in a multicentre setting, while two trials were single‐centre studies. The setting was not reported for one trial (Provenzano 2001). Only two of the multicentre trials reported the number of study centres involved, which was 59 (Raskin 2000) and 88 centres (Home 2000). All trials were either funded commercially (Ferguson 2001; Home 2000; Iwamoto 2001; Raskin 2000; Z011 2007; Z013 2007; Z015 2007), or the funding was not reported (Provenzano 2001; Recasens 2003).
One trial was carried out in Japan (Iwamoto 2001), while the rest of the trials were predominantly carried out in North America and Europe, but two trials also included study centres in South Africa and Australia (Z011 2007; Z013 2007). Five multicentre trials had an outpatient setting (Home 2000; Raskin 2000; Z011 2007; Z013 2007; Z015 2007). The other trials, even though not always explicitly stated, can be assumed to have been also carried out in an outpatient setting, but at a single centre.
The duration of intervention ranged from 24 to 52 weeks with a mean of about 37 weeks. Seven of the trials reported a run‐in period lasting from two to six weeks in order to achieve stable metabolic conditions. None of the trials had to be terminated before the planned end of follow‐up.
Participants
There was some diversity of the participant populations included in the different trials. For example, one trial only included participants with an impaired awareness of hypoglycaemia who had been diagnosed with type 1 diabetes for at least five years (Ferguson 2001), while another trial only included people who were newly diagnosed (Recasens 2003). Overall, the weighted mean age of the participants was 37 years with the mean age ranging between 23 and 46 years across trials. Forty‐seven per cent of all participants were female and the mean body mass was 25 kg/m2 with the trial means ranging from 22 kg/m2 to 26 kg/m2. The mean disease duration across trials ranged from 0.2 to 26 years with a mean disease duration of all participants of 14 years. The participants' mean HbA1c was 8.0%, but the trials' mean baseline HbA1c varied between 7.5% and 11.0%. The trials did not report data on disease severity, co‐morbidities or co‐medications. Three trials provided information on ethnicity (Home 2000; Raskin 2000; Iwamoto 2001). In Iwamoto 2001, all participants were Asian, in Home 2000, 99% of participants were white and in Raskin 2000, 94% of the participants were white.
Criteria for entry into the individual trials are outlined in the Characteristics of included studies table. Major exclusion criteria were insulin pump therapy and advanced diabetic complications.
Diagnosis
All participants in all trials had with type 1 diabetes mellitus. Most trials confirmed the diagnosis of type 1 diabetes against standard diagnostic criteria; two trials against World Health Organization (WHO) 1994 criteria (Home 2000; Raskin 2000), three trials against WHO 1980 criteria (Z011 2007; Z013 2007; Z015 2007), and one trial (Recasens 2003) against the criteria of the National Diabetes Data Group (National Diabetes Data Group 1979). Ferguson 2001 reported to have used the diagnostic criteria of the WHO, but did not specify a year. The other two trials did not provide any information regarding their diagnostic criteria (Iwamoto 2001; Provenzano 2001).
Interventions
All trials tried to apply a comparable insulin regimen throughout the investigation period, but usually, insulin therapy was left somewhat flexible with the aim to reach the best possible glycaemic control. Six of the trials had defined pre‐ and post‐prandial blood glucose targets (Home 2000; Raskin 2000; Recasens 2003; Z011 2007; Z013 2007; Z015 2007). Pre‐prandial targets varied between less than 126 mg/dL and less than 144 mg/dL across trials, while post‐prandial targets were always defined as less than 180 mg/dL. All trials administered insulin by injection: insulin analogues or RHI was usually given before every meal, whereby participants taking RHI were instructed to take the insulin 30 to 40 minutes before the meal. Furthermore, all participants took an additional slower‐acting insulin once or twice a day. Most trials used NPH as basal insulin, one trial used Ultralente insulin (Z011 2007), and another trial allowed both, NPH or Ultralente insulin (Z015 2007). Two trials did not specify the type of slow‐acting insulin (Iwamoto 2001; Provenzano 2001).
All but two trials reported on the treatment before the start of the trial: in Home 2000, Raskin 2000, and Iwamoto 2001, participants had been treated with insulin for at least one year; in Provenzano 2001, Z011 2007, Z013 2007, and Z015 2007, participants had received insulin treatment for at least two months.
Outcomes
Only four trials clearly defined a primary study endpoint (Ferguson 2001; Home 2000; Iwamoto 2001; Raskin 2000). For Ferguson 2001, the primary endpoint was severe hypoglycaemia, for the other trials it was glycaemic control. The trials Z011 2007, Z013 2007, and Z015 2007 provided inconsistent information regarding primary study endpoints. The original study reports referred to "postprandial blood glucose levels" as the "primary efficacy variable" while the study protocol referred to the variables "postprandial glucose excursions", "hypoglycaemia episodes in relation to glycaemic control" and "metabolic control" as "primary efficacy variables". Furthermore, the power analysis was carried out based on the variables pre‐prandial blood glucose, HbA1c and hypoglycaemia. The remaining trials did not explicitly specify a primary study endpoint. None of the trials explicitly defined secondary outcomes.
For a summary of all outcomes assessed in each study, see Appendix 5.
Excluded studies
Overall, we excluded 37 records after full‐text screening. Reasons for exclusion of records are given in the Characteristics of excluded studies table. The main reasons for exclusion were not type 1 diabetes, follow‐up duration was too short and not an RCT (for details see Figure 1).
Risk of bias in included studies
For details on the risk of bias of included trials see the Characteristics of included studies table. For an overview of review authors' judgments about each risk of bias item for individual trials and across all trials, see Figure 2 and Figure 3.
2.
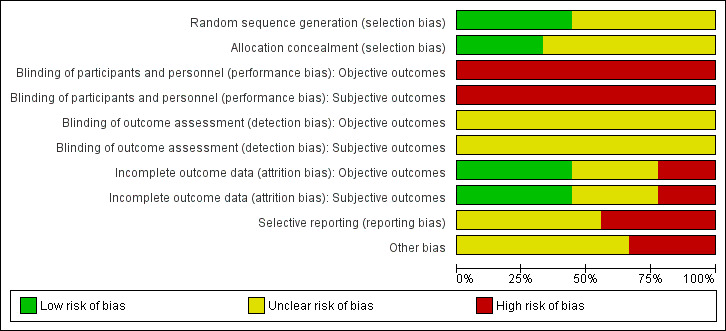
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
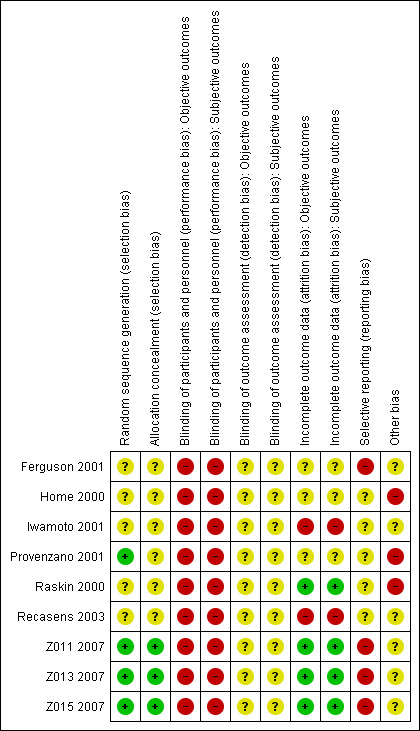
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We investigated performance bias, detection bias and attrition bias separately for objective and subjective outcome measures. We defined HbA1c, all‐cause mortality, diabetes‐related mortality, costs and diabetic complications as objective outcome measures. We defined hypoglycaemia, health‐related quality of life and adverse events as subjective outcome measures.
Allocation
The generation of the allocation sequence and allocation concealment before randomisation was adequate in three trials (Z011 2007; Z013 2007; Z015 2007). In Provenzano 2001, the sequence generation was adequate, while not enough information was provided on allocation concealment. The other trials did not provide sufficient information on their methods (Ferguson 2001; Home 2000; Iwamoto 2001; Raskin 2000; Recasens 2003).
Blinding
None of the trials blinded their participants and personnel. This open‐label design was commonly chosen because RHI needs to be injected 30 to 45 minutes before a meal, while the short‐acting insulin analogue should be injected immediately before the meal. None of the trials explicitly described blinding of outcome assessment. However, six trials described that blood samples were analysed in a central laboratory, which we assumed to be blinded (Home 2000; Iwamoto 2001; Raskin 2000; Z011 2007; Z013 2007; Z015 2007). For the other objective outcomes and all subjective outcomes, the information regarding the blinding of outcome assessment was insufficient in all included trials so that we considered the risk of bias to be unclear.
Incomplete outcome data
Most trials provided information on the number of study withdrawals. Loss to follow‐up ranged from 0% to 13% across trials. None of the trials addressed incomplete outcome data according to current recommended practice using techniques such as multiple imputation. However, considering that most of the trials were carried out in the early to late 2000s, we considered the treatment of incomplete outcome data adequate if the amount of missing data and the treatment of these data in the analysis was sufficiently described and not considered problematic (e.g. high number of missing values or comparison of inconsistent numbers of participants). We judged four trials to have a low risk of bias regarding incomplete outcome assessment (Raskin 2000; Z011 2007; Z013 2007; Z015 2007). We considered two trials to have a high risk of attrition bias (Iwamoto 2001; Recasens 2003). For the other trials, there was insufficient information to make a judgement (Ferguson 2001; Home 2000; Provenzano 2001). The information on the methods of analysis and missing values regarding individual outcomes was usually not detailed enough to judge the risk of bias for every outcome separately.
Selective reporting
Because there were no study protocols available, it was generally difficult to judge risk of bias due to selective reporting. However, for all trials, we found outcomes mentioned in the abstract, methods section or other documents related to the trial to be insufficiently reported in the results section. Therefore, we judged all trials at unclear or high risk of bias regarding selective reporting (see detailed comments in the table 'Risk of bias' section of the Characteristics of included studies table to support the choice of unclear or high risk of bias).
Other potential sources of bias
Under other potential sources of bias, we considered the lack of definition of a primary outcome, the inconsistent or clearly erroneous presentation of data and the commercial funding of a study. All but one trial received funding from a commercial sponsor or the funding situation was unclear. In three trials, the presentation of the data contained substantial inconsistencies so that we judged these trials at high risk of bias in this category (Home 2000; Provenzano 2001; Raskin 2000).
Effects of interventions
See: Table 1
Baseline characteristics
For details on baseline characteristics, see Appendix 3 and Appendix 4.
Primary outcomes
All‐cause mortality
None of the trials was designed to investigate the effect of short‐acting insulin compared to RHI on all‐cause mortality. Therefore, also considering the relatively short follow‐up periods of the trials, all trials were underpowered regarding all‐cause mortality. Six trials reported on the number of deaths in the two study groups. Overall, there was only one death across these six trials, which occurred in the treatment arm. For Provenzano 2001 and Recasens 2003, we concluded from the text that no deaths occurred during follow‐up. In the case of Iwamoto 2001, the information was insufficient.
Microvascular and macrovascular complications
None of the included trials reported results on microvascular or macrovascular complications.
Severe hypoglycaemic episodes
All included trials reported severe hypoglycaemic episodes, but only one trial defined it as a primary outcome (Ferguson 2001); in the other studies, severe hypoglycaemia was reported as an additional outcome or as part of the description of adverse events. The definitions of severe hypoglycaemic episodes varied strongly across studies. In one study, hypoglycaemic episodes were only reported based on the symptoms that were associated with them, so there was no special category for severe hypoglycaemic episodes (Iwamoto 2001). However, we could extract data on the number of participants who experienced a hypoglycaemic coma, which occurred for only one participant in the treatment arm and no participants in the control arm. Provenzano 2001 classified hypoglycaemic episodes into five different categories (hypoglycaemic symptoms and signs with spontaneous resolution, resolution after glucose ingestion, resolution after glucagon injection, resolution after intravenous glucose and coma). The results were only presented as the total number of episodes experienced in the two treatment groups, so that we did not include these data in any meta‐analyses. Overall, considering only the last three categories as severe, there were four hypoglycaemic episodes (two events of hypoglycaemic coma and one episode requiring glucagon injection and intravenous glucose) in the insulin lispro group and two episodes that were resolved after intravenous glucose in the RHI group.
The trials by Recasens 2003 and Ferguson 2001 defined a severe hypoglycaemic episode as one that required the help of another person. For the remaining five trials, there were (according to IQWIG 2007) inconsistencies between the information provided in the published articles and the original study reports (Home 2000; Raskin 2000; Z011 2007; Z013 2007; Z015 2007). Home 2000 divided severe hypoglycaemic episodes into grade A and B with grade A being defined through the need for help from another person, whereas grade B also required the infusion or injection of glucose or glucagon. The data presented in the publication and the original study report were inconsistent, but in neither case was the difference between the treatment groups statistically significant. Raskin 2000 defined severe hypoglycaemic episodes differently in the original study report and the publication. In the publication, they defined a severe hypoglycaemic episode as a hypoglycaemic event that the participant could not treat himself/herself or required administration of parenteral glucose or glucagon, whereas the study report's definition required typical symptoms of hypoglycaemia associated with a disturbance of consciousness that required either the assistance of another person or hospital admission. Furthermore, only the study report presented detailed results on severe hypoglycaemia. As in Home 2000, they divided episodes into grade A and B, whereas in the publication it was only briefly stated that major hypoglycaemic episodes were experienced by about 20% of the participants in each treatment arm.
For the trials Z011 2007, Z013 2007, and Z015 2007, we obtained data on severe hypoglycaemic episodes from the original study reports (as published in IQWIG 2007) and from a previous review (Brunelle 1998). The study reports provided separate results on the number of participants who experienced a hypoglycaemic coma, treatment with intravenous glucose or glucagon, but did not provide information on the number of participants who experienced at least one episode of severe hypoglycaemia (i.e. any of the three events above). However, such results were published in Brunelle 1998. However, since the numbers show inconsistencies with those presented in the original study report of Z011 2007, these results should be interpreted with caution.
Analysis 1.1 combines the results of all trials, for which data on the number of participants who experienced at least one episode of severe hypoglycaemia was available (see Figure 4). We excluded Iwamoto 2001, because the data provided were limited to hypoglycaemic coma and Provenzano 2001 because data were only presented in the form of total number of episodes experienced in each treatment arm. Because one of the remaining trials used a cross‐over design (Ferguson 2001), we used OR as an effect measure to include the Becker‐Balagtas OR for the cross‐over study in the pooled analysis (Becker 1993; Curtin 2002; Elbourne 2002; Stedman 2009). As the information provided in Ferguson 2001 was insufficient, we estimated the within‐subject correlation using the smallest correlation of several other cross‐over studies on severe hypoglycaemia presented in Elbourne 2002. The analysis showed no substantial difference between the treatment and control group (OR 0.89, 95% CI 0.71 to 1.12; P value = 0.31; 2459 participants; 7 trials; very low quality evidence).
1.1. Analysis.
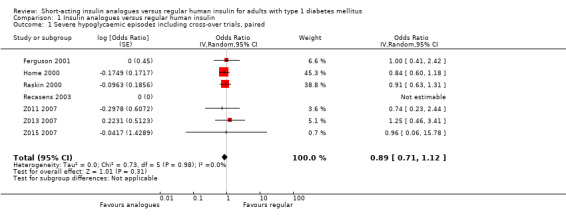
Comparison 1 Insulin analogues versus regular human insulin, Outcome 1 Severe hypoglycaemic episodes including cross‐over trials, paired.
4.
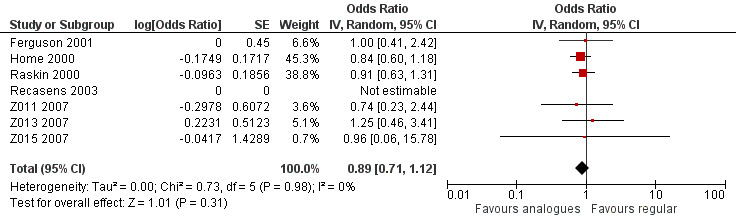
Forest plot of comparison: 1 Insulin analogues versus regular human insulin, outcome: 1.1 Severe hypoglycaemic episodes including cross‐over trials, paired.
Leaving out the cross‐over trial, using an unpaired effect estimate, or taking the largest correlation presented in Elbourne 2002 to estimate the within‐subject variance led to comparable results (Analysis 1.2: OR 0.88, 95% CI 0.70 to 1.12; P value = 0.30; 2426 participants; 6 trials; Analysis 1.3: OR 0.89, 95% CI 0.71 to 1.12; P value = 0.31; 2492 participants; 7 trials; Analysis 1.4: OR 0.89, 95% CI 0.71 to 1.12; P value = 0.32, 2492 participants; 7 trials). Furthermore, using a fixed‐effect model instead of a random‐effects model had no impact on the effect estimate (Analysis 1.5: OR 0.89, 95% CI 0.71 to 1.12; P value = 0.31; 2492 participants; 7 trials).
1.2. Analysis.
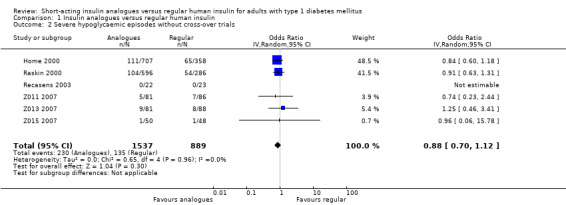
Comparison 1 Insulin analogues versus regular human insulin, Outcome 2 Severe hypoglycaemic episodes without cross‐over trials.
1.3. Analysis.
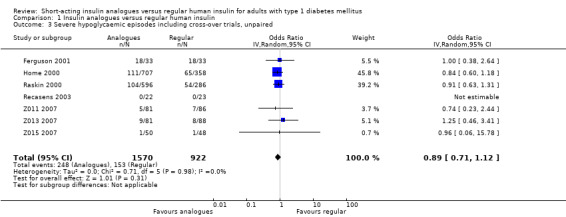
Comparison 1 Insulin analogues versus regular human insulin, Outcome 3 Severe hypoglycaemic episodes including cross‐over trials, unpaired.
1.4. Analysis.
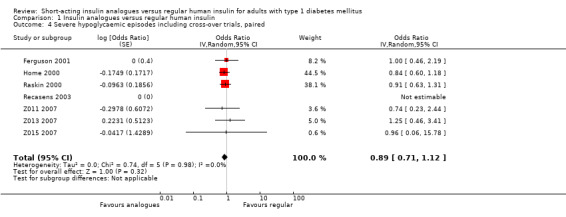
Comparison 1 Insulin analogues versus regular human insulin, Outcome 4 Severe hypoglycaemic episodes including cross‐over trials, paired.
1.5. Analysis.
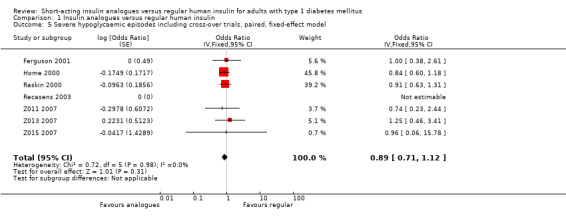
Comparison 1 Insulin analogues versus regular human insulin, Outcome 5 Severe hypoglycaemic episodes including cross‐over trials, paired, fixed‐effect model.
The cross‐over trial by Ferguson 2001 also stood out from the other trials, because it included only participants with an impaired awareness of hypoglycaemia and therefore showed a much higher frequency of severe hypoglycaemic episodes compared to the other trials. However, consistent with the overall result, this trial also found no substantial difference between the two treatment groups when considering the number of participants experiencing severe hypoglycaemic episodes in general.
Carrying out separate analyses for all trials using insulin aspart or insulin lispro, we found no relevant treatment effect on severe hypoglycaemic episodes independently of which insulin analogue was used (Analysis 1.6: insulin lispro: OR 1.00, 95% CI 0.56 to 1.80; 512 participants; 5 trials; insulin aspart: OR 0.87, 95% CI 0.68 to 1.11; 1947 participants; 2 trials).
1.6. Analysis.
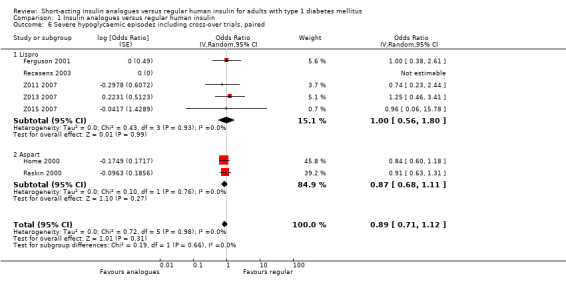
Comparison 1 Insulin analogues versus regular human insulin, Outcome 6 Severe hypoglycaemic episodes including cross‐over trials, paired.
Secondary outcomes
Glycaemic control
All included studies provided data on the HbA1c. The group difference of the mean HbA1c at the end of follow‐up was ‐0.15% (95% CI ‐0.21 to ‐0.08; P value < 0.00001; 2608 participants; 9 trials, low quality evidence; Analysis 1.7; Figure 5).
1.7. Analysis.
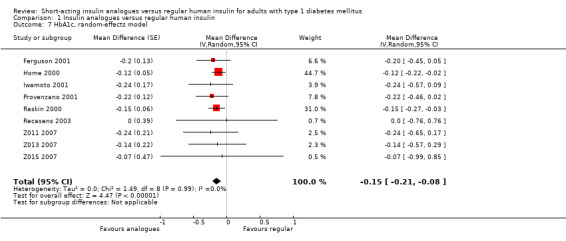
Comparison 1 Insulin analogues versus regular human insulin, Outcome 7 HbA1c, random‐effects model.
5.
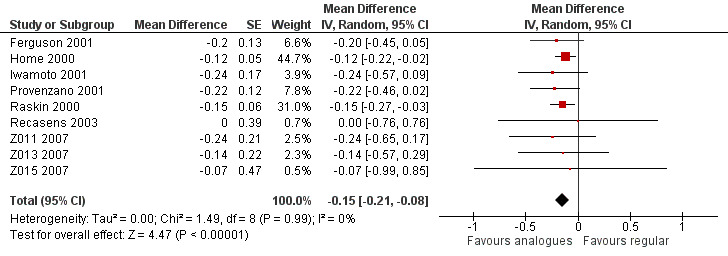
Forest plot of comparison: 1 Insulin analogues versus regular human insulin, outcome: 1.7 HbA1c, random‐effects model.
The effect persisted in a separate analysis of insulin aspart (Home 2000; Raskin 2000), and insulin lispro (Ferguson 2001; Provenzano 2001; Recasens 2003; Z011 2007; Z013 2007; Z015 2007) trials (Analysis 1.8: insulin lispro: ‐0.20%, 95% CI ‐0.34 to ‐0.05; insulin aspart: ‐0.14%, 95% CI ‐0.21 to ‐0.06). Analysis 1.7 and Analysis 1.8 included the cross‐over trials making use of the paired data available in the publications. In Ferguson 2001, we could estimate the within‐subject variance based on the reported results of a paired t‐test. Provenzano 2001 only provided the mean HbA1c in the two treatment conditions. In this case, we used the within‐subject correlation from Ferguson 2001 to estimate the standard error of the mean HbA1c difference. We also carried out a separate analysis of cross‐over (Ferguson 2001; Provenzano 2001) and parallel trials (Home 2000; Iwamoto 2001; Raskin 2000; Recasens 2003; Z011 2007; Z013 2007; Z015 2007) (Analysis 1.9), as well as a pooled analysis in which cross‐over trials were included using unpaired effect estimates (Analysis 1.10), and found similar results. In addition, using a fixed‐effect model instead of a random‐effects model did not substantially affect the results (Analysis 1.11).
1.8. Analysis.
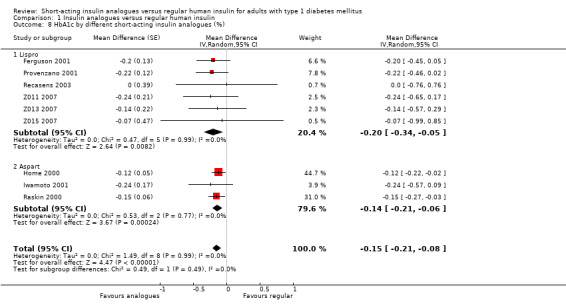
Comparison 1 Insulin analogues versus regular human insulin, Outcome 8 HbA1c by different short‐acting insulin analogues (%).
1.9. Analysis.
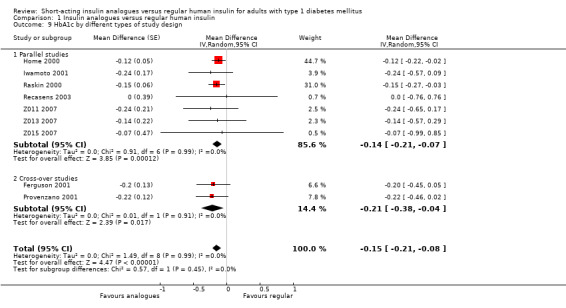
Comparison 1 Insulin analogues versus regular human insulin, Outcome 9 HbA1c by different types of study design.
1.10. Analysis.
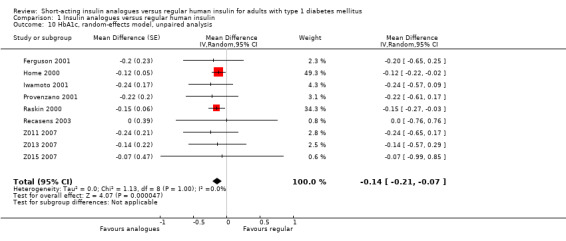
Comparison 1 Insulin analogues versus regular human insulin, Outcome 10 HbA1c, random‐effects model, unpaired analysis.
1.11. Analysis.
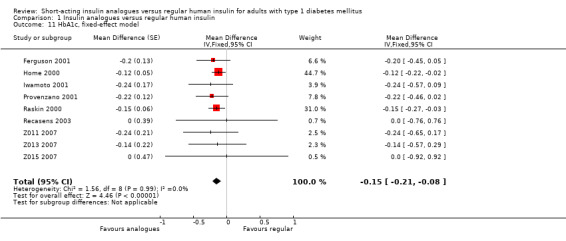
Comparison 1 Insulin analogues versus regular human insulin, Outcome 11 HbA1c, fixed‐effect model.
There were inconsistencies in the data presentation between the publications and the original study reports for the studies by Home 2000 and Raskin 2000. In Home 2000, the HbA1c values in the publication text were different from the values reported in the table (by 0.02%). This difference is possibly due to values in the table being adjusted for "baseline value and centre". Furthermore, there were inconsistencies between the results reported in Home 2000 and those reported in FDA documents (for more details see IQWIG 2007). The IQWIG report (IQWIG 2007) also mentioned inconsistencies regarding the baseline HbA1c between the publication, the study report and Lindholm 2002. IQWIG 2007 further criticises that, according to the original study reports, both Home 2000 and Raskin 2000 were planned as non‐inferiority studies with a non‐inferiority margin of 0.6%. However, the publications only tested for superiority and described a significant effect of insulin aspart over RHI, even though the size of the effect was smaller than the 0.6% margin described in the report.
Adverse events
All hypoglycaemic episodes
Apart from severe hypoglycaemic episodes, all trials also assessed hypoglycaemia in general, including weaker episodes, which were usually defined by any symptoms associated with hypoglycaemia (for details see Appendix 8). Six trials further specified a hypoglycaemic episode as any time a participant measured a blood glucose value below 36 mg/dL to below 65 mg/dL, depending on the trial (Ferguson 2001; Home 2000; Recasens 2003; Z011 2007; Z013 2007; Z015 2007). Eight trials found no substantial differences between the treatment and control group regarding the occurrence of hypoglycaemic episodes in general. Only Provenzano 2001 reported a significantly lower hypoglycaemia rate with insulin lispro compared to RHI. However, it was unclear what exactly the authors referred to when they reported the "monthly mean of hypoglycaemic episodes" to be 0.047 in the RHI group and 0.028 in the insulin lispro group. Furthermore, the numbers of hypoglycaemic episodes presented in table 3 of their publication did not add up correctly.
Overall, none of the trials assessed hypoglycaemia in a blinded manner. Since the reporting of symptoms and the decision to carry out a blood glucose measurements are highly subjective, the results were at a high risk of bias and therefore are not presented in more detail here.
Severe nocturnal hypoglycaemia
Three of the included trials specifically compared the frequency of severe nocturnal hypoglycaemic episodes (Ferguson 2001; Home 2000; Raskin 2000). All three trials concluded that insulin analogues might be beneficial regarding the avoidance of nocturnal hypoglycaemia. However, no trial provided convincing results to support this claim. In Ferguson 2001, the authors reported a 47% lower incidence of severe nocturnal hypoglycaemic episodes with insulin lispro compared to RHI. However, the result was not statistically significant (25 episodes with insulin lispro versus 47 episodes with RHI, P value = 0.11). The publication of Ferguson 2001 defined a nocturnal hypoglycaemic episode as any episode occurring between 0:00 and 8:00 am. According to IQWIG 2007, the study report used a different definition (between 0:00 and 6:00 am). Using this time period, the difference between the two treatments was smaller.
There were also inconsistent definitions of nocturnal hypoglycaemic episodes between the publication and the original study report for Home 2000 and Raskin 2000. While the original study report and EMA documents referred to the period between 0:00 and 8:00 am, the publications were based on the period between 0:00 and 6:00 am. In the publications, both trials reported a significantly lower risk for participants in the insulin aspart group compared to the RHI group. In Raskin 2000, 4% of participants with insulin aspart versus 8% of participants with RHI experienced at least one major hypoglycaemic episode during the night (P value = 0.013). In Home 2000, 1.3% of participants with insulin aspart versus 3.4% of participants with RHI experienced a major hypoglycaemic nocturnal event of grade B (P value < 0.05). Comparing the groups regarding major nocturnal hypoglycaemic episodes grade A or all major nocturnal episodes, the results were not statistically significant. According to IQWIG 2007, the data presentation in the original study reports was not transparent, so that it was difficult to either confirm the results presented in the publications or come to firm conclusions regarding the effect of insulin aspart on the risk of nocturnal hypoglycaemia.
Weight gain
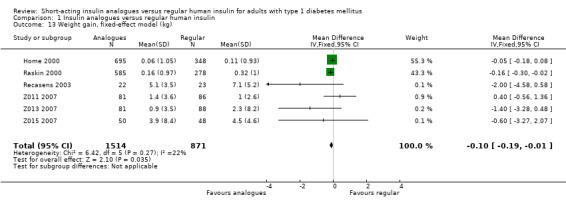
Seven trials reported results on weight gain (Home 2000; Provenzano 2001; Raskin 2000; Recasens 2003; Z011 2007; Z013 2007; Z015 2007). Provenzano 2001 only reported that there were no observed statistically or clinically significant differences. The other six trials provided the mean weight change from baseline in the two treatment groups. Combining the results of these six trials in a meta‐analysis showed an MD of ‐0.11 kg (95% CI ‐0.25 to 0.04; P value = 0.14; 2385 participants; 6 trials; moderate quality evidence; Analysis 1.12; Figure 6). Using a fixed‐effect model instead of a random‐effects model showed similar results (Analysis 1.13). A stratified analysis by type of insulin analogue revealed no substantial differences for the analogues lispro and aspart (Analysis 1.14).
1.12. Analysis.
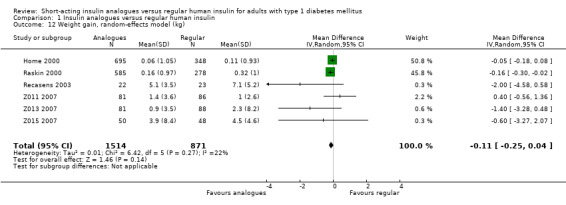
Comparison 1 Insulin analogues versus regular human insulin, Outcome 12 Weight gain, random‐effects model (kg).
6.
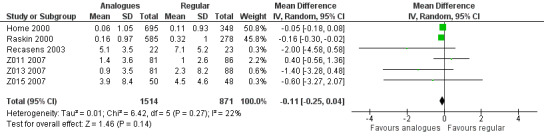
Forest plot of comparison: 1 Insulin analogues versus regular human insulin, outcome: 1.12 Weight gain, random‐effects model.
1.13. Analysis.
Comparison 1 Insulin analogues versus regular human insulin, Outcome 13 Weight gain, fixed‐effect model (kg).
1.14. Analysis.
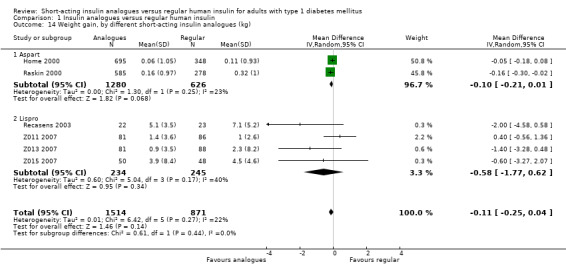
Comparison 1 Insulin analogues versus regular human insulin, Outcome 14 Weight gain, by different short‐acting insulin analogues (kg).
Other adverse events
Most publications presented little information on their assessment of other adverse events. Four trials had a short section mentioning the most common adverse events with a statement that there were no differences between the two treatment groups (Home 2000; Provenzano 2001; Raskin 2000; Anderson 1997). Iwamoto 2001 presented a table specifically listing the frequencies of adverse events with probable or possible relation to the trial treatment; the safety profile was considered similar in the two treatment groups. According to IQWIG 2007, the original study reports contained more detailed information on other adverse events. However, for the studies Z011 2007, Z013 2007, and Z015 2007, the information was not presented in a transparent way. While the report contained the statement that there were no significant differences between the treatment groups, it reported no detailed results. Two trials did not report on other adverse events (Ferguson 2001; Recasens 2003).
More detailed information on other adverse events was available in the original study reports of the trials Home 2000; Ferguson 2001; Raskin 2000; Z011 2007; Z013 2007 and Z015 2007 (IQWIG 2007), but overall, the results were again comparable for both treatment groups.
Six trials reported information on the number of study withdrawals due to adverse events (Home 2000; Ferguson 2001;Raskin 2000; Z011 2007; Z013 2007; Z015 2007); the percentage of participants leaving the trial due to adverse events ranged from 0% to 4% with no substantial significant differences between the treatment groups in any of the trials.
Health‐related quality of life
Three of the included trials provided information regarding the health‐related quality of life (Ferguson 2001; Home 2000; Z015 2007). The publication of Ferguson 2001 presented results on the Hypoglycaemia Fear Survey (HFS). IQWIG 2007 reported that according to the original study report, the Well Being Questionnaire (WBQ) was also used. Overall, no detailed results were presented: in the publication it was only briefly stated that there were no significant group differences and the study report presented only information on the individual study periods, but no appropriate cross‐over analysis was carried out. Z015 2007 used the Diabetes Quality of Life Clinical Trial Questionnaire (DQOLCTQ). The presented results only covered the participant populations from the USA and Canada, even though according to the study protocol it was planned to apply this questionnaire in European study centres (IQWIG 2007). Results were only presented in a qualitative manner without a description of the type of analysis carried out; there was no significant group difference.
In the study on the insulin analogue aspart (Home 2000), results on health‐related quality of life were published in Bott 2003, but only for the German subpopulation. The publication by Home 2000 mentioned the assessment of health‐related quality of life, but presented no results. IQWIG 2007 further reported that the original study report did not contain any information on measuring health‐related quality of life, so it is unclear whether data were only assessed for the German participant subgroup or also in other study centres. According to Bott 2003, the Diabetes‐Specific Quality of Life Scale (DSQOLS) was used. The overall questionnaire score showed no statistically significant group difference; an analysis on the subscales showed a statistically significant effect for the subscale 'diet restrictions' in favour of insulin aspart, but no statistically significant group differences for the subscales 'burden of hypoglycaemia' and 'blood glucose fluctuations'. It was unclear whether the analysis on the subscale level had been planned a priori. The baseline data presented in Bott 2003 showed a difference between the two treatment groups regarding the variable gender (50% in the insulin aspart group versus 38% in the RHI group), which raises concerns on whether this subgroup of participants was still appropriately randomised; the methods section provided insufficient information on this issue.
Costs
None of the included trials reported on the costs related to the treatment with short‐acting insulin analogues versus treatment with RHI.
Subgroup analyses
We could not perform most of the planned subgroup analyses because the available data were insufficient. For the outcomes for which we carried out meta‐analyses (severe hypoglycaemic episodes, HbA1c and weight gain), we performed separate analyses for the two insulin analogues lispro and aspart. The results are presented in the sections on the respective outcomes.
Sensitivity analyses
We performed sensitivity analyses using the fixed‐effect model instead of random‐effects models. The results of these analyses are described in the sections on the respective outcomes.
Assessment of reporting bias
We did not draw funnel plots due to limited number of studies for a particular outcome (nine studies).
Discussion
Summary of main results
This review could not provide any reliable results regarding long‐term patient‐relevant outcomes of short‐acting insulin analogues compared to RHI in adults with type 1 diabetes. None of the included trials had a follow‐up period that was longer than one year and none of the trials measured the development or progression of any microvascular or macrovascular complication. Although mortality was usually assessed as an adverse event, only one death occurred across all of the studies, so that no analysis regarding this outcome was possible.
Therefore, based on the data available in this review, we have to rely on glycaemic control as a surrogate measure for late complications of diabetes. Our meta‐analysis found a small reduction in HbA1c for a therapy using short‐acting insulin analogues compared to RHI. This effect was similar for the insulin analogues lispro and aspart. None of the included studies investigated the effects of insulin glulisine.
The effects of insulin analogues on hypoglycaemia were inconclusive. There was no substantial difference between the treatment and control groups regarding the occurrence of severe hypoglycaemic episodes. For general hypoglycaemia, also taking into account mild forms of hypoglycaemia, the data were generally of low quality, but also did not indicate relevant group differences. For nocturnal severe hypoglycaemic episodes, two trials reported statistically significant effects in favour of insulin aspart. However, due to inconsistent reporting in publications and study reports, the validity of the result remains questionable.
For health‐related quality of life, the presentation of results was often incomplete and questionnaires were frequently only presented for a subgroup of participants. Overall, the results provided no clear evidence that insulin therapy using insulin analogues as opposed to RHI had a marked effect on health‐related quality of life.
Our meta‐analysis on weight gain showed some evidence for RHI treatment being associated with a higher weight gain compared to insulin analogues; however, the difference was not statistically significant. None of the trials reported firm evidence regarding any other adverse event. None of the trials assessed costs of treatment.
Overall completeness and applicability of evidence
In contrast to the previous review, we restricted this update to include only studies with a follow‐up duration of at least 24 weeks. This restriction intended to focus better on the effects of insulin analogues on participant‐relevant outcomes. In order to come to definite conclusions on long‐term outcomes such as mortality or microvascular or macrovascular complications of diabetes, trials with a follow‐up duration of several years would be required. The longest trials found in our systematic search had a follow‐up duration of 12 months and none of the included trials investigated the effects of insulin analogues on microvascular or macrovascular complications.
For a reliable assessment of metabolic control, trials should also be long enough to obtain a valid measure that can be interpreted in relation to the occurrence of hypoglycaemic events. However, since we excluded trials with short follow‐up durations, the number of trials that we could include in this review was low, so that on many outcomes we could make no firm conclusions. None of the included trials compared the costs of treatment with insulin analogues versus RHI and the data regarding health‐related quality of life as well as many adverse events, such as potential carcinogenic effects, were insufficient or non‐existing. The results presented in these trials did not allow us to investigate whether effects were different for various subgroups.
Some of the included trials found effects on post‐prandial glucose values. We did not investigate this outcome in this review because analyses of post‐prandial glucose values leave a lot of leeway for subjective analysis and are often carried out posthoc.
The results of this review may not generalise to all people with type 1 diabetes. Many trials had restrictive participant selection criteria excluding participants with diabetic complications. There were also no trials in this review in which participants were using continuous subcutaneous insulin infusion (CSII). As it has been shown that insulin pumps might be associated with favourable long‐term outcomes and are increasingly used by participants, this is a major gap in the evidence on short‐acting insulin analogues in this review (Colquitt 2003; Johnson 2013; Pozzilli 2015). However, there are trials with follow‐up periods shorter than 24 weeks that show a beneficial effect of short‐acting insulin analogues on glycaemic control compared to RHI (Bode 2002; Johansson 2000; Renner 1999; Zinman 1997).
This review does not provide any information regarding the use of insulin analogues in children or pregnant women, as we explicitly excluded these groups.
Overall, our results were based on trials identified through an extensive and systematic literature search, including articles in all languages. We also searched trial registers to find potentially relevant but not yet published trials. However, due to our restrictions to only include longer trials and only RCTs, the number of trials is very low and, therefore, the insight gained on many outcomes is mostly inconclusive. On the one hand, this clearly shows the lack of firm evidence available regarding many outcomes, but, on the other hand, a large number of shorter trials or trials using observational designs are ignored. To gain a better idea on the value of short‐acting insulin analogues in general, all available evidence should be taken into account.
Furthermore, this review focuses only on a narrow question comparing short‐acting insulin analogues to RHI with all other diabetes‐related medication being the same in both groups. While this allows us to single out the effect that can be obtained by the use of short‐acting insulin analogues alone, some effects might become only evident if full analogue insulin regimens (combining short‐acting and long‐acting insulin analogues) are compared to RHI‐only regimens (Ashwell 2006; Hermansen 2004; Home 2012; Pedersen‐Bjergaard 2014).
It also should be noted that the majority of participants in this review used NPH as basal insulin. Therefore, little can be said about whether the observed effects would be the same if a long‐acting insulin analogue was used instead.
Quality of the evidence
Since none of the studies was carried out in a blinded manner, they were all at a risk of performance bias, especially for subjective outcomes such as hypoglycaemia. All trials were also at high risk of bias in one other risk of bias domain.
There were several inconsistencies regarding the reporting of methods and results. Both studies on insulin aspart showed inconsistencies in the reporting of the results on the HbA1c and severe hypoglycaemic events (Home 2000; Raskin 2000). In Home 2000, the reported HbA1c value in the text of the publication differed from the value presented in the table. This discrepancy was likely to be due to one of the values being adjusted for other baseline variables; however, there was no clear explanation. For Raskin 2000, a different baseline HbA1c value was reported in Lindholm 2002, a review article that was published by one of the co‐authors of the original trial. Furthermore, the definition of hypoglycaemia and also the time frame used to define nocturnal hypoglycaemia showed inconsistencies: in the publication of Raskin 2000, hypoglycaemic events were divided up into minor (blood glucose value less than 45 mg/dL or classical symptoms of hypoglycaemia) and major events (need of assistance or administration of parenteral glucose or glucagon), while the original study report described a further distinction of major events into grade A and B. Both Home 2000 and Raskin 2000 defined nocturnal hypoglycaemia as a hypoglycaemic episode that occurred between 0:00 and 8:00 am in the original study report, but between 0:00 and 6:00 am in the publications (IQWIG 2007). The same discrepancy regarding the definition of nocturnal hypoglycaemia occurred with Ferguson 2001. There were inconsistent results regarding severe hypoglycaemia in the study report of Z011 2007 and the review by Brunelle 1998, a co‐author of the original trial.
A commonly used criterion for the definition of severe hypoglycaemic episodes was the need for assistance from a third person. However, this type of definition is highly subjective and therefore prone to bias. A more robust definition, such as 'injection of glucose or glucagon by another person' may have resulted in more reliable data (Muehlhauser 1998).
Caution is also needed in the interpretation of the results regarding health‐related quality of life. For insulin aspart, results were only presented in the publication by Bott 2003 while the outcome was not mentioned in the original study report. The publication by Home 2000 reported that health‐related quality of life was assessed, but did not report any results (see IQWIG 2007). Bott 2003 only reported results for the German subpopulation. Overall, it was unclear whether this was a retrospective analysis. Differences in the baseline data of the two treatment groups in Bott 2003 raised the question whether the groups were adequately randomised. The limited results could not show firm evidence that the use of insulin analogues instead of RHI had an effect on participants' health‐related quality of life. Only the subscale 'diet restrictions' showed a statistically significant difference between the two treatment groups. However, it was not clear whether an analysis on the subscale level had been planned a priori. Furthermore, as participants in the regular insulin group were instructed to take insulin at least 20 minutes ahead of their meals, while participants treated with insulin analogues could apply insulin directly with the meal, the observed effect might rather be related to these treatment instructions. This is also important to note, because the scientific literature does not show clear evidence that such a longer insulin‐meal time difference is beneficial when using RHI (Müller 2013; Scheen 1999).
Overall, the limited methodological quality of the included trials allowed only a cautious interpretation of the results.
Potential biases in the review process
Our review process was potentially hampered by insufficient and inconsistent information provided in the available publications. Although we contacted all trial authors, we obtained no further information to complete missing information. The lack of information regarding study design and applied methods made it difficult to judge the risks of bias in many cases so that we frequently had to judge the risk of bias as unclear.
Furthermore, we frequently found inconsistencies in the reporting of outcome definitions and trial results. If we found substantial inconsistencies, we downgraded the quality of the evidence.
For severe hypoglycaemia, we combined data from several trials in a meta‐analysis. However, it is important to keep in mind that the definitions of severe hypoglycaemic episodes as well as the trial population varied across trials. For example, the majority of the trials excluded people with hypoglycaemia unawareness (Home 2000; Iwamoto 2001; Provenzano 2001; Raskin 2000; Z011 2007; Z013 2007; Z015 2007), while one trial explicitly included only these participants (Ferguson 2001). Potentially, a beneficial effect of insulin analogues on avoiding severe hypoglycaemic effects would become more apparent in participants at particular risk of hypoglycaemia. However, since most trials excluded these participants, we did not have sufficient data to investigate this question. Furthermore, as mentioned above, due to the lack of blinding and subjective definitions of hypoglycaemia, the results were at a high risk of performance bias. The data on severe hypoglycaemia reported in the included trials were also not detailed enough to do a more thorough meta‐analysis. We only looked at participants who experienced at least one severe hypoglycaemic episode, but did not take into account the total number of episodes experienced in the two groups.
Agreements and disagreements with other studies or reviews
Our finding of a small improvement of glycaemic control with short‐acting insulin analogues over RHI was confirmed by the results of other published literature reviews (Banerjee 2007; Garg 2010; Rys 2011; Singh 2009). Regarding hypoglycaemia, we agreed with other reviews that did not find substantial differences between insulin analogues and RHI regarding severe hypoglycaemic episodes (Banerjee 2007; Heller 2013; Rys 2011). Several reviews, which in contrast to us also included studies of shorter duration, reported a reduction of nocturnal hypoglycaemia under the treatment with insulin analogues (Banerjee 2007; Heller 2013; Rys 2011). Due to the low number of studies investigating nocturnal hypoglycaemia in our review, as well as the inconsistencies in reporting on the definition of nocturnal hypoglycaemia, we did not carry out a meta‐analysis on this outcome and suggest interpreting the results with caution.
Because we found no RCTs that compared the costs of treatment with short‐acting insulin analogues and RHI, our review did not allow any conclusions on the issue of cost‐effectiveness. In the political debate around the wide use of insulin analogues, the higher costs of insulin analogues combined with only little improvement of glycaemic control is one of the main arguments against the wide use of insulin analogues (Davidson 2014). Grunberger 2014 points out the complexity of assessing cost‐effectiveness on this issue, especially if one considers that insulin prices are highly dependent on the health system and vary immensely over time and across different countries.
Regarding health‐related quality of life, there are shorter studies not included in this review that suggest an improved outcome for insulin analogues compared to RHI (Annuzzi 2001; Holleman 1997b; Renner 1999). However, this result is almost always related to insulin analogues being perceived as more convenient due to the fact that they can just be taking together with meals instead of 20 to 30 minutes beforehand. As described above, it is not clear whether this insulin‐meal interval is really necessary for RHI.
Overall, there was also a lack of observational studies reporting on the long‐term benefits and harms of short‐term insulin analogues. In trials on the effect of insulin analogues on cancer, the results usually do not distinguish between long‐acting and short‐acting insulin analogues. However, while for some long‐acting insulin analogues the literature presents inconsistent results on the risk of cancer, there are to date no studies suggesting an increased risk of cancer associated with the use of short‐acting insulin analogues (Sciacca 2012; Smith 2009).
Authors' conclusions
Implications for practice.
Our analysis can only show a minor clinical benefit with regard to glycaemic control of short‐acting insulin analogues in people with type 1 diabetes. This result only applies to people on multiple daily injection therapy: furthermore, the evidence is mostly based on people using neutral protamine Hagedorn (NPH) as basal insulin.
Implications for research.
For safety purposes, high‐quality studies with a long‐term follow‐up of large numbers of participants who use short‐acting insulin analogues are needed. There is insufficient information on the development of long‐term adverse events such as potential carcinogenic effects. Furthermore, there is need for more research on the effects of short‐acting insulin analogues on mortality, the development of long‐term complications, and health‐related quality of life and cost‐effectiveness of this treatment.
Future research will have to take into account new, even faster‐acting insulins, that are currently being developed and tested (Heise 2014; Kaye 2013; Krasner 2012). In addition, the methods of insulin application will likely become more variable in the future. People are increasingly using different types of insulin pumps and new research shows that modulation of the injection site or other needle‐free applications can have effects on the pharmacokinetic and pharmacodynamic profiles of short‐acting insulins (Engwerda 2011; Landau 2014; Pfützner 2014).
What's new
| Date | Event | Description |
|---|---|---|
| 27 June 2019 | Amended | Conflict of interest statement in published Cochrane Review and Conflict of Interest form were harmonised. |
History
Review first published: Issue 6, 2016
| Date | Event | Description |
|---|---|---|
| 29 February 2016 | New citation required but conclusions have not changed | The conclusion drawn from the first update on the original systematic review remained unchanged |
| 29 February 2016 | New search has been performed | This review is an update of the former Cochrane review "Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus" which has been withdrawn and split into two Cochrane reviews on short acting insulin analogues versus regular human insulin for type 1 and 2 diabetes mellitus. |
| 21 September 2005 | New search has been performed | This review is an update of the review published in issue 4, 2004 (second update of the original version). A highly sensitive search applying the same search strategy as used for the original review was performed from 01/10/2003 to 21/09/2005 (adding the search terms for glulisine, which is new on the market) : 386 potentially relevant abstracts were identified and screened for retrieval. 375 of these were excluded by consensus. Eleven publications were potentially appropriate to be included in this systematic review, of which further 4 were excluded by consensus because of not being randomised, no comparable insulin regimen were used or analogues were not compared with regular insulin. Finally, seven new studies fulfilled the criteria to be included into this systematic review. For further details see figure 9 presenting the flow chart according the QUOROM statement. After including the 7 new studies in the analyses the conclusion drawn from the first systematic review remained unchanged. |
| 31 December 2003 | New search has been performed | First update |
Notes
The former Cochrane review "Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus" has been withdrawn and split into the following Cochrane reviews: 'Short‐acting insulin analogues versus regular human insulin for adults with type 1 diabetes mellitus' and 'Short‐acting insulin analogues versus regular human insulin for type 2 diabetes mellitus'.
Acknowledgements
We would like to thank Mirjam Seitz for help with data extraction.
Appendices
Appendix 1. Search strategies
| Search through the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid in MEDLINE and EMBASE |
| 1 (Lyspro$ or Lispro$).ti,ab,ot.
2 (Lys$B28 or B28Lys$ or (lys$ adj1 B28)).ti,ab,ot.
3 (Pro$B29 or B29Pro$ or (pro$ adj1 B29)).ti,ab,ot.
4 humalog$.ti,ab,ot,tn.
5 133107‐64‐9.rn.
6 or/1‐5
7 (insulin$ adj1 aspart$).ti,ab,ot.
8 (Asp$B28 or B28Asp$ or (asp$ adj1 B28)).ti,ab,ot.
9 (Novorapid$ or Novolog$).ti,ab,ot,tn.
10 116094‐23‐6.rn.
11 or/7‐10
12 (Glulisin$ or Glulysin$).ti,ab,ot.
13 (Glu$B29 or B29Glu$ or (glu$ adj1 B29)).ti,ab,ot.
14 (Lys$B3 or B3Lys$ or (lys$ adj1 B3)).ti,ab,ot.
15 Apidra$.ti,ab,ot,tn.
16 207748‐29‐6.rn.
17 or/12‐16
18 6 or 11 or 17
19 (insulin$ adj6 (analog$ or derivat$)).ti,ab,ot.
20 ((shortacting or fastacting or rapidacting) adj6 insulin$).ti,ab,ot.
21 ((short$ or fast$ or rapid$) adj1 acting adj6 insulin$).ti,ab,ot.
22 ((novel or new) adj6 insulin$).ti,ab,ot.
23 or/19‐22
24 exp insulin/aa
25 Insulin Derivative/ or insulin aspart/ or insulin glulisine/ or insulin lispro/ or recombinant human insulin/ or short acting insulin/ or synthetic insulin/
26 or/24‐25
27 23 or 26
28 exp Diabetes Mellitus/
29 diabet$.ti,ab,ot.
30 mellitu$.ti,ab,ot.
31 IDDM.ti,ab,ot.
32 MODY.ti,ab,ot.
33 NIDDM.ti,ab,ot.
34 (T1DM or T2DM or ((T1 or T2) adj1 DM)).ti,ab,ot.
35 (insulin$ depend$ or insulin?depend$ or noninsulin$ or noninsulin?depend$).ti,ab,ot.
36 ((matury or late) adj onset$ adj6 diabet$).ti,ab,ot.
37 (typ$ adj6 diabet$).ti,ab,ot.
38 or/30‐37
39 exp Diabetes Insipidus/
40 insipid$.ti,ab,ot.
41 or/39‐40
42 28 or 38
43 42 or (29 not (41 not 42))
44 (18 or 27) and 43
45 44 use pmoz
46 44 use emed
47 44 use cctr
48 randomized controlled trial.pt.
49 controlled clinical trial.pt.
50 randomized.ab.
51 placebo.ab.
52 clinical trials as topic.sh.
53 randomly.ab.
54 trial.ti.
55 or/48‐54
56 exp animals/ not humans.sh.
57 55 not 56
58 crossover procedure/
59 Double Blind Procedure/
60 Randomized Controlled Trial/
61 Single Blind Procedure/
62 random$.ti,ab.
63 factorial$.ti,ab.
64 (crossover$ or cross‐over$).ti,ab.
65 placebo$.ti,ab.
66 (doubl$ adj blind$).ti,ab.
67 (singl$ adj blind$).ti,ab.
68 assign$.ti,ab.
69 allocat$.ti,ab.
70 volunteer$.ti,ab.
71 or/58‐70
72 45 and 57
73 46 and 71
74 47 or 72 or 73 75 remove duplicates from 74 |
Appendix 2. Description of interventions
| Trial | Intervention(s) (route, frequency, total dose/day) | Adequatea intervention | Comparator(s) (route, frequency, total dose/day) | Adequatea comparator |
| Ferguson 2001 | Insulin lispro and human NPH insulin: participants advised to inject insulin lispro immediately before meals Blood glucose targets: no formal blood glucose targets were requested or advised |
Yes | RHI and NPH insulin: participants advised to inject RHI 30 min before meals Blood glucose targets: no formal blood glucose targets were requested or advised |
Yes |
| Home 2000 | Insulin aspart (100 U/mL) subcutaneously (by pen) in the anterior abdominal wall immediately before meals + NPH administered once or twice daily (determined by participant's previous practice) Target blood glucose values:
|
Yes | RHI (100 IU/mL) subcutaneously (by pen) 30 minutes before meals + NPH administered once or twice daily (determined by participant's previous practice) Target blood glucose values:
|
Yes |
| Iwamoto 2001 | Insulin aspart as meal‐related insulin immediately before meals and basal insulin was administered once or twice daily Blood glucose targets: ‐ Route of administration: ‐ |
Yes | RHI as meal‐related insulin 30 min before meals and basal insulin was administered once or twice daily Blood glucose targets: ‐ Route of administration: ‐ |
Yes |
| Provenzano 2001 | Insulin lispro just before each main meal and a dose of slow‐acting insulin at bed‐time Blood glucose targets: ‐ Route of administration: subcutaneous injections For the first 3 months, participants were on a normal diet followed by a standardised Mediterranean diet for 3 months |
Yes | RHI just before each main meal and a dose of slow‐acting insulin at bed‐time Blood glucose targets: ‐ Route of administration: subcutaneous injections For the first 3 months, participants were on a normal diet followed by a standardised Mediterranean diet for 3 months |
Yes |
| Raskin 2000 | Insulin aspart immediately before meals; NPH as a single bed‐time dose (if necessary additional morning dose) Blood glucose targets:
|
Yes | RHI 30 min before meals; NPH as a single bed‐time dose (if necessary additional morning dose) Blood glucose targets:
|
Yes |
| Recasens 2003 | Lispro insulin 3‐5 daily doses () immediately before meals and NPH before dinner/bed‐time Extra dose of NPH insulin before breakfast or lunch when necessary according to pre‐meal glucose targets Blood glucose targets:
|
Yes | RHI 3‐5 daily doses (subcutaneous) 30 min before meals and NPH before dinner/bed‐time Extra dose of NPH insulin before breakfast or lunch when necessary according to pre‐meal glucose targets Blood glucose targets:
|
Yes |
| Z011 2007 | Insulin lispro before every mealb; ultralente 1‐2 times a day Blood glucose targets:
|
Yes | RHI before every mealb; ultralente 1‐2 times a day Blood glucose targets:
|
Yes |
| Z013 2007 | Insulin lispro before every mealb; NPH 1‐2 times a day Blood glucose targets:
|
Yes | RHI before every mealb; RHI 1‐2 times a day Blood glucose targets:
|
Yes |
| Z015 2007 | Insulin lispro before every mealb; NPH or ultralente 1‐2 times a day Blood glucose targets:
|
Yes | RHI before every mealb; RHI or ultralente 1‐2 times a day Blood glucose targets:
|
Yes |
| "‐" denotes not reported aThe term 'adequate' refers to sufficient use of the intervention/comparator with regard to dose, dose escalation, dosing scheme, provision for contraindications and other features necessary to establish a fair contrast between intervention and comparator bInconsistent information regarding the exact timing: according to the study report, insulin lispro was taken immediately before meals and RHI was taken 30 minutes before meals; according to Garg 1996, insulin lispro was applied 5, 10 or 15 minutes before meals and RHI 20, 30 or 40 minutes before meals depending on the current blood glucose measurement C: comparator; h: hour; I: intervention; IU: international unit; min: minute; NPH: neutral protamine Hagedorn; RHI: regular human insulin; U: unit | ||||
Appendix 3. Baseline characteristics (I)
| Trial | Intervention(s) and comparator(s) | Duration of intervention | Population | Study period (year to year) | Country | Setting | Ethnic groups (%) | Duration of diabetes (mean years (SD), or as reported) |
| Ferguson 2001 | I: lispro | 24 weeks | People with type 1 diabetes and impaired awareness of hypoglycaemia | ‐ | Scotland | Outpatient clinic of the Department of Diabetes, Royal Infirmary of Edinburgh | ‐ | 26 (10)/10‐45 |
| C: RHI | ||||||||
| Home 2000 | I: aspart | 6 months | Adults with type 1 diabetes | 1997 | Austria, Denmark, Finland, Germany, Norway, Sweden, Switzerland, UK | Multicentre, outpatients | White: 99 | 15 (10) |
| C: RHI | White: 99 | 15 (10) | ||||||
| Iwamoto 2001 | I: aspart | 168 days | People with type 1 diabetes | ‐ | Japan | Hospital outpatients; physicians' clinics | Asian: 100 | 11 (7) |
| C: RHI | Asian: 100 | 11 (6) | ||||||
| Provenzano 2001 | I: lispro | 6 monthsa | People with type 1 diabetes | ‐ | Italy | ‐ | ‐ | 12 (3‐20)b |
| C: RHI | ||||||||
| Raskin 2000 | I: aspart | 6 months | People with type 1 diabetes | 1997 | US, Canada | Multicentre, outpatients | White: 94 | 16 (10) |
| C: RHI | White: 93 | 16 (9) | ||||||
| Recasens 2003 | I: lispro | 12 months | People newly diagnosed with type 1 diabetes | ‐ | Spain | ‐ | ‐ | 8.1 (3.8) weeks from diagnosis |
| C: RHI | 8.1 (8.0) weeks from diagnosis | |||||||
| Z011 2007 | I: lispro | 12 months | People with type 1 diabetes | 1992‐1993 | North America, Europe, South Africa | Multicentre, outpatients | ‐ | 12 |
| C: RHI | ‐ | 13 | ||||||
| Z013 2007 | I: lispro | 12 months | People with type 1 diabetes | 1992‐1993 | North America, Europe, South Africa, Australia | Multicentre, outpatients | ‐ | 13 |
| C: RHI | ‐ | 11 | ||||||
| Z015 2007 | I: lispro | 12 months | People with type 1 diabetes who had been on insulin therapy for < 2 months | 1993‐1994 | North America, Europe | Multicentre, outpatients | ‐ | 0.2 |
| C: RHI | ‐ | 0.2 | ||||||
|
aCross‐over study with total duration of one year, insulin was changed after six months (the six‐month long blocks were divided into two x three‐month blocks with normal and Mediterranean diet)
b"12 (3‐2)" in the publication. We assume this might be a typing mistake and should be 12 (3‐20). "‐" denotes not reported C: comparator; I: intervention; RHI: regular human insulin; SD: standard deviation | ||||||||
Appendix 4. Baseline characteristics (II)
| Trial | Intervention(s) and control(s) | Sex (female %) | Age (mean/range years (SD)) | HbA1c (mean % (SD)) | BMI (mean kg/m2 (SD)) | Comedications/Cointerventions | Comorbidities |
| Ferguson 2001 | I: lispro | 45 | 46 (11)/19‐65 | 9.0 (1.1) | 25 (3) | Twice daily free‐mixed insulin: 11 (33%) Multiple injection regimen: 22 (67%) |
‐ |
| C: RHI | |||||||
| Home 2000 | I: aspart | 45 | 38 (11)/ ‐ | 8.0 (1.2) | 25.1 (3.1) | ‐a | ‐ |
| C: RHI | 44 | 38 (12)/ ‐ | 8.0 (1.2) | 24.9 (3.0) | ‐a | ‐ | |
| Iwamoto 2001 | I: aspart | 59 | 34 (16)/ ‐ | 7.5 (1.1) | 22 (3) | ‐ | ‐ |
| C: RHI | 66 | 32 (13)/ ‐ | 7.6 (1.1) | 22 (2) | ‐ | ‐ | |
| Provenzano 2001 | I: lispro | 58 | 28 (‐)/14‐44 | 7.6 (0.5) | 23 (3) | ‐ | ‐ |
| C: RHI | |||||||
| Raskin 2000 | I: aspart | 49 | 39 (11)/ ‐ | 7.9 (1.1) | 26 (4) | ‐b | ‐ |
| C: RHI | 47 | 40 (12)/ ‐ | 8.0 (1.3) | 26 (3) | ‐b | ‐ | |
| Recasens 2003 | I: lispro | 36 | 24 (6)/ ‐ | 10.5 (2.4) | 22 (1) | ‐ | ‐ |
| C: RHI | 39 | 23 (5)/ ‐ | 11.4 (1.9) | 21 (3) | ‐ | ‐ | |
| Z011 2007 | I: lispro | 49 | 29 (‐)/ ‐ | 8.2 (1.4) | 24 (‐) | ‐ | ‐ |
| C: RHI | 55 | 32 (‐)/ ‐ | 8.3 (1.7) | 25 (‐) | ‐ | ‐ | |
| Z013 2007 | I: lispro | 49 | 35 (‐)/ ‐ | 8.3 (1.6) | 24 (‐) | ‐ | ‐ |
| C: RHI | 48 | 32 (‐)/ ‐ | 8.1 (1.6) | 24 (‐) | ‐ | ‐ | |
| Z015 2007 | I: lispro | 44 | 24 (‐)/ ‐ | 9.2 (2.2) | 23 (‐) | ‐ | ‐ |
| C: RHI | 33 | 25 (‐)/ ‐ | 8.8 (2.2) | 23 (‐) | ‐ | ‐ | |
|
aAccording to IQWIG (2005), the original study reports mentioned 2 participants who took acarbose and 1 participant who took metformin during the study period. These participants were included in the per‐protocol analysis
bThe list of co‐medications was not included in the study report provided for the IQWIG report. "‐" denotes not reported BMI: body mass index; C: comparator; HbA1c: glycosylated haemoglobin A1c; I: intervention; RHI: regular human insulin; SD: standard deviation | |||||||
Appendix 5. Matrix of study endpoints (publications)
| Trial | Characteristic | Endpoint reported in publication | Endpoint not reported in publication | Time of measurementa |
| Ferguson 2001 | Review's primary outcomes | |||
| All‐cause mortality | ‐ | x | ‐ | |
| Macrovascular complications | ‐ | x | ‐ | |
| Microvascular complications | ‐ | x | ‐ | |
| Severe hypoglycaemic episodes (P) | x | ‐ | 4, 8, 12, 16, 20, 24 weeks | |
| Review's secondary outcomes | ||||
| Glycaemic control (HbA1c) (O) | x | ‐ | 0, 24 weeks | |
| Adverse events (O) | x | ‐ | 24 weeks | |
| Health related quality of life (O) | xc | ‐ | 0, 24 weeks | |
| Costs | ‐ | x | ‐ | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| Participant satisfaction (O), pre‐ and post‐prandial blood glucose (O) | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| Home 2000 | Review's primary outcomes | |||
| All‐cause mortality (O) | x | ‐ | 6 months | |
| Macrovascular complications | ‐ | x | ‐ | |
| Microvascular complications | ‐ | x | ‐ | |
| Severe hypoglycaemic episodes (O) | x | ‐ | 6 months | |
| Review's secondary outcomes | ||||
| Glycaemic control (HbA1c) (P) | x | ‐ | 0, 2 weeks, 1, 2, 3, 4, 5, 6 months | |
| Adverse events (O) | x | ‐ | 6 months | |
| Health‐related quality of life (O) | xd | ‐ | 0, 3, 6 months | |
| Costs | ‐ | x | ‐ | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| Treatment satisfaction (O) | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| Iwamoto 2001 | Review's primary outcomes | |||
| All‐cause mortality | ‐ | x | ‐ | |
| Macrovascular complications | ‐ | x | ‐ | |
| Microvascular complications | ‐ | x | ‐ | |
| Severe hypoglycaemic episodes | ‐ | x | ‐ | |
| Review's secondary outcomes | ||||
| Glycaemic control (HbA1c) (P) | x | ‐ | 0, 4, 8, 16, 20, 24 weeks | |
| Adverse events (O) | x | ‐ | 0, 4, 8, 16, 20, 24 weeks | |
| Health‐related quality of life | ‐ | x | ‐ | |
| Costs | ‐ | x | ‐ | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| Weight (O), insulin antibodies (O), insulin dose (O), blood pressure (O), fasting and post‐prandial blood glucose (O) | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| Provenzano 2001 | Review's primary outcomes | |||
| All‐cause mortality (O) | x | ‐ | ‐ | |
| Macrovascular complications | ‐ | x | ‐ | |
| Microvascular complications | ‐ | x | ‐ | |
| Severe hypoglycaemic episodes | ‐ | x | ‐ | |
| Review's secondary outcomes | ||||
| Glycaemic control (HbA1c) (O) | x | ‐ | ‐2, 0, 6, 12, 18, 24 weeks | |
| Adverse events (O) | x | ‐ | every 15 days | |
| Health related quality of life | ‐ | x | ‐ | |
| Costs | ‐ | x | ‐ | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| ‐ | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| Raskin 2000 | Review'sprimary outcomes | |||
| All‐cause mortality | ‐ | x | ‐ | |
| Macrovascular complications | ‐ | x | ‐ | |
| Microvascular complications | ‐ | x | ‐ | |
| Severe hypoglycaemic episodes (O) | x | ‐ | 12 months | |
| Review'ssecondary outcomes | ||||
| Glycaemic control (HbA1c) (P) | x | ‐ | 0, 12 months | |
| Adverse events (O) | x | ‐ | 12 months | |
| Health‐related quality of life | ‐ | x | ‐ | |
| Costs | ‐ | x | ‐ | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| Post‐prandial blood glucose (O) | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| Recasens 2003 | Review'sprimary outcomes | |||
| All‐cause mortality | ‐ | x | ‐ | |
| Macrovascular complications | ‐ | x | ‐ | |
| Microvascular complications | ‐ | x | ‐ | |
| Severe hypoglycaemic episodes (O) | x | ‐ | 12 months | |
| Review'ssecondary outcomes | ||||
| Glycaemic control (HbA1c) (O) | x | ‐ | 0, 12 months | |
| Adverse events (O) | x | ‐ | 12 months | |
| Health‐related quality of life | ‐ | x | ‐ | |
| Costs | ‐ | x | ‐ | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| ‐ | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| Z011 2007 | Review'sprimary outcomes | |||
| All‐cause mortality (O) | x | ‐ | 12 months | |
| Macrovascular complications | ‐ | x | ‐ | |
| Microvascular complications | ‐ | x | ‐ | |
| Severe hypoglycaemic episodes (O) | x | ‐ | 12 months | |
| Review'ssecondary outcomes | ||||
| Glycaemic control (HbA1c) (O) | x | ‐ | 0, 3, 6, 12 months | |
| Adverse events (O) | x | ‐ | 12 months | |
| Health‐related quality of life | ‐ | x | ‐ | |
| Costs | ‐ | x | ‐ | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| ‐ | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| Z013 2007 | Review'sprimary outcomes | |||
| All‐cause mortality (O) | x | ‐ | 12 months | |
| Macrovascular complications | ‐ | x | ‐ | |
| Microvascular complications | ‐ | x | ‐ | |
| Severe hypoglycaemic episodes (O) | x | ‐ | 12 months | |
| Review'ssecondary outcomes | ||||
| Glycaemic control (HbA1c) (O) | x | ‐ | 0, 3, 6, 12 months | |
| Adverse events (O) | x | ‐ | 12 months | |
| Health‐related quality of life | ‐ | x | ‐ | |
| Costs | ‐ | x | ‐ | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| ‐ | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| Z015 2007 | Review'sprimary outcomes | |||
| All‐cause mortality (O) | x | ‐ | 12 months | |
| Macrovascular complications | ‐ | x | ‐ | |
| Microvascular complications | ‐ | x | ‐ | |
| Severe hypoglycaemic episodes (O) | x | ‐ | 12 months | |
| Review'ssecondary outcomes | ||||
| Glycaemic control (HbA1c) (O) | x | ‐ | 0, 3, 6, 12 months | |
| Adverse events (O) | x | ‐ | 12 months | |
| Health‐related quality of life | ‐ | x | ‐ | |
| Costs | ‐ | x | ‐ | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| ‐ | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
|
aUnderlined data denote times of measurement for primary and secondary review outcomes, if measured and reported in the results section of the publication (other times represent planned but not reported points in time)
b(P) Primary or (S) secondary endpoint(s) refer to verbatim statements in the publication, (O) other endpoints relate to outcomes that were not specified as 'primary' or 'secondary' outcomes in the publication
cThe publication reports on only one questionnaire, while the trial documents described two different questionnaires
dOnly reported for the German subpopulation (Bott 2003) HbA1c: glycosylated haemoglobin A1c; O: other endpoint; P: primary endpoint; S: secondary endpoint | ||||
Appendix 6. Examination of outcome reporting bias
| Trial | Outcome | Clear that outcome was measured and analyseda (trial report stated that outcome was analysed but only reported that result was not significant) | Clear that outcome was measured and analysedb (trial report stated that outcome was analysed but no results reported) | Clear that outcome was measuredc (clear that outcome was measured but not necessarily analysed (judgement says likely to have been analysed but not reported because of non‐significant results)) | Unclear whether the outcome was measuredd (not mentioned but clinical judgement says likely to have been measured and analysed but not reported because of non‐significant results) |
| Ferguson 2001 | Other adverse events | ‐ | ‐ | ‐ | x |
| Home 2000 | N/A | ||||
| Iwamoto 2001 | N/A | ||||
| Provenzano 2001 | N/A | ||||
| Raskin 2000 | N/A | ||||
| Recasens 2003 | N/A | ||||
| Z011 2007 | Severe hypoglycaemia | ‐ | ‐ | ‐ | x |
| Ketoacidosis | ‐ | ‐ | ‐ | x | |
| Z013 2007 | Severe hypoglycaemia | ‐ | ‐ | ‐ | x |
| Ketoacidosis | ‐ | ‐ | ‐ | x | |
| Z015 2007 | N/A | ||||
| 'High risk of bias' categories for outcome reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) study classification system for missing or incomplete outcome reporting in reports of randomised trials (Kirkham 2010) aClassification 'A' (table 2, Kirkham 2010) bClassification 'D' (table 2, Kirkham 2010) cClassification 'E' (table 2, Kirkham 2010) dClassification 'G' (table 2, Kirkham 2010) N/A: not applicable | |||||
Appendix 7. Definition of endpoint measurement (I)
| Trial | Diabetic complications: myocardial infarction | Diabetic complications: stroke | Diabetic complications: heart failure | Diabetic complications: PVD | Diabetic complications: blindness | Diabetic complications: retinopathy |
| Ferguson 2001 | N/I | N/I | N/I | N/I | N/I | N/I |
| Home 2000 | N/I | N/I | N/I | N/I | N/I | N/I |
| Iwamoto 2001 | N/I | N/I | N/I | N/I | N/I | N/I |
| Provenzano 2001 | N/I | N/I | N/I | N/I | N/I | N/I |
| Raskin 2000 | N/I | N/I | N/I | N/I | N/I | N/I |
| Recasens 2003 | N/I | N/I | N/I | N/I | N/I | N/I |
| Z011 2007 | N/I | N/I | N/I | N/I | N/I | N/I |
| Z013 2007 | N/I | N/I | N/I | N/I | N/I | N/I |
| Z015 2007 | N/I | N/I | N/I | N/I | N/I | N/I |
| N/I: not investigated; PVD: peripheral vascular disease | ||||||
Appendix 8. Definition of endpoint measurement (II)
| Trial | Diabetic complications: amputation | Diabetic complications: end stage renal disease | Costs | Health‐related quality of life | Hypoglycaemia | Ketoacidosis | Other adverse events |
| Ferguson 2001 | N/I | N/I | N/I | Hypoglycaemia Fear Survey (HFS), Well‐being questionnaire (WBQ)a | All: values of ≤ 3.5 mmol/L (65 mg/dL) were recorded as evidence of biochemical hypoglycaemia; capillary blood glucose concentrations in this range, irrespective of whether accompanied by symptoms of hypoglycaemia, were recorded as hypoglycaemia episodes; symptomatic episodes were recorded independent of blood glucose measurements Severe: severe hypoglycaemia was defined as any episode of hypoglycaemia for which a person required external (third party) assistance to facilitate recovery Nocturnal: definition as 'all' but at night Severe nocturnal: definition as 'severe' but at night | ND | N/I |
| Home 2000 | N/I | N/I | N/I | N/I | Minorb: symptomatic events dealt with by the participant Major grade Ab: requiring third party help Major grade Bb: parenteral glucose or glucagon administration | ND | N/I |
| Iwamoto 2001 | N/I | N/I | N/I | N/I | All: hypoglycaemia symptoms reported by practitioners (not participants) Severe: ‐ Nocturnal: ‐ Severe nocturnal: ‐ SAE: ‐ | N/I | N/I |
| Provenzano 2001 | N/I | N/I | N/I | N/I | All: hypoglycaemic episodes were classified according to signs and symptoms in 5 degrees: hypoglycemic symptoms and signs with spontaneous resolution (S1); resolution after glucose ingestion (S2); after glucagon injections (S3); after intravenous glucose (S4); coma (S5) Severe: ‐ Nocturnal: ‐ Severe nocturnal: ‐ SAE: ‐ | ND | ND |
| Raskin 2000 | N/I | N/I | N/I | N/I | Allc: hypoglycaemic events were defined as minor when the participants had a blood glucose value < 45 mg/dL (2.5 mmol/L) or had classical symptoms of hypoglycaemia (such as sweating, strong hunger, dizziness and tremor) and were able to deal with the episode on their own Severe: a major hypoglycaemic event was one that the participant could not treat by himself/herself or require administration of parenteral glucose or glucagon Nocturnal: 0:00 to 6:00 amd Severe nocturnal: ‐ SAE: ‐ | ND | ND |
| Recasens 2003 | N/I | N/I | N/I | N/I | All: classified as severe or mild and estimated from participants' diaries of self capillary blood glucose monitoring Severe: severe hypoglycaemic events were defined as those associated with neuroglycopenia severe enough to require treatment from a third party Mild: mild hypoglycaemic events were defined as symptoms or signs associated with hypoglycaemia experienced by the participant and self treated without the need of assistance from a third party or a blood glucose measurement of < 3.3 mmol/L Nocturnal: ‐ Severe nocturnal: as mild, but assistance of a third person required SAE: ‐ | N/I | ND |
| Z011 2007 | N/I | N/I | N/I | N/I |
Alle: blood glucose measurement < 36 mg/dL (2.0 mmol/L) or hypoglycaemic symptoms, but also treatment with glucagon or intravenous glucose or hypoglycaemic coma Severe: ‐ Nocturnal: ‐ Severe nocturnal: ‐ SAE: ‐ |
ND | ND |
| Z013 2007 | N/I | N/I | N/I | N/I |
Alle: blood glucose measurement < 36 mg/dL (2.0 mmol/L) or hypoglycaemic symptoms, but also treatment with glucagon or intravenous glucose or hypoglycaemic coma Severe: ‐ Nocturnal: ‐ Severe nocturnal: ‐ SAE: ‐ |
ND | ND |
| Z015 2007 | N/I | N/I | N/I | DQOLCTQ (Diabetes Quality of Life Clinical Questionnaire) |
Alle: blood glucose measurement < 63 mg/dL or hypoglycaemic symptoms, but also treatment with glucagon or intravenous glucose or hypoglycaemic coma Severe: ‐ Nocturnal: ‐ Severe nocturnal: ‐ SAE: ‐ |
ND | ND |
|
a WBQ only reported in original study report (IQWIG 2007)
bReported in categories 'all’ and 'night’, but 'night' was not defined in more detail
cOriginal study report further defines grade A and grade B hypoglycaemias (IQWIG 2007)
dDefinition in original study report: 0:00 to 8:00 am (IQWIG 2007)
eFrom IQWIG 2007 ND: not defined; N/I: not investigated; SAE: serious adverse event | |||||||
Appendix 9. Adverse events (I)
| Trial | Intervention(s) and comparator(s) | Randomised/safety (N) | Deaths (n/N) | All adverse events (n/N (%)) | Severe/serious adverse events (n/N (%)) | Drop‐outs due to adverse events (n/N (%)) | Hypoglycaemic episodes, all (n/N (%)) | Hypoglycaemic episodes, severe (n/N (%)) |
| Ferguson 2001 | I: lispro | 39/35 | 0/33 | ‐ | ‐ | 0/33 (0) | ‐ | 18/33 (55) |
| C: RHI | 0/33 | ‐ | ‐ | 0/33 (0) | ‐ | 18/33 (55) | ||
| Home 2000 | I: aspart | 708/707 | 1/707 | 484/707 (68) | 31/707 (4) | 6/707 (1) | ‐ | 111/707 (16) |
| C: RHI | 362/358 | 0/358 | 233/358 (65) | 21/358 (6) | 3/358 (1) | ‐ | 65/358 (18) | |
| Iwamoto 2001 | I: aspart | 145a/143 | ‐ | 102/143 (71) | Severe event: 13/143 (9) Very severe event: 3/143 (2) |
‐ | 67/143 (47)c | 1/143 (1)d |
| C: RHI | 64b/62 | ‐ | 47/62 (73) | Severe event: 1/62 (2) Very severe event: 1/62 (2) |
‐ | 33/62 (53)c | 0/62 (0)d | |
| Provenzano 2001 | I: lispro | 12/‐ | 0/12 | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: RHI | 0/12 | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Raskin 2000e | I: aspart | 597/596 | 0/596 | ‐ | ‐ | 3/596 (1) | ‐ | 104/596 (17)f |
| C: RHI | 287/286 | 0/286 | ‐ | ‐ | 2/286 (1) | ‐ | 54/286 (19)f | |
| Recasens 2003 | I: lispro | 22/22 | ‐ | ‐ | ‐ | ‐ | ‐ | 0/22 (0) |
| C: RHI | 23/23 | ‐ | ‐ | ‐ | ‐ | ‐ | 0/23 (0) | |
| all: | 45/45 | ‐ | ‐ | ‐ | ‐ | ‐ | 0/45 (0) | |
| Z011 2007 | I: lispro | 81/81 | 0/81 | ‐ | ‐ | 2/81 (3) | ‐ | 5/81 (1) |
| C: RHI | 86/86 | 0/86 | ‐ | ‐ | 1/86 (1) | ‐ | 7/86 (1) | |
| all: | 167/167 | 0/167 | ‐ | 12/167 (7)g | 3/167 (2) | ‐ | 12/167 (1) | |
| Z013 2007 | I: lispro | 81/81 | 0/81 | ‐ | ‐ | 4/81 (5) | ‐ | 9/81 (11) |
| C: RHI | 88/88 | 0/88 | ‐ | ‐ | 3/88 (3) | ‐ | 8/88 (9) | |
| all: | 169/169 | 0/169 | ‐ | 11/169 (7)g | 7/169 (4) | ‐ | 17/169 (10) | |
| Z015 2007 | I: lispro | 50/50 | 0/50 | ‐ | ‐ | 1/50 (2) | ‐ | 1/50 (2) |
| C: RHI | 48/48 | 0/48 | ‐ | ‐ | 1/48 (2) | ‐ | 1/48 (2) | |
| all: | 98/98 | 0/98 | ‐ | 5/98 (5)g | 2/98 (2) | ‐ | 2/98 (2) | |
|
aTwo participants not exposed to treatment
bOne participant not exposed to treatment, one participant removed because of protocol violation
cInconsistent information in translated text and table of publication
dOnly hypoglycaemic coma
eAccording to original study report, 884 participants were randomised, but only 882 received treatment (IQWIG 2007)
fBased on data from original study report as described in IQWIG 2007
gExcluding hypoglycaemic events from Brunelle 1998 "‐" denotes not reported C: comparator; I: intervention; RHI: regular human insulin | ||||||||
Appendix 10. Adverse events (II)
| Trial | Intervention(s) and comparator(s) | Randomised/Safety (N) | Hypoglycaemic episodes, severe nocturnal (n/N (%)) | Hypoglycaemic episodes, SAE (n/N (%)) | Hypoglycaemic episodes, nocturnal (n/N (%)) | Hyperglycaemic/ketoacidotic episodes (n/N (%)) |
| Ferguson 2001 | I: lispro | 39/35 | ‐ | ‐ | ‐ | ‐ |
| C: RHI | ‐ | ‐ | ‐ | ‐ | ||
| Home 2000 | I: aspart | 708/707 | 54/707 (8) | ‐ | ‐ | 3/707 (0) |
| C: RHI | 362/358 | 39/358 (11) | ‐ | ‐ | 3/358 (1) | |
| Iwamoto 2001 | I: aspart | 145a/143 | ‐ | ‐ | ‐ | ‐ |
| C: RHI | 64b/62 | ‐ | ‐ | ‐ | ‐ | |
| Provenzano 2001 | I: lispro | 12/12 | ‐ | ‐ | ‐ | ‐ |
| C: RHI | ‐ | ‐ | ‐ | ‐ | ||
| Raskin 2000c | I: aspart | 597/596 | ‐/‐ (4) | ‐ | ‐ | 2/596 (0)d |
| C: RHI | 287/286 | ‐/‐ (8) | ‐ | ‐ | 2/286 (1)d | |
| Recasens 2003 | I: lispro | 22/22 | 0/22 (0) | ‐ | ‐ | ‐ |
| C: RHI | 23/23 | 0/23 (0) | ‐ | ‐ | ‐ | |
| Z011 2007 | I: lispro | 81/81 | ‐ | ‐ | ‐ | Ketoacidosis: 0/81 (0) Other: 0/81 (0) |
| C: RHI | 86/86 | ‐ | ‐ | ‐ | Ketoacidosis: 2/86 (2) Other: 0/86 (0) | |
| Z013 2007 | I: lispro | 81/81 | ‐ | ‐ | ‐ | Ketoacidosis: 0/81 (0) Other: 0/81 (0) |
| C: RHI | 88/88 | ‐ | ‐ | ‐ | Ketoacidosis: 0/88 (0) Other: 2/88 (2) | |
| Z015 2007 | I: lispro | 50/50 | ‐ | ‐ | ‐ | Ketoacidosis: 1/50 (2) Other: 0/50 (0) |
| C: RHI | 48/48 | ‐ | ‐ | ‐ | Ketoacidosis: 0/48 (0) Other: 0/48 (0) | |
|
aTwo participants not exposed to treatment
bOne participant not exposed to treatment, one participant removed because of protocol violation
cAccording to original study report, 884 participants were randomised, but only 882 received treatment (IQWIG 2007)
dOnly ketoacidotic events "‐" denotes not reported C: comparator; I: intervention; RHI: regular human insulin | ||||||
Appendix 11. Survey of trial investigators providing information on included trials
| Trial | Date trial author contacted | Date trial author replied | Date trial author asked for additional information (short summary) | Date trial author provided data (short summary) |
| Ferguson 2001 | 24 January 2013 | No reply | N/A | N/A |
| Home 2000 | 19 March 2013 | 19 March 2013 | Not easy to access protocols and data. Author provided some information based on what he remembered | N/A |
| Iwamoto 2001 | 24 January 2013 | No reply | N/A | N/A |
| Provenzano 2001 | 5 March 2013 | No reply | N/A | N/A |
| Raskin 2000 | 11 February 2013 | No reply | N/A | N/A |
| Recasens 2003 | 11 February 2013 | No reply | N/A | N/A |
| Z011 2007 | 28 November 2012 | No reply | N/A | N/A |
| Z013 2007 | 28 November 2012 | No reply | N/A | N/A |
| Z015 2007 | 28 November 2012 | No reply | N/A | N/A |
| N/A: not applicable | ||||
Data and analyses
Comparison 1. Insulin analogues versus regular human insulin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe hypoglycaemic episodes including cross‐over trials, paired | 7 | Odds Ratio (Random, 95% CI) | 0.89 [0.71, 1.12] | |
| 2 Severe hypoglycaemic episodes without cross‐over trials | 6 | 2426 | Odds Ratio (IV, Random, 95% CI) | 0.88 [0.70, 1.12] |
| 3 Severe hypoglycaemic episodes including cross‐over trials, unpaired | 7 | 2492 | Odds Ratio (IV, Random, 95% CI) | 0.89 [0.71, 1.12] |
| 4 Severe hypoglycaemic episodes including cross‐over trials, paired | 7 | Odds Ratio (Random, 95% CI) | 0.89 [0.71, 1.12] | |
| 5 Severe hypoglycaemic episodes including cross‐over trials, paired, fixed‐effect model | 7 | Odds Ratio (Fixed, 95% CI) | 0.89 [0.71, 1.12] | |
| 6 Severe hypoglycaemic episodes including cross‐over trials, paired | 7 | Odds Ratio (Random, 95% CI) | 0.89 [0.71, 1.12] | |
| 6.1 Lispro | 5 | Odds Ratio (Random, 95% CI) | 1.00 [0.56, 1.80] | |
| 6.2 Aspart | 2 | Odds Ratio (Random, 95% CI) | 0.87 [0.68, 1.11] | |
| 7 HbA1c, random‐effects model | 9 | Mean Difference (Random, 95% CI) | ‐0.15 [‐0.21, ‐0.08] | |
| 8 HbA1c by different short‐acting insulin analogues (%) | 9 | Mean Difference (Random, 95% CI) | ‐0.15 [‐0.21, ‐0.08] | |
| 8.1 Lispro | 6 | Mean Difference (Random, 95% CI) | ‐0.20 [‐0.34, ‐0.05] | |
| 8.2 Aspart | 3 | Mean Difference (Random, 95% CI) | ‐0.14 [‐0.21, ‐0.06] | |
| 9 HbA1c by different types of study design | 9 | Mean Difference (Random, 95% CI) | ‐0.15 [‐0.21, ‐0.08] | |
| 9.1 Parallel studies | 7 | Mean Difference (Random, 95% CI) | ‐0.14 [‐0.21, ‐0.07] | |
| 9.2 Cross‐over studies | 2 | Mean Difference (Random, 95% CI) | ‐0.21 [‐0.38, ‐0.04] | |
| 10 HbA1c, random‐effects model, unpaired analysis | 9 | Mean Difference (Random, 95% CI) | ‐0.14 [‐0.21, ‐0.07] | |
| 11 HbA1c, fixed‐effect model | 9 | Mean Difference (Fixed, 95% CI) | ‐0.15 [‐0.21, ‐0.08] | |
| 12 Weight gain, random‐effects model (kg) | 6 | 2385 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.25, 0.04] |
| 13 Weight gain, fixed‐effect model (kg) | 6 | 2385 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 14 Weight gain, by different short‐acting insulin analogues (kg) | 6 | 2385 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.25, 0.04] |
| 14.1 Aspart | 2 | 1906 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.21, 0.01] |
| 14.2 Lispro | 4 | 479 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.77, 0.62] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ferguson 2001.
| Methods | Cross‐over randomised controlled clinical trial | |
| Participants |
Inclusion criteria: participants with type 1 diabetes for > 5 years; reported reduction in hypoglycaemia warnings symptoms for ≥ 2 years; experienced ≥ 2 episodes of severe hypoglycaemia in the 2 years preceding participation, HbA1c of less than double the local non‐diabetic reference range (HbA1c: 5.0‐6.6%); aged 19‐65 years Exclusion criteria: systemic, renal or hepatic disease; pregnant participants; active, proliferative retinopathy (untreated) Diagnostic criteria: WHOa |
|
| Interventions |
Number of study centres: 1 Treatment before study: either twice‐daily free‐mixed insulin or multiple injection regimen Titration period: 12‐months (2 treatment periods each lasting 24 weeks) Insulin lispro vs. RHI (see Appendix 2) |
|
| Outcomes |
Outcomes reported in abstract of publication: HbA1c, 8‐point blood glucose profile, frequency and severity of hypoglycaemic episodes and quality of life Primary outcome(s): frequency of severe hypoglycaemic episodesb Secondary outcome(s): ‐ Other outcome(s): treatment satisfaction (Diabetes Treatment Satisfaction Questionnaire), aspects of quality of life (Hypoglycaemia Fear Survey), hypoglycaemia (mild, severe nocturnal) |
|
| Study details |
Run‐in period: 4 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: commercial (Eli Lilly) Publication status: peer‐reviewed journal/full article |
|
| Stated aim for study | Quote from publication: "The aim of this study was to compare treatment with insulin lispro and regular human insulin in a cohort of patients with type 1 diabetes who had impaired awareness of hypoglycaemia and a history of frequent severe hypoglycaemia. The two insulins were compared with respect to the frequency of mild and severe hypoglycaemia, glycaemic control, and quality of life measures" | |
| Notes |
aAccording to the study protocol, but no year given (information obtained from IQWIG report) bThe quality of glycaemic control was described as a primary outcome in Ferguson 2001, but not in the original study report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomised to receive treatment either with insulin lispro and human NPH [neutral protamine Hagedorn] insulin, or alternatively with regular human insulin and NPH insulin" Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: study diaries were validated against data stored in participants' blood glucose meters |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not described, but considered unlikely |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: participants dropped out before treatment or were excluded for data validity reasons ‐ no drop‐outs from ITT population. Unclear whether there were missing values and how they were addressed |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: participants dropped out before treatment or were excluded for data validity reasons ‐ no drop‐outs from ITT population. Unclear whether there were missing values and how they were addressed |
| Selective reporting (reporting bias) | High risk | Comment: primary outcomes were differently defined in publication and study report; incomplete reporting on health‐related quality of life results, baseline data only provided for overall participant population |
| Other bias | Unclear risk | Comment: not enough information to judge |
Home 2000.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 2:1 |
|
| Participants |
Inclusion criteria: adults, type 1 diabetes, diabetes duration ≥ 2 years, treated with insulin for 1 year, BMI < 35.0 kg/m2, HbA1c ≤ 11.0% Exclusion criteria: active proliferative retinopathy, nephropathy (serum creatinine > 150 mmol/L), recurrent severe hypoglycaemia, significant cardiovascular disease, systemic corticosteroid treatment, requiring > 1.4 U/(kg*day) insulin, pregnant, drug abuse Diagnostic criteria: type 1 diabetes according to WHO 1994 |
|
| Interventions |
Number of study centres: 88 Treatment before study: insulin for 1 year Titration period: 6 months Insulin aspart vs. RHI (see Appendix 2) |
|
| Outcomes |
Outcomes reported in abstract of publication: HbA1c, 8‐point blood glucose profiles, insulin dose, quality of life, hypoglycaemia, adverse events, treatment satisfaction Primary outcome(s): HbA1c Secondary outcome(s): ‐ Other outcome(s): hypoglycaemia (mild, severe, severe nocturnal), adverse events, quality of lifea, treatment satisfactionb |
|
| Study details |
Run‐in period: 4 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: commercial (Novo Nordisk) Publication status: peer‐reviewed journal/full article |
|
| Stated aim for study | Quote from publication: "To compare the efficacy of insulin aspart, a rapid‐acting insulin analogue, with that of unmodified human insulin on long‐term blood glucose control in Type 1 diabetes mellitus" | |
| Notes |
aResults only reported for the German subpopulations (published in Bott 2003) bOnly for participants in the UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: "open‐labelled" Comment: no blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "open‐labelled" Comment: no blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Quote: "Safety haematology and biochemistry tests, drugs‐of‐abuse screen, HbA1c and serum lipids were measured using standard laboratory techniques at a central laboratory" Comment: HbA1c and other biochemical analyses performed at central laboratory ‐ likely to be blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not described, but considered unlikely |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: not described |
| Selective reporting (reporting bias) | Unclear risk | Comment: health‐related quality of life only mentioned in abstract |
| Other bias | High risk | Comment: discrepancies between tables and text for hypoglycaemia and HbA1c |
Iwamoto 2001.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 2:1 |
|
| Participants |
Inclusion criteria: diabetes duration > 2 years, insulin treatment > 1 year, capable of coping with hypoglycaemia, 1‐3 times insulin injection, blood glucose ‐ self monitoring, HbA1c < 11%, BMI < 30, aged > 12 years Exclusion criteria: ‐ Diagnostic criteria: ‐ |
|
| Interventions |
Number of study centres: multicentre (number of centres not reported) Treatment before study: insulin treatment > 1 year Titration period: 168 days (24 weeks) Insulin aspart vs. RHI (see Appendix 2) |
|
| Outcomes |
Outcomes reported in abstract of publication: change in HbA1c, blood glucose 90 minutes after breakfast, adverse events, insulin antibodies Primary outcome(s): Secondary outcome(s): Other outcome(s): |
|
| Study details |
Run‐in period: 6 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: Japanese Funding: commercial (Novo Nordisk) Publication status: peer‐reviewed journal/full article |
|
| Stated aim for study | Quote from publication: "The efficacy and safety of insulin aspart, a rapid‐acting insulin, were investigated in type 1 diabetes patients treated in basal‐bolus regimen compared to soluble human insulin" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: unblinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: unblinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: HbA1c measured at a central laboratory |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not known |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: number of drop‐outs reported, but no details; analysis seems to be complete case |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: number of drop‐outs reported, but no details; analysis seems to be complete case |
| Selective reporting (reporting bias) | Unclear risk | Comment: not enough information to make judgement on selective reporting |
| Other bias | Unclear risk | Comment: not enough information on data analysis |
Provenzano 2001.
| Methods | Cross‐over randomised controlled clinical trial | |
| Participants |
Inclusion criteria: optimum compliance with diabetic diet and insulin therapy, human insulin therapy for ≥ 2 months prior to enrolment Exclusion criteria: total dose of insulin therapy > 2.0 U/kg, continuous s.c. insulin infusion pump, history of clinically significant hypoglycaemia unawareness Diagnostic criteria: ‐ |
|
| Interventions |
Number of study centres: ‐ Treatment before study: s.c. doses of human insulin for ≥ 2 months prior to their enrolment Titration period: 6 months (3 months on normal diet, 3 months on Mediterranean diet) Insulin lispro vs. RHI (see Appendix 2) |
|
| Outcomes |
Outcomes reported in abstract of publication: glycaemic control (HbA1c), incidence and frequency of hypoglycaemic episodes, adverse events, pre‐ and post‐prandial glycaemic and insulinaemic profiles Primary outcome(s): not defined Secondary outcome(s): not defined Other outcome(s): HbA1c, hypoglycaemia, adverse events, pre‐ and post‐prandial glycaemic and insulinaemic profiles |
|
| Study details |
Run‐in period: 4 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: ‐ Publication status: full article in a peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The aim of this randomised, cross‐over study was to evaluate whether LP [lispro] insulin is appropriate in insulin‐treated diabetic patients on a MD [Mediterranean diet] in regard to glycaemic control and patients´ quality of life" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into two groups of 6 patients (groups A and B). Each group´s treatment was determined using a computer‐generated randomisation table which created two treatment sequences" Comment: considered adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: not described |
| Selective reporting (reporting bias) | Unclear risk | Comment: it was stated as an aim of the study to investigate the participants' health‐related quality of life. However, no results were reported |
| Other bias | High risk | Comment: no power analysis, no clearly defined primary endpoint, reporting of results on hypoglycaemia inconsistent |
Raskin 2000.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 2:1 |
|
| Participants |
Inclusion criteria: aged 18‐75 yearsa, type 1 diabetes for ≥ 18 monthsb, BMI ≤ 35.0 kg/m2, HbA1c ≤ 11% Exclusion criteria: impaired hepatic, renal, or cardiac function; recurrent major hypoglycaemia; active proliferative retinopathy; total daily insulin dose ≥ 1.4 IU/kg; women were excluded if they were pregnant, breastfeeding or not practicing contraception Diagnostic criteria: WHO 1994 |
|
| Interventions |
Number of study centres: 59 (in the US and Canada) Treatment before study: ≥ 1 year of therapy Titration period: 6 monthsc Insulin aspart vs. RHI (see Appendix 2) |
|
| Outcomes |
Outcomes reported in abstract of publication: 8‐point blood glucose profiles, HbA1c, adverse events, overall hypoglycaemic episodes Primary outcome(s): HbA1c Secondary outcome(s): not specified Other outcome(s): hypoglycaemia (mild, severe, nocturnald), adverse events |
|
| Study details |
Run‐in period: 4‐ to 5‐week run‐in period Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: commercial (Novo Nordisk) Publication status: full article in a peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "To compare long‐term glycaemic control and safety of using insulin aspart (IAsp) with that of regular human insulin (HI)" | |
| Notes | aAccording to study report ≥ 18 years bAccording to study report ≥ 24 months cParticipants were treated with insulin aspart or RHI for 6 months, but could continue their assigned treatment in a 6‐month extension of the study dNot defined as an outcome in the methods section of the study report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the run‐in period, subjects were randomised, in a 2:1 ratio, to receive either IAsp [insulin aspart] or HI [RHI] as their mealtime insulin" Comment: no details |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: HbA1c analysed in central laboratory, therefore likely to be blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Quote: "The last observation carried forward approach was used for missing data in most analyses" Comment: for blood glucose profiles values before and after all 3 meals were required for inclusion in the analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Quote: "The last observation carried forward approach was used for missing data in most analyses" Comment: for blood glucose profiles values before and after all 3 meals were required for inclusion in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: inconsistently reported data between different publications (also see IQWIG 2007), weight gain results reported after 12 months, but not after 6 months |
| Other bias | High risk | Comment: inconsistencies regarding the data in different publications (also see IQWIG 2007), number of drop‐outs reported, but reasons not clearly stated |
Recasens 2003.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: people with newly diagnosed type 1 diabetes Exclusion criteria: ‐ Diagnostic criteria: National Diabetes Data Group 1979 |
|
| Interventions |
Number of study centres: ‐ Treatment before study: ‐ Titration period: ‐ Insulin lispro vs. RHI (see Appendix 2) |
|
| Outcomes |
Outcomes reported in abstract of publication: HbA1c, proportion of participants with HbA1c < 6%, daily blood glucose profiles, mild hypoglycaemic episodes, β‐cell function Primary outcome(s): not defined Secondary outcome(s): not defined Other outcome(s): HbA1c, hypoglycaemia (mild, severe) |
|
| Study details |
Run‐in period: ‐ Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: ‐ Publication status: full article in a peer‐reviewed journal |
|
| Stated aim for study | Quote: "The aim of the study was to examine the effects of intensive insulin therapy using lispro on metabolic control, immunogenicity and β‐cell function of newly diagnosed type 1 diabetic subjects in comparison with intensive insulin therapy using regular insulin" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to intensive insulin therapy using insulin lispro or intensive insulin therapy using regular insulin" Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: "open‐label" Comment: not blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "open‐label" Comment: not blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Comment: reasons for drop‐outs not described, handling of missing values in analysis unclear |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Comment: reasons for drop‐outs not described, handling of missing values in analysis unclear |
| Selective reporting (reporting bias) | Unclear risk | Comment: not enough information to make judgement |
| Other bias | Unclear risk | Comment: no primary endpoint defined |
Z011 2007.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: IDDM, aged 12‐70 years, insulin therapy for ≥ 2 months before study entry, optimal compliance with diet and insulin therapy Exclusion criteria: any other severe disease, current use of insulin infusion devices, hypoglycaemia unawareness, > 2 hospital admissions due to hypoglycaemia in the previous year Diagnostic criteria: WHO 1980 |
|
| Interventions |
Number of study centres: multicentre (number of centres unknown) Treatment before study: human insulin therapy for ≥ 2 months before study Titration period: ‐ Insulin lispro vs. RHI (see Appendix 2) |
|
| Outcomes |
Outcomes reported in abstract of publication: 1 h and 2 h post‐prandial rise in serum glucose, HbA1c Primary outcome(s): uncleara Secondary outcome(s): not defined Other outcome(s): HbA1c, hypoglycaemia (mild, severe, nocturnal), adverse events |
|
| Study details |
Run‐in period: 2‐4 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: commercial (Eli Lilly) Publication status: full article in a peer‐reviewed journal (pooled analysis of Z011 2007 and Z013 2007 in Anderson 1997, pooled analysis of Z011 2007, Z013 2007, and Z015 2007 in Garg 1996) |
|
| Stated aim for study | Quote from publication: "We examined the safety and efficacy of insulin lispro in the premeal treatment of patients with diabetes mellitus"b | |
| Notes |
aStudy report described post‐prandial glucose values as primary endpoint, but used HbA1c, pre‐prandial blood sugar and hypoglycaemia for power analysis; study protocol mentioned several primary endpoints: post‐prandial blood sugar excursions, hypoglycaemia in relation to glycaemic control and metabolic control bFrom Anderson 1997 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: not described in Anderson 1997, but considered adequate in IQWIG 2007 based on information from original study reports |
| Allocation concealment (selection bias) | Low risk | Comment: not described in Anderson 1997, but considered adequate in IQWIG 2007 based on information from original study reports |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Quote: "Blood samples were taken at 3‐month intervals for the determination of glycated haemoglobin (HbA1c) levels and analysed by a central laboratory" Comment: laboratory parameters were likely to be blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Quote: "The treatment comparison was performed using the last measurement (end point) observed for each patient, thus including the patients not completing the study" Comment: considered adequate in IQWIG 2007 based on information from original study reports |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Quote: "The treatment comparison was performed using the last measurement (end point) observed for each patient, thus including the patients not completing the study" Comment: considered adequate in IQWIG 2007 based on information from original study reports |
| Selective reporting (reporting bias) | High risk | Comment: primary outcome unclear because of inconsistent information in publication, study report and study protocol |
| Other bias | Unclear risk | Comment: not enough information to judge |
Z013 2007.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: IDDM, aged 12‐70 years, insulin therapy for ≥ 2 months before study entry, optimal compliance with diet and insulin therapy Exclusion criteria: any other severe disease, current use of insulin infusion devices, hypoglycaemia unawareness, > 2 hospital admissions due to hypoglycaemia in the previous year Diagnostic criteria: WHO 1980 |
|
| Interventions |
Number of study centres: multicentre (number of centres not known) Treatment before study: human insulin therapy for ≥ 2 months before study Titration period: ‐ Insulin lispro vs. RHI (see Appendix 2) |
|
| Outcomes |
Outcomes reported in abstract of publication: 1 h and 2 h post‐prandial rise in serum glucose, HbA1c Primary outcome(s): uncleara Secondary outcome(s): not defined Other outcome(s): HbA1c, hypoglycaemia (mild, severe, nocturnal), adverse events |
|
| Study details |
Run‐in period: 2‐4 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: commercial (Eli Lilly) Publication status: full article in a peer‐reviewed journal (pooled analysis of Z011 2007 and Z013 2007 in Anderson 1997, pooled analysis of Z011 2007, Z013 2007, and Z015 2007 in Garg 1996) |
|
| Stated aim for study | Quote from publication: "We examined the safety and efficacy of insulin lispro in the premeal treatment of patients with diabetes mellitus"b | |
| Notes |
aStudy report described post‐prandial glucose values as primary endpoint, but used HbA1c, pre‐prandial blood sugar and hypoglycaemia for power analysis; study protocol mentioned several primary endpoints: post‐prandial blood sugar excursions, hypoglycaemia in relation to glycaemic control and metabolic control bFrom Anderson 1997 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: not described in Anderson 1997, but considered adequate in IQWIG 2007 based on information from original study reports |
| Allocation concealment (selection bias) | Low risk | Comment: not described in Anderson 1997, but considered adequate in IQWIG 2007 based on information from original study reports |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Quote: "Blood samples were taken at 3‐month intervals for the determination of glycated haemoglobin (HbA1c) levels and analyzed by a central laboratory" Comment: laboratory parameters were likely to be blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Quote: "The treatment comparison was performed using the last measurement (end point) observed for each patient, thus including the patients not completing the study" Comment: considered adequate in IQWIG 2007 based on information from original study reports |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Quote: "The treatment comparison was performed using the last measurement (end point) observed for each patient, thus including the patients not completing the study" Comment: considered adequate in IQWIG 2007 based on information from original study reports |
| Selective reporting (reporting bias) | High risk | Comment: primary outcome unclear because of inconsistent information in publication, study report and study protocol |
| Other bias | Unclear risk | Comment: not enough information to judge |
Z015 2007.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: IDDM, aged 12‐70 years, insulin therapy for ≥ 2 months before study entry Exclusion criteria: any other severe disease, current use of insulin infusion devices, hypoglycaemia unawareness, > 2 hospital admissions due to hypoglycaemia in the previous year Diagnostic criteria: WHO 1980 |
|
| Interventions |
Number of study centres: multicentre (number of centres not known) Treatment before study: human insulin therapy for ≥ 2 months before study Titration period: ‐ Insulin lispro vs. RHI (see Appendix 2) |
|
| Outcomes |
Outcomes reported in abstract of publication: no abstract available Primary outcome(s): uncleara Secondary outcome(s): not defined Other outcome(s): HbA1c, hypoglycaemia (mild, severe, nocturnal), adverse events, quality of life |
|
| Study details |
Run‐in period: 2‐4 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: commercial (Eli Lilly) Publication status: full article in a peer‐reviewed journal (pooled analysis of Z011 2007, Z013 2007, and Z015 2007 in Garg 1996) |
|
| Stated aim for study | Quote from publication: "The purpose of the present 1‐year prospective randomised clinical trial was to compare HumulinRb to the human insulin analogue lispro, with respect to postprandial glucose excursions, frequency of hypoglycaemic episodes, glucose control, body weight, body mass index (BMI), and safety in subjects with type 1 diabetes"c | |
| Notes | aStudy report described post‐prandial glucose values as primary endpoint, but used HbA1c, pre‐prandial blood sugar and hypoglycaemia for power analysis; study protocol mentioned several primary endpoints: post‐prandial blood sugar excursions, hypoglycaemia in relation to glycaemic control and metabolic control bRHI cFrom Garg 1996 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: considered adequate in IQWIG 2007 based on information from original study reports |
| Allocation concealment (selection bias) | Low risk | Comment: considered adequate in IQWIG 2007 based on information from original study reports |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote: "open‐label". Comment: no blinding |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "open‐label" Comment: no blinding |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: laboratory parameters assessed in central laboratory ‐ HbA1c assessment likely to be blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: considered adequate in IQWIG 2007 based on information from original study reports |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: considered adequate in IQWIG 2007 based on information from original study reports |
| Selective reporting (reporting bias) | High risk | Comment: primary outcome unclear because of inconsistent information in publication, study report and study protocol |
| Other bias | Unclear risk | Comment: not enough information to judge |
"‐" denotes not reported.
BMI: body mass index; h: hour; HbA1c: glycosylated haemoglobin A1c; IDDM: insulin‐dependent diabetes mellitus; ITT: intention‐to‐treat; IU: international unit; RHI: regular human insulin; s.c.: subcutaneous; U: unit; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altuntas 2003 | Not type 1 diabetes |
| Bastyr 2000 | Not type 1 diabetes |
| Bi 2007 | Follow‐up duration too short |
| Boehm 2004 | Not type 1 diabetes |
| Boivin 1999 | Not a comparison of short‐acting insulin analogue vs. RHI |
| Caixàs 1998 | Follow‐up duration too short |
| Chan 2004 | Follow‐up duration too short |
| Chen 2011 | Follow‐up duration too short |
| Chlup 2004 | Not an RCT |
| Cypryk 2004 | Not an RCT |
| Dailey 2004 | Not type 1 diabetes |
| Fineberg 1996 | Pooled data of 4 trials |
| Gao 2009 | Follow‐up duration too short |
| Garg 2000 | Not an RCT |
| Gram 2011 | Not a comparison of short‐acting insulin analogue vs. RHI |
| Herrmann 2013 | Not type 1 diabetes |
| Holleman 1997 | Follow‐up duration too short |
| Iwamoto 2002 | Not type 1 diabetes |
| Kaplan 2004 | Not a comparison of short‐acting insulin analogue vs. RHI |
| Lalli 1999 | Different additional anti‐hyperglycaemic medication in the study arms |
| Laube 1996 | Follow‐up duration too short |
| Lindholm 1999 | Follow‐up duration too short |
| Lindholm 2002 | Pooled data of 4 trials |
| Loukovaara 2003 | Not an RCT |
| Perriello 2005 | Not type 1 diabetes |
| Persson 2002 | Only pregnant women |
| Pfützner 2013 | Not type 1 diabetes |
| Pérez‐Maraver 2013 | Different additional anti‐hyperglycaemic medication in the study arms |
| Rami 1997 | Follow‐up duration too short |
| Rayman 2007 | Not type 1 diabetes |
| Roach 2001 | Different additional anti‐hyperglycaemic medication in the study arms |
| Ross 2001 | Not type 1 diabetes |
| Schernthaner 2004 | Not type 1 diabetes |
| Skrha 2002 | Follow‐up duration too short |
| Tubiana‐Rufi 1997 | Follow‐up duration too short |
| Vignati 1997 | Follow‐up duration too short |
| Yanagisawa 2013 | Not an RCT |
RCT: randomised controlled trial; RHI: regular human insulin.
Differences between protocol and review
The former Cochrane review "Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus" has been withdrawn and split into the following Cochrane reviews: 'Short‐acting insulin analogues versus regular human insulin for adults with type 1 diabetes mellitus' and 'Short‐acting insulin analogues versus regular human insulin for type 2 diabetes mellitus'.
We implemented several methodological improvements, such as the integration of a 'Summary of findings' table as demanded by the Cochrane Metabolic & Endocrine Disorders (CMED) Group, in this review update.
A major change from the original review was that we changed the minimum duration of intervention from four weeks in the former review to 24 weeks. Because we focused our review update on participant‐important outcome measures such as microvascular and macrovascular complications, a longer time period of interventions appeared meaningful. This also concurs with the requirement of the European Medicines Agency for confirmatory studies in the treatment of diabetes mellitus (EMA 2002).
Contributions of authors
Birgit Fullerton (BF) ‐ for the update of the review: literature screening, data extraction, data analysis, manuscript draft and review of manuscript.
Andrea Siebenhofer (AS) ‐ for the update of the review: protocol development, literature screening and review of manuscript.
Klaus Jeitler (KJ) ‐ for the update of the review: protocol development, searching for trials, literature screening and review of manuscript.
Karl Horvath (KH) ‐ for the update of the review: literature screening and review of manuscript.
Thomas Semlitsch (TS) ‐ for the update of the review: literature screening and review of manuscript.
Andrea Berghold (AB): protocol development, data analysis, development of final review, for the update of the review: data analysis and review of manuscript.
Johannes Plank (JP): searching for trials, quality assessment of trials, data extraction and development of final review.
Thomas R. Pieber (TRP): protocol development, quality assessment of trials and development of final review.
Ferdinand M. Gerlach (FMG): protocol development and development of final review.
Sources of support
Internal sources
Department of Internal Medicine, University Hospital Graz; Institute for Medical Informatics, Statistics and Documentation; University of Graz, Austria.
External sources
No sources of support supplied
Declarations of interest
BF: none known.
AS: was involved in the preparation of the report on short‐acting insulin analogues for the treatment of type 1 diabetes mellitus for the Institute for Quality and Efficiency in Health Care (www.iqwig.de).
KJ: was involved in the preparation of the report on short‐acting insulin analogues for the treatment of type 1 diabetes mellitus for the Institute for Quality and Efficiency in Health Care (www.iqwig.de).
KH: was involved in the preparation of the report on short‐acting insulin analogues for the treatment of type 1 diabetes mellitus for the Institute for Quality and Efficiency in Health Care (www.iqwig.de). KH has received payment for lectures, travel/accommodations/meeting expenses and consultancy from various sources (Novartis, Medtronic, Eli Lilly, Novo Nordisk, Sanofi Aventis, Merck Sharp & Dohme).
TS: none known.
AB: none known.
JP: none known.
TRP: the Medical University of Graz (employee of Thomas Pieber) has received unrestricted grants from AstraZenaca and Novo Nordisk.Thomas Pieber has received personal fees for advisory boards and steering committees for AstraZeneca, Eli Lilly and Novo Nordisk; and has received personal fees in speaker bureaus for AstraZeneca and Novo Nordisk.
FMG: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Ferguson 2001 {published data only}
- Ferguson SC, Strachan MWJ, Janes JM, Frier BM. Severe hypoglycaemia in patients with type 1 diabetes and impaired awareness of hypoglycaemia: a comparative study of insulin lispro and regular human insulin. Diabetes/Metabolism Research and Reviews 2001;17(4):285‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Short acting insulin analogues for the treatment of type 1 diabetes mellitus, 2007. www.iqwig.de/download/A05‐02_Abschlussbericht_Kurzwirksame_Insulinanaloga_bei_Diabetes_mellitus_Typ_1.pdf. Köln, (accessed 29 January 2016).
Home 2000 {published data only}
- Bott U, Ebrahim S, Hirschberger S, Skovlund SE. Effect of the rapid‐acting insulin analogue insulin aspart on quality of life and treatment satisfaction in patients with type 1 diabetes. Diabetic Medicine 2003;20:626‐34. [DOI] [PubMed] [Google Scholar]
- Home PD, Hallgren P, Usadel KH, Sane T, Faber J, Grill V, et al. Pre‐meal insulin aspart compared with pre‐meal soluble human insulin in type 1 diabetes. Diabetes Research and Clinical Practice 2006;71(2):131‐39. [DOI] [PubMed] [Google Scholar]
- Home PD, Lindholm A, Riis A. Insulin aspart vs. human insulin in the management of long‐term blood glucose control in type 1 diabetes mellitus: a randomized controlled trial. Diabetic Medicine 2000;17(11):762‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Short acting insulin analogues for the treatment of type 1 diabetes mellitus, 2007. www.iqwig.de/download/A05‐02_Abschlussbericht_Kurzwirksame_Insulinanaloga_bei_Diabetes_mellitus_Typ_1.pdf. Köln, (accessed 29 January 2016).
Iwamoto 2001 {published data only}
- Iwamoto Y, Akanuma Y, Niimi H, Sasaki N, Tajima N, Kawamori R, et al. Comparison between insulin aspart and soluble human insulin in type 1 diabetes (IDDM) patients treated with basal‐bolus insulin therapy ‐ Phase III clinical trial in Japan. Journal of the Japan Diabetes Society 2001;44(10):799‐811. [Google Scholar]
Provenzano 2001 {published data only}
- Provenzano C, Vero R, Oliva A, Leto G, Puccio L, Vecci E, et al. Lispro insulin in type 1 diabetic patients on a Mediterranean or normal diet: a randomized, cross‐over comparative study with regular insulin. Diabetes, Nutrition & Metabolism 2001;14(3):133‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Raskin 2000 {published data only}
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Short acting insulin analogues for the treatment of type 1 diabetes mellitus, 2007. www.iqwig.de/download/A05‐02_Abschlussbericht_Kurzwirksame_Insulinanaloga_bei_Diabetes_mellitus_Typ_1.pdf. Köln, (accessed 29 January 2016).
- Raskin P, Guthrie RA, Leiter L, Riis A, Jovanovic L. Use of insulin aspart, a fast‐acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care 2000;23(5):583‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Recasens 2003 {published data only}
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Short acting insulin analogues for the treatment of type 1 diabetes mellitus, 2007. www.iqwig.de/download/A05‐02_Abschlussbericht_Kurzwirksame_Insulinanaloga_bei_Diabetes_mellitus_Typ_1.pdf. Köln, accessed 29 January 2016.
- Recasens M, Aguilera E, Morinigo R, Casamitjana R, Nicoletti F, Gomis R, et al. Insulin lispro is as effective as regular insulin in optimising metabolic control and preserving β‐cell function at onset of type 1 diabetes mellitus. Diabetes Research and Clinical Practice 2003;60:153‐9. [DOI] [PubMed] [Google Scholar]
Z011 2007 {published data only}
- Anderson JH Jr, Brunelle RL, Koivisto VA, Trautmann ME, Vignati L, DiMarchi R. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Clinical Therapeutics 1997;19(1):62‐72. [DOI] [PubMed] [Google Scholar]
- Garg SK, Carmain JA, Braddy KC, Anderson JH, Vignati L, Jennings MK, et al. Pre‐meal insulin analogue insulin lispro vs humulin insulin treatment in young subjects with type 1 diabetes. Diabetic Medicine 1996;13(1):47‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Short acting insulin analogues for the treatment of type 1 diabetes mellitus, 2007. www.iqwig.de/download/A05‐02_Abschlussbericht_Kurzwirksame_Insulinanaloga_bei_Diabetes_mellitus_Typ_1.pdf. Köln, (accessed 29 January 2016).
Z013 2007 {published data only}
- Anderson JH Jr, Brunelle RL, Koivisto VA, Trautmann ME, Vignati L, DiMarchi R. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Clinical Therapeutics 1997;19(1):62‐72. [DOI] [PubMed] [Google Scholar]
- Garg SK, Carmain JA, Braddy KC, Anderson JH, Vignati L, Jennings MK, et al. Pre‐meal insulin analogue insulin lispro vs humulin insulin treatment in young subjects with type 1 diabetes. Diabetic Medicine 1996;13(1):47‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Short acting insulin analogues for the treatment of type 1 diabetes mellitus, 2007. www.iqwig.de/download/A05‐02_Abschlussbericht_Kurzwirksame_Insulinanaloga_bei_Diabetes_mellitus_Typ_1.pdf (accessed 29 January 2016).
Z015 2007 {published data only}
- Garg SK, Carmain JA, Braddy KC, Anderson JH, Vignati L, Jennings MK, et al. Pre‐meal insulin analogue insulin lispro vs Humulin R insulin treatment in young subjects with type 1 diabetes. Diabetic Medicine 1996;13(1):47‐52. [DOI] [PubMed] [Google Scholar]
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Short acting insulin analogues for the treatment of type 1 diabetes mellitus, 2007. www.iqwig.de/download/A05‐02_Abschlussbericht_Kurzwirksame_Insulinanaloga_bei_Diabetes_mellitus_Typ_1.pdf. Köln, (accessed 29 January 2016).
References to studies excluded from this review
Altuntas 2003 {published data only}
- Altuntas Y, Ozen B, Ozturk B, Sengul A, Ucak S, Ersoy O, et al. Comparison of additional metformin or NPH insulin to mealtime insulin lispro therapy with mealtime human insulin therapy in secondary OAD failure. Diabetes, Obesity & Metabolism 2003;5(6):371‐8. [DOI] [PubMed] [Google Scholar]
Bastyr 2000 {published data only}
- Bastyr III EJ, Huang Y, Brunelle RL, Vignati L, Cox DJ, Kotsanos JG. Factors associated with nocturnal hypoglycaemia among patients with type 2 diabetes new to insulin therapy: experience with insulin lispro. Diabetes Obesity and Metabolism 2000;2(1):39‐46. [DOI] [PubMed] [Google Scholar]
Bi 2007 {published data only}
- Bi YF, Zhao LB, Li XY, Wang WQ, Sun SY, Chen YH, et al. A 2‐way cross‐over, open‐labeled trial to compare efficacy and safety of insulin aspart and Novolin R delivered with CSII in 21 Chinese diabetic patients. Chinese Medical Journal 2007;120(19):1700‐3. [PubMed] [Google Scholar]
Boehm 2004 {published data only}
- Boehm BO, Vaz JA, Brøndsted L, Home PD. Long‐term efficacy and safety of biphasic insulin aspart in patients with type 2 diabetes. European Journal of Internal Medicine 2004;15(8):496‐502. [DOI] [PubMed] [Google Scholar]
Boivin 1999 {published data only}
- Boivin S, Belicar P, Melki V. Assessment of in vivo stability of a new insulin preparation for implantable insulin pumps. A randomized multicenter prospective trial. Diabetes Care 1999;22(12):2089‐90. [DOI] [PubMed] [Google Scholar]
Caixàs 1998 {published data only}
- Caixàs A, Pérez A, Payés A, Otal C, Carreras G, Ordóñez‐Llanos J, et al. Effects of a short‐acting insulin analog (Insulin Lispro) versus regular insulin on lipid metabolism in insulin‐dependent diabetes mellitus. Metabolism: Clinical & Experimental 1998;47(4):371‐6. [DOI] [PubMed] [Google Scholar]
Chan 2004 {published data only}
- Chan WB, Chow CC, Yeung VT, Chan JC, So WY, Cockram CS. Effect of insulin lispro on glycaemic control in Chinese diabetic patients receiving twice‐daily regimens of insulin. Chinese Medical Journal 2004;117(9):1404‐7. [PubMed] [Google Scholar]
Chen 2011 {published data only}
- Chen SF, Li H. Comparison on the efficacy of biphasic insulin aspart 30 and premixed human insulin 30/70 through continuous glucose monitoring system [Chinese]. Chung‐Hua Liu Hsing Ping Hsueh Tsa Chih Chinese Journal of Epidemiology 2011;32(8):827‐9. [PubMed] [Google Scholar]
Chlup 2004 {published data only}
- Chlup R, Zapletalová J, Seckar P, Chlupová L, Táncosová S, Reznícková M. Benefits of insulin aspart vs phosphate‐buffered human regular insulin in persons with type 1 Diabetes treated by means of an insulin pump. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc, Czech Republic 2004;148(1):27‐32. [PubMed] [Google Scholar]
Cypryk 2004 {published data only}
- Cypryk K, Sobczak M, Petynska‐Marczewska M, Zawodniak‐Szalapska M, Szymczak W, Wilczynski J, et al. Pregnancy complications and perinatal outcome in diabetic women treated with Humalog (insulin lispro) or regular human insulin during pregnancy. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 2004;10(2):29‐32. [PubMed] [Google Scholar]
Dailey 2004 {published data only}
- Dailey G, Rosenstock J, Moses RG, Ways K. Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care 2004;27(10):2363‐8. [DOI] [PubMed] [Google Scholar]
Fineberg 1996 {published data only}
- Fineberg NS, Fineberg SE, Anderson JH, Birkett MA, Gibson RG, Hufferd S. Immunologic effects of insulin lispro [Lys (B28), Pro (B29) human insulin] in IDDM and NIDDM patients previously treated with insulin. Diabetes 1996;45(12):1750‐4. [DOI] [PubMed] [Google Scholar]
Gao 2009 {published data only}
- Gao Y, Pan CY, Zou DJ, Xu ZR, Liu XM, Guo XH. Postprandial glycemic control using insulin aspart with NPH in inadequately controlled diabetics [Chinese]. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2009;89(28):1960‐3. [PubMed] [Google Scholar]
Garg 2000 {published data only}
- Garg SK, Anderson JH, Gerard LA, Mackenzie TA, Gottlieb PA, Jennings MK, et al. Impact of insulin lispro on HbA1c values in insulin pump users. Diabetes Obesity and Metabolism 2000;2(5):307‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gram 2011 {published data only}
- Gram J, Henriksen JE, Grodum E, Juhl H, Hansen TB, Christiansen C, et al. Pharmacological treatment of the pathogenetic defects in type 2 diabetes: the randomized multicenter South Danish Diabetes Study. Diabetes Care 2011;34(1):27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrmann 2013 {published data only}
- Herrmann BL, Kasser C, Keuthage W, Huptas M, Dette H, Klute A. Comparison of insulin aspart vs. regular human insulin with or without insulin detemir concerning adipozytokines and metabolic effects in patients with type 2 diabetes mellitus. Experimental & Clinical Endocrinology & Diabetes 2013;121(4):210‐3. [DOI] [PubMed] [Google Scholar]
Holleman 1997 {published data only}
- Holleman F, Schmitt H, Symanowski S, Rees A, Rottiers R, Anderson J. Pre‐meal therapy with lispro insulin and regular insulin in IDDM patients. The Netherlands Journal of Medicine 1997;50(5):A20‐1. [Google Scholar]
Iwamoto 2002 {published data only}
- Iwamoto Y. An open‐labelled, randomised, parallel group, multicentre, safety and efficacy study of NN‐X14Mix50 (BIAsp50) in a twice daily regimen in type 2 diabetic subjects, 2002. novonordisk‐trials.com/website/pdf/registry/bin_20080410‐024437‐672.pdf (last accessed 25 June 2015).
Kaplan 2004 {published data only}
- Kaplan W, Rodriguez LM, Smith OE, Haymond MW, Heptulla RA. Effects of mixing glargine and short‐acting insulin analogs on glucose control. Diabetes Care 2004;27(11):2739‐40. [DOI] [PubMed] [Google Scholar]
Lalli 1999 {published data only}
- Lalli C, Ciofetta M, Sindaco P, Torlone E, Pampanelli S, Compagnucci P, et al. Long‐term intensive treatment of type 1 diabetes with the short‐acting insulin analog lispro in variable combination with NPH insulin at mealtime. Diabetes Care 1999;22(3):468‐77. [DOI] [PubMed] [Google Scholar]
Laube 1996 {published data only}
- Laube H, Heller M, Liersch J, Mäser E, Linn Th. Experience with lispro‐insulin in the intensified therapy of IDDM and NIDDM patients. Diabetes und Stoffwechsel 1996;5(6):273‐6. [Google Scholar]
Lindholm 1999 {published data only}
- Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart. A randomized double‐blind cross‐over trial in type 1 diabetes. Diabetes Care 1999;22(5):801‐5. [DOI] [PubMed] [Google Scholar]
Lindholm 2002 {published data only}
- Lindholm A, Jensen LB, Home PD, Raskin P, Boehm BO, Rastam J. Immune responses to insulin aspart and biphasic insulin aspart in people with type 1 and type 2 diabetes. Diabetes Care 2002;25(5):876‐82. [DOI] [PubMed] [Google Scholar]
Loukovaara 2003 {published data only}
- Loukovaara S, Immonen I, Teramo KA, Kaaja R. Progression of retinopathy during pregnancy in type 1 diabetic women treated with insulin lispro. Diabetes Care 2003;26(4):1193‐8. [DOI] [PubMed] [Google Scholar]
Pérez‐Maraver 2013 {published data only}
- Pérez‐Maraver M, Caballero‐Corchuelo J, Boltana A, Insa R, Soler J, Montanya E. Comparison of human insulin and insulin analogues on hypoglycaemia and metabolic variability in type 1 diabetes using standardized measurements (HYPO score and Lability Index). Acta Diabetologica 2013;50(4):529‐35. [DOI] [PubMed] [Google Scholar]
Perriello 2005 {published data only}
- Perriello G, Pampanelli S, Porcellati F, Avogaro A, Bosi E, Petrella G, et al. Insulin aspart improves meal time glycaemic control in patients with type 2 diabetes: a randomized, stratified, double‐blind and cross‐over trial. Diabetic Medicine 2005;22(5):606‐11. [DOI] [PubMed] [Google Scholar]
Persson 2002 {published data only}
- Persson B, Swahn ML, Hjertberg R, Hanson U, Nord E, Nordlander E, et al. Insulin lispro therapy in pregnancies complicated by type 1 diabetes mellitus. Diabetes Research & Clinical Practice 2002;58(2):115‐21. [DOI] [PubMed] [Google Scholar]
Pfützner 2013 {published data only}
- Pfützner A, Forst T, Mitri M, Löffler A, Heise J, Forkel C, et al. Impact of short‐acting insulin analogs on biomarkers of oxidative stress and chronic systemic inflammation in patients with type 2 diabetes: results from a pilot study. Diabetes. 2013; Vol. 62 Suppl:A235‐6.
Rami 1997 {published data only}
- Rami B, Schober E. Postprandial glycaemia after regular and lispro insulin in children and adolescents with diabetes. European Journal of Pediatrics 1997;156(11):838‐40. [DOI] [PubMed] [Google Scholar]
Rayman 2007 {published data only}
- Rayman G, Profozic V, Middle M. Insulin glulisine imparts effective glycaemic control in patients with type 2 diabetes. Diabetes Research & Clinical Practice 2007;76(2):304‐12. [DOI] [PubMed] [Google Scholar]
Roach 2001 {published data only}
- Roach P, Strack T, Arora V, Zhao Z. Improved glycemic control with the use of self‐prepared mixtures of insulin lispro and insulin lispro protamine suspension in patients with types 1 and 2 diabetes. International Journal of Clinical Practice 2001;55(3):177‐82. [PubMed] [Google Scholar]
Ross 2001 {published data only}
- Ross SA, Zinman B, Campos RV, Strack T. A comparative study of insulin lispro and human regular insulin in patients with type 2 diabetes mellitus and secondary failure of oral hypoglycemic agents. Clinical & Investigative Medicine ‐ Medecine Clinique et Experimentale 2001;24(6):292‐8. [PubMed] [Google Scholar]
Schernthaner 2004 {published data only}
- Schernthaner G, Kopp HP, Ristic S, Muzyka B, Peter L, Mitteregger G. Metabolic control in patients with type 2 diabetes using humalog mix 50 injected three times daily: crossover comparison with human insulin 30/70. Hormone and Metabolic Research 2004;36(3):188‐93. [DOI] [PubMed] [Google Scholar]
Skrha 2002 {published data only}
- Skrha J, Smahelova A, Andĕl M, Vrtovec M, Subić J, Kreze A, et al. Insulin lispro improves postprandial glucose control in patients with diabetes mellitus. Sbornik Lekarsky 2002;103(1):15‐21. [PubMed] [Google Scholar]
Tubiana‐Rufi 1997 {published data only}
- Tubiana‐Rufi N, Munz‐Licha G. Lispro analog and quality of life. Diabetes & Metabolism 1997;3:58‐62. [PubMed] [Google Scholar]
Vignati 1997 {published data only}
- Vignati L, Anderson JH Jr, Iversen PW. Efficacy of insulin lispro in combination with NPH human insulin twice per day in patients with insulin‐dependent or non‐insulin dependent diabetes. Clinical Therapeutics 1997;19(6):1408‐21. [DOI] [PubMed] [Google Scholar]
Yanagisawa 2013 {published data only}
- Yanagisawa K, Yamagishi S, Ashihara J, Obara S, Wada N. Evaluation of efficacy, safety and QoL of glulisine on Japanese type 1 diabetes. Diabetes 2013;62 Suppl:A654. [Google Scholar]
Additional references
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22(Suppl 1):S5‐19. [DOI] [PubMed] [Google Scholar]
ADA 2008
- American Diabetes Association. Standards of medical care in diabetes ‐ 2008. Diabetes Care 2008;31(Suppl 1):S12‐54. [PUBMED: 18165335] [DOI] [PubMed] [Google Scholar]
Anderson 1997
- Anderson JH Jr, Brunelle RL, Koivisto VA, Trautmann ME, Vignati L, DiMarchi R. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Clinical Therapeutics 1997;19(1):62‐72. [DOI] [PubMed] [Google Scholar]
Annuzzi 2001
- Annuzzi G, Prato S, Arcari R, Bellomo Damato A, Benzi L, Bruttomesso D, et al. Preprandial combination of lispro and NPH insulin improves overall blood glucose control in type 1 diabetic patients: a multicenter randomized crossover trial. Nutrition, Metabolism, and Cardiovascular Diseases 2001;11:168‐75. [PubMed] [Google Scholar]
Ashwell 2006
- Ashwell SG, Amiel SA, Bilous RW, Dashora U, Heller SR, Hepburn DA, et al. Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross‐over trial in people with type 1 diabetes. Diabetic Medicine 2006;23:285‐92. [DOI] [PubMed] [Google Scholar]
Banerjee 2007
- Banerjee S, Tran K, Li H, Cimon K, Daneman D, Simpson S, et al. Short‐Acting Insulin Analogues for Diabetes Mellitus: Meta‐analysis of Clinical Outcomes and Assessment of Cost Effectiveness (Technology Report No. 87). Ottawa: Canadian Agency for Drugs and Technologies in Health, 2007. [Google Scholar]
Becker 1993
- Becker MP, Balagtas CC. Marginal modeling of binary cross‐over data. Biometrics 1993;49:997‐1009. [PubMed] [Google Scholar]
Berger 2001
- Berger M, Jörgens V. Insulintherapie in der Praxis. Berlin, Heidelberg: Springer Verlag, 2001. [Google Scholar]
Bode 2002
- Bode B, Weinstein R, Bell D, McGill J, Nadeau D, Raskin P, et al. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion. Diabetes Care 2002;25(3):439‐44. [DOI] [PubMed] [Google Scholar]
Bott 2003
- Bott U, Ebrahim S, Hirschberger S, Skovlund SE. Effect of the rapid‐acting insulin analogue insulin aspart on quality of life and treatment satisfaction in patients with type 1 diabetes. Diabetic Medicine 2003;20:626‐34. [DOI] [PubMed] [Google Scholar]
Bradley 1990
- Bradley C, Lewis KS. Measures of psychological well‐being and treatment satisfaction developed from the responses of people with tablet‐treated diabetes. Diabetic Medicine 1990;7(5):445‐51. [DOI] [PubMed] [Google Scholar]
Brunelle 1998
- Brunelle RL, Llewelyn J, Anderson JH Jr, Gale EAM, Koivisto VA. Meta‐analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care 1998;21(10):1726‐31. [DOI] [PubMed] [Google Scholar]
Brunner 2000
- Brunner GA, Hirschberger S, Sendlhofer G, Wutte A, Ellmerer M, Balent B, et al. Post‐prandial administration of the insulin analogue insulin aspart in patients with type 1 diabetes mellitus. Diabetic Medicine 2000;17(5):371‐5. [DOI] [PubMed] [Google Scholar]
Cameron 2009
- Cameron CG, Bennett HA. Cost‐effectiveness of insulin analogues for diabetes mellitus. CMAJ 2009;180(4):400‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2011
- Cohen 2011. The prickly problem of access to insulin. BMJ 2011;343:d5782. [DOI] [PubMed] [Google Scholar]
Colquitt 2003
- Colquitt J, Royle P, Waugh N. Are analogue insulins better than soluble in continuous subcutaneous insulin infusion? Results of a meta‐analysis. Diabetic Medicine 2003;20:863‐6. [DOI] [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Altman DG, Elbourne DR. Meta‐analysis combining parallel and cross‐over clinical trials II: binary outcomes. Statistics in Medicine 2002;21:2145‐59. [DOI] [PubMed] [Google Scholar]
Davidson 2014
- Davidson MB. Insulin analogs ‐ is there a compelling case to use them? No!. Diabetes Care 2014;37(6):1771‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
DCCT 1993
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. New England Journal of Medicine 1993;329:977‐86. [DOI] [PubMed] [Google Scholar]
DeWitt 2003
- DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA 2003;289(17):2254‐64. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analysis involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
EMA 2002
- European Medicines Agency. Note for guidance on clinical investigation of medicinal products in the treatment of diabetes mellitus, 2002. www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003262.pdf (accessed 14 April 2016).
Engwerda 2011
- Engwerda EE, Abbink EJ, Tack C J, Galan BE. Improved pharmacokinetic and pharmacodynamic profile of rapid‐acting insulin using needle‐free jet injection technology. Diabetes Care 2011;34(8):1804‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frick 2008
- Frick M, Knollmeyer J, Riederer H, Heinemann C. Modern insulins ‐ comment on facts and assumptions in a recent Editorial. Diabetologia 2008;51(4):689‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gale 2011
- Gale, EAM. Commentary: politics of affordable insulin. BMJ 2011;343:d5675. [DOI] [PubMed] [Google Scholar]
Garg 1996
- Garg SK, Carmain JA, Braddy KC, Anderson JH, Vignati L, Jennings MK, et al. Pre‐meal insulin analogue insulin lispro vs humulin insulin treatment in young subjects with type 1 diabetes. Diabetic Medicine 1996;13(1):47‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garg 2010
- Garg S, Ampudia‐Blasco FJ, Pfohl M. Rapid‐acting insulin analogues in basal‐bolus regimens in type 1 diabetes mellitus. Endocrine Practice 2010;16(3):486‐505. [DOI] [PubMed] [Google Scholar]
Gerstein 2001
- Gerstein HC, Haynes RB. Evidence‐Based Diabetes Care. Hamilton: BC Decker Inc, 2001. [Google Scholar]
Gough 2007
- Gough SC. A review of human and analogue insulin trials. Diabetes Research and Clinical Practice 2007;77(11):1‐15. [DOI] [PubMed] [Google Scholar]
Grant 1993
- Grant MB, Mames RN, Fitzgerald C, Ellis EA, Aboufrikha M, Guy J. Insulin‐like growth factor I acts as an angiogenic agent in rabbit cornea and retina: comparative studies with basic fibroblast growth factor. Diabetologia 1993;36:282‐91. [DOI] [PubMed] [Google Scholar]
Grunberger 2014
- Grunberger G. Insulin analogs ‐ are they worth it? Yes!. Diabetes Care 2014;37(6):1767‐70. [DOI] [PubMed] [Google Scholar]
Haffner 1998
- Haffner SM. The importance of hyperglycemia in the nonfasting state to the development of cardiovascular disease. Endocrine Reviews 1998;19(5):583‐92. [DOI] [PubMed] [Google Scholar]
Heinemann 1996
- Heinemann L, Kapitza C, Starke AAR, Heise T. Time‐action profile of the insulin analogue B28Asp. Diabetic Medicine 1996;13:683‐4. [DOI] [PubMed] [Google Scholar]
Heise 2014
- Heise T, Haahr H, Jensen L, Erichsen L, Hompesch M. Faster‐acting insulin aspart improves postprandial glycaemia versus insulin aspart in patients with type 1 diabetes mellitus. Diabetes 2014;63(Suppl 1):A34. [Google Scholar]
Heller 2013
- Heller S, Bode B, Kozlovski P, Svendsen AL. Meta‐analysis of insulin aspart versus regular human insulin used in a basal‐bolus regimen for the treatment of diabetes mellitus. Journal of Diabetes 2013;5:482‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hermansen 2004
- Hermansen K, Fontaine P, Kukolja KK, Peterkova V, Leth G, Gall MA. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal‐bolus therapy for patients with type 1 diabetes. Diabetologia 2004;47(4):622‐29. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holden 2011
- Holden SE, Poole CD, Morgan CL, Currie CJ. Evaluation of the incremental cost to the National Health Service of prescribing analogue insulin. BMJ Open 2011;1(2):e000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holleman 1997b
- Holleman F, Schmitt H, Rottiers R, Rees A, Symanowski S, Anderson JH. Reduced frequency of severe hypoglycemia and coma in well‐controlled IDDM patients treated with insulin lispro. Diabetes Care 1997;20(12):1827‐32. [DOI] [PubMed] [Google Scholar]
Holleman 2007a
- Holleman F, Gale EAM. Nice insulins, pity about the evidence. Diabetologia 2007;50:1783‐90. [DOI] [PubMed] [Google Scholar]
Home 2012
- Home, PD. The pharmacokinetics and pharmacodynamics of rapid‐acting insulin analogues and their clinical consequences. Diabetes, Obesity and Metabolism 2012;14(9):780‐88. [DOI] [PubMed] [Google Scholar]
Howey 1994
- Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28),Pro(B29)]‐human insulin A rapidly absorbed analogue of human insulin. Diabetes 1994;43:396‐402. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
IQWIG 2007
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Short acting insulin analogues for the treatment of type 1 diabetes mellitus, 2007. www.iqwig.de/download/A05‐02_Abschlussbericht_Kurzwirksame_Insulinanaloga_bei_Diabetes_mellitus_Typ_1.pdf. Köln, (accessed 29 January 2016).
Johansson 2000
- Johansson UB, Adamson UCK, Lins PES, Wredling RAM. Improved blood glucose variability, HbA1c Insuman Infusat and less insulin requirement in IDDM patients using insulin lispro in CSII. The Swedish multicenter lispro insulin study. Diabetes Metabolism 2000;26(3):192‐6. [PubMed] [Google Scholar]
Johnson 2013
- Johnson SR, Cooper MN, Jones TW, Davis EA. Long‐term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population‐based case‐control study. Diabetologia 2013;56(11):2392‐400. [DOI] [PubMed] [Google Scholar]
Jørgensen 1992
- Jørgensen LN, Didriksen LH, Drejer K. Carciogenic effect of the human insulin analogue B10 Asp in female rats. Diabetologia 1992;35 Suppl 1:A3. [Google Scholar]
Kaye 2013
- Kaye J, Krasner A, Canney L, Pichotta P, Simms P, Krishnarajah J, et al. Novel formulations BIOD‐238 and BIOD‐250 result in more rapid absorption and declines from peak than Humalog. Proceeding of the 49th Annual Meeting of the European Association for the Study of Diabetes; 2013 Sept 23‐27; Barcelona. Barcelona, Spain: EASD, 2013:56 S413.
King 1985
- King GL, Goodman AD, Buzney S, Moses A, Kahn CR. Receptors and growth‐promoting effects of insulin and insulin‐like growth factors on cells from bovine retinal capillaries and aorta. Journal of Clinical Investigation 1985;75:1028‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Krasner 2012
- Krasner A, Pohl R, Simms P, Pichotta P, Hauser R, Souza E. A review of a family of ultra‐rapid‐acting insulins: formulation development. Journal of Diabetes Science and Technology 2012;6(4):786‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurtzhals 2000
- Kurtzhals P, Schäffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, et al. Correlation of receptor binding and metabolic and metogenic potencies of insulin analogues designed for clinical use. Diabetes 2000;49:999‐1005. [DOI] [PubMed] [Google Scholar]
Landau 2014
- Landau Z, Klonoff D, Nayberg I, Feldman D, Levit SB, Lender D, et al. Improved pharmacokinetic and pharmacodynamic profiles of insulin analogues using InsuPatch, a local heating device. Diabetes/Metabolism Research and Reviews 2014;30(8):686‐92. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 1999
- Marshall SM, Home PD, Rizza RA. The Diabetes Annual 12. Amsterdam: Elsevier Science Ltd, 1999. [Google Scholar]
Mosekilde 1989
- Mosekilde E, Skovbo JK, Binder C, Pramming S, Thorsteinsson B. Modeling absorption kinetics of subcutaneous injected soluble insulin. Journal of Pharmacokinetics and Biopharmaceutics 1989;17(1):67‐87. [DOI] [PubMed] [Google Scholar]
Muehlhauser 1998
- Muehlhauser I, Overmann H, Bender R, Bott U, Berger M. Risk factors of severe hypoglycaemia in adult patients with type 1 diabetes ‐ a prospective population based study. Diabetologia 1998;41:1274‐82. [DOI] [PubMed] [Google Scholar]
Müller 2013
- Müller N, Frank T, Kloos C, Lehmann T, Wolf G, Müller UA. Randomized crossover study to examine the necessity of an injection‐to‐meal interval in patients with type 2 diabetes and human insulin. Diabetes Care 2013;36:1865‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
National Diabetes Data Group 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039‐57. [DOI] [PubMed] [Google Scholar]
Overmann 1999
- Overmann H, Heinemann L. Injection‐meal interval: recommendations of diabetologists and how patients handle it. Diabetes Research and Clinical Practice 1999;43:137‐42. [DOI] [PubMed] [Google Scholar]
Pedersen‐Bjergaard 2014
- Pedersen‐Bjergaard U, Kristensen PL, Beck‐Nielsen H, Norgaard K, Perrild H, Christiansen JS, et al. Effect of insulin analogues on risk of severe hypoglycaemia in patients with type 1 diabetes prone to recurrent severe hypoglycaemia (HypoAna trial): a prospective, randomised, open‐label, blinded‐endpoint crossover trial. The Lancet Diabetes & Endocrinology 2014;2(7):S84. [DOI] [PubMed] [Google Scholar]
Pfützner 2014
- Pfützner A, Dissel S, Forkel C, Grenningloh M, Bitton G, Nagar R, et al. Standardized modulation of the injection site allows for insulin dose reduction without deterioration of metabolic control. Current Medical Research & Opinion 2014;30(10):2001‐8. [DOI] [PubMed] [Google Scholar]
Pozzilli 2015
- Pozzilli P, Battelino T, Danne T, Hovorka R, Jarosz‐Chobot P, Renard E. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes/Metabolism Research and Reviews 2015;32(1):21‐39. [DOI: 10.1002/dmrr.2653] [DOI] [PMC free article] [PubMed] [Google Scholar]
Renner 1999
- Renner R, Pfützner A, Trautmann ME, Harzer O, Sauter K, Landgraf R. Use of insulin lispro in continuous subcutaneous insulin infusion treatment. Diabetes Care 1999;22(5):784‐8. [DOI] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Russell‐Jones 2007
- Russell‐Jones D, Khan R. Insulin‐associated weight gain in diabetes ‐ causes, effects and coping strategies. Diabetes, Obesity and Metabolism 2007;9(6):799‐812. [DOI] [PubMed] [Google Scholar]
Rys 2011
- Rys P, Pankiewicz O, Łach K, Kwaskowski A, Skrzekowska‐Baran I, Malecki MT. Efficacy and safety comparison of rapid‐acting insulin aspart and regular human insulin in the treatment of type 1 and type 2 diabetes mellitus: a systematic review. Diabetes & Metabolism 2011;37(3):190‐200. [DOI] [PubMed] [Google Scholar]
Sawicki 2011
- Sawicki PT. Commentary: does additional benefit justify additional costs of insulin analogues?. BMJ 2011;343:d5858. [DOI] [PubMed] [Google Scholar]
Scheen 1999
- Scheen AJ, Letiexhe MR, Lefebvre PJ. Minimal influence of the time interval between injection of regular insulin and food intake on blood glucose control of type 1 diabetic patients on a basal‐bolus insulin scheme. Diabetes & Metabolism 1999;25(2):157‐62. [PubMed] [Google Scholar]
Schernthaner 1998
- Schernthaner G, Wein W, Sandholzer K, Equiluz‐Bruck S, Bates PC, Birkett MA. Postprandial insulin lispro: a new therapeutic option for type 1 diabetic patients. Diabetes Care 1998;21(4):570‐3. [DOI] [PubMed] [Google Scholar]
Sciacca 2012
- Sciacca L, Moli R, Vigneri R. Insulin analogs and cancer. Frontiers in Endocrinology 2012;3:21. [DOI: 10.3389/fendo.2012.00021] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siebenhofer 2006
- Siebenhofer A, Plank J, Berghold A, Jeitler K, Horvath K, Narath M, et al. Short acting insulin analogues versus regular human insulin inpatients with diabetes mellitus. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003287.pub4] [DOI] [PubMed] [Google Scholar]
Singh 2009
- Singh SR, Ahmad F, Lal A, Yu C, Bai Z, Bennett H. Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta‐analysis. CMAJ 2009;180(4):385‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2009
- Smith U, Gale EAM. Does diabetes therapy influence the risk of cancer?. Diabetologia 2009;52(9):1699‐708. [DOI] [PubMed] [Google Scholar]
Stedman 2009
- Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2009;40(6):1732‐4. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Torlone 1994
- Torlone E, Fanelli C, Rambotti AM, Kassi G, Modarelli F, Vicenzo A, et al. Pharmacokinetics, pharmacodynamics and glucose counterregulation following subcutaneous injection of the monomeric insulin analogue [Lys(B28),Pro(B29)] in IDDM. Diabetologia 1994;37(7):713‐20. [DOI] [PubMed] [Google Scholar]
WHO 1980
- World Health Organization Expert Committee on Diabetes Mellitus. Second report. WHO Technical Report Series 1980; Vol. No. 646. [PubMed]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. 18th Expert Committee on the Selection and Use of Essential Medicines. Review of the evidence comparing insulin (human or animal) with analogue insulins; 2011 Mar 21‐25; Accra, Ghana. 2011.
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zinman 1989
- Zinman B. The physiological replacement of insulin: an elusive goal. New England Journal of Medicine 1989;321(6):363‐70. [DOI] [PubMed] [Google Scholar]
Zinman 1997
- Zinman B, Tildesley H, Chiasson JL, Tsui E, Strack T. Insulin lispro in CSII: results of a double‐blind crossover study. Diabetes 1997;46(3):440‐3. [DOI] [PubMed] [Google Scholar]


