Abstract
Oxidative stress is elevated in the recipients of allogeneic hematopoietic cell transplantation (allo-HCT) and likely contributes to the development of graft-versus-host disease (GVHD). GVHD is characterized by activation, expansion, cytokine production, and migration of alloreactive donor T cells, and remains a major cause of morbidity and mortality after allo-HCT. Hence, strategies to limit oxidative stress in GVHD are highly desirable. Thioredoxin-1 (Trx1) counteracts oxidative stress by scavenging ROS and regulating other enzymes that metabolize H2O2. The present study sought to elucidate the role of Trx1 in the pathophysiology of GVHD. Using murine and xenograft models of allogeneic bone marrow transplantation (allo-BMT) and genetic (human Trx1-Tg) as well as pharmacological (human recombinant Trx1 [RTrx1]) strategies, we found that Trx1-Tg donor T cells or administration of RTrx1 to the recipients significantly reduced GVHD severity. Mechanistically, we observed that RTrx1 reduced ROS accumulation and cytokine production of mouse and human T cells in response to alloantigen stimulation in vitro. In allo-BMT settings, we found that Trx1-Tg or RTrx1 decreased downstream signaling molecules, including NF-κB activation and T-bet expression, and reduced proliferation, IFN-γ production, and ROS accumulation in donor T cells within GVHD target organs. More importantly, administration of RTrx1 did not impair the graft-versus-leukemia effect. Taken together, the current work provides a strong rationale for, and demonstrates the feasibility of, targeting the ROS pathway, which can be readily translated to the clinic.
Keywords: Immunology, Transplantation
Keywords: Adaptive immunity, Hematopoietic stem cells, Immunotherapy
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is an effective therapeutic procedure to treat hematopoietic malignancy such as leukemia through donor lymphocyte–mediated antitumor response, known as the graft-versus-leukemia (GVL) effect (1, 2). Unfortunately, the nonspecific immune response that contributes to the desired GVL effect is also responsible for the induction of deleterious graft-versus-host disease (GVHD) (3). GVHD is a pathological process caused by an exaggerated and undesirable immune response in which donor lymphocytes encounter host antigens and undergo extensive clonal expansion and differentiation. This process damages the recipient target organs, including skin, intestines, liver, and lung (4). Inflammatory cytokines, such as IFN-γ (4) and TNF-α (5), are elevated after allo-HCT and perpetuate GVHD through direct cytotoxic effects on host tissues via priming and activation of immune effector cells (6). The immunological mediators of GVHD have been investigated extensively; however, biochemical and subcellular changes like oxidative stress that precede and are mechanistically linked to T cell activation and cytokine dysregulation are not well defined.
Oxidative stress has been considered an unavoidable consequence of allo-HCT and may be an important factor in exacerbating GVHD (7). Owing to the contributions of preexisting disease conditions and the requirement for conditioning regimens that increase cellular reactive oxygen species (ROS), oxidative stress is elevated in all HCT recipients (8, 9). Excess nitric oxide (NO) production was previously observed in both clinical GVHD (10, 11) and experimental models (11, 12). Oxidative membrane lipids, proteins, and nucleic acids are known as damage-associated molecular patterns (DAMPs) and are ligands for innate immune cell activation (13, 14). Triggering DAMP receptors may facilitate alloantigen presentation and donor T cell activation required for GVHD initiation. It is also known that alloantigen-activated T cells exhibit higher cellular mitochondrial ROS generation (6, 15), suggesting an important role for redox-sensing molecules such as thioredoxin-1 (Trx1) in regulating oxidative stress during allo-HCT and thereby delaying or alleviating GVHD.
Trx1 is a ubiquitously expressed enzyme that counteracts oxidative stress by scavenging ROS and regulating other enzymes that metabolize H2O2 (16). In humans, increased Trx1 production in naturally occurring regulatory T cells (Tregs) confers enhanced tolerance to oxidative stress (17). Trx1, originally cloned as a soluble factor named adult T cell leukemia-derived factor (18, 19), is one of the most important molecules controlling the redox regulation system and contains a redox-active disulfide/dithiol within the conserved active site sequence Cys32-Gly-Pro-Cys35. Trx1 has a pivotal role in scavenging ROS with peroxiredoxins, which prevents apoptosis of various cells, such as lymphocytes, monocytes, and epithelial cells (12). Moreover, intracellular Trx1 reduces DNA binding of several transcription factors, including p53, NF-κB, and activator protein-1 (13). In addition, circulating Trx1 inhibits neutrophil infiltration into the sites of inflammation in an air pouch model (20, 21). These results suggest that Trx1 is not only an antioxidant and antiapoptotic molecule but is also an antiinflammatory molecule.
The current study sought to dissect the role of human Trx1 in the development of inflammation, with particular emphasis on the pathophysiology of GVHD in murine and xenograft models due to Trx1 being highly conserved with 90% homology between human and mouse (22). We demonstrated that the overexpression of human Trx1 in donor T cells or administration of human recombinant Trx1 (RTrx1) significantly reduced GVHD development. Thioredoxin-1–Tg (Trx1-Tg) overexpression or administration of RTrx1 significantly reduced the in vivo accumulation of ROS in the donor T cells in both secondary lymphoid organ (spleen) and GVHD target organ (liver) after allogeneic stimulation. This in turn was associated with a significant decrease in the downstream signaling molecules of Trx1, including NF-κB, T-bet, and chemokine receptor CXCR3. More importantly, administration of RTrx1 did not impair the GVL effect. These findings provide a strong rationale and potential means to prevent GVHD while preserving the GVL effect in the clinic.
Results
Trx1 is critical in regulating T cell alloresponse.
The administration of Trx1 has been demonstrated to be antiinflammatory and prevents autoimmune disease (23). To investigate the role of oxidative stress and redox metabolism in the induction of GVHD, we used Trx1-Tg T cells as donor T cells in murine models of allogeneic bone marrow transplantation (allo-BMT). Given that regulation of activation and function of primary T cells via Trx1 is largely undefined, we initially evaluated the role of overexpressed Trx1 in T cell homeostasis, development, and phenotype in Trx1-Tg mice compared with WT mice on a C57BL/6 (B6) background.
In the thymus, Trx1-Tg had no significant effect on the CD4+CD8+ T cell population, but significantly decreased the frequency of CD4+CD8– and increased CD4–CD8+ cell frequency (Supplemental Figure 1, A and B; supplemental material available online with this article; https://doi.org/10.1172/JCI122899DS1). In addition, a significant increase in percentages of CD4+CD25+Foxp3+ T cells (nTregs) was observed in Trx1-Tg mice (Supplemental Figure 1, A and B). In the spleen, Trx1-Tg CD4+ and CD8+ T cells and nTregs had trends similar to those seen in the thymus compared with their WT counterparts (Supplemental Figure 1, C and D). Furthermore, percentages of naive CD4+ T cells (CD44–CD62L+) were decreased, whereas effector and central memory CD4+ T cells (CD44+CD62L–) were increased, in Trx1-Tg mice (Supplemental Figure 1, E and F). These data suggest that Trx1 affects T cell development and homeostasis. Because Trx1 impacts T cell phenotypes, we focused our study on naive T cells, and thus purified naive CD4+ and CD8+ T cells (CD25–CD44–CD62L+) separately and pooled them with a 2:1 (CD4/CD8) ratio throughout the experiments.
In the current study, we sought to test how Trx1 affects T cell activation and function. Because Trx1 is known to counteract ROS, we first measured ROS accumulation. Upon in vitro stimulation with anti-CD3 plus anti-CD28 antibodies for 48 hours, Trx1-Tg T cells had significantly reduced ROS accumulation as compared with WT cells (Supplemental Figure 2, A and C). Since ROS promotes T cell activation (24), we then asked the impact of abundant Trx1 on T cell activation. It is commonly believed that glutamine provides fuel for rapidly dividing cells, including lymphocytes (25), and a heterodimeric amino acid transporter (CD98) is crucial for glutamine uptake (26). We thus measured and found that activated Trx1-Tg T cells expressed lower surface CD98 than WT controls (Supplemental Figure 2, B and D). Trx1-Tg T cells also took up significantly less glutamine as compared with the WT controls (Supplemental Figure 2E). These results prompted us to examine the impact of Trx1 on their downstream signaling molecules. Given that Trx1 is known to modulate NF-κB activity and T-bet expression (27), we stimulated WT or Trx1-Tg cells with anti-CD3 and anti-CD28 and measured the phospho-p65 subunit of NF-κB and T-bet expression at multiple time points after stimulation. We observed that both NF-κB activity and T-bet expression were significantly reduced in Trx1-Tg T cells as compared with WT counterparts at various time points (Supplemental Figure 2, F–K).
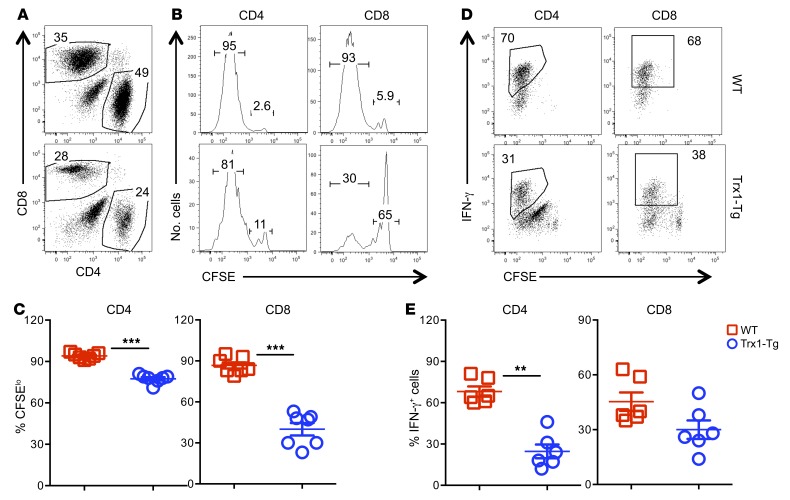
These results prompted us to evaluate the impact of Trx1-Tg on T cell activation and proliferation. Upon alloantigen stimulation in vitro, Trx1-Tg T cells had a substantially reduced ability to proliferate and produce IFN-γ compared with WT counterparts as reflected by the percentage of CFSE-diluted cells (Supplemental Figure 2, L, M, and O) and intracellular IFN-γ production (Supplemental Figure 2, N and P). Trx1-Tg T cells also had significantly reduced apoptosis after alloantigen stimulation in vitro (Supplemental Figure 2, R and S). To further evaluate T cell responses in vivo, we transferred T cells into irradiated allogeneic recipients and observed that the Trx1-Tg T cells had significantly reduced proliferation (Figure 1, A–C) and IFN-γ production (Figure 1, D and E) compared with WT counterparts. These results indicate that Trx1 negatively regulates T cell activation, proliferation, cytokine production, and apoptosis in response to alloantigens.
Figure 1. Trx1 regulates the T cell response to alloantigen in vivo.
Naive CD4+ and CD8+ T cells (CD25–CD44–CD62L+) were purified separately from WT and Trx1-Tg mice on B6 background and pooled with 2:1 (CD4/CD8) ratio. Combined T cells were labeled with CFSE and injected i.v. into lethally irradiated BALB/c mice at 2 × 106 per mouse. Four days after cell transfer, spleens were collected from recipient mice and subjected to cell counting and FACS staining. (A) CD4+ and CD8+ cells are shown among gated live donor cells (H2Kb+). (B and D) CFSE dilution and percentage IFN-γ+ cells are on gated donor CD4+ or CD8+ cells. (C and E) Data shown are from 2 combined experiments of 6–7 mice per group. The mean ± SD is depicted for 6–7 mice per group. Significance was determined by Student’s t test. **P < 0.01 and ***P < 0.001.
Trx1 regulates T cell oxidative stress and alleviates GVHD after allo-BMT.
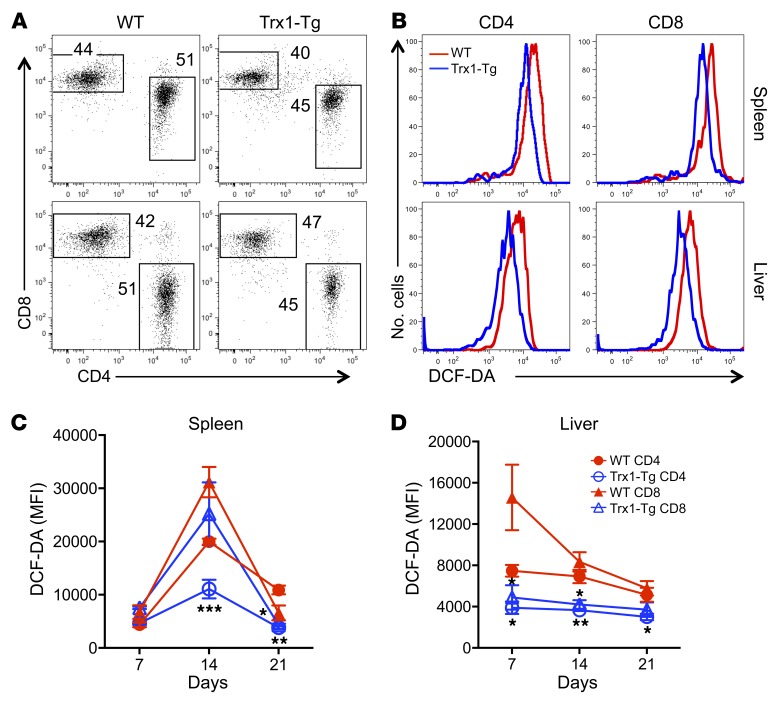
ROS activates hepatic stellate cells, leading to an increase of proliferation, contributing to fibrosis and cirrhosis (28), which is associated with inflammation and destruction of hepatocytes. Because tissues are susceptible to oxidative damage and inflammation, we investigated how Trx1 overexpression impacted ROS accumulation in the donor T cells that infiltrated into GVHD target organs, especially the liver. To do so, we transferred naive WT and Trx1-Tg T cells into irradiated allogeneic recipients and measured ROS levels among donor T cells in recipients at various time points (Figure 2, A and B). Trx1-Tg T cells in recipient spleen and liver had significantly less ROS accumulation compared with WT T cells, especially on days 14 and 21 after BMT (Figure 2, C and D).
Figure 2. Trx1 modulates ROS concentration after allogeneic T cell response.
Purified T cells from WT and Trx1-Tg mice were injected i.v. into lethally irradiated BALB/c mice at 0.5 × 106 per mouse. Recipient spleens and livers were collected 7, 14, and 21 days after transplant and subjected to cell counting and FACS staining. (A) CD4 and CD8 expression is shown on donor-derived (H2Kb+) live cells. (B) Cells were washed and stained with DCF-DA gated on CD4+ and CD8+ donor cells. The representative figure shown is from day 14. (C and D) Data shown are from 1 representative experiment with mean fluorescence intensity (MFI) ± SD of 3–4 mice per group. Two replicate experiments were performed for a total of 6–8 mice. Significance was determined by Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
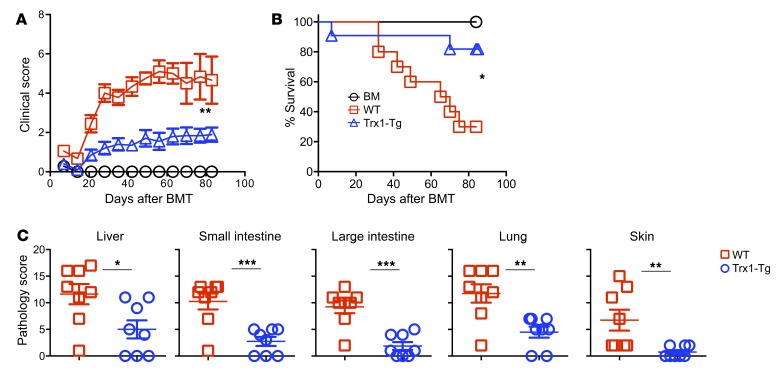
Given that Trx1-Tg T cells displayed a reduced level of ROS production and reduced allogeneic response in vitro and in vivo, we further hypothesized that Trx1 overexpression in T cells would alleviate GVHD. Using an MHC-mismatched B6→BALB/c BMT model, we found that the recipients of WT T cells developed severe and lethal GVHD, whereas the majority of the recipients with transplanted Trx1-Tg T cells survived long-term with significantly less weight loss and lower clinical scores (Figure 3, A and B). Premorbid state was defined when animals reach a clinical score of 8 or higher (10 as the highest) or had 30% or more weight loss compared with before BMT. Clinical manifestations were confirmed with pathological analysis in multiple GVHD target organs (Figure 3C).
Figure 3. Overexpression of Trx1 in T cells reduces GVHD mortality after allo-BMT.
BALB/c mice were lethally irradiated and underwent transplantation with 5 × 106 per mouse T cell–depleted bone marrow cells (TCD-BM, Ly5.1+) with or without purified T cells (Ly5.2+) (0.5 × 106 per mouse) from WT and Trx1-Tg mice. (A and B) Recipients were monitored for survival and clinical score until 80 days after BMT (n = 10 per group). (C) Three weeks after BMT, liver, lung, small intestine, colon, and skin were collected from the recipients for H&E staining and were scored for microscopic GVHD severity by a pathologist blinded to the treatment groups. Pathological score, means ± SD, of GVHD target organs is depicted. Data shown are from 2 combined experiments. For comparison of recipient survival among groups, the log-rank test was used to determine statistical significance. Clinical scores were compared using a nonparametric Mann-Whitney U test. For pathology, significance was determined by Student’s t test (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001.
We wished to extend our study using loss-function strategy as well; however, a Trx1-knockout strain is currently not available. Evidence suggests that the function of Trx1 is similar to that of glutaredoxin-1 (Grx1) (29). Glutaredoxins are small enzymes that use glutathione (GSH) as a cofactor. Early shifts in hepatic oxidative stress and plasma GSH loss preceded a statistically significant rise in TNF-α that is associated with clinical GVHD pathogenesis (30). Thus, we decided to test the effect of Grx1 deficiency on T cell response to alloantigens. To evaluate their T cell responses in vivo, we transferred T cells into irradiated allogeneic recipients and observed that Grx1-deficient CD4+ T cells produced a significantly higher level of IFN-γ than WT counterparts (Supplemental Figure 3, A–C). Furthermore, Grx1-deficient T cells induced an increase in lethal GVHD after allo-BMT as compared with WT T cells (Supplemental Figure 3D). These results indicate that antioxidants in general and Trx1 in particular negatively regulate T cell responses to alloantigen and GVHD development.
Trx1 impacts donor T cell distribution in allo-BMT recipients.
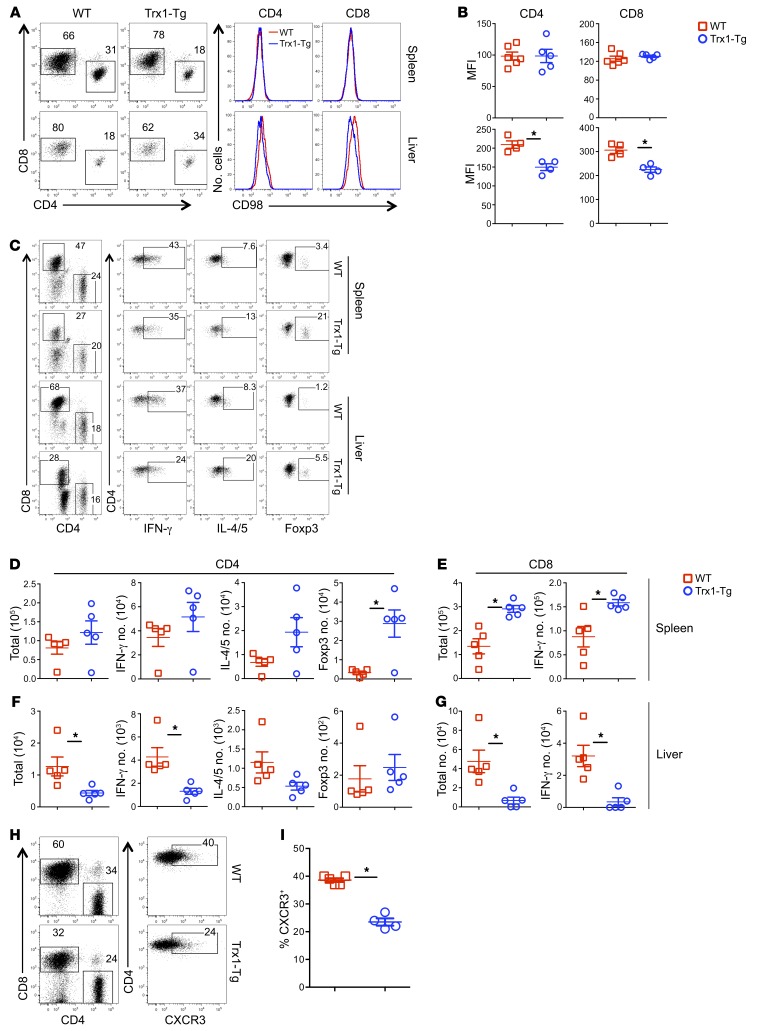
We found that Trx1 reduced ROS accumulation, CD98 upregulation, and glutamine uptake in vitro (Supplemental Figure 2). Furthermore, ROS accumulation was also reduced in Trx1-Tg T cells in vivo (Figure 2). Given that CD98 is critical for glutamine uptake, we measured CD98 expression and found that Trx1-Tg T cells expressed significantly lower levels of CD98 than WT T cells in recipient liver but not in spleens (Figure 4, A and B). Development of GVHD requires donor T cell expansion in lymphoid organs and migration into target organs (31). Hence, we asked how Trx1 affects T cell activation, expansion, and infiltration in target organs. Three weeks after allo-BMT, comparable numbers and percentage of donor CD4+ T cells were found in recipient spleens, regardless of donor type (Figure 4, C and D; and Supplemental Figure 4A). Trx1-Tg CD4+ T cells produced comparable levels of IFN-γ but significantly higher levels of IL-4/5 and Foxp3 (Supplemental Figure 4A). In terms of absolute numbers, Tregs were the only significantly increased subset observed in spleens of the recipients of Trx1-Tg compared with those of WT T cells (Figure 4D). On the other hand, the numbers of total and IFN-γ–producing CD8+ Trx1-Tg T cells were significantly more than those of WT counterparts in recipient spleens (Figure 4E), although the percentage of IFN-γ–producing CD8+ Trx1-Tg T cells was lower than that of WT T cells (Supplemental Figure 4B). In contrast to the spleen, significantly fewer CD4+ or CD8+ Trx1-Tg T cells were found in recipient liver compared with WT counterparts (Figure 4, F and G, and Supplemental Figure 4, C and D). Furthermore, significantly reduced percentage and numbers of IFN-γ–producing CD4+ and CD8+ T cells, but increased numbers of Foxp3+ Tregs, were found in livers of the recipients of Trx1-Tg T cells compared with those of WT T cells (Figure 4, F and G, and Supplemental Figure 4C).
Figure 4. Effect of Trx1 overexpression on donor T cell expansion and migration after allo-BMT.
BMT was carried out as outlined in Figure 3 using BALB/c mice as the recipients. Three weeks after BMT, recipient spleens and livers were collected and mononuclear cells were isolated and subjected to cell counting and FACS staining. (A) CD98 expression is shown on live donor-derived (H2Kb+) CD4+ and CD8+ cells in 1 representative recipient. (B) The MFI of CD98 on donor-derived T cells is summarized in recipient spleen and liver, respectively. Data shown here are from 1 of 2 representative experiments. (C) CD4 and CD8 expression is shown on gated donor cells among live spleen or liver cells. IFN-γ, IL-4/5, and Foxp3 expression is shown on gated donor CD4+ cells from a representative mouse from each group. (D–G) The absolute numbers of IFN-γ+, IL-4/5+, or Foxp3+ donor (H2Kb+Ly5.1–) CD4+ (D and F) and CD8+ cells (E and G) in recipient spleen and liver, respectively. (H) CXCR3 expression is shown on donor-derived (H2Kb+) CD4+ cells in 1 representative recipient. (I) Percentage CXCR3+ cells is summarized on donor-derived CD4+ cells in recipient spleen. Data shown here are from 1 of 2 representative experiments. Significance was determined by Student’s t test. *P < 0.05.
Given that more Trx1-Tg T cells were found in recipient spleens but fewer in recipient liver (Figure 4 and Supplemental Figure 4), we further hypothesized that Trx1 reduces T cell migration to GVHD target organs. Indeed, Trx1-Tg CD4+ T cells had significantly reduced expression of CXCR3 compared with WT counterparts in recipient spleens (Figure 4, H and I). Taking these results together, we interpret that although overexpression of Trx1 reduced T cell activation and proliferation, these suboptimally activated T cells did not acquire full migratory potential, so they accumulated in spleens rather than infiltrating into target organs such as liver.
We next asked whether Trx1 overexpression could attenuate GVHD while maintaining T cell–mediated GVL activity. To test this, we used a haploidentical B6→BDF1 BMT model with p815 mastocytoma. p815 mastocytoma was injected after irradiation and at the same time as other donor cells into the recipients. We titrated the dose of T cells from Trx1-Tg mice with 2.5 × 106 and 4.0 × 106 T cells, while the WT T cell dose was kept constant at 2.5 × 106 given that any higher dose of WT T cells would cause severe and early GVHD lethality. All the recipients of p815 without T cell infusion died from leukemia relapse within 20–25 days after BMT (Supplemental Figure 5C), whereas most of the recipients with transplanted WT T cells died from GVHD, reflected by 70%–80% lethality by day 60 after BMT with high clinical scores (Supplemental Figure 5, A–C). In contrast, the majority of the recipients with Trx1-Tg T cells at 2.5 × 106 died from tumor mortality (Supplemental Figure 5C). However, 70% of the recipients of Trx1-Tg T cells at 4.0 × 106 survived long-term with attenuated GVHD reflected by significantly reduced clinical score as compared with WT counterparts while maintaining T cell–mediated GVL activity (Supplemental Figure 5, A–C). Taken together, these data indicate that overexpression of Trx1 in donor T cells compromised GVL response. However, increasing doses of Trx1-Tg T cells in the graft are able to maintain the GVL response without inducing severe GVHD, and therefore improve overall survival in recipients after allogeneic BMT.
RTrx1 treatment reduces ROS accumulation and allogeneic T cell responses.
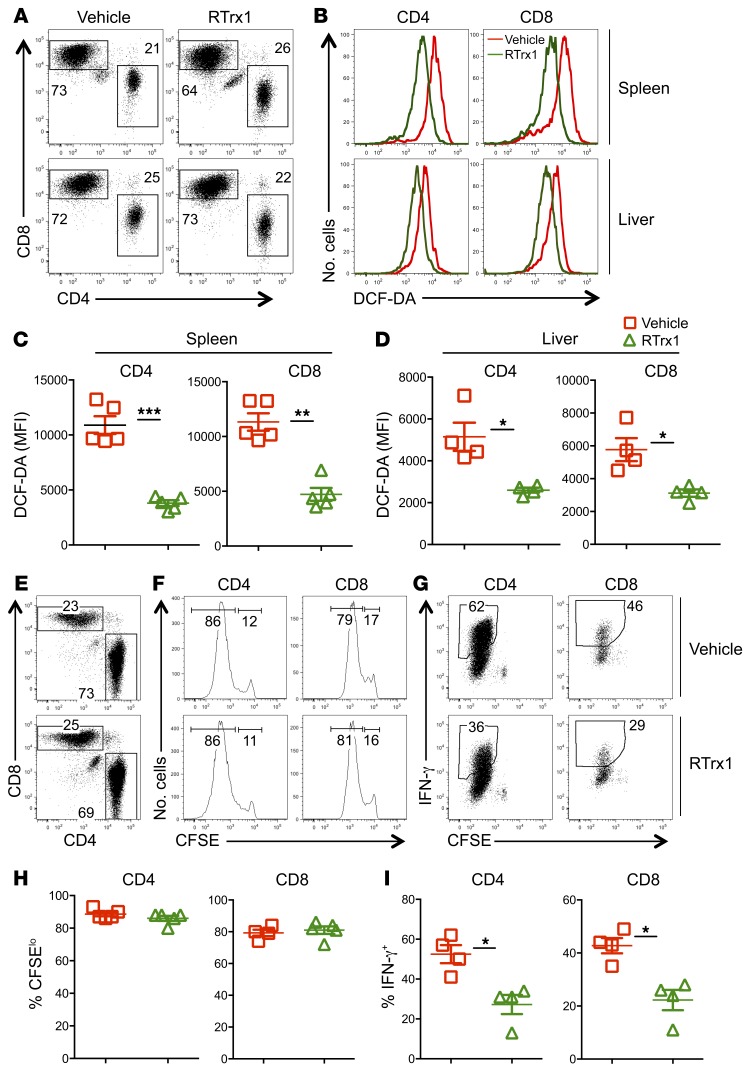
The data presented thus far indicate that overexpression of Trx1 reduces T cell activation and function as well as attenuates GVHD development. To translate the findings into potential applications in the clinic, we tested whether recombinant (R) Trx1 could mediate a similar effect as compared with transgenic overexpression. First we asked whether RTrx1 can be transported into the cells or whether it works through extracellular mechanisms. Upon in vitro stimulation with anti-CD3 plus anti-CD28 antibodies in the presence and absence of RTrx1, RTrx1 was detected inside of the T cells (Supplemental Figure 6, A and B) but not on cell surface (data not shown). We next investigated the effect of RTrx1 on T cell activation and function. Upon in vitro stimulation with anti-CD3 plus anti-CD28 antibodies, RTrx1 significantly reduced ROS production and CD98 expression of CD4+ and CD8+ T cells (Supplemental Figure 6, C–F). Furthermore, RTrx1 substantially reduced IFN-γ production from CD4+ but not CD8+ T cells (Supplemental Figure 6, G–K). We next evaluated the effect of RTrx1 in vivo by transferring WT T cells into irradiated allogeneic recipients and treated them with RTrx1. Consistent with in vitro results, treatment of recipients with RTrx1 for 2 weeks significantly reduced ROS accumulation in donor T cells in recipient spleen and liver (Figure 5, A–D). Although treatment of recipients with RTrx1 for 4 days in an in vivo mixed lymphocyte reaction did not affect donor T cell proliferation (Figure 5, E, F, and H), it significantly reduced the ability of donor T cells to produce IFN-γ (Figure 5, G and I). These results indicate that administration of RTrx1 mimics overexpression of Trx1 in diminishing ROS accumulation and modulating T cell activation and function.
Figure 5. RTrx1 affects T cell response to alloantigen in vivo.
Purified T cells from B6 mice were injected i.v. into lethally irradiated BALB/c mice at 0.5 × 106 per mouse. One group of recipient mice were injected i.p. with human RTrx1 at 5 μg/mouse/day from day –1 to day 14. Recipient spleens and liver were collected 21 days after transplant and subjected to cell counting and FACS staining. (A) CD4 and CD8 expression is shown on donor-derived (H2Kb+) live spleen or liver cells, respectively. (B–D) The MFI of DCF-DA is shown on gated donor CD4+ and CD8+ cells (B), with quantified data shown in C and D. Purified T cells from B6 mice were labeled with CFSE and injected i.v. into lethally irradiated BALB/c mice at 2 × 106 per mouse. One group of recipient mice was injected i.p. with recombinant human RTrx1 at 5 μg/mouse/day from day –1 to day 3. Four days after cell transfer, spleens were collected from recipient mice and subjected to cell counting and FACS staining. (E) CD4 and CD8 expression is shown on donor-derived (H2Kb+) live splenocytes. (F and G) CFSE dilution and percentage of IFN-γ+ cells are shown on gated donor CD4+ or CD8+ cells. (H and I) The mean ± SD is depicted for 4–5 mice per group. Data shown are from 1 representative experiment with mean ± SD of 5 mice per group. Two replicate experiments were performed with a total of 8 mice. Significance was determined by Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
RTrx1 treatment attenuates GVHD severity.
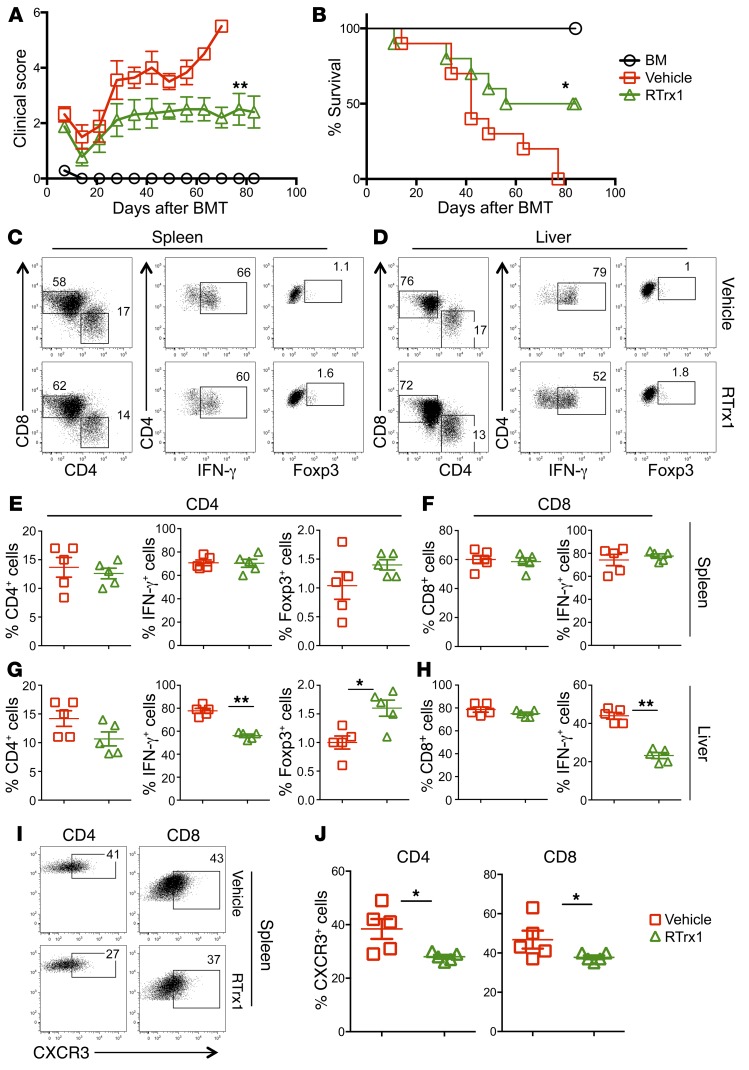
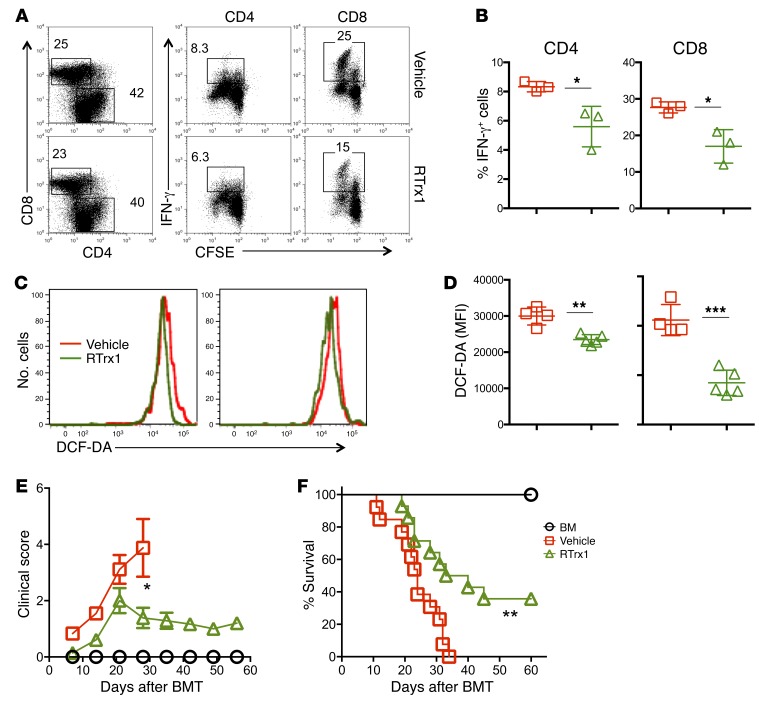
Given that RTrx1 treatment reduced ROS accumulation and activation of T cells in response to alloantigens in vitro and in vivo, we further hypothesized that RTrx1 treatment would attenuate GVHD. Using an MHC-mismatched B6→BALB/c BMT model, we found that treatment of recipients with RTrx1 significantly reduced clinical score and prevented GVHD mortality by 50% (Figure 6, A and B). We next asked how RTrx1 affected T cell activation, expansion, and migration. Two weeks after allo-BMT, we observed comparable absolute numbers and percentages of CD4+ or CD8+ donor T cells as well as IFN-γ+ and Foxp3+ T cells in recipient spleens regardless of treatment (Figure 6, C, E, and F; and Supplemental Figure 7, A and B). In contrast, RTrx1 treatment significantly reduced the numbers of CD4+ and CD8+ donor T cells and those that produced IFN-γ in recipient liver (Figure 6, D, G, and H; and Supplemental Figure 7, C and D). However, RTrx1 treatment significantly increased the percentage of Foxp3+ donor T cells but not the absolute number in recipient liver (Figure 6, D, G, and H; and Supplemental Figure 7C). Given that donor T cell migration into target organs is required for the development of GVHD, we measured expression of several migratory receptors, and found that RTrx1 treatment significantly reduced expression of CXCR3 on donor T cells in recipient spleen (Figure 6, I and J). These data suggest that RTrx1 treatment reduced GVHD severity by inhibiting IFN-γ production and migration of allogeneic T cells while promoting Treg differentiation/expansion.
Figure 6. Trx1 treatment reduces GVHD mortality by modulating T cell expansion and migration after allo-BMT.
BALB/c mice were lethally irradiated and underwent transplantation with 5 × 106 per mouse T cell–depleted bone marrow cells (TCD-BM, Ly5.1+) with or without purified T cells (Ly5.2+) (0.5 × 106 per mouse) from B6 mice. One group of recipient mice was injected with human RTrx1 at 5 μg/mouse/day from day –1 to day 14. (A and B) Recipients were monitored for survival and clinical score until 80 days after BMT (n = 10 per group). Data shown here are from 2 combined experiments. For comparison of recipient survival among groups, the log-rank test was used to determine statistical significance. Clinical scores were compared using a nonparametric Mann-Whitney U test. In separate experiments with the same setting, recipient spleens and livers were collected 2 weeks after BMT, and mononuclear cells were subjected to cell counting and FACS staining. (C and D) CD4 and CD8 expression is shown on gated donor cells among live spleen or liver cells. IFN-γ and Foxp3 expression is shown on gated donor CD4+ cells from a representative mouse from each group. (E–H) Percentage IFN-γ+ or Foxp3+ donor (H2Kb+Ly5.1–) CD4+ (E and G) and CD8+ cells (IFN-γ) (F and H) is summarized in recipient spleen and liver, respectively. (I) CXCR3 expression is shown on donor-derived (H2Kb+) CD4+ and CD8+ cells in 1 representative recipient. (J) Percentage CXCR3+ cells is summarized on donor-derived CD4+ and CD8+ in recipient spleen. The data are from 1 representative of 2 independent experiments. Significance was determined by Student’s t test. *P < 0.05, **P < 0.01.
RTrx1 treatment affects donor T cell expansion and migration.
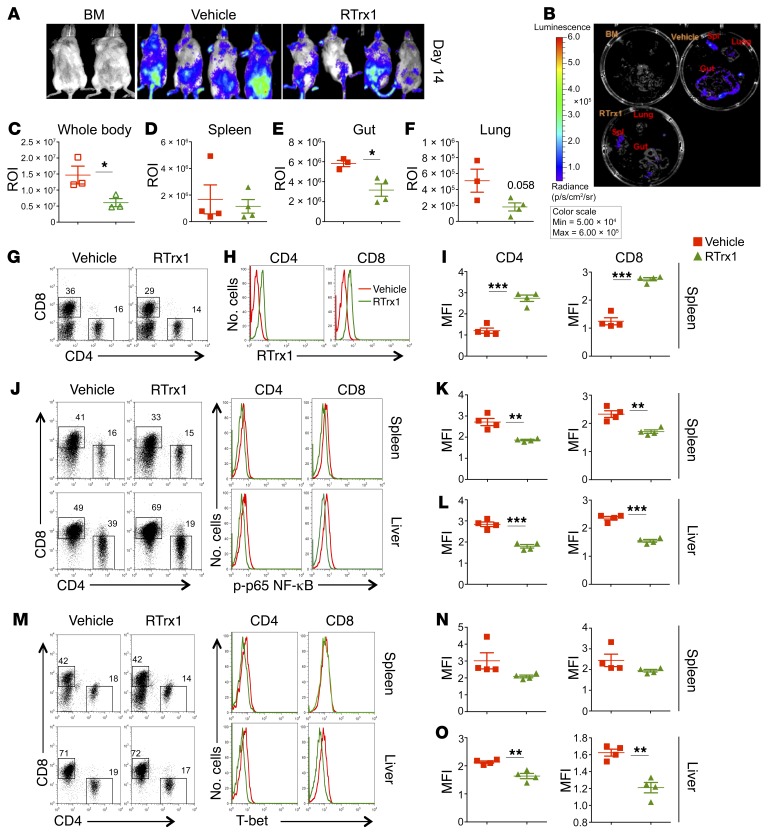
Given the fact that RTrx1 treatment reduced GVHD severity, we further asked whether RTrx1 impacts T cell expansion and/or migration. β-Actin luciferase transgenic T cells from B6 donors were transferred into irradiated allogeneic recipients, and expansion and migration of donor T cells was monitored using bioluminescent imaging (BLI) on day 14. Treatment of RTrx1 significantly reduced the abundance of donor T cells in the recipients, reflected by total-body BLI signal strength (Figure 7, A and C). Furthermore, the treatment significantly decreased donor T cells in target organs including gut and lung (substantial decrease) but not spleen (Figure 7, B and D–F). These results suggest that RTrx1 reduced T cell expansion as well as migration to GVHD target organs. As in the in vitro culture (Supplemental Figure 6), we detected RTrx1 expression intracellularly in donor T cells in the recipients, suggesting that the RTrx1 protein was transported into the T cells and most likely functioned intracellularly (Figure 7, G–I). To further elucidate underlying molecular mechanisms, we next tested how RTrx1 affects downstream signaling. Two weeks after allo-BMT, we observed that donor T cells in the recipients treated with RTrx1 had significantly reduced NF-κB activity in both the lymphoid (spleen) and GVHD target organs (liver) (Figure 7, J–L). Similarly, we found that the expression of T-bet was also significantly reduced in donor T cells in the recipient liver upon RTrx1 treatment (Figure 7, M–O). Taken together (Figures 6 and 7), these data indicate that RTrx1 treatment was able to reduce T cell expansion and migration to target organs by modulating downstream signaling molecules as well as chemokine receptor CXCR3.
Figure 7. Trx1 treatment modulates T cell expansion and migration after allo-BMT.
BALB/c mice were lethally irradiated and underwent transplantation with 5 × 106 per mouse T cell–depleted bone marrow cells (TCD-BM, Ly5.1+) with or without purified β-actin luciferase transgenic T cells (Ly5.2+) (0.75 × 106 per mouse) from B6 mice. The recipient mice were injected with vehicle alone or human RTrx1 at 5 μg/mouse/day from day –1 to day 14. T cell expansion and migration were monitored using bioluminescent imaging (BLI). (A–F) Macrophotos are shown for BLI of total-body (A) and individual organs (B) with a region of interest (ROI) summary (C–F). In separate experiments with the same setting, the last dose of human RTrx1 was given on day 14, two hours before mice were euthanized. The recipient spleens were collected, and mononuclear cells were subjected to cell counting and FACS staining. (G) CD4 and CD8 expression is shown on gated donor cells among live spleen cells. (H and I) Intracellular expression of human RTrx1 is displayed on CD4+ or CD8+ donor T cells. (J–O) Intracellular p-p65 subunit of NF-κB (J–L) and T-bet (M–O) was displayed on gated donor CD4+ and CD8+ T cells in recipient spleens and livers, respectively. The data analyzed using the MFI shown are from 3–4 mice per group. Significance was determined by Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
RTrx1 treatment does not impair GVL activity.
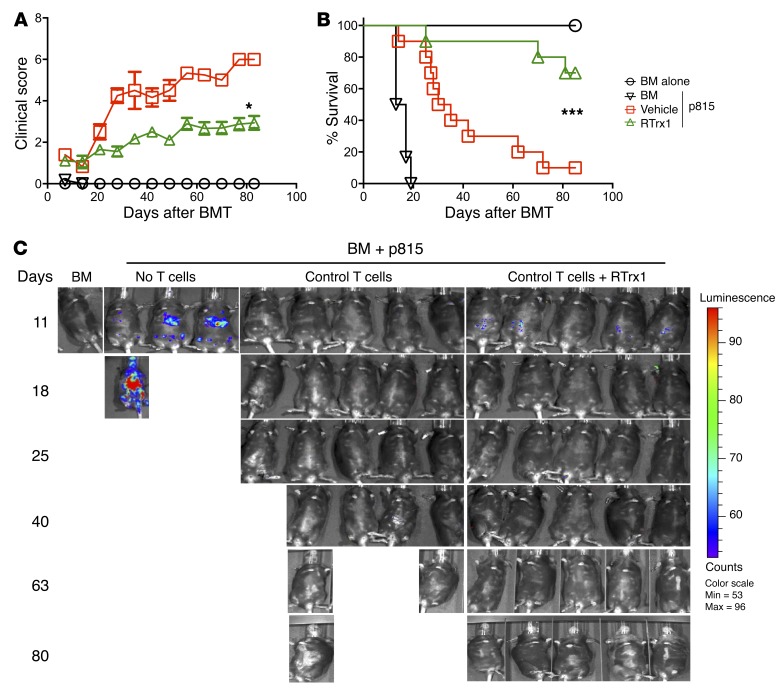
We next asked the critical question of whether treatment with RTrx1 could attenuate GVHD while maintaining T cell–mediated GVL activity. To test this, we used a haploidentical B6→BDF1 BMT model with aggressive p815 mastocytoma. All the recipients of p815 without T cell infusion died from leukemia relapse within 20 days after BMT (Figure 8, B and C), whereas the recipients treated with vehicle control died from GVHD, reflected by 90% lethality, severe clinical score, and no tumor signal (Figure 7, A–C). In contrast, the vast majority of the recipients treated with RTrx1 survived with mild GVHD and without tumor relapse (Figure 8, A–C). To evaluate the impact of RTrx1 on GVL activity more quantitatively, we next titrated T cell doses down to 1 × 106 to 2 × 106 with the same number of tumor cells. Given lower doses of donor T cells, the recipients developed mild or moderate GVHD, and had approximately 70% or 40% survival after allo-BMT with 1 × 106 or 2 × 106 donor T cells, respectively. Treatment with RTrx1 significantly reduced GVHD severity and mortality of the recipients of 2 × 106 donor T cells (Supplemental Figure 8, A and B). All the recipients of p815 without T cell infusion died from leukemia relapse within 40 days after BMT (Supplemental Figure 8C), whereas only approximately 20% of the recipients that had different T cell doses transplanted and were treated with vehicle or RTrx1 died from leukemia relapse (Supplemental Figure 8C).
Figure 8. RTrx1 attenuates GVHD and preserves GVL activity.
BDF1 mice were lethally irradiated and underwent transplantation with 5 × 106 per mouse TCD-BM with or without purified T cells (3 × 106 per mouse) from B6 mice. One group of recipients was injected with RTrx1 at 5 μg/mouse/day from day –1 to day 14. Recipient mice were also infused with luciferase-transduced p815 cells (5000 cells per mouse) at the day of BMT. (A and B) Recipients were monitored for clinical scores and post-BMT survival. (C) Tumor growth was monitored using BLI on the dates indicated. Data shown here are from 2 combined experiments (n = 10). The BLI (C) is from 1 representative experiment. For comparison of recipient survival among groups, the log-rank test was used to determine statistical significance. Clinical scores were compared using a nonparametric Mann-Whitney U test. *P < 0.05 and ***P < 0.001.
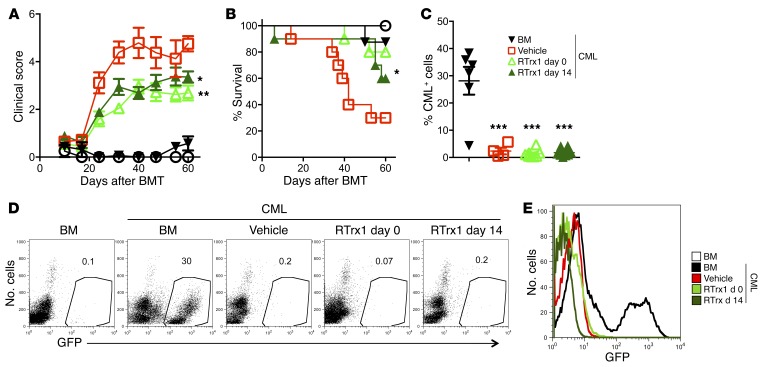
Because preservation of GVL activity is critically important, we extended our study to another leukemia model to determine whether RTrx1 can effectively control GVHD without impairing GVL activity. Using an MHC-mismatched B6→BALB/c BMT model, we infused chronic myeloid leukemia (CML) into BALB/c recipients and treated recipients with RTrx1 starting day 0 or 14 for 2 weeks. Either treatment schedule significantly reduced GVHD severity and mortality, although the delayed treatment was less effective (Figure 9, A and B). Furthermore, we observed that treatment with RTrx1 preserved GVL activity in either regimen, because all the recipients transplanted with donor T cells were leukemia free regardless of treatment (Figure 9, C–E). Taken together, these data indicate that RTrx1 treatment was able to markedly attenuate GVHD while preserving the GVL effect.
Figure 9. Delayed RTrx1 treatment ameliorates GVHD while preserving the GVL activity.
Lethally irradiated BALB/c mice underwent transplantation with BM or BM plus 0.5 × 106 T cells per mouse from B6 donors together with recipient type of GFP+ CML. The recipients were injected with vehicle alone or RTrx1 at 5 μg/mouse/day started from either day 0 or day 14 for 2 weeks. (A–C) Mice were monitored for clinical score (A), survival (B), and percentage of GFP+ CML among white cells in peripheral blood (C). Percentages of CML (GFP+CD11b+) are shown for 1 representative mouse in each group. (D) Representative flow cytometric plots and (E) overlay of GFP (CML) expression for individual groups. Data shown are from 2 combined experiments (n = 10). For comparison of recipient survival among groups, the log-rank test was used to determine statistical significance. Clinical scores were compared using a nonparametric Mann-Whitney U test. Significance was determined by ANOVA multiparametric analysis for percentage positive tumor expression. *P < 0.05, **P < 0.01, and ***P < 0.001.
Effect of RTrx1 on human-to-mouse xenograft GVHD model.
For translational purposes, we extended our study to human T cells. By stimulating human T cells with allogeneic antigen-presenting cells in vitro, we observed that RTrx1 significantly reduced IFN-γ production as well as ROS accumulation in human T cells (Figure 10, A–D). To further test the effects of RTrx1 on human T cells in vivo, we irradiated NSG-A2+ mice and transplanted HLA-A2– human PBMCs into them. Indeed, treatment of recipient mice with RTrx1 significantly reduced clinical score and GVHD mortality (Figure 10, E and F). Thus, RTrx1 can also modulate human T cell alloresponses and alleviate GVHD induced by human T cells in vivo.
Figure 10. Preventative treatment with RTrx1 reduces GVHD in NSG xenograft mouse model.
CFSE-labeled human T cells were stimulated with human T cell–depleted antigen-presenting cells isolated from an HLA-mismatched donor for 5 days in the presence or absence of 2 μg/ml RTrx1. Cells were subjected to FACS staining and analyzed for IFN-γ production. (A) Intracellular IFN-γ expression is shown on gated CD4+ or CD8+ cells. (B) The mean ± SD of IFN-γ+ cells is shown for CD4+ and CD8+ T cells, respectively. (C and D) In the same setting, cells were washed and stained with DCF-DA (C), and the MFI ± SD for DCF-DA+ CD4+ and CD8+ is shown, respectively (D). Data shown here are from 1 of 2 independent experiments. Significance was determined by Student’s t test. In separate experiments, NSG-A2+ mice were irradiated (250 cGy) and underwent transplantation with HLA-A2– human PBMCs (10 × 106 to 13 × 106). Recipient mice were injected with vehicle alone or with human RTrx1 at 5 μg/mouse/day from day –1 to day 14. (E and F) Recipients were monitored for clinical score (E) and survival (F) until 60 days after transplantation. Data shown here are from 2 combined experiments (n = 13–14 per group). For comparison of recipient survival among groups, the log-rank test was used to determine statistical significance. Clinical scores were compared using a nonparametric Mann-Whitney U test. *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
The present study demonstrated that overexpression of Trx1 or administration of RTrx1 decreased the pathogenicity of T cells to induce GVHD in mice as shown by clinical, histological, and immunological parameters. Moreover, administration of RTrx1 not only reduced the T cell alloresponses and GVHD severity but also preserved the GVL response to different types of leukemia. Ability of RTrx1 to attenuate GVHD was also extended into GVHD induced by human T cells in a xenograft model, implying that RTrx1 could be translated into clinical applications for patients undergoing allo-HCT. Mechanistically, Trx1 reduced ROS accumulation in donor T cells and decreased Trx1 downstream molecules including NF-κB and T-bet, which restrained the ability of T cells to activate, expand, and migrate to the target organs in response to alloantigens in vivo.
The contributions of preexisting disease conditions and the conditioning regimens increase cellular ROS, which correlates with elevated oxidative stress in all BMT recipients (8, 9). Alloantigen-activated T cells exhibit higher cellular mitochondrial ROS generation and contain less antioxidant than their non-alloreactive counterparts (15). The increased ROS levels and oxidative damage that occur in inflamed mucosa of patients with inflammatory bowel disease (32) suggest an important role of antioxidants in regulating ROS in particular and inflammation in general. Although moderate concentrations of ROS act as signaling messengers and modify protein function or structure by oxidation, under a proinflammatory environment, T cell response induces ROS production that in turn “fires up” T cell activation, proliferation, and effector functions (33–35). In the present study, we observed that overexpression or administration of Trx1 significantly decreased ROS accumulation in donor T cells in recipient lymphoid organs (Figure 2). Interestingly, ROS accumulation of donor T cells was even more profound in GVHD target organs such as liver as compared with lymphoid organs, suggesting an important role for antioxidants such as Trx1 in regulating ROS production and inflammation in GVHD target organs (Figures 2 and 5). Reduced ROS accumulation was associated with reduced NF-κB activity and T-bet expression (Figure 7 and Supplemental Figure 2) consistent with previous observations (36) that showed that RTrx1 was capable of reducing the proinflammatory cytokine IL-1β through reduction of both p50 and p65 subunits of NF-κB activation on one hand and the induction of IκBα on the other hand. Consistently, overexpression of Trx1 in donor T cells significantly reduced pathology in recipient GVHD target organs including liver, lung, skin, and gut (Figure 3C). Therefore, the maintenance of Trx1 levels in T cells likely serves as a host defense against oxidative stress but is dysregulated in the recipients after allo-BMT.
Along with diminished ROS accumulation, we also found that a high abundance of Trx1 reduced the expression of CD98, an important amino acid transporter induced on activated T cells (35), on the T cell surface. Furthermore, reduced expression of CD98 was correlated with diminished glutamine uptake by T cells (Figure 2), which likely accounted for reduced T cell allogeneic response and GVHD pathogenesis. Our results are in line with the report by Sena et al., who convincingly demonstrated that mitochondrial ROS signaling was required for antigen-specific T cell activation (24). However, extremely high levels of ROS accumulation due to glutathione deficiency also compromised T cell metabolic reprogramming and inflammatory responses (35). Taking these results together, we interpret that tight regulation of ROS production to maintain intermediate levels of ROS accumulation is critical for productive T cell responses, whereas too low or too high ROS accumulation is detrimental as elucidated previously by others (37, 38).
Development of GVHD requires donor T cell expansion in lymphoid organs and migration into target organs. Chemokine receptors and integrins play important roles in T cell migration to GVHD target organs (39). Previous studies have reported that a significant decline in antioxidants status occurs after chemotherapy and BMT (8). Additionally, overexpression or administration of antioxidants reduced oxidative stress in experimental colitis models (40, 41). The administration of RTrx1 is internalized or transported into cells through lipid raft–mediated endocytosis, which affects ROS directly and inflammation indirectly (42). In the present study we clearly demonstrated that the overexpression or administration of RTrx1 significantly reduced ROS accumulation in the donor T cells of target organs, especially liver. Donor Trx1-Tg T cells or RTrx1 treatment also significantly reduced T cell ability to migrate to recipient liver and produce IFN-γ (Figures 4 and 6). The reduced migration was related to the decreased Trx1 downstream molecules, including NF-κB activity and T-bet expression. T-bet is known to upregulate chemokine receptors, especially CXCR3, in T cells (43). Thus, reduced T-bet led to decreased CXCR3, which is one of the key receptors driving T cell migration to target organs (Figures 4 and 6).
Our current observations, together with previous reports (44, 45), suggest that Trx1 plays a critical role in regulating different immune cells, including macrophages and T cells, which in turn can modulate different autoimmune diseases such as colitis, experimental autoimmune myocarditis, and GVHD. The detrimental effect of ROS on T cells is well established in many chronic inflammatory diseases (46, 47). Our study establishes that treatment with RTrx1 significantly ameliorates GVHD without hampering the GVL effect. Several mechanisms may account for the GVL preservation upon RTrx1 administration. First, Trx1 scavenges ROS and thus counteracts oxidative stress (47), which may in turn limit malignant progression (48). Second, Trx1 significantly increased the frequency of induced Tregs (Figures 4 and 6), and these induced Tregs may be prone to suppress GVH over GVL responses by largely sparing the perforin killing pathway (49). Third, our collaborators showed that promoting thiol expression increases the durability of antitumor T cell functions (50). It is possible that thiol expression can facilitate T cell memory development that contributes to GVL response. Finally, treatment with RTrx1 significantly reduced migration of donor T cells into target organs (e.g., liver), potentially through the NF-κB, T-bet, and CXCR3 axis, whereas it did not impact T cell expansion or cytokine production in the lymphoid organs (Figure 6). Thus, activated T cells in the refined tissues could still exert their GVL response without causing severe injury in parenchymal tissues. We therefore propose that maintenance of Trx1 controls ROS accumulation that could preferentially suppress alloantigen-driven responses while preserving T cell homeostasis and avoiding a broad immune suppression in patients undergoing allo-HCT.
In conclusion, we demonstrated that Trx1 exerts antioxidative and antiinflammatory effects, downregulates T cell alloresponses, and alleviates GVHD development in both murine allo-HCT and xenograft transplant models. We anticipate that the effect of Trx1 can be extended to human T cells and that RTrx1 treatment has a translational potential in patients with hematological malignancies undergoing allo-HCT. Furthermore, RTrx1 can potentially be applied in the treatment of other inflammatory and autoimmune diseases.
Methods
Mice.
C57BL/6 (B6; H-2b, CD45.2), B6.Ly5.1 (CD45.1), BD2F1 (H-2b/d), and BALB/c (H-2d) NSG-A2+ mice were purchased from the National Cancer Institute (Frederick, Maryland, USA) or The Jackson Laboratory. The generation and maintenance of Trx1-Tg mice were described previously (51). All animals were housed in the Medical University of South Carolina. Luciferase-transduced p815 mastocytoma was provided by Pavan Reddy (University of Michigan, Ann Arbor, Michigan, USA); chronic myeloid leukemia (CML) was provided by Warren Shlomchik (University of Pittsburgh, Pittsburgh, Pennsylvania, USA).
Experimental procedures and materials.
Mixed lymphocyte reaction, murine BMT, GVHD scoring, treatment with RTrx1, flow cytometry, cytokine measurement, and histopathology are described in previously published work (52–59) and in Supplemental Methods, available online with this article. Briefly, naive CD4+ and CD8+ T cells (CD25–CD44–CD62L+) were purified separately through negative selection and pooled at a 2:1 (CD4/CD8) ratio throughout the experiments.
Mixed lymphocyte reaction.
Naive CD4+ and CD8+ T cells (CD25–CD44–CD62L+) were purified separately by negative selection and pooled with 2:1 (CD4/CD8) ratio throughout the study. The cocktail of antibodies used to purify T cells (eBioscience) included anti–mouse CD25–biotin (catalog 13-0251-85), anti–mouse CD49b–biotin (catalog 13-5971-85), anti–mouse TER-119–biotin (catalog 13-5921-85), anti–human/mouse CD45R–biotin (catalog 13-0452-85), anti–mouse CD11b–biotin (catalog 13-0112-85), anti–human/mouse CD44–biotin (catalog 13-0441-85), anti–mouse CD4–biotin (catalog 13-0041-86), and anti–mouse CD8a–biotin (catalog 13-0081-86); anti-biotin microbeads were from Miltenyi Biotec (catalog 130-090-485). Purified T cells from B6 or Trx1-Tg mice were labeled with CFSE (Invitrogen, Molecular Probes Inc.). For in vitro experiments, 0.2 × 106 T cells were cocultured with 0.6 × 106 T cell–depleted splenocytes for 5 days. T cells were depleted from total splenocytes by positive selection using anti-CD4 and anti-CD8 biotin-conjugated antibodies (catalog 13-0041-86 and 13-0081-86, respectively, eBioscience) followed by anti-biotin–conjugated beads (Miltenyi Biotec). T cell–depleted splenocytes were used as antigen-presenting cells. For in vivo experiments, 2 × 106 CFSE-labeled purified T cells were injected i.v. into irradiated recipient mice, and analyzed after 4 days. For long-term GVHD, T cells were injected at a lower number to avoid early deaths (0.5 × 106). In some in vivo experiments, recipient mice were also injected with RTrx1 intraperitoneally at 5 μg/mouse/day from day –1 to day 3. Recipient spleens were excised, and the cells were stained for surface molecules and intracellular cytokines by flow cytometry.
Bone marrow transplantation.
T cells were purified from spleen and lymph node cells by negative selection using magnetic beads. MHC-mismatched (B6→BALB/c) and haploidentical (B6→BD2F1) BMT models were used. The mice were lethally irradiated at the dose of 700 cGy for BALB/c and 1100 cGy (split) for B2DF1 mice (x-ray source, X-RAD 320). In some experiments, RTrx1 or vehicle was administered to the recipients. Recipient mice were monitored for weight loss and other clinical signs of GVHD twice per week. Clinical scores were tabulated based on 5 parameters: weight loss, posture, activity, fur texture, and skin integrity. Individual mice were scored from 0 to 2 for each criterion and from 0 to 10 overall. Recipients at the premorbid stage were euthanized and counted toward lethality. Recipients at the premoribund stage were euthanized and counted for lethality. The GVL model p815 mastocytoma was established. Tumor growth was measured with bioluminescent imaging (BLI) using a Xenogen IVIS 200 preclinical in vivo imaging system (PerkinElmer) and analyzed by Living Image software (PerkinElmer). Tumor and GVHD mortality were distinguished by BLI signal intensity and clinical manifestation of GVHD. Representative samples of GVHD target organs were excised from recipients 21 days after BMT and subjected to pathology scoring.
Intracellular ROS.
T cells either were stimulated in vitro with anti-CD3 (catalog BE0001-1, Bio X Cell) plus anti-CD28 (catalog BE0015-1, Bio X Cell) antibodies at 1 μg/ml each for 48 hours or were mononuclear cells isolated from recipient spleen or liver as previously described (57, 59, 60). These cells were first stained for surface markers and then incubated with 5 μM dichlorofluorescein diacetate (DCF-DA, Sigma-Aldrich) for 30 minutes. Cells were analyzed by flow cytometry using standard flow cytometric protocols.
Glutamine uptake assay.
T cells were stimulated in vitro with anti-CD3 plus anti-CD28 antibodies at 1 μg/ml each for 48 hours. The cells were washed with PBS followed by 2 washes with glutamine-free RPMI 1640 media (catalog 21870-076, Thermo Fisher Scientific). These were starved in glutamine-free RPMI 1640 media for 15 minutes before being incubated with l-2,3,4-[3H]glutamine (0.5 mCi; PerkinElmer) for 10 minutes at room temperature. Cells were lysed in 500 μl of lysis buffer (Sigma-Aldrich), and radioactivity was measured by liquid scintillation.
Intracellular phospho–NF-κB p65.
T cells were either stimulated in vitro with anti-CD3 plus anti-CD28 antibodies at 1 μg/ml each for 48 hours, or isolated from recipient spleen or liver as previously described (57, 59, 60). These cells were first stained for surface markers, washed, and then fixed in formaldehyde for 15 minutes at room temperature. Cells were permeabilized by adding ice-cold 100% methanol slowly to prechilled cells, while gently vortexing, to a final concentration of 90% methanol. Cells were then incubated on ice for 30 minutes, washed, and stained for intracellular primary antibody phospho–NF-κB p65 for 1 hour followed by 30 minutes for secondary antibody. Cells were thoroughly washed and analyzed by flow cytometry.
Statistics.
For comparison of recipient survival among groups in GVHD experiments, the log-rank test was used to determine statistical significance. Clinical scores and body weight loss were compared using a nonparametric Mann-Whitney U test. To compare pathology scores and cytokine levels, a 2-tailed Student’s t test was performed. A P value less than 0.05 was considered significant. Statistical tests were performed in each experiment between WT and Trx1-Tg or between vehicle and RTrx1 treatment, and P values between these groups are indicated. If statistical significance is not indicated, the relevant groups were compared but statistical significance was not reached (P > 0.05).
Study approval.
The present studies in animals and xenograft models were reviewed and approved by the IACUC (protocol nos. 446 and 397) and the Institutional Biosafety Committee (IBC) of the Medical University of South Carolina. Experiments were carried out under protocols approved by the IACUC of the Medical University of South Carolina.
Author contributions
MHS, SM, and XZY participated in designing research studies. MHS, YW, MD, SDS, SPS, AD, HN, DB, JHV, and SI participated in conducting experiments and acquiring data. CL performed pathological analysis. MHS and XZY participated in analyzing and interpreting data and drafted the manuscript. MHS, YW, SS, BO, JJ, NM, SM, and XZY participated in writing and editing the manuscript.
Supplementary Material
Acknowledgments
The authors thank the Flow Cytometry Core, Small Animal Imaging Core, and Cell and Molecular Imaging Facilities at the Medical University of South Carolina for their assistance. The research presented in this article was supported in part by NIH grants R01-CA169116, R01-AI118305, and R01-HL137373 and the SmartState Endowment in Cancer Stem Cell Biology and Therapy Program (to XZY); NIH grants P01-CA20362, CA173687, CA214461, DE016572, and DE016572 (to BO); and NIH grants R01-CA138930 and P01-CA154778 (to SM). The project described was supported by the NIH National Center for Advancing Translational Sciences through grants TL1-TR001451 and UL1-TR001450.
Version 1. 05/02/2019
In-Press Preview
Version 2. 06/10/2019
Electronic publication
Version 3. 07/01/2019
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2019;129(7):2760–2774.https://doi.org/10.1172/JCI122899.
Contributor Information
M. Hanief Sofi, Email: sofi@musc.edu.
Yongxia Wu, Email: wuyyo@musc.edu.
Steven D. Schutt, Email: schutts@musc.edu.
Min Dai, Email: berrydai2003@aliyun.com.
Anusara Daenthanasanmak, Email: anusara.daenthanasanmak@nih.gov.
Jessica Heinrichs Voss, Email: jessica.heinrichs@northwestern.edu.
Hung Nguyen, Email: nguyenhd@musc.edu.
David Bastian, Email: bastian@musc.edu.
Supinya Iamsawat, Email: iamsawat@musc.edu.
Shanmugam Panneer Selvam, Email: panneers@musc.edu.
Chen Liu, Email: cl1063@njms.rutgers.edu.
Nilanjana Maulik, Email: nmaulik@neuron.uchc.edu.
Besim Ogretmen, Email: ogretmen@musc.edu.
Junfei Jin, Email: 2945556572@qq.com.
Shikhar Mehrotra, Email: mehrotr@musc.edu.
Xue-Zhong Yu, Email: yux@musc.edu.
References
- 1.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411(6835):385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 3.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 4.Paczesny S, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113(2):273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI, Negrin RS. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood. 2011;118(8):2342–2350. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda Y, et al. Both perforin and Fas ligand are required for the regulation of alloreactive CD8+ T cells during acute graft-versus-host disease. Blood. 2005;105(5):2023–2027. doi: 10.1182/blood-2004-08-3036. [DOI] [PubMed] [Google Scholar]
- 7.Amer J, Weiss L, Reich S, Shapira MY, Slavin S, Fibach E. The oxidative status of blood cells in a murine model of graft-versus-host disease. Ann Hematol. 2007;86(10):753–758. doi: 10.1007/s00277-007-0321-7. [DOI] [PubMed] [Google Scholar]
- 8.Jonas CR, et al. Plasma antioxidant status after high-dose chemotherapy: a randomized trial of parenteral nutrition in bone marrow transplantation patients. Am J Clin Nutr. 2000;72(1):181–189. doi: 10.1093/ajcn/72.1.181. [DOI] [PubMed] [Google Scholar]
- 9.Sabuncuoğlu S, Kuşkonmaz B, Uckun Çetinkaya D, Ozgüneş H. Evaluation of oxidative and antioxidative parameters in pediatric hematopoietic SCT patients. Bone Marrow Transplant. 2012;47(5):651–656. doi: 10.1038/bmt.2011.145. [DOI] [PubMed] [Google Scholar]
- 10.Weiss G, et al. Nitric oxide formation as predictive parameter for acute graft-versus-host disease after human allogeneic bone marrow transplantation. Transplantation. 1995;60(11):1239–1244. doi: 10.1097/00007890-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Ellison CA, Natuik SA, McIntosh AR, Scully SA, Danilenko DM, Gartner JG. The role of interferon-γ, nitric oxide and lipopolysaccharide in intestinal graft-versus-host disease developing in F1-hybrid mice. Immunology. 2003;109(3):440–449. doi: 10.1046/j.1365-2567.2003.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S, et al. Effects of oxidant stress on inflammation and survival of iNOS knockout mice after marrow transplantation. Am J Physiol Lung Cell Mol Physiol. 2001;281(4):L922–L930. doi: 10.1152/ajplung.2001.281.4.L922. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatza E, et al. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci Transl Med. 2011;3(67):67ra8. doi: 10.1126/scitranslmed.3001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396(1):120–124. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 17.Mougiakakos D, Johansson CC, Jitschin R, Böttcher M, Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117(3):857–861. doi: 10.1182/blood-2010-09-307041. [DOI] [PubMed] [Google Scholar]
- 18.Tagaya Y, et al. ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO J. 1989;8(3):757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakasugi N, et al. Adult T cell leukemia-derived factor/thioredoxin, produced by both human T-lymphotropic virus type I- and Epstein-Barr virus-transformed lymphocytes, acts as an autocrine growth factor and synergizes with interleukin 1 and interleukin 2. Proc Natl Acad Sci U S A. 1990;87(21):8282–8286. doi: 10.1073/pnas.87.21.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura H, et al. Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2001;98(26):15143–15148. doi: 10.1073/pnas.191498798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura H, et al. Chronic elevation of plasma thioredoxin: inhibition of chemotaxis and curtailment of life expectancy in AIDS. Proc Natl Acad Sci U S A. 2001;98(5):2688–2693. doi: 10.1073/pnas.041624998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Hadri K, et al. Thioredoxin-1 promotes anti-inflammatory macrophages of the M2 phenotype and antagonizes atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(6):1445–1452. doi: 10.1161/ATVBAHA.112.249334. [DOI] [PubMed] [Google Scholar]
- 23.Chernatynskaya AV, Looney B, Hu H, Zhu X, Xia CQ. Administration of recombinant human thioredoxin-1 significantly delays and prevents autoimmune diabetes in nonobese diabetic mice through modulation of autoimmunity. Diabetes Metab Res Rev. 2011;27(8):809–812. doi: 10.1002/dmrr.1232. [DOI] [PubMed] [Google Scholar]
- 24.Sena LA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38(2):225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakaya M, et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40(5):692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicklin P, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-κB by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20(15):3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diesen DL, Kuo PC. Nitric oxide and redox regulation in the liver: part II. Redox biology in pathologic hepatocytes and implications for intervention. J Surg Res. 2011;167(1):96–112. doi: 10.1016/j.jss.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahedi Avval F, Holmgren A. Molecular mechanisms of thioredoxin and glutaredoxin as hydrogen donors for Mammalian s phase ribonucleotide reductase. J Biol Chem. 2009;284(13):8233–8240. doi: 10.1074/jbc.M809338200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh JH, et al. Thiol/redox metabolomic profiling implicates GSH dysregulation in early experimental graft versus host disease (GVHD) PLoS One. 2014;9(2):e88868. doi: 10.1371/journal.pone.0088868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Socié G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease — radicals or ridiculous? Aliment Pharmacol Ther. 2002;16(12):1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 33.Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med. 2002;195(1):59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5(8):818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 35.Mak TW, et al. Glutathione primes T cell metabolism for inflammation. Immunity. 2017;46(4):675–689. doi: 10.1016/j.immuni.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Billiet L, et al. Extracellular human thioredoxin-1 inhibits lipopolysaccharide-induced interleukin-1β expression in human monocyte-derived macrophages. J Biol Chem. 2005;280(48):40310–40318. doi: 10.1074/jbc.M503644200. [DOI] [PubMed] [Google Scholar]
- 37.Kesarwani P, Murali AK, Al-Khami AA, Mehrotra S. Redox regulation of T-cell function: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2013;18(12):1497–1534. doi: 10.1089/ars.2011.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarosz EL, Chang CH. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw. 2018;18(1):e14. doi: 10.4110/in.2018.18.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105(11):4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krieglstein CF, et al. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J Exp Med. 2001;194(9):1207–1218. doi: 10.1084/jem.194.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araki Y, Andoh A, Fujiyama Y. The free radical scavenger edaravone suppresses experimental dextran sulfate sodium-induced colitis in rats. Int J Mol Med. 2003;12(1):125–129. [PubMed] [Google Scholar]
- 42.Kondo N, et al. Lipid raft-mediated uptake of cysteine-modified thioredoxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1. Antioxid Redox Signal. 2007;9(9):1439–1448. doi: 10.1089/ars.2007.1665. [DOI] [PubMed] [Google Scholar]
- 43.Lord GM, et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106(10):3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamaki H, et al. Human thioredoxin-1 ameliorates experimental murine colitis in association with suppressed macrophage inhibitory factor production. Gastroenterology. 2006;131(4):1110–1121. doi: 10.1053/j.gastro.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Liu W, et al. Thioredoxin-1 ameliorates myosin-induced autoimmune myocarditis by suppressing chemokine expressions and leukocyte chemotaxis in mice. Circulation. 2004;110(10):1276–1283. doi: 10.1161/01.CIR.0000141803.41217.B6. [DOI] [PubMed] [Google Scholar]
- 46.Gringhuis SI, Leow A, Papendrecht-Van Der Voort EA, Remans PH, Breedveld FC, Verweij CL. Displacement of linker for activation of T cells from the plasma membrane due to redox balance alterations results in hyporesponsiveness of synovial fluid T lymphocytes in rheumatoid arthritis. J Immunol. 2000;164(4):2170–2179. doi: 10.4049/jimmunol.164.4.2170. [DOI] [PubMed] [Google Scholar]
- 47.Li W, et al. NK cell apoptosis in coronary artery disease: relation to oxidative stress. Atherosclerosis. 2008;199(1):65–72. doi: 10.1016/j.atherosclerosis.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 48.Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7(6):e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edinger M, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 50.Kesarwani P, et al. Promoting thiol expression increases the durability of antitumor T-cell functions. Cancer Res. 2014;74(21):6036–6047. doi: 10.1158/0008-5472.CAN-14-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adluri RS, et al. Thioredoxin 1 enhances neovascularization and reduces ventricular remodeling during chronic myocardial infarction: a study using thioredoxin 1 transgenic mice. J Mol Cell Cardiol. 2011;50(1):239–247. doi: 10.1016/j.yjmcc.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sofi MH, et al. Ceramide synthesis regulates T cell activity and GVHD development. JCI Insight. 2017;2(10):91701. doi: 10.1172/jci.insight.91701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Betts BC, et al. Targeting JAK2 reduces GVHD and xenograft rejection through regulation of T cell differentiation. Proc Natl Acad Sci U S A. 2018;115(7):1582–1587. doi: 10.1073/pnas.1712452115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schutt SD, et al. Inhibition of the IRE-1α/XBP-1 pathway prevents chronic GVHD and preserves the GVL effect in mice. Blood Adv. 2018;2(4):414–427. doi: 10.1182/bloodadvances.2017009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Y, et al. MicroRNA-17-92 is required for T-cell and B-cell pathogenicity in chronic graft-versus-host disease in mice. Blood. 2018;131(17):1974–1986. doi: 10.1182/blood-2017-06-789321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu Y, et al. c-Rel is an essential transcription factor for the development of acute graft-versus-host disease in mice. Eur J Immunol. 2013;43(9):2327–2337. doi: 10.1002/eji.201243282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang Y, et al. Beta2 integrins separate graft-versus-host disease and graft-versus-leukemia effects. Blood. 2008;111(2):954–962. doi: 10.1182/blood-2007-05-089573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haarberg KM, et al. Pharmacologic inhibition of PKCα and PKCθ prevents GVHD while preserving GVL activity in mice. Blood. 2013;122(14):2500–2511. doi: 10.1182/blood-2012-12-471938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valenzuela JO, et al. PKCθ is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. J Clin Invest. 2009;119(12):3774–3786. doi: 10.1172/JCI39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Y, et al. Prevention of GVHD while sparing GVL effect by targeting Th1 and Th17 transcription factor T-bet and RORγt in mice. Blood. 2011;118(18):5011–5020. doi: 10.1182/blood-2011-03-340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.