Abstract
Rationale
There is a paucity of information about the association of seizure severity and quality of life in people living with epilepsy (PLWE) in sub-Saharan Africa. We evaluated the relationship between seizure severity and health-related quality of life of patients with epilepsy being followed up in an outpatient neurology clinic in urban central Uganda
Methods
Forty-eight PLWE who met the study inclusion criteria were enrolled. The study questionnaire was comprised of the Chalfont Seizure Severity Scale, and the Quality of Life Inventory in Epilepsy (QOLIE-31). Spearman’s rank correlation coefficient was used to determine the association between seizure severity and quality of life score.
Results
The median age of the study participants was 25 years, with median age (IQR) of epilepsy onset of 12 (6−18) years. Over 57.4% of the study participants were unemployed. The mean (SD) of QOLIE-31 and Seizure Severity Score was 62.5 (14.5) and 62.4 (1. 6), respectively. There was no gender difference in the seizure severity scores (P = 0. 451). An inverse relationship existed between seizure severity and the total QOLIE-31 score (Spearman’s rank correlation coefficient, r = −0.48, p = 0.001), and seizure worry (r = −0.31, p=0.030),
Conclusions
In this Ugandan sample seizure severity is unacceptably high, and directly impacts the life of PLWE. Interventions that reduce seizure severity are urgently needed in our settings to reduce seizures and improve the quality of life in PLWE.
Keywords: Epilepsy, seizure, severity, quality of life
Introduction
Epilepsy is a common chronic brain disorder globally, and affects people of all ages. It is estimated that it affects more than 70 million people with epilepsy worldwide, and 80% of them live in developing countries (1, 2). Epilepsy significantly impairs the quality of life (QoL) of people living with epilepsy (PLWE). There are various reasons for this, including the chronicity and long-duration of disease, seizure complications, poorly controlled seizures, stigma, coexisting chronic diseases and social isolation which all negatively impact QoL (3–7). Additionally, various social – cultural beliefs held both by the PLWE and their immediate caretakers or family, may impact QoL. While quality of life in PLWE remains one of the critical outcomes during epilepsy care, few studies in Africa have evaluated the health-related quality of life (HRQoL) of PLWE. (8–10)
A key focus of epilepsy care is to reduce the number of seizures. Both seizure frequency (a numerical count) and seizure severity (which characterizes the duration of seizures, the type of seizure and impact on patient’s social functioning) impact the quality of life in PLWE (11, 12). Understanding the relationship between quality of life and seizure frequency is important in caring for PLWE and development of a targeted self-management approach for PLWE in our settings where the highest burden of the disease occurs. We assessed the impact of seizure severity on the QoL of PLWE, with the aim of ascertaining the socio-demographic and clinical determinants of seizure severity.
Materials and methods
The study was carried out at Mulago National referral and teaching hospital in Kampala, Uganda, between November 2017 and February 2018. Two study sites at Mulago were used to screen for study recruitment: the neurology outpatient clinic and mental health outpatient clinic. These clinics serve as a secondary and tertiary referral center for patients with epilepsy in the district surrounding the hospital.
Forty-eight (48) PLWE attending the neurology and mental health clinics were consecutively enrolled.
Study design
This was a descriptive cross-sectional study using a convenient sampling method. Consecutive patients seen at the outpatient departments with a diagnosis of epilepsy (i.e., at least two unprovoked stereotyped afebrile seizures with eye witness corroboration with/without supportive inter ictal electroencephalographic findings) were approached for potential recruitment. Seizures were defined using the 2017 International League Against Epilepsy (ILAE) classification (13), Recruitment criteria
Adults aged 18 years and above with a clinical diagnosis of epilepsy that gave written informed consent were recruited, those who could not provide reliable history or could not communicate, respond to questions, had mental retardation or dementia and had no attendant available were excluded. Additionally, patients with mental retardation, dementia and those who did not give informed consent were excluded. Of the 60 patients screened, 12 patients (eight of whom could not respond or communicate, 2 had mental retardation and two objected to participate in the study due to conflicting schedules) were excluded.
Measures:
Participants completed a composite questionnaire comprising socio-demographic data that included age, sex, marital status, highest formal educational level, employment status, ethnic group, and religion, and selected disease-related variables such as epilepsy type. Medical records were reviewed to extract additional clinical information pertaining to the date of initiation of antiepileptic drugs (AEDs) and type of therapy (poly-therapy or mono-therapy).
Quality of Life in Epilepsy-31
The Quality of Life in Epilepsy-31 (QOLIE-31) instrument is a self- administered questionnaire. The QOLIE-31 assesses seven subscales: Overall Quality of Life, Seizure Worry, Emotional Well-Being, Energy/Fatigue, Cognitive, Medication Effects, and Social Function. These responses can yield seven individual scores (per subtest) and a total (composite) score (14). The QOLIE-31 has been used in Ugandan studies before and found to be applicable (10). The QOLIE-31 subscales and the overall quality of life scores were summarized using means and standard deviation.
Seizure severity scale
The Chalfont seizure severity scale was used to assess seizure severity of the study participants. It measures seizure severity and assesses the components of seizures most disturbing or disruptive to the patient. It has 11 questions with fractionated scores with a total of the scores for a given seizure type. It has high interrater and test–retest reliability; a change of 10 points or more on CSSS is considered clinically significant (15). An attending physician administered the Chalfont seizure severity scale during an interview with a patient and a person who is a witness to the seizures. A seizure severity score was generated for each seizure type the patient experienced. For this study, the analyses of seizure severity, the outcome was the total CSSS score (we selected the total CSSS score rather than the CSSS score for the specific seizure type because of the stipulation above regarding potential misclassification of seizure types reported by caregivers or patients). The Chalfont seizure severity score has been used in one epilepsy study in similar settings (16). It provides features of both a patient-based and an observer-based scale obtained by open interview of patients and caregivers with factor weightings were affected by the views of patients, caregivers and health care providers. An observer completes the scale and is readily employed in non-specialized outpatient clinics, and completed in a few minutes by a doctor or nurse practitioner.
Seizure frequency
Seizure frequency was arbitrary determined as the number of seizures reported by the PLWE or caregiver over a period of one year. The patients or immediate caregivers provided the seizure frequency information retrospectively, which we categorized to facilitate some statistical analyses: no seizures 1 year, one – nine seizures a year, having 10 – 20 seizures in a year and having more than 20 seizures in a year.
Statistical analysis
The socio-demographic and clinical variables were summarized using proportion and percentages for discrete variables and median for continuous variables. Spearman’s rank correlation coefficient (r) was used to correlate seizure severity scores and HRQoL. Associations with p values less than 5% were considered statistically significant. All analyses were performed using Stata version 14 (StataCorp.2015. Stata Statistical Software: Release 14, StataCorp LP, College Station, TX, USA).
Results
Demographic characteristics
Males comprised 56.3% (27/48) of the study participants. The median age in years (IQR) was 25 (19–34) years in PLWE. More than half of the study participants (56.3%) were unemployed, and 54.2% attained up to a primary education. The majority (79%) of participants were not married (38/48).
Clinical characteristics
As noted in Table 1, generalized seizures were the predominant seizure type, seen in 39 (83%) of study participants and these primarily were generalized tonic-clonic seizures with no significant gender differences. In the preceding four weeks before enrolment 34.0% (16/47) of the study participants reported a warning aura before the occurrence of a seizure. Over 83% (39/47) reported epilepsy onset before the age of 20 years and 60% (27/47) had epilepsy for over 10 years. The median age for epilepsy diagnosis was 12 (interquartile range: 6–18) years with no significant gender differences (p=0.434). Only three participants (6.4%) had epilepsy onset after 30 years of age. Only 11.4% (5/47) were receiving one AED, while the average number of AED as 2 (SD 1.2). The median seizure severity score (IQR) was 26 (12–88).
Table 1:
Clinical characteristics of the study participants
| n | Median (IQR) | |
|---|---|---|
| Female sex | 21 | 43.8% |
| Age onset of epilepsy in years | 48 | 12 (6 −18) |
| Seizure severity score | 48 | 28.5 (12 – 88) |
| Epilepsy type | n | % |
| Generalized | 40 | 83.3 |
| Partial | 8 | 16.7 |
| Seizure frequency/episodes per year | ||
| No seizure | 10 | 20.8 |
| 1–9 | 20 | 41.7 |
| 10–20 | 3 | 6.3 |
| ≥ 21 | 15 | 31.3 |
| Medication type | ||
| Monotherapy | 5 | 11.1 |
| Polytherapy | 40 | 88.9 |
| Duration of epilepsy | ||
| <2 years | 2 | 4.4 |
| 2 to <5 years | 6 | 13.0 |
| 5 to <10 years | 11 | 23.9 |
| ≥10 years | 27 | 58.7 |
Seizure frequency
Ten study participants (20.8%) reported no seizures over a period of 1 year, while nearly 42% (20/48) reported 1–9 seizures/year, three (6.3%) had 10 – 20, while 31.3% (15/48) had nearly two seizures per month.
On bivariate analysis, the QOLIE-31 scores were not associated with age in years (p=0.715), age at epilepsy onset (p=0.659), gender (p=0.076), marital status (p=0.500), employment (p=0.322), education status (p=0.735) and duration of epilepsy (p=0.380). None of the demographic characteristics was associated with severity of epilepsy.
Correlations between Chalfont seizure severity score and quality of life
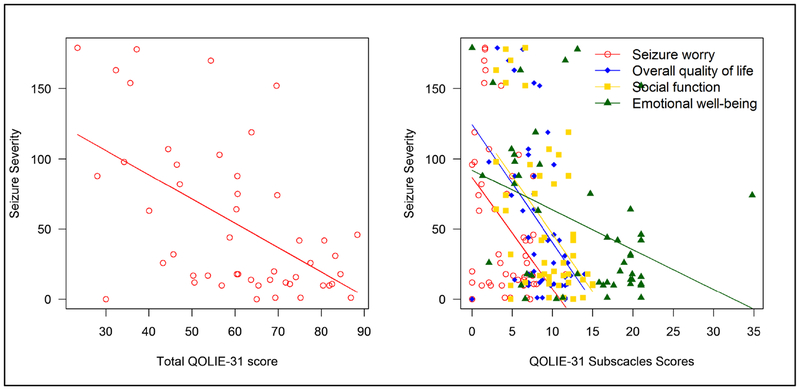
The median (IQR) of the total Chalfont seizure severity score was 26 (12 – 88). There was no gender difference in the Chalfont seizure severity score scores (p = 0.592). When correlation analysis was conducted between Chalfont seizure severity score and QOLIE-31 subscales, an inverse relationship existed between Chalfont seizure severity score and the total QOLIE-31 score (TS) (Spearman’s rank correlation coefficient, r = −0.48, p = 0.001), seizure worry (r = −0.31, p=0.030), overall quality of life (r = −0.44, p = 0.002), and social function (r =−0.35, p = 0.014), emotional well-being (r = −0.46, p = 0.001), energy fatigue (r = −0.49, p = 0.001) subscales of the QOLIE-31. Both cognitive function and medication effect were not correlated to Chalfont seizure severity score. See Table 2 showing the correlation analysis of seizure severity with the quality of life domains.
Table 2:
Correlation analysis of seizure severity with the quality of life domains
| Quality of life domains | Seizure severity score | |
|---|---|---|
| Spearman coefficient correlation | P-value | |
| Seizure worry (SW) | −0.31 | 0.030 |
| Overall quality of life (QOL) | −0.44 | 0.002 |
| Emotional well-being (EWB) | −0.46 | 0.001 |
| Energy fatigue (EF) | −0.49 | 0.001 |
| Cognitive function (CF) | −0.12 | 0.423 |
| Medication effect (ME) | −0.07 | 0.650 |
| Social function (SF) | −0.35 | 0.014 |
| Total QOLIE-31 score (TS) | −0.48 | 0.001 |
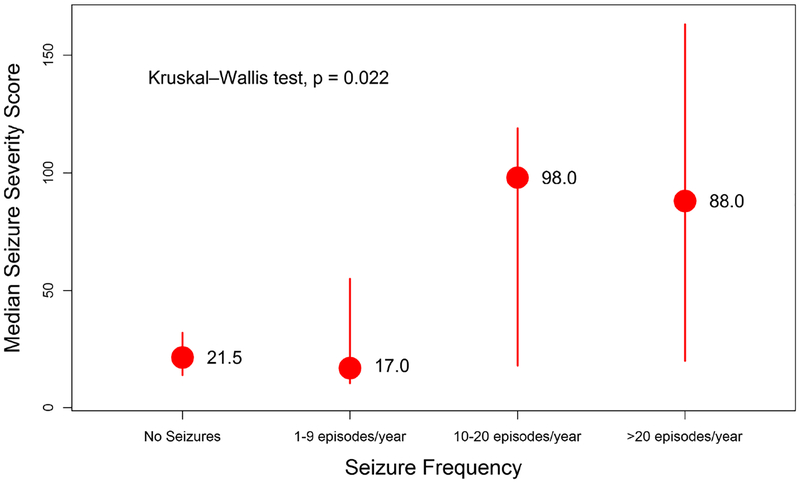
There were significant differences among study subjects where those with 10 or more seizures per year had a higher median Chalfont seizure severity score compared to those who had no seizures or experienced less than 10 seizures in a year, Kruskall Wallis test (p = 0.022). See Figure 1 showing the relationship between seizure frequency and Chalfont seizure severity score. There was an inverse relationship between QOLIE-31 score and the frequency of seizures. Overall participants with a higher seizure severity score the lower the total QOLIE-31 score. This was also reflected in the QOLIE-31 sub-scales scores. See figure 2 showing the relationship between Chalfont seizure severity score and subscales of the QOLIE-31
Figure 1.
Showing the relationship between seizure frequency and the median Chalfont seizure severity score
Figure 2.
Showing the relationship between the Chalfont seizure severity score and subscales of the QOLIE-31
Discussion
Whereas seizure frequency is an important primary treatment outcome, few studies in Africa have studied it. This is the first study to explore the effects of seizure severity on quality of life of PLWE in Uganda. Our findings revealed that people living with epilepsy who have a higher Chalfont seizure severity score had a lower overall QOLIE-31 score, and lower subscale scores for the various domains in the quality of life. Majority of health care providers often ask patients how many seizures they had in a given period of time and what the seizure was like to guide provision of care. Whereas both seizure frequency and seizure severity scores are important in guiding seizure control in PLWE, a focused seizure severity score represents a more comprehensive view of how outcomes related to seizures impact health more broadly.
Seizure frequency and seizure severity are interrelated yet they measure different aspects of epilepsy and both impact the quality of life. Earlier studies have reported associations between seizure frequency and quality of life (4, 6, 10, 17).
Seizure severity may worsen worrying about the occurrence of seizures and subsequently lead to social avoidant behaviors. An earlier study has reported that seizure severity is significantly correlated with several domains of the QOLIE-31 even when depression is controlled for (12). PLWE with more severe seizures especially those associated with generalization and falls were more likely to be worried about their seizure (18). In this study, seizure worry, overall quality of life, emotional wellbeing, energy fatigue and social function were all correlated with seizure severity. Seizure worry subscale assesses the PLWE’s worry about future seizures and anxiety over future injury arising from seizures, social embarrassment over having seizures, and anxiety over adverse side effects of medications. Perhaps not surprisingly, individuals with more frequent seizures (and more severe seizures) had higher levels of seizure worry.
The increased seizure severity impacts day to day activities and the social and leisure time activities such as visiting friends and relatives. This isolating behavior may reduce the amount and quality of interpersonal relationships and may increase the sense of being stigmatized. This might be attributed to the worry of seizing during these interactions which drives out personal comfort and peace, potentiating anxiety. The limited interpersonal relationships also increase the risk of developing depression and resignation to epilepsy morbidity. Cognitive function and medication effect were not correlated to Chalfont seizure severity score. Majority of the study participants had received post primary education and were receiving two or more drugs, majority of the study participants often run out of anti-epileptic drugs and have no out of pocket to refill them. This might be a plausible explanation for the lack of correlation between seizure severity, medication effect and cognitive function.
Therefore developing interventions that reduce seizure severity and increase personal autonomy could subsequently improve the quality of lives of PLWE.
A number of studies have reported an association of some determinants of seizure severity (such as falls, longer time to recovery, and generalized type of seizures) with quality of life (4, 9, 11, 12, 18); this suggests that improving these determinants could be an important intervention in epilepsy care. This seems to be a feasible and achievable target of targeted self-management programs in epilepsy care such as those in the Managing Epilepsy well (MEW network) (19). These programs aim at increasing PLWE and their caregivers’ knowledge, skills and confidence in monitoring disease symptoms, problem solving, decision-making, goal setting, appropriate communication as well as adopting healthful behaviors to improve health and quality of life. Therefore developing tailored interventions aimed at improving health related outcomes including reducing seizure frequency and/or severity provides a good achievable measure and practical attempt to improve the quality of life in PLWE especially within sub-Saharan Africa where majority of the epilepsy occurs. This study has a number of limitations, including relatively small sample size, the hospital-based setting which may be biased in favor of PWLE who have more severe or poorly controlled illness, the cross-sectional design which may not allow for generalization all Ugandan PLWE. A community-based longitudinal study with a larger sample size might be able to address these concerns. The seizure frequency was self-reported and the seizure frequency classifications were determined arbitrary. However, strengths of our study include the heterogeneous group of PLWE in our sample with a broad age range, variability in seizure control and use of standardized assessments of seizure severity.
In conclusion, this study found associations’ of Chalfont seizure severity score and the QOLIE-31 total score. Developing culturally feasible strategies to improve some determinants of seizure severity could be an important measure in epilepsy care in Uganda especially among those with difficult to control seizures. Understanding these determinants is a critical first step to developing and building targeted programs of epilepsy care in Uganda.
Highlights.
Epilepsy still remains a big challenge
Seizure severity remains unacceptably high in our settings
It directly impacts the quality of life of PLWE and their immediate families.
Interventions that reduce seizure severity are urgently needed in our settings.
Acknowledgements –
Not applicable
Funding - The study was supported by a grant from the National Institutes Health (K43TW010401 NINDS and Fogarty International Center (FIC) to Mark Kaddumukasa. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate
The institutional review boards (IRB) of Makerere University, College of Health Sciences’ School of Medicine (Rec Ref: 2017–112) and Uganda National Council of Science and Technology (UNCST), SS4486 approved the study. All participants provided written informed consent.
Consent for publication - Not applicable
Availability of data and material - All data generated or analyzed during this study are included in this published article.
Competing interests - The authors declare that they have no competing interests.
References
- 1.World Health Organisation, editor. Epilepsy Geneva: WHO; 2009. [Google Scholar]
- 2.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51(5):883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagarathnam M, Vengamma B, Shalini B, Latheef S. Stigma and Polytherapy: Predictors of Quality of Life in Patients with Epilepsy from South India. Annals of Indian Academy of Neurology. 2017;20(3):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leidy NK, Elixhauser A, Vickrey B, Means E, Willian MK. Seizure frequency and the health-related quality of life of adults with epilepsy. Neurology. 1999;53(1):162–6. [DOI] [PubMed] [Google Scholar]
- 5.van Hout B, Gagnon D, Souetre E, Ried S, Remy C, Baker G, et al. Relationship between seizure frequency and costs and quality of life of outpatients with partial epilepsy in France, Germany, and the United Kingdom. Epilepsia. 1997;38(11):1221–6. [DOI] [PubMed] [Google Scholar]
- 6.Baker GA, Jacoby A, Buck D, Stalgis C, Monnet D. Quality of life of people with epilepsy: a European study. Epilepsia. 1997;38(3):353–62. [DOI] [PubMed] [Google Scholar]
- 7.Kobau R, Cui W, Kadima N, Zack MM, Sajatovic M, Kaiboriboon K, et al. Tracking psychosocial health in adults with epilepsy--estimates from the 2010 National Health Interview Survey. Epilepsy & behavior: E&B. 2014;41:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nubukpo P, Clement JP, Houinato D, Radji A, Grunitzky EK, Avode G, et al. Psychosocial issues in people with epilepsy in Togo and Benin (West Africa) II: quality of life measured using the QOLIE-31 scale. Epilepsy & behavior: E&B. 2004;5(5):728–34. [DOI] [PubMed] [Google Scholar]
- 9.Imam I, Talabi OA, Sanya EO, Ogunniyi A. The quality of life of chronic epileptic patients in Ibadan, Nigeria. African journal of medicine and medical sciences. 2003;32(4):367–9. [PubMed] [Google Scholar]
- 10.Nabukenya AM, Matovu JK, Wabwire-Mangen F, Wanyenze RK, Makumbi F. Health-related quality of life in epilepsy patients receiving anti-epileptic drugs at National Referral Hospitals in Uganda: a cross-sectional study. Health and quality of life outcomes. 2014;12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bautista RE, Glen ET. Seizure severity is associated with quality of life independent of seizure frequency. Epilepsy & behavior: E&B. 2009;16(2):325–9. [DOI] [PubMed] [Google Scholar]
- 12.Harden CL, Maroof DA, Nikolov B, Fowler K, Sperling M, Liporace J, et al. The effect of seizure severity on quality of life in epilepsy. Epilepsy & behavior: E&B. 2007;11(2):208–11. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–30. [DOI] [PubMed] [Google Scholar]
- 14.Cramer JA, Perrine K, Devinsky O, Bryant-Comstock L, Meador K, Hermann B. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia. 1998;39(1):81–8. [DOI] [PubMed] [Google Scholar]
- 15.Duncan JS, Sander JW. The Chalfont Seizure Severity Scale. Journal of neurology, neurosurgery, and psychiatry. 1991;54(10):873–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Rashidy OF, Nassar MF, El Gendy YG, Deifalla SM, Gaballa S. Experience with MAD on children with epilepsy in Egypt after classic KD failure. Acta Neurol Scand. 2018;137(2):195–8. [DOI] [PubMed] [Google Scholar]
- 17.Birbeck GL, Hays RD, Cui X, Vickrey BG. Seizure reduction and quality of life improvements in people with epilepsy. Epilepsia. 2002;43(5):535–8. [DOI] [PubMed] [Google Scholar]
- 18.Adebayo PB, Akinyemi RO, Ogun SA, Ogunniyi A. Seizure severity and health-related quality of life of adult Nigerian patients with epilepsy. Acta neurologica Scandinavica. 2014;129(2):102–8. [DOI] [PubMed] [Google Scholar]
- 19.Helmers SL, Kobau R, Sajatovic M, Jobst BC, Privitera M, Devinsky O, et al. Self-management in epilepsy: Why and how you should incorporate self-management in your practice. Epilepsy & behavior: E&B. 2017;68:220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]