Abstract
Proinflammatory immune responses to Gram-negative bacterial lipopolysaccharides (LPS) are crucial to innate host defenses but can also contribute to pathology. How host cells sensitively detect structural features of LPS was a mystery for years, especially given that a portion of the molecule essential for its potent proinflammatory properties – lipid A – is buried in the bacterial membrane. Studies of responses to extracellular and vacuolar LPS revealed a crucial role for accessory proteins that specifically bind LPS-rich membranes and extract LPS monomers to generate a complex of LPS, MD-2, and toll-like receptor 4 (TLR4). These insights provided means to understand better both the remarkable host sensitivity to LPS and the means whereby specific LPS structural features are discerned. More recently, the noncanonical inflammasome, consisting of caspases-4/5 in humans and caspase-11 in mice, has been demonstrated to mediate responses to LPS that has reached the host cytosol. Precisely how LPS gains access to cytosolic caspases – and in what form – is not well characterized, and understanding this process will provide crucial insights into how the noncanonical inflammasome is regulated during infection. Herein, we briefly review what is known about LPS detection by cytosolic caspases-4/5/11, focusing on lessons derived from studies of the better-characterized TLR4 system that might direct future mechanistic questions.
Keywords: caspase, inflammasome, outer membrane vesicle
Summary Sentence:
How is activating LPS delivered to and recognized by cytosolic pro-caspases of the noncanonical inflammasome?
Graphical Abstract
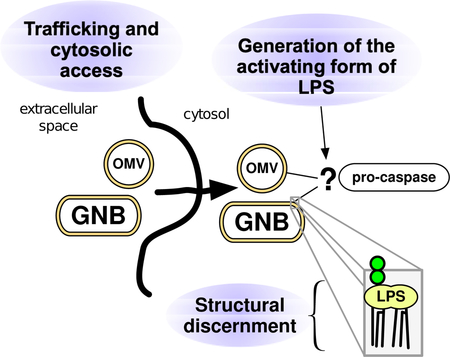
Introduction
Lipopolysaccharides (LPS) are structurally unique glycolipids that comprise much of the outer membrane of Gram-negative bacteria (GNB) and provide a prominent target for host pattern-recognition systems that mediate vital pro-inflammatory responses to infection [1, 2]. In the past 2–3 decades, many of the molecular mechanisms required for sensitive host responses to extracellular or intra-vacuolar LPS via toll-like receptor 4 (TLR4) have been elucidated [3, 4]. More recently, a novel cytosolic LPS-recognition system has been identified – the caspases of the noncanonical inflammasome [5] – but many of the molecular requirements for delivery and engagement of activating LPS by this system are still to be discerned. Herein, we briefly review the current understanding of cytosolic LPS sensing by the noncanonical inflammasome, focusing upon applying important concepts derived from studies of the TLR4 system.
Lessons from studies of MD-2 and toll-like receptor 4 (TLR4)
Richard Pfeiffer’s discovery in the late 19th century of a heat-stable “endotoxin” derived from the membrane of Vibrio cholerae laid the groundwork for seminal contributions to the understanding of innate immunity [1, 2]. Subsequent decades of chemical characterization revealed that the “endotoxic” properties of LPS were dependent on its lipid A portion [2, 6]. Lipid A containing the appropriate number, length, and configuration of fatty acids and charged constituents can activate mammalian innate immune responses at pM concentrations [7], whereas subtle deviations from this structure can render LPS less toxic, antagonistic, or even inert [8]. Initial research focused on the major species of LPS found in clinically-relevant Enterobacteriaceae, but subsequent decades of research have revealed marked structural diversity at different levels. Structures within a given bacterium are heterogeneous and vary depending upon environmental cues, and each extension of structural characterization to new pathogenic, commensal, and environmental bacteria has revealed a remarkable diversity of structures [8–11]. Efforts to better understand how variables in the structure of the LPS molecule that are buried in the bacterial membrane or expressed at the membrane interface affect TLR4 activation have revealed the capacity of LPS to engage in a variety of non-covalent interactions with distinct LPS-recognition proteins. Potent activation of TLR4 by LPS requires initial interactions of LPS-binding protein (LBP) and CD14 with LPS-rich interfaces followed by extraction and transfer of individual LPS monomers first to CD14 and then to MD-2/TLR4 [7, 12, 13]. Both the potency of LPS-induced activation and the signaling pathways induced depend on the efficiency of formation of LPS monomer.CD14 complexes and of dimerized LPS.MD-2.TLR4 ternary complexes [14–18]. Variables in the rates of either of these processes along with uptake of the LPS-rich particle likely determine the extent to which activated LPS.MD-2.TLR4 dimers form at the host cell surface to trigger MyD88-dependent signaling or within endosomes to trigger TRIF-dependent signaling [19–21] (reviewed in [4]). The ultimate response of the host MD-2/TLR4 to LPS is thus determined by interactions between multiple proteins and varied forms of LPS, at diverse tissue sites and in diverse cells, providing multiple points at which LPS recognition is regulated dynamically during Gram-negative infections.
Taken together, the understanding gained from studies of LPS-induced MD-2/TLR4 activation suggests organizing questions and concepts that can be applied to the recently described noncanonical inflammasome. These include: How is activating LPS delivered to the non-canonical inflammasome (i.e., the cytosolic pro-caspase)? Are cellular responses different when activating LPS is derived from internalized bacteria, replicating cytosolic bacteria, or bacteria-free LPS-rich particles, such as outer membrane vesicles (OMVs)? Are other host LPS-recognition proteins needed to promote delivery or generation of activating LPS? Does LPS activate the noncanonical inflammasome as it activates MD-2/TLR4, by engaging with LPS monomers or instead by providing an LPS-rich interface? And finally, how do variables in lipid A structure modify the agonist properties of LPS toward the resting pro-caspase?
Noncanonical inflammasome caspases as cytosolic LPS-sensors
Inflammasomes are multiprotein complexes that assemble in the cytosol in response to microbial invasion or cell damage and induce lytic cell death and release of IL-1ß (“pyroptosis”) [22, 23] or cell hyperactivation (i.e. IL-1ß release without cell death [24, 25]). Ligand specificity of canonical inflammasomes is conferred by receptor proteins that associate with accessory proteins that, in turn, bind and orient caspase-1 molecules to induce autocatalytic activation [22, 23]. By contrast, caspases of the noncanonical inflammasome (caspase-11 in mice, and −4 and −5 in humans) can directly bind to – and be activated by – purified LPS aggregates without need for accessory proteins [5]. In fact, the classic model of endotoxic shock can be rendered caspase-11-dependent, and TLR4-independent, if experimental animals are first exposed to stimuli that induce caspase-11 expression [26, 27]. Studies using both purified LPS and whole bacteria illustrate the vital role of cytosolic localization of LPS, achieved either by transfection of LPS into host cells in vitro [26, 27] or by infection with Gram-negative bacteria with a cytosolic phase of their life cycle or secretion systems that disrupt the integrity of the phagosome [28–32]. More recently, it was appreciated that extracellular outer membrane vesicles (OMVs), which are shed from Gram-negative bacteria during replication, can gain access to and activate cytosolic caspases, whereas purified LPS cannot [33–36]. Given that OMV generation appears to be a broadly conserved phenomenon, OMV-induced pyroptosis may be a general means whereby the host detects and responds to replicating extracellular bacteria [33]. Noncanonical inflammasomes thus appear to be able to respond to a variety of forms of activating cytosolic LPS in diverse contexts, and we now consider the emerging data on how this system might function.
How does LPS gain access to cytosolic caspases?
Transfected LPS and Gram-negative bacteria that disrupt the phagosome activate the noncanonical inflammasome [26–32, 37], consistent with the crucial role of cytosolic localization of LPS. Interferon (IFN) signaling enhances noncanonical inflammasome activation by several Gram-negative bacteria [29, 30, 37–41], raising the possibility that induction of one or more IFN-stimulated genes (ISGs) contributes to cytosolic localization of ingested LPS. Among the ISGs are guanylate-binding proteins (GBPs), which are dynamin-like GTPases involved in a variety of membrane trafficking events [42]. One report found that GBPs were required for disruption of Gram-negative pathogen-containing vacuoles (PVs) and activation of caspase-11 and that the need for GBPs could be bypassed by transfecting LPS into the cytosol [30]. However, other reports have found GBPs to enhance caspase-11 activation independently of PV rupture [40, 43, 44] and to enhance noncanonical inflammasome activation by transfected LPS [34, 40, 43]. IFN signaling – and GBPs, specifically – also contribute to activation of the noncanonical inflammasome by OMVs [34–36], although how OMV-derived LPS accesses the cytosol remains unknown. Thus, the preponderance of the data indicates that GBPs enhance caspase engagement by cytosolic LPS and do not mediate vacuolar disruption, though other IFN-induced factors may be important to cytosolic localization [43].
LPS is an amphipathic glycolipid and in aqueous environments is thus found in either bacterial membranes (e.g. an OMV or intact bacterium) or as high-molecular weight aggregates (e.g. purified LPS) [7, 45]. Not all LPS-rich membranes/aggregates are equivalent with respect to their abilities to activate the noncanonical inflammasome in whole cells. OMVs, but not purified LPS or heat-killed bacteria, can activate caspase-4/5/11-dependent pyroptosis via clathrin-dependent uptake [33], suggesting that as-of-yet undefined structural or compositional features of the OMV stimulate trafficking of activating LPS to the cytosol. The characteristic size of OMVs (20–250 nm) may engage specific mechanisms of uptake [46] in ways that facilitate LPS trafficking to the cytosol. In addition, the composition and lipid configuration of the outer leaflet vary between intact bacteria and OMVs, as suggested by the greater than four-fold greater potency of the latter in the Limulus amebocyte assay, even when normalized for LPS content [47]. OMVs, but not intact bacteria, are susceptible to the action of phospholipases, suggesting alteration of the typical membrane asymmetry of the outer membrane to place phospholipids in the outer leaflet [14]. Studies of their ability to deliver protein virulence factors suggest that OMVs can deliver luminal or membrane contents via fusion with host membranes [48, 49]. If combined with flipping of LPS to the cytosolic surface, such fusion events could potentially generate complex LPS-rich surfaces for caspase binding and activation. Given the variety of means whereby distinct forms of LPS might gain access to the cytosol, it becomes apparent that different forms of LPS might traffic distinctly within cells and that, as described next, the form of LPS presented to cytosol caspases may vary markedly in different physiologic settings.
What is the form of LPS that engages cytosolic pro-caspase-4/5/11?
A combination of careful biochemical, crystallographic, and microscopic approaches have been used to demonstrate that monomers of LPS are required for pM binding and activation of MD-2/TLR4 [7, 12, 13, 15–18]. These findings helped explain the key roles of LBP and CD14 in LPS-induced MD-2/TLR4 activation as well as the role of specific variables in lipid A structure on dimerization of LPS.MD-2.TLR4 ternary complexes and receptor activation. A similar level of mechanistic understanding of engagement and activation of pro-caspase-4/5/11 by LPS has not yet been achieved. By analogy to the binding of LPS monomers to MD-2/TLR4, many have speculated that initial engagement of LPS by pro-caspase is also via LPS monomers but without any experimental evidence as yet to support this view [50, 51]. It is noteworthy that LPS binding experiments with pro-caspase-11 implicated basic residues within the CARD domain as instrumental in LPS binding [5]. This is in contrast to the dominant role of the deep hydrophobic pocket of MD-2 in LPS monomer binding [16, 52, 53], but reminiscent of the structural determinants of LPS binding by LBP (and the closely-related bactericidal/permeability-increasing protein) that preferentially engage LPS-rich interfaces and not LPS monomers [54, 55]. As judged by gel electrophoresis and size-exclusion chromatography, binding of caspase-4/5/11 to purified LPS yields high molecular weight caspase/LPS complexes, consistent with binding of caspase to the naturally aggregated form of LPS [5]. Using catalytically-inactive purified recombinant pro-caspase-4 to model the initial interactions with LPS, we have shown that recombinant pro-caspase-4 interacts with high affinity both to purified LPS aggregates and to OMVs but not to protoplasts of Gram-positive bacteria or complexes of LPS monomers with CD14 [56]. Taken together, these findings seem most compatible with preferential interactions of pro-caspase-4/5/11 with the multiple anionic groups (e.g. phosphates, acidic sugars) of LPS clustered in close proximity at an LPS-rich interface [1, 57].
Since the mechanisms mediating delivery of activating LPS (monomers) to MD-2/TLR4 occur largely in the extracellular space, it has been possible to study this process cell-free with defined extracellular fluids or purified proteins (e.g., LBP and soluble CD14; [7, 14, 18, 58, 59]). In contrast, the trafficking and generation of caspase-activating LPS from ingested GNB and OMV occurs within largely inaccessible intracellular vacuoles. Consequently, the search for cytosolic and/or intra-vacuolar host proteins that promote this process has relied mainly on targeted deletion of specific proteins and protein families, including IRGB10 and GBPs that can apparently interact directly with intracellular GNB [41, 43] and OMVs [34, 35] and enhance noncanonical inflammasome activation [34–36, 41, 43]. These observations have relied on microscopy to infer the association of these proteins with ingested LPS, but the possible role of these intracellular interactions in the generation of caspase-activating forms of LPS cannot yet be judged. Techniques employed to date have also not been able to detect potential modifications of the structure or composition of ingested LPS-rich membranes. Finally, studies of MD-2/TLR4 indicate that ligand binding, receptor endocytosis, and signaling are distinct events that can be regulated by specific host proteins (e.g. CD14) and LPS and outer membrane structural features [14, 21, 60]. Whether ligand-binding and enzymatic activation of cytosolic caspases are also distinct events that are regulated independently remains to be determined. Thus, the diverse array of possible intracellular routes and forms of LPS generated following uptake of GNB or OMVs (Fig. 1) suggests that novel experimental approaches will be needed to directly identify and characterize caspase-activating LPS.
Figure 1.
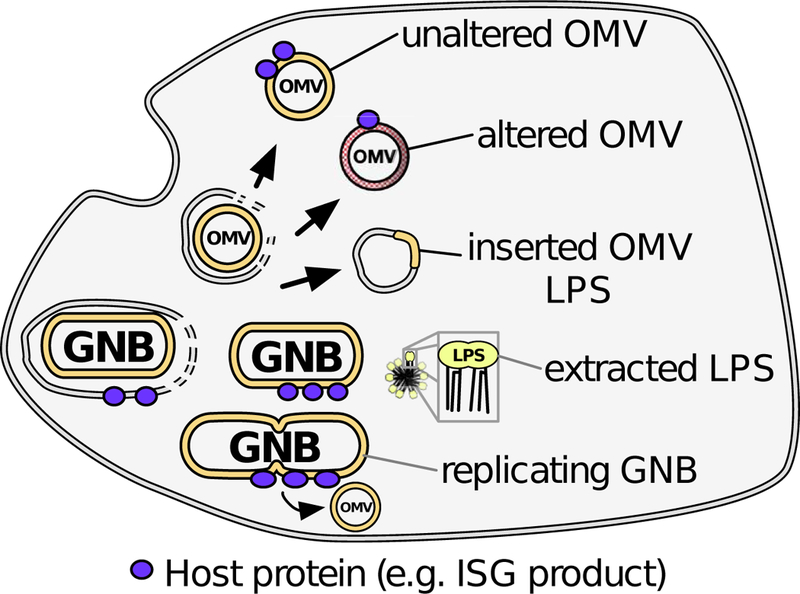
Schematic representation of diverse trafficking and processing of GNB and OMV that may take place after ingestion and yield caspase-activating LPS. Some Gram-negative bacteria (GNB) can escape to the cytosol, exposing their LPS-rich surfaces to cytosolic proteins and caspases. Extracellular OMVs could potentially emerge into the cytosol in their native state or altered in lipid content. Membrane extraction or fusion events could also generate structures with combinations of host and microbial lipid or even LPS-enriched aggregates. Finally, bacteria replicating in the cytosol may shed distinct OMVs or possess novel membrane surfaces.
How do noncanonical inflammasome caspases discern the structure of lipid A/LPS?
Both MD-2/TLR4 and noncanonical inflammasome caspases are preferentially activated by LPS structures with similar features – namely phosphorylated hexa-acyl >> penta- or tetra-acyl lipid A [5, 15–17, 26, 27, 61–63]. However, whereas the effect of differences in LPS acylation on MD-2/TLR4 activation is on the efficiency of dimerization of ternary complexes containing LPS monomers [15–18], we speculate that differences in LPS acylation affect the efficiency of caspase-4/5/11 activation by affecting the geometry of LPS-rich surfaces. Presumably, activation of the noncanonical inflammasome is achieved by circumstances in which pro-caspase binding to LPS brings caspase molecules into sufficient proximity to stimulate auto-catalytic activation. Studies with purified LPS have shown that variations in lipid A acylation can markedly alter the supramolecular arrangement and intermolecular packing of LPS within LPS aggregates [64, 65]. It is thus plausible that within physiologic asymmetric membranes – like OMVs, intact bacteria, or host vacuoles that have fused with bacterial membrane – variables in the packing of LPS at LPS-rich membrane interfaces could affect the spacing of bound caspase and hence the probability of autocatalytic activation (Fig. 2). In this way, LPS-induced caspase-4/5/11 activation could resemble that of Limulus factor C zymogen, in which productive engagement of an LPS-rich surface causes conformational alterations that induce proteolytic activity and intermolecular activation of neighboring LPS-bound zymogen molecules [5, 66, 67].
Figure 2.
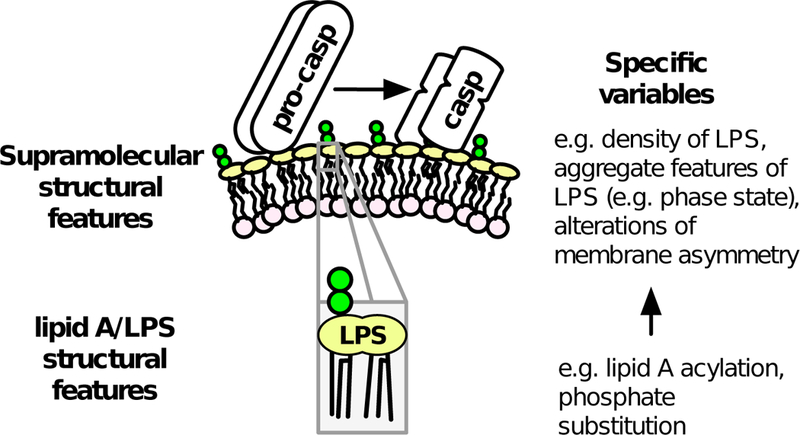
Potential effects of lipid A and LPS structure on physical characteristics of LPS-rich membranes. Specific features of individual LPS molecules could affect the membrane interface in ways that affect binding and activation of caspase-4/5/11.
Concluding remarks
The original discovery of LPS-sensing caspases was prompted by a search for a novel cytosolic LPS response system that was triggered by GNB that could establish intracellular infection and delivery of LPS to the host cell cytosol. However, ingested LPS-containing bacteria and bacteria-derived membranes can take multiple potential forms, each of which might have different capacities for trafficking to and activating the noncanonical inflammasome (Fig. 1). Whether the noncanonical inflammasome responds differently to Gram-negative bacteria replicating in the cytosol (and any associated shed OMVs) as compared to extracellular bacteria or OMVs that have been ingested and trafficked through membrane compartments is not known. As noted above, shed OMVs are approximately 4x more potent than the corresponding intact Gram-negative bacteria (normalized for LPS content) as activators of Limulus factor C [47], an ancient (horseshoe crab) LPS-regulated protease, indicating that all LPS-rich membranes may not be equivalent with regard to engagement and activation of host LPS-binding zymogens. For instance, if OMVs generated in the cytosol possess unique structural or compositional characteristics, then it is possible that their ability to traffic to and engage noncanonical inflammasome caspases is no longer as dependent upon TRIF- and IFN-inducible co-factors needed for noncanonical inflammasome activation by extracellular OMVs and Gram-negative bacteria. It also seems likely that additional extracellular regulators of the intracellular actions of OMVs and Gram-negative bacteria will be discovered as already suggested by the recent finding that HMGB1 that may promote the delivery and access of activating LPS to its cytosolic target [68]. Thus, the remarkable diversity of contexts in which caspases-4/5/11 are engaged by diverse forms of LPS will require novel conceptual and technical approaches to understand the regulation of this system during infection.
Acknowledgments.
This work was supported by funding from the Office of Extramural Research, National Institutes of Health (R01AI104728).
Abbreviations
- ISG
Interferon-stimulated gene
- GBP
guanylate-binding protein
- GNB
Gram-negative bacteria
- IFN
interferon
- ISG
interferon-stimulated gene
- IRGB10
Immunity-related GTPase B10
- LBP
lipopolysaccharide-binding protein
- MD-2
lymphocyte antigen 96
- MyD88
Myeloid differentiation primary response protein 88
- OMV
outer membrane vesicle
- TLR4
toll-receptor 4
- TRIF
toll or interleukin receptor (TIR) domain-containing adaptor-inducing interferon-ß
Footnotes
Conflict of Interest Disclosure. The authors declare no conflict of interest.
References
- 1.Raetz CR, Whitfield C 2002. Lipopolysaccharide endotoxins. Annu Rev Biochem 71: 635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler B, Rietschel ET 2003. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol 3: 169–176. [DOI] [PubMed] [Google Scholar]
- 3.Rosadini CV, Kagan JC 2017. Early innate immune responses to bacterial LPS. Curr Opin Immunol 44: 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss J, Barker J 2018. Diverse pro-inflammatory endotoxin recognition systems of mammalian innate immunity. F1000Res 7: 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514: 187–192. [DOI] [PubMed] [Google Scholar]
- 6.Westphal O, Lüderitz O, Rietschel ET, Galanos C 1981. Bacterial lipopolysaccharide and its lipid A component: some historical and some current aspects. Biochem Soc Trans 9: 191–195. [DOI] [PubMed] [Google Scholar]
- 7.Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP 2004. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A 101: 4186–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao X, Sankaranarayanan K, Khosla C 2017. Biosynthesis and structure-activity relationships of the lipid a family of glycolipids. Curr Opin Chem Biol 40: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trent MS, Stead CM, Tran AX, Hankins JV 2006. Diversity of endotoxin and its impact on pathogenesis. J Endotoxin Res 12: 205–223. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Quinn PJ 2010. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog Lipid Res 49: 97–107. [DOI] [PubMed] [Google Scholar]
- 11.Molinaro A, Holst O, Di Lorenzo F, Callaghan M, Nurisso A, D’Errico G, Zamyatina A, Peri F, Berisio R, Jerala R, Jiménez-Barbero J, Silipo A, Martín-Santamaría S 2015. Chemistry of lipid A: at the heart of innate immunity. Chemistry 21: 500–519. [DOI] [PubMed] [Google Scholar]
- 12.Prohinar P, Re F, Widstrom R, Zhang D, Teghanemt A, Weiss JP, Gioannini TL 2007. Specific high affinity interactions of monomeric endotoxin.protein complexes with Toll-like receptor 4 ectodomain. J Biol Chem 282: 1010–1017. [DOI] [PubMed] [Google Scholar]
- 13.Ryu JK, Kim SJ, Rah SH, Kang JI, Jung HE, Lee D, Lee HK, Lee JO, Park BS, Yoon TY, Kim HM 2017. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 46: 38–50. [DOI] [PubMed] [Google Scholar]
- 14.Post DM, Zhang D, Eastvold JS, Teghanemt A, Gibson BW, Weiss JP 2005. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J Biol Chem 280: 38383–38394. [DOI] [PubMed] [Google Scholar]
- 15.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO 2009. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458: 1191–1195. [DOI] [PubMed] [Google Scholar]
- 16.Ohto U, Fukase K, Miyake K, Satow Y 2007. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science 316: 1632–1634. [DOI] [PubMed] [Google Scholar]
- 17.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO 2007. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130: 906–917. [DOI] [PubMed] [Google Scholar]
- 18.Gioannini TL, Teghanemt A, Zhang D, Esparza G, Yu L, Weiss J 2014. Purified monomeric ligand.MD-2 complexes reveal molecular and structural requirements for activation and antagonism of TLR4 by Gram-negative bacterial endotoxins. Immunol Res 59: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagan JC, Medzhitov R 2006. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125: 943–955. [DOI] [PubMed] [Google Scholar]
- 20.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R 2008. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan Y, Zanoni I, Cullen TW, Goodman AL, Kagan JC 2015. Mechanisms of Toll-like Receptor 4 Endocytosis Reveal a Common Immune-Evasion Strategy Used by Pathogenic and Commensal Bacteria. Immunity 43: 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Place DE, Kanneganti TD 2018. Recent advances in inflammasome biology. Curr Opin Immunol 50: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubois H, Wullaert A, Lamkanfi M 2016. General Strategies in Inflammasome Biology. Curr Top Microbiol Immunol 397: 1–22. [DOI] [PubMed] [Google Scholar]
- 24.Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC 2018. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 48: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanoni I, Tan Y, Di Gioia M, Springstead JR, Kagan JC 2017. By Capturing Inflammatory Lipids Released from Dying Cells, the Receptor CD14 Induces Inflammasome-Dependent Phagocyte Hyperactivation. Immunity 47: 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, Dixit VM 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341: 1246–1249. [DOI] [PubMed] [Google Scholar]
- 27.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA 2013. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341: 1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA 2013. Caspase-11 protects against bacteria that escape the vacuole. Science 339: 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, Zamboni DS, Roy CR 2013. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A 110: 1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meunier E, Dick MS, Dreier RF, Schürmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, Yamamoto M, Broz P 2014. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509: 366–370. [DOI] [PubMed] [Google Scholar]
- 31.Baker PJ, Boucher D, Bierschenk D, Tebartz C, Whitney PG, D’Silva DB, Tanzer MC, Monteleone M, Robertson AA, Cooper MA, Alvarez-Diaz S, Herold MJ, Bedoui S, Schroder K, Masters SL 2015. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J Immunol 45: 2918–2926. [DOI] [PubMed] [Google Scholar]
- 32.Casson CN, Copenhaver AM, Zwack EE, Nguyen HT, Strowig T, Javdan B, Bradley WP, Fung TC, Flavell RA, Brodsky IE, Shin S 2013. Caspase-11 activation in response to bacterial secretion systems that access the host cytosol. PLoS Pathog 9: e1003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, Rathinam VAK 2016. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 165: 1106–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos JC, Dick MS, Lagrange B, Degrandi D, Pfeffer K, Yamamoto M, Meunier E, Pelczar P, Henry T, Broz P 2018. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J 37: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finethy R, Luoma S, Orench-Rivera N, Feeley EM, Haldar AK, Yamamoto M, Kanneganti TD, Kuehn MJ, Coers J 2017. Inflammasome Activation by Bacterial Outer Membrane Vesicles Requires Guanylate Binding Proteins. MBio 8: e01188–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu L, Meng R, Tang Y, Zhao K, Liang F, Zhang R, Xue Q, Chen F, Xiao X, Wang H, Wang H, Billiar TR, Lu B 2018. Toll Like Receptor 4 Signaling Licenses the Cytosolic Transport of Lipopolysaccharide from Bacterial Outer Membrane Vesicles. Shock [DOI] [PubMed] [Google Scholar]
- 37.Aachoui Y, Kajiwara Y, Leaf IA, Mao D, Ting JP, Coers J, Aderem A, Buxbaum JD, Miao EA 2015. Canonical Inflammasomes Drive IFN-γ to Prime Caspase-11 in Defense against a Cytosol-Invasive Bacterium. Cell Host Microbe 18: 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurung P, Malireddi RK, Anand PK, Demon D, Vande Walle L, Liu Z, Vogel P, Lamkanfi M, Kanneganti TD 2012. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem 287: 34474–34483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casson CN, Yu J, Reyes VM, Taschuk FO, Yadav A, Copenhaver AM, Nguyen HT, Collman RG, Shin S 2015. Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc Natl Acad Sci U S A 112: 6688–6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J 2014. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci U S A 111: 6046–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Man SM, Karki R, Sasai M, Place DE, Kesavardhana S, Temirov J, Frase S, Zhu Q, Malireddi RKS, Kuriakose T, Peters JL, Neale G, Brown SA, Yamamoto M, Kanneganti TD 2016. IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell 167: 382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daumke O, Praefcke GJ 2016. Invited review: Mechanisms of GTP hydrolysis and conformational transitions in the dynamin superfamily. Biopolymers 105: 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu BC, Sarhan J, Panda A, Muendlein HI, Ilyukha V, Coers J, Yamamoto M, Isberg RR, Poltorak A 2018. Constitutive Interferon Maintains GBP Expression Required for Release of Bacterial Components Upstream of Pyroptosis and Anti-DNA Responses. Cell Rep 24: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finethy R, Jorgensen I, Haldar AK, de Zoete MR, Strowig T, Flavell RA, Yamamoto M, Nagarajan UM, Miao EA, Coers J 2015. Guanylate binding proteins enable rapid activation of canonical and noncanonical inflammasomes in Chlamydia-infected macrophages. Infect Immun 83: 4740–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richter W, Vogel V, Howe J, Steiniger F, Brauser A, Koch MH, Roessle M, Gutsmann T, Garidel P, Mäntele W, Brandenburg K 2011. Morphology, size distribution, and aggregate structure of lipopolysaccharide and lipid A dispersions from enterobacterial origin. Innate Immun 17: 427–438. [DOI] [PubMed] [Google Scholar]
- 46.Turner L, Bitto NJ, Steer DL, Lo C, D’Costa K, Ramm G, Shambrook M, Hill AF, Ferrero RL, Kaparakis-Liaskos M 2018. Helicobacter pylori Outer Membrane Vesicle Size Determines Their Mechanisms of Host Cell Entry and Protein Content. Front Immunol 9: 1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoppe Parr KA, Hađina S, Kilburg-Basnyat B, Wang Y, Chavez D, Thorne PS, Weiss JP 2017. Modification of sample processing for the Limulus amebocyte lysate assay enhances detection of inflammogenic endotoxin in intact bacteria and organic dust. Innate Immun 23: 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog 5: e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jäger J, Keese S, Roessle M, Steinert M, Schromm AB 2015. Fusion of Legionella pneumophila outer membrane vesicles with eukaryotic membrane systems is a mechanism to deliver pathogen factors to host cell membranes. Cell Microbiol 17: 607–620. [DOI] [PubMed] [Google Scholar]
- 50.Yi YS 2017. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 152: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demon D, Vande Walle L, Lamkanfi M 2014. Sensing the enemy within: how macrophages detect intracellular Gram-negative bacteria. Trends Biochem Sci 39: 574–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teghanemt A, Re F, Prohinar P, Widstrom R, Gioannini TL, Weiss JP 2008. Novel roles in human MD-2 of phenylalanines 121 and 126 and tyrosine 131 in activation of Toll-like receptor 4 by endotoxin. J Biol Chem 283: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 53.Resman N, Vasl J, Oblak A, Pristovsek P, Gioannini TL, Weiss JP, Jerala R 2009. Essential roles of hydrophobic residues in both MD-2 and toll-like receptor 4 in activation by endotoxin. J Biol Chem 284: 15052–15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamping N, Hoess A, Yu B, Park TC, Kirschning CJ, Pfeil D, Reuter D, Wright SD, Herrmann F, Schumann RR 1996. Effects of site-directed mutagenesis of basic residues (Arg 94, Lys 95, Lys 99) of lipopolysaccharide (LPS)-binding protein on binding and transfer of LPS and subsequent immune cell activation. J Immunol 157: 4648–4656. [PubMed] [Google Scholar]
- 55.Beamer LJ, Carroll SF, Eisenberg D 1998. The BPI/LBP family of proteins: a structural analysis of conserved regions. Protein Sci 7: 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wacker MA, Teghanemt A, Weiss JP, Barker JH 2017. High-affinity caspase-4 binding to LPS presented as high molecular mass aggregates or in outer membrane vesicles. Innate Immun 23: 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikaido H 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67: 593–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitchens RL, Thompson PA 2005. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res 11: 225–229. [DOI] [PubMed] [Google Scholar]
- 59.Teghanemt A, Weiss JP, Gioannini TL 2013. Radioiodination of an endotoxin·MD-2 complex generates a novel sensitive, high-affinity ligand for TLR4. Innate Immun 19: 545–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC 2011. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147: 868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lagrange B, Benaoudia S, Wallet P, Magnotti F, Provost A, Michal F, Martin A, Di Lorenzo F, Py BF, Molinaro A, Henry T 2018. Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11. Nat Commun 9: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS 2013. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc Natl Acad Sci U S A 110: 1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teghanemt A, Zhang D, Levis EN, Weiss JP, Gioannini TL 2005. Molecular basis of reduced potency of underacylated endotoxins. J Immunol 175: 4669–4676. [DOI] [PubMed] [Google Scholar]
- 64.Schromm AB, Brandenburg K, Loppnow H, Moran AP, Koch MH, Rietschel ET, Seydel U 2000. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur J Biochem 267: 2008–2013. [DOI] [PubMed] [Google Scholar]
- 65.Schromm AB, Brandenburg K, Loppnow H, Zähringer U, Rietschel ET, Carroll SF, Koch MH, Kusumoto S, Seydel U 1998. The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J Immunol 161: 5464–5471. [PubMed] [Google Scholar]
- 66.Shibata T, Kobayashi Y, Ikeda Y, Kawabata SI 2018. Intermolecular autocatalytic activation of serine protease zymogen factor C through an active transition state responding to lipopolysaccharide. J Biol Chem 293: 11589–11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kobayashi Y, Shiga T, Shibata T, Sako M, Maenaka K, Koshiba T, Mizumura H, Oda T, Kawabata S 2014. The N-terminal Arg residue is essential for autocatalytic activation of a lipopolysaccharide-responsive protease zymogen. J Biol Chem 289: 25987–25995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, Zhao X, Liu J, Tang C, Liu Z, Huang Y, Peng H, Xiao L, Tang D, Scott MJ, Wang Q, Liu J, Xiao X, Watkins S, Li J, Yang H, Wang H, Chen F, Tracey KJ, Billiar TR, Lu B 2018. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 49: 740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]


