Abstract
Belatacept, the CD28-B7 costimulation pathway inhibitor, has been approved as a calcineurin inhibitor (CNI) alternative in kidney transplantation. Although costimulation blockade (CoB) allows for CNI avoidance, it is associated with increased rates of early rejection, prompting a search for agents to pair with belatacept. Methotrexate (MTX) is an antimetabolite that has been found to be complimentary with abatacept, a lower affinity CD28-B7-specific analogue of belatacept, in the treatment of rheumatoid arthritis (RA). We examined whether this synergy would extend to prevention of kidney allograft rejection. Rhesus macaques underwent kidney transplantation treated with abatacept maintenance therapy with either a steroid taper, MTX, or both. The combination of abatacept maintenance with steroids prolonged graft survival compared to untreated historical controls and previous reports of abatacept monotherapy. The addition of MTX did not provide additional benefit. These data demonstrate that abatacept with adjuvant therapy may delay the onset of acute rejection, but fail to show synergy between abatacept and MTX beyond that of steroids. These findings indicate that MTX is unlikely to be a suitable adjuvant to CoB in kidney transplantation, but also suggest that with further modification, a CoB regimen used for advanced RA may suffice for RA patients requiring kidney transplantation.
Keywords: methotrexate, costimulation, non-human primate
Introduction
Since the introduction of cyclosporine, and later, tacrolimus, calcineurin inhibitors (CNIs) have formed the backbone of maintenance immunosuppression in clinical kidney transplantation. Recently, the development of the B7-CD28 targeted fusion protein, abatacept (CTLA4-Ig), and more importantly the approval of the second-generation molecule, belatacept, has offered an opportunity to replace CNIs and their inherent side effects. Indeed, the BENEFIT (1) and BENEFIT-EXT (2) trials showed improved graft function in belatacept treated groups compared to cyclosporine with similar graft survival. Extended follow-up further suggests those gains in renal function translate to improved graft survival in the long term (3). Despite these promising results, early acute cellular rejection has been seen with increased frequency in patients treated with belatacept (1–4), and this costimulation blockade (CoB) resistant rejection has tended to be more severe than early rejection episodes in CNI treated patients.
Significant effort has been put forth to understand CoB resistant rejection and find additional agents that may ameliorate the associated early rejection without sacrificing long-term salutary effects. Much of this work has focused on targeting other immunomodulatory pathways. Combining CD28-based agents with monoclonal antibodies targeting the CD40-CD154 interaction has met with significant success in animal models (5–10); however, translation to human studies has been hampered by thrombotic events associated with anti-CD154 antibodies (11). Our group recently reported that the combination of belatacept with LFA-1 blockade, a regimen shown to be quite efficacious in models of islet transplantation (12), did not share the same success in non-human primate renal transplantation (13). These targeted biologics have met with only modest success and come with their own set of potential side effects and costs.
Methotrexate (MTX) is a competitive inhibitor of dihydrofolate reductase, halting DNA synthesis and cell division by reducing the availability of donor methyl groups, particularly in the generation of the DNA precursor thymidylate. Methotrexate has a multitude of clinical uses, primarily in autoimmunity such as rheumatoid arthritis (RA) (14–16), asthma (17,18), and psoriasis (19,20), but also in oncology (21–23) as well. It has long been used in hematopoietic transplantation in the prevention of graft-versus-host disease (24–26). Several limited studies in solid organ transplantation also have been completed. Multiple small studies showed MTX to reverse recalcitrant rejection successfully in cardiac (27–31) and lung (32–34) transplantation. Dosing in these studies varied, and was often higher than used for treatment of autoimmunity. One study using methotrexate in addition to cyclosporine and steroid maintenance in renal transplantation studies showed reduction in rejection episodes at 6 months and lower serum creatinine at 12 months (35).
In the treatment of RA, MTX has been one of the most commonly used agents. However, a proportion of patients will continue to have active disease despite appropriate MTX dosing. The addition of CoB using abatacept has been shown to be beneficial to this patient group (36–38). There is reason to believe this combination may also be effective in transplantation. Methotrexate is known to be pro-apoptotic for mitogen activated T cells while leaving resting cells alone, and has been shown to prevent recall responses in T cells exposed to alloantigen for which they were previously sensitized (39). It is this latter effect that is particularly notable, as memory responses are thought to play a major role in early CoB-resistant rejection. Methotrexate also is known to decrease expression of surface adhesion molecules important for lymphocyte homing and exit into tissues (40). Furthermore, as an anti-proliferative agent, MTX may have synergistic effects with CoB similar to those seen with the anti-purine medications such as mycophenolate or azathioprine. Although belatacept is the approved CoB agent for use in transplantation, abatacept is mechanistically identical, differing only by affinity. Additionally, it is available in a subcutaneous formulation, which could offer an option for patients with poor vascular access or lack of a suitable infusion center. Therefore, we sought to determine whether the addition of MTX to a maintenance regimen of abatacept and methylprednisolone would prolong graft survival in a non-human primate (NHP) model of renal transplantation. We show that abatacept prolongs graft survival in this pre-clinical model, but show no benefit from the addition of MTX beyond that seen with steroids. Our results highlight the potential for prolonged graft survival with CoB monotherapy if early rejection can be avoided, reiterate the efficacy of abatacept in kidney transplantation, and suggest therapeutic options for patients with RA requiring kidney transplantation.
Materials and Methods
Protocols for the care of all experimental animals in this study were approved by the Emory University Institutional Animal Care and Use Committee and designed to comply with the principles laid out in The Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, DHHS (41). Veterinary staff were actively involved in the care of all animals, examining them on a regular basis. Rhesus macaques (Macaca mulatta) were obtained from breeding colonies at AlphaGenesis, Inc. (Yemassee, SC, USA) or Yerkes National Primate Research Center (Lawrenceville, GA, USA). Class I and class II MHC typing by 454 pyrosequencing (University of Wisconsin, Madison, WI, USA) was obtained for each individual. Donor-recipient pairs were selected for matched size and maximal MHC disparity. Transplantation was performed in a domino fashion to maximize the utility of the available animals, with each animal serving as a kidney donor prior to receiving a transplant, while avoiding bilateral retroperitoneal dissection within a single operation. Left donor nephrectomy was performed at least 3 weeks prior to transplantation in order to allow sufficient recovery time prior to a second laparotomy. Renal transplantation was performed as previously described (10), with concomitant right nephrectomy leaving each animal entirely dependent on its allograft. Post-transplant monitoring consisted of daily clinical assessment by veterinary staff. Laboratory studies including serum chemistry and complete blood count were performed at least weekly, or more often as dictated by the animal’s clinical course.
Experimental Groups
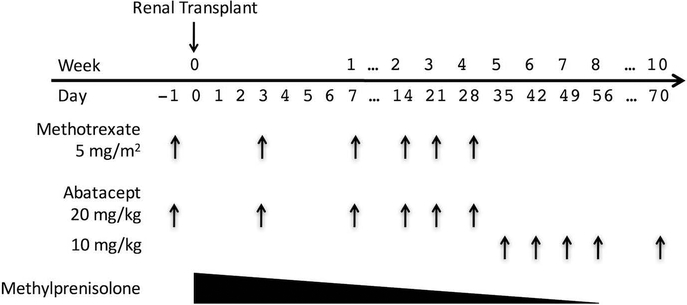
There were three experimental groups (Figure 1): Group 1 received abatacept and methylprednisolone, Group 2 received abatacept and methotrexate, and Group 3 received abatacept, methylprednisolone, and methotrexate. Abatacept was dosed at 20mg/kg on days −1, 3, 7, 14, 21, and 28 relative to the day of transplantation. Abatacept maintenance therapy was then continued at 10mg/kg weekly until day 56, then biweekly indefinitely. Two groups received a methylprednisolone taper beginning on the day of transplantation with a dose of 15 mg/kg IV. The dose was converted to IM and reduced by half daily until a maintenance dose of 0.5mg/kg daily was reached. Termination of steroid therapy was based on the animals’ clinical appearance and ranged from day 30 to day 60. Methotrexate was dosed at 5mg/m2 on days −1, 3, 7, 14, 21, and 28. Body surface area (BSA) for a rhesus macaque was calculated using the DuBois equation, substituting head-to-anus length for height and multiplying by a factor of 1.147 (42).
Figure 1:
Experimental Plan. All animals received maintenance abatacept. Groups 1 and 3 received the steroid taper. Groups 2 and 3 received 4 weeks of methotrexate.
Flow Cytometric Analysis
Analysis of circulating immune cell phenotypes was performed both prior to transplant and at regular intervals following transplantation. Cell frequencies from flow cytometric analysis were combined with complete blood counts to calculate total numbers of circulating T cells and various T cell subsets. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density centrifugation (BD Biosciences, Franklin Lakes, NJ) within 6 hours of phlebotomy. PBMCs (1.5×10^6) were then incubated with antibody mixtures at the appropriate titer for 15 minutes and washed twice. For assessment of intracellular markers, cells were fixed and permeabilized with BD Cytofix/Cytoperm (BD Biosciences) according to the manufacturer’s direction following surface staining. Flow cytometric data was acquired immediately using a BD LSR II multicolor flow cytometer (BD Biosciences). All flow data was analyzed using FlowJo (Tree Star, San Carlos, CA).
Surface markers were stained with the following monoclonal antibodies (mAbs): CD3 PacBlue, CD3 APC-Cy7, CD4 PerCP-Cy5.5, CD8 V500, CD28 PE-Cy7, CD127 PE-Cy7, PD-1 APC, LFA-1 APC, CD20 APC-Cy7 (all BD Biosciences), CD95 PacBlue, CD69 FITC (Invitrogen, Grand Island, NY), and CD25 PE (Miltenyi Biotech, San Diego, CA). Intracellular staining for FoxP3 was performed using FoxP3 Alexa488 (Biolegend, San Diego, CA).
Viral Monitoring
In order to assess treatment effect on protective immunity, weekly analysis of rhesus cytomegalovirus (rhCMV) viral loads was performed by quantitative real-time polymerase chain reaction using DNA isolated from whole blood as previously described (43). Levels of greater than 10,000copies/mL were considered significant and were treated with ganciclovir (6mg/kg IM twice daily) until resolution of the viremia. No routine viral prophylaxis was administered.
Statistics
All statistical analyses were performed in Prism 5 (GraphPad Software, La Jolla, CA, USA). Survival statistics were calculated using the Mantel-Cox method. For all analyses, a two-tailed p-value of < 0.05 was considered statistically significant.
Results
The Tested Regimens are Nondepleting and Preserve Protective Immunity.
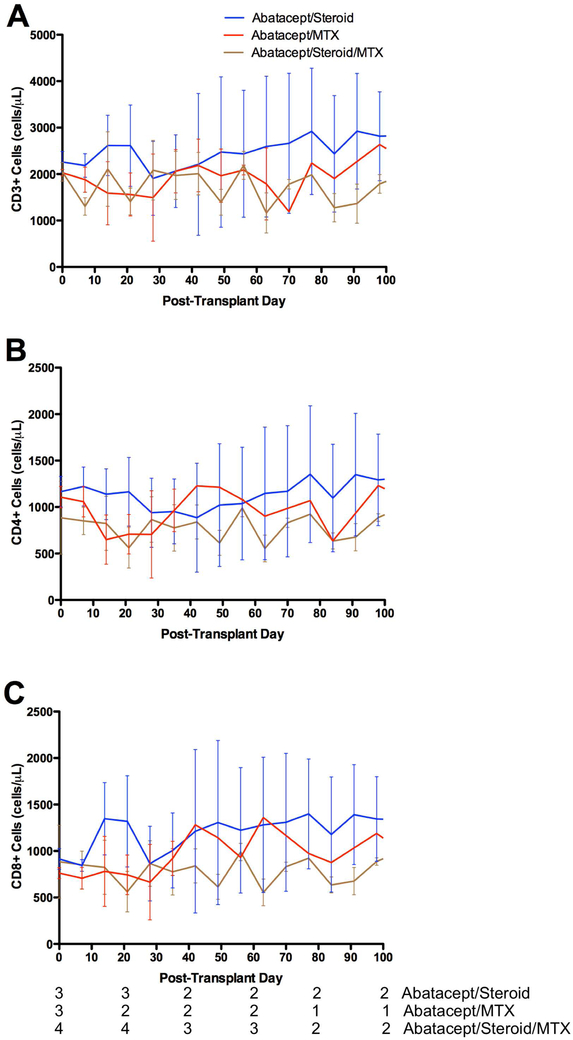
Weekly flow cytometry was performed to measure circulating numbers of immune cells. The number of circulating CD3+ cells was not significantly different between any of the treatment groups (Figure 2A). Furthermore, there were no significant changes in the numbers of circulating CD4+ or CD8+ cells (Figures 2B, 2C). As a measure of the effect of these regimens on protective immunity, rhCMV levels were monitored weekly. No animals had significant CMV viremia over the treatment course (Figure S1), and no animals required antiviral treatment.
Figure 2:
Absolute counts of (A) CD3+, (B) CD4+, and (C) CD8+ cells were relatively stable over the treatment course and were not affected by treatment regimen.
Maintenance Abatacept with Steroid Induction Prolongs Renal Allograft Survival.
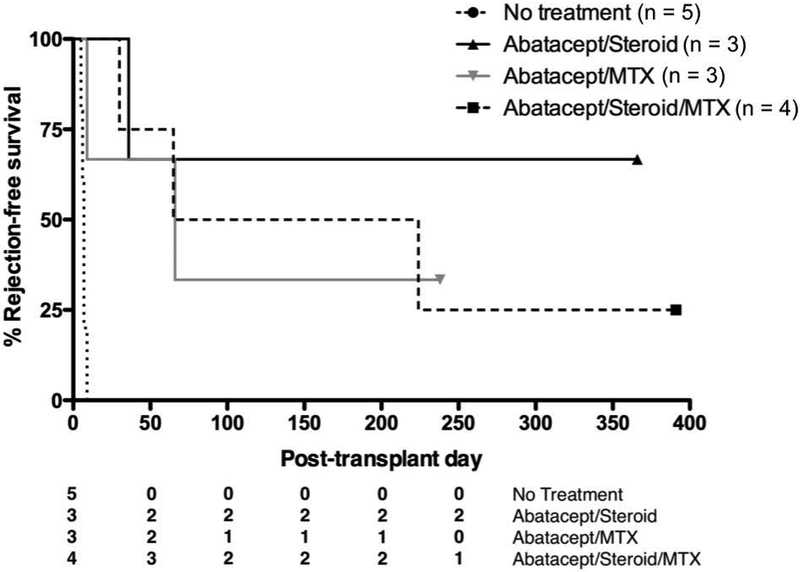
Rejection-free survival was measured for all animals (Table 1, Figure 3). Three animals received a combination of abatacept and methylprednisolone. One animal had rejection at 36 days. The two remaining animals had prolonged graft survival and were sacrificed with functioning grafts at 1 year. Each had a serum creatinine less than 1mg/dL (Figure S2). Historical controls from our lab that were given no immunosuppression had mean graft survival of 6.8 days (p = 0.01 for comparison with Group 1) (10).
Table 1:
Rejection-free survival for each treatment group.
| Treatment Group | Rejection-Free Survival (days) |
|---|---|
| No Treatment (Historic Control) | 5, 6, 7, 7, 9 |
| Group 1: Abatacept + Steroid | 36, >366, >366 |
| Group 2: Abatacept + Methotrexate | 9, 66, >238 |
| Group 3: Abatacept + Steroid + Methotrexate | 30, 65, 224, >391 |
Figure 3:
Survival curves for treatment groups. All regimens prolonged graft survival versus no therapy. There was no statistical difference between any of the treatment groups.
The Addition of a Short Course of Methotrexate Does Not Provide Additional Benefit.
Two additional groups received a four-week course of MTX, either instead of, or in addition to, the methylprednisolone taper. In the group that received abatacept and MTX, there was one long-term graft survival of greater than 386 days. Two animals rejected their grafts at 9 and 66 days. Four animals were transplanted in the group receiving all three medications. There was one long-term graft survival (>391 days). The remaining animals had graft rejection at 30, 65, and 224 days. Neither group had graft survival significantly different than the group receiving abatacept and methylprednisolone (Figure 3, p = 0.49 for Group 1 vs. Group 2, p = 0.47 for Group 1 vs. Group 3).
Memory Cell Populations Are Not Affected by Treatment.
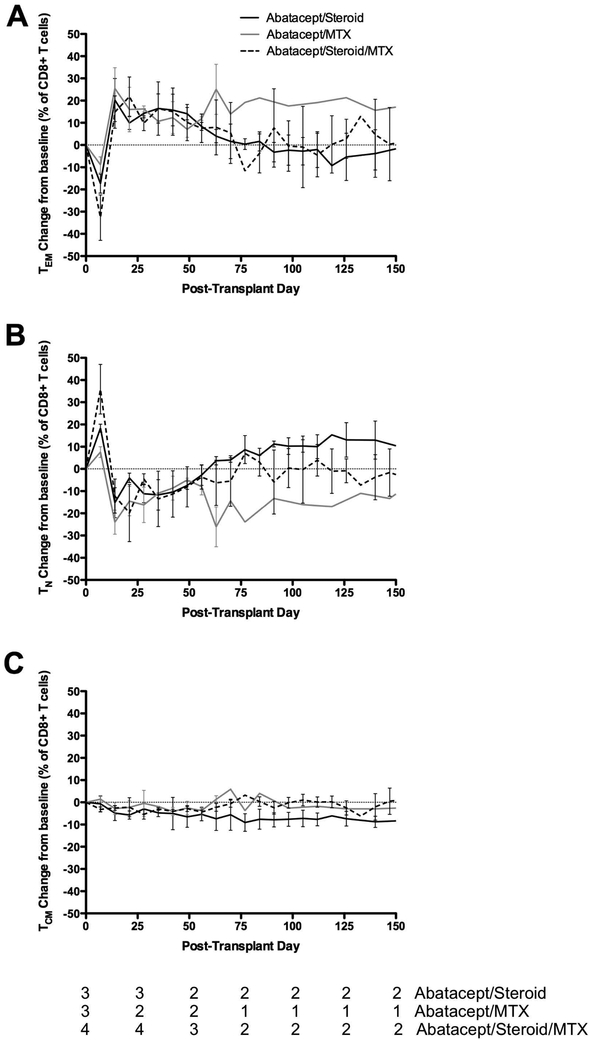
The phenotypes of circulating T cells also were measured by flow cytometry. One week following transplant, there was a decline in the relative abundance of circulating CD8+ effector memory T cells (TEM, Figure 4A). This was matched by an increase in the relative abundance of naive CD8+ T cells (TN, Figure 4B). This effect was reversed completely by post-operative day 14, and CD8+ TEM abundance remained above baseline through post-operative day 70. The kinetics of these changes were essentially similar regardless of treatment regimen, but the amplitude of changes seen in the first week was greatest in the group receiving triple therapy. Over the first 10 weeks, there was a slight downward trend in the percentage of circulating CD8+ central memory T cells (TCM) in all populations (Figure 4C). Interestingly, the groups that received methotrexate had a stabilization in the central memory compartment, while the group that did not get MTX saw continued declines. The relative abundance of these memory subpopulations in CD4+ was unchanged over time and also unaffected by treatment regimen (data not shown).
Figure 4:
Memory subpopulations of CD8+ T cells: (A) TEM, (B) TN, and (C) TCM. The kinetics of changes in memory subpopulations was not affected by treatment regimen
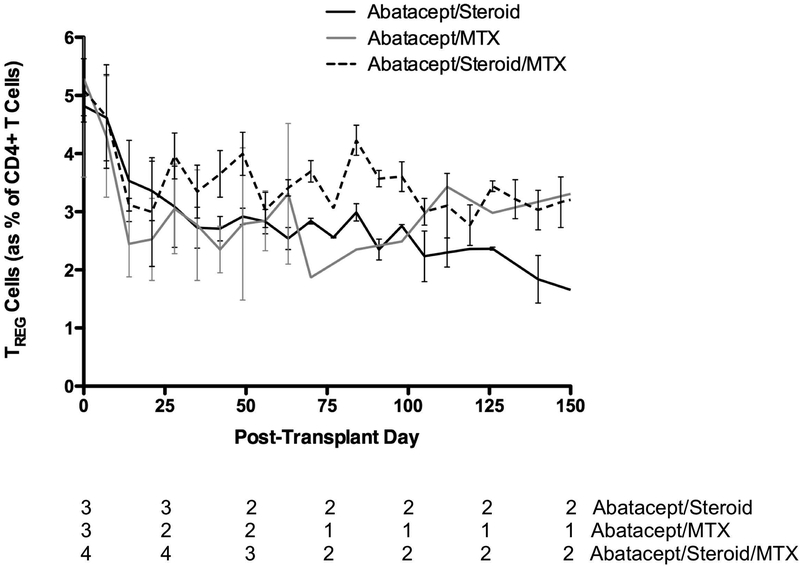
Regulatory T Cells Are Reduced in All Treatment Groups.
To assess the effect of these treatment regimens on circulating regulatory T cells (TREG), flow cytometric analysis of this population was performed weekly. Lymphocytes were gated for CD3, CD4, and FoxP3. Over the first four weeks, all animals saw decreased levels of circulating TREG cells, both by percentage of circulating CD4+ T cells (Figure 5) and by absolute cell counts (data not shown). Levels of TREG cells remained stable at this reduced level for the duration of the experiment. This effect was seen in all treatment groups and irrespective of the presence of methylprednisolone or MTX.
Figure 5:
TREG cells were decreased in all treatment arms.
Discussion
This study was designed to evaluate the effectiveness of a combination of abatacept and MTX in renal transplantation. Several factors provided justification for choosing abatacept. First, abatacept is mechanistically identical to belatacept, albeit with poorer binding affinity (44), and it is already FDA approved for use in combination with MTX in RA. While belatacept would presumably exhibit the same synergy, this has not been proven. Its availability as a subcutaneous injection removes the potential logistic and vascular access barriers inherent to belatacept administration. We sought to determine whether the efficacy of MTX combined with abatacept in the autoimmune disease setting would extend to transplantation.
Transplant pairs were selected to maximize MHC disparity in order to test these regimens in as rigorous a model as possible. It is possible that lesser degrees of MHC mismatch would result in longer graft survival, although it is unknown whether this effect would be independent of immunosuppression regimen. Prior to the availability of Rhesus MHC typing, mixed lymphocyte reactions were typically used to confirm disparity in non-human primate studies. While this was able to confirm the alloreactive response, we feel the direct sequencing of the Rhesus MHC locus allows for better mismatching of the donor-recipient pairs, particularly since the animals come from a small number of colonies and are frequently related.
The clinical dosing of MTX varies significantly with the indication, with some protocols using a simple dose titrated to effect, while others use weight-based or BSA-based calculations. We sought to approximate the dosing typically used in autoimmune disease. Dosing for RA or severe psoriasis starts at 7.5-10mg weekly and can be titrated up to effect. Given an average BSA of adult 1.8m2(45), this range is 4.2-5.5mg/m2. Therefore, we chose a dose of 5mg/m2. Oncologic use of MTX is often at a much higher dose, and it is possible a higher dosing scheme would yield substantially different results. Similarly, lengthening the treatment course of methotrexate similar to its use in autoimmunoty may have changed the outcomes of those treatment groups. However, we sought to examine the effect of methotrexate as an adjuvant in the early post-transplant period when the risk of rejection is highest and whether avoidance of early rejection would provide a lasting benefit, therefore the course of methotrexate was limited. The dosing of abatacept used here is higher than typically used for treatment of autoimmunity in humans (20mg/kg vs 10mg/kg), but we elected to use the higher dose for consistency with previously published reports using abatacept in the non-human primate model.
Our data show that the use of abatacept with an adjuvant agent was effective at prolonging graft survival when compared to untreated historic controls. Some prolongation of graft survival in these groups was expected based on prior reports of animals receiving abatacept monotherapy having graft survival of 5-58 days depending on the dose used (10,44). Given these previous results, abatacept monotherapy is not a clinically relevant regimen, and a monotherapy group was not performed here. In this study, one animal that received abatacept and steroids rejected its graft at 36 days, similar to those in the aforementioned studies. However, the two remaining animals in that group both had graft survival over 1 year. Additionally, the one animal in the group receiving abatacept and MTX that avoided rejection in this early period also had good long-term survival. This suggests that if early rejection can be prevented prolonged graft survival with CoB monotherapy is possible.
The prolonged graft survival seen in the abatacept/steroid group was a surprising result. Failure of abatacept monotherapy prompted the development of belatacept by Larsen et al. (44), and our results compare favorably to the belatacept cohorts in that study. However, several distinctions can be made. In this study, animals received a slightly higher dose of abatacept, and this was continued indefinitely, whereas the prior study stopped abatacept at day 16 and belatacept at day 70. The steroid taper used here was also significantly higher than the steroid taper used by Larsen et al. It is possible the higher steroid dosing compensated for the lower binding affinity of abatacept compared to belatacept.
The interpretation of the results of our study is limited by a few factors. There are a small number of animals in each cohort. This is further exaggerated by several early rejections, so later time points may only have one or two individuals. The surprising success of the abatacept/steroid group also limits the usefulness of this group as a comparator, as it unlikely any regimen tested would show any significant additional benefit within the design of this study.
Replacing steroids with MTX as the adjuvant to abatacept provided no benefit in terms of graft survival. In fact, this group had the earliest rejection in the study, at 9 days. Addition of MTX to the abatacept/steroid treatment to create a three-agent regimen also provided no graft survival advantage versus abatacept and steroids. Review of the survival curves appears to show increased numbers of rejections seen in the groups receiving MTX. However, given the small sample sizes, it is difficult to determine whether this truly represents a negative effect of MTX. Comparison of the abatacept/steroid group versus all animals receiving MTX, with or without steroids, also failed to show any statistically significant difference (p = 0.42, curves not shown).
Interestingly, in the group receiving triple therapy, there was one late rejection at 224 days. It is unclear why this animal had a relatively late rejection, while other animals rejected early or not at all. Analysis of T cell memory subsets during this period revealed that at most time points, compared to the long-term survivor, the late rejector had higher numbers of circulating CD3+ cells and higher percentages of CD4+ and CD8+ TEM (Figure S3). Whether this truly is related to the late rejection experienced by this animal is unclear. Some differences in the relative abundance of the various T cell subsets between treatment groups was seen at later time points, but this did not affect the overall performance of each regimen. The interpretation of these data is limited severely by a low number of animals reaching the later time points.
Intense immunosuppression can lead to opportunistic infections such as CMV or BK virus, both of which have been associated with increased rates of rejection (46,47). The NHP model is very sensitive to a loss of protective viral immunity, as we have shown (41). Although BK virus or SV40 were not specifically tested, we detected no significant CMV viremia to suggest major impairment of protective immunity in any animal.
The effect of these regimens on TREG populations was expected. There is ample data suggesting that CoB of the CD28 pathway via competitive binding of CD80 and CD86 is deleterious to TREG function (48,49), likely owing to the importance of the complimentary CTLA4-CD80/86 pathway on TREG development and homeostasis (50,51). Progress towards direct inhibition of CD28, which would avoid concomitant blockade of CTLA4, was crippled by the disastrous results of the TGN1412 study (52). However, the development of monovalent antibody fragments which maintain their inhibitory ability but lack the ability to crosslink their targets or act as super antigens has renewed interest in direct CD28 blockade (53,54). Preclinical trials utilizing these new agents are underway (55).
In summary, we have shown that CoB with abatacept can foster long-term graft survival when paired with an adjuvant agent to reduce the risk of early rejection. The choice of agent, either methylprednisolone or MTX, offered no statistical advantage. These data are relevant to the potential use of abatacept in kidney transplantation, and may be of use in designing or considering transplant regimens for patients with RA who are well controlled on a regimen of abatacept and MTX.
Supplementary Material
Figure S1: No significant rhCMV viremia occurred in any treatment group.
Figure S2: Serum creatinine values for all animals included in the study. Generally, creatinine levels rapidly approached normal post-transplantation where they remained until time of graft loss or sacrifice with a functioning graft.
Figure S3: Comparison of T cells between animals experiencing late rejection or long-term survival in the abatacept, steroid, and methotrexate treatment group. At most time points, the animal that rejected had higher numbers of (A) CD3+ cells, (B) CD8+ TEM cells, and (C) CD4+ TEM cells.
Acknowledgments
Funding for this research was provided by a grant from the NIH, 1U01AI079223, U01AI084150 and 2U19AI051731-11. Further grant support of the Yerkes National Primate Research Center was provided by the National Center for Research Resources P51RR165 and is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132. Dr. Anderson was partially supported by the ASTS-Genentech Scientist Scholarship. The authors would like to thank the research support staff of the Emory Transplant Center and the veterinary staff at the Yerkes National Primate Research Center for their efforts on our behalf.
Abbreviations:
- BSA
body surface area
- CNI
calcineurin inhibitor
- CoB
costimulation blockade
- NHP
non-human primate
- rhCMV
rhesus cytomegalovirus
- PBMC
peripheral blood mononuclear cell
- TN
naïve T cell
- TCM
central memory T cell
- TEM
effector memory T cell
- TREG
regulatory T cell
- MTX
methotrexate
Footnotes
Disclosure
Dr. Larsen has received funding from Bristol-Myers-Squibb for clinical trials and preclinical studies. The remaining authors of this manuscript have no conflicts of interest to disclose as described by Clinical Transplantation.
Supporting Information
Additional supporting information may be found in the online version of this article.
References
- 1.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010;10(3):535–46. [DOI] [PubMed] [Google Scholar]
- 2.Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 2010;10(3):547–57. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med 2016. January 28;374(4):333–43. [DOI] [PubMed] [Google Scholar]
- 4.Adams AB, Goldstein J, Garrett C, Zhang R, Patzer RE, Newell KA, et al. Belatacept Combined With Transient Calcineurin Inhibitor Therapy Prevents Rejection and Promotes Improved Long-Term Renal Allograft Function. Am J Transplant. 2017. November;17(11):2922–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badell IR, Thompson PW, Turner AP, Russell MC, Avila JG, Cano JA, et al. Nondepleting anti-CD40-based therapy prolongs allograft survival in nonhuman primates. Am J Transplant 2012;12(1):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson TC, Trambley J, Odom K, Anderson DC, Cowan SR, Bray R, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation 2002;74(7):933–40. [DOI] [PubMed] [Google Scholar]
- 7.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996. May 30;381(6581):434–8. [DOI] [PubMed] [Google Scholar]
- 8.Adams AB, Shirasugi N, Jones TR, Durham MM, Strobert EA, Cowan SR, et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol 2005;174(1):542–50. [DOI] [PubMed] [Google Scholar]
- 9.Kumagai-Braesch M, Ekberg H, Wang F, Osterholm C, Ehrnfelt C, Sharma A, et al. Anti-LFA-1 improves pig islet xenograft function in diabetic mice when long-term acceptance is induced by CTLA4Ig/anti-CD40L. Transplantation 2007;83(9):1259–67. [DOI] [PubMed] [Google Scholar]
- 10.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A 1997;94(16):8789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med 2000;6(2):114. [DOI] [PubMed] [Google Scholar]
- 12.Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest 2010;120(12):4520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson DJ, Lo DJ, Leopardi F, Song M, Turgeon NA, Strobert EA, et al. Anti-Leukocyte Function-Associated Antigen 1 Therapy in a Nonhuman Primate Renal Transplant Model of Costimulation Blockade-Resistant Rejection. Am J Transplant 2016. May;16(5):1456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swierkot J, Szechiński J. Methotrexate in rheumatoid arthritis. Pharmacol Rep 2006;58(4):473–92. [PubMed] [Google Scholar]
- 15.Weinblatt ME, Trentham DE, Fraser PA, Holdsworth DE, Falchuk KR, Weissman BN, et al. Long-term prospective trial of low-dose methotrexate in rheumatoid arthritis. Arthritis Rheum 1988;31(2):167–75. [DOI] [PubMed] [Google Scholar]
- 16.Cutolo M, Sulli A, Pizzorni C, Seriolo B, Straub RH. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis 2001;60(8):729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comet R, Domingo C, Larrosa M, Morón A, Rué M, Amengual MJ, et al. Benefits of low weekly doses of methotrexate in steroid-dependent asthmatic patients. A double-blind, randomized, placebo-controlled study. Respir Med 2006. March;100(3):411–9. [DOI] [PubMed] [Google Scholar]
- 18.Bush A, Saglani S. Management of severe asthma in children. Lancet 2010. September 4;376(9743):814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CRESS RH, DEAVER NL. METHOTREXATE IN THE MANAGEMENT OF SEVERE PSORIASIS AND ARTHRITIS: REPORT OF A CASE. South Med J 1964;57:1088–90. [DOI] [PubMed] [Google Scholar]
- 20.Heydendael VMR, Spuls PI, Opmeer BC, de Borgie CAJM, Reitsma JB, Goldschmidt WFM, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med 2003;349(7):658–65. [DOI] [PubMed] [Google Scholar]
- 21.Moore AE, Stock CC, Sugiura K, Rhoads CP. Inhibition of Development of Sarcoma 180 by 4-Amino-N10-Methyl Pteroylglutamic Acid. Proc Soc Exp Biol Med 1949;70(3):396–8. [DOI] [PubMed] [Google Scholar]
- 22.Meyer LM, Miller FR, Rowen MJ, Bock G, Rutzky J. Treatment of Acute Leukemia with Amethopterin (4-amino, 10-methyl pteroyl glutamic acid). Acta Haematol 1950;4(3):157–67. [DOI] [PubMed] [Google Scholar]
- 23.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet 2005;366(9493):1303–14. [DOI] [PubMed] [Google Scholar]
- 24.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and Cyclosporine Compared with Cyclosporine Alone for Prophylaxis of Acute Graft versus Host Disease after Marrow Transplantation for Leukemia. N Engl J Med 1986. March 20;314(12):729–35. [DOI] [PubMed] [Google Scholar]
- 25.Torlen J, Ringden O, Garming-Legert K, Ljungman P, Winiarski J, Remes K, et al. A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation. Haematologica 2016;101(11):1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byun JM, Kim H-L, Shin D-Y, Koh Y, Yoon S-S, Seong M-W, et al. The Impact of Methylenetetrahydrofolate Reductase C677T Polymorphism on Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation with Methotrexate Prophylaxis Katoh M, editor. PLoS One 2016. October 26;11(10):e0163998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costanzo-Nordin MR, Grusk BB, Silver MA, Sobotka PA, Winters GL, O’Connell JB, et al. Reversal of recalcitrant cardiac allograft rejection with methotrexate. Circulation 1988;78(5 Pt 2):III47–57. [PubMed] [Google Scholar]
- 28.Olsen SL, O’Connell JB, Bristow MR, Renlund DG. Methotrexate as an adjunct in the treatment of persistent mild cardiac allograft rejection. Transplantation 1990;50(5):773–5. [DOI] [PubMed] [Google Scholar]
- 29.Ferraro P, Carrier M, White M, Pelletier GB, Pelletier LC. Antithymocyte globulin and methotrexate therapy of severe or persistent cardiac allograft rejection. Ann Thorac Surg 1995;60(2):372–6. [DOI] [PubMed] [Google Scholar]
- 30.Costanzo MR, Koch DM, Fisher SG, Heroux AL, Kao WG, Johnson MR. Effects of methotrexate on acute rejection and cardiac allograft vasculopathy in heart transplant recipients. J Heart Lung Transplant 1997;16(2):169–78. [PubMed] [Google Scholar]
- 31.Chinnock R, Emery J, Larsen R, Baum M, Janner D, Razzouk A, et al. Methotrexate therapy for complex graft rejection in pediatric heart transplant recipients. J Heart Lung Transplant 1995;14(4):726–33. [PubMed] [Google Scholar]
- 32.Boettcher H, Costard-Jäckle A, Möller F, Hirt SW, Cremer J. Methotrexate rescue therapy in lung transplantation. Transplant Proc 2002;34(8):3255–7. [DOI] [PubMed] [Google Scholar]
- 33.Dusmet M, Maurer J, Winton T, Kesten S. Methotrexate can halt the progression of bronchiolitis obliterans syndrome in lung transplant recipients. J Heart Lung Transplant 1996;15(9):948–54. [PubMed] [Google Scholar]
- 34.Cahill BC, O’Rourke MK, Strasburg KA, Savik K, Jessurun J, Bolman RM, et al. Methotrexate for lung transplant recipients with steroid-resistant acute rejection. J Heart Lung Transplant 1996;15(11):1130–7. [PubMed] [Google Scholar]
- 35.Michael B, Francos GC, Burke JF, Gaughan WI. Methotrexate is effective in preventing acute and potentially chronic renal allograft rejection. Transplant Proc 1994;26(5):3046–7. [PubMed] [Google Scholar]
- 36.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med 2003;349(20):1907–15. [DOI] [PubMed] [Google Scholar]
- 37.Westhovens R, Kremer JM, Moreland LW, Emery P, Russell AS, Li T, et al. Safety and efficacy of the selective costimulation modulator abatacept in patients with rheumatoid arthritis receiving background methotrexate: a 5-year extended phase IIB study. J Rheumatol 2009;36(4):736–42. [DOI] [PubMed] [Google Scholar]
- 38.Orencia Package Insert.
- 39.Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest 1998;102(2):322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnston A, Gudjonsson JE, Sigmundsdottir H, Runar Ludviksson B, Valdimarsson H. The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol 2005;114(2):154–63. [DOI] [PubMed] [Google Scholar]
- 41.Guide for Care and Use of Laboratory Animals.
- 42.Liu CT, Higbee GA. Determination of body surface area in the rhesus monkey. J Appl Physiol 1976. January 1;40(1):101–4. [DOI] [PubMed] [Google Scholar]
- 43.Lo DJ, Anderson DJ, Weaver TA, Leopardi F, Song M, Farris AB, et al. Belatacept and sirolimus prolong nonhuman primate renal allograft survival without a requirement for memory T cell depletion. Am J Transplant 2013;13(2):320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant 2005;5(3):443–53. [DOI] [PubMed] [Google Scholar]
- 45.Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: A multicentre retrospective study. PLoS One. 2010;5(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin-Gandul C, Mueller NJ, Pascual M, Manuel O. The impact of infection on chronic allograft dysfunction and allograft survival after solid organ transplantation. Am J Transplant 2015. December;15(12):3024–40. [DOI] [PubMed] [Google Scholar]
- 47.Zeng G, Huang Y, Huang Y, Lyu Z, Lesniak D, Randhawa P. Antigen-specificity of T-cell Infiltrates in Biopsies with T-cell Mediated Rejection and BK Polyomavirus Viremia: Analysis by Next Generation Sequencing. Am J Transplant 2016. November;16(10):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riella LV, Liu T, Yang J, Chock S, Shimizu T, Mfarrej B, et al. Deleterious Effect of CTLA4-Ig on a Treg-Dependent Transplant Model. Am J Transplant 2012;12(4):846–55. [DOI] [PubMed] [Google Scholar]
- 49.Charbonnier LM, Vokaer B, Lemaître PH, Field KA, Leo O, Le Moine A. CTLA4-Ig Restores Rejection of MHC Class-II Mismatched Allografts by Disabling IL-2-Expanded Regulatory T Cells. Am J Transplant 2012;12(9):2313–21. [DOI] [PubMed] [Google Scholar]
- 50.Magee CN, Boenisch O, Najafian N. The Role of Costimulatory Molecules in Directing the Functional Differentiation of Alloreactive T Helper Cells. Am J Transplant 2012;12(10):2588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol 2003;171(7):3348–52. [DOI] [PubMed] [Google Scholar]
- 52.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 2006;355(10):1018–28. [DOI] [PubMed] [Google Scholar]
- 53.Poirier N, Azimzadeh AM, zhang tianshu, Dilek N, Mary C, Nguyen B, et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med 2010;2(17):17ra10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suchard SJ, Davis PM, Kansal S, Stetsko DK, Brosius R, Tamura J, et al. A Monovalent Anti-Human CD28 Domain Antibody Antagonist: Preclinical Efficacy and Safety. J Immunol 2013. November 1;191(9):4599–610. [DOI] [PubMed] [Google Scholar]
- 55.Poirier N, Blancho G, Hiance M, Mary C, Van Assche T, Lempoels J, et al. First-in-Human Study in Healthy Subjects with FR104, a Pegylated Monoclonal Antibody Fragment Antagonist of CD28. J Immunol 2016. December 15;197(12):4593–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: No significant rhCMV viremia occurred in any treatment group.
Figure S2: Serum creatinine values for all animals included in the study. Generally, creatinine levels rapidly approached normal post-transplantation where they remained until time of graft loss or sacrifice with a functioning graft.
Figure S3: Comparison of T cells between animals experiencing late rejection or long-term survival in the abatacept, steroid, and methotrexate treatment group. At most time points, the animal that rejected had higher numbers of (A) CD3+ cells, (B) CD8+ TEM cells, and (C) CD4+ TEM cells.