Abstract
Study Design
Cross-sectional cohort study of chronic low back pain (CLBP) patients and matched controls.
Objective
To explore the interplay between vertebral endplate damage and adjacent paraspinal muscle (PSM) quality, and to test their association in a cohort of patients with chronic low back pain (CLBP) and matched controls.
Summary of Background Data
Non-specific CLBP is challenging to diagnose, in part, due to uncertainty regarding the source of pain. Delineating interactions among potential CLBP mechanisms may enhance diagnosis and treatment customization.
Methods
We collected advanced MRI imaging on 52 adult subjects, including 38 CLBP patients and 14 age- and sex-matched asymptomatic control subjects. Mean multifidus and erector spinae fat fraction (FF) was measured throughout the spine using an IDEAL MRI sequence. Presence of cartilage endplate (CEP) defects was determined at each disc level using UTE MRI. Logistic regression was used to test association of PSM FF, CEP defects, modic changes (MC), disc degeneration, and their interplay.
Results
We observed that CEP defects were the strongest predictor of non-specific CLBP (OR: 14.1, p<0.01) even after adjusting for MC and disc degeneration (OR: 26.1, p=0.04). PSM quality did not independently distinguish patient and control groups, except for patients with high self-reported disability.
At specifically L4L5, CEP damage was most prevalent and CEP damage was significantly associated with CLBP (OR: 3.7, 95%CI: 1.2-21.5, p=0.03). CEP damage at L4L5 was predictive of CLBP when adjacent to PSMs with greater FF (MF, OR 14.7, p=0.04; ES, OR: 17.3, p=0.03), but not when PSM FF was lower and comparable to values in control, asymptomatic subjects.
Conclusions
These results demonstrate the clinically important reciprocity between passive and dynamic spinal stabilizers, and support the notion that therapies targeting the PSMs may provide clinical benefit even in the presence of other spinal pathologies.
Keywords: Endplate Pathology, Lumbar Spine, Paraspinal Muscles, Multifidus, Chronic Low Back Pain, Modic Changes, MRI Phenotypes
INTRODUCTION
Chronic low back pain (CLBP) represents a substantial and growing clinical challenge that represents the most common, non-cancer reason for opioid prescription in the US.1 Effective CLBP care is hindered by its multifactorial etiology, multiple involved tissues, and lack of sensitive diagnostic tools to help customize treatments to individual patients. Yet, there is growing evidence that certain tissue pathologies in the spinal column highly associate with chronic pain, for which imaging evidence may provide diagnostic and prognostic value. For example, innervated damage of the vertebral endplate with co-occurring bone marrow edema (seen on MRI as Modic changes, MC) can strongly correlate with back pain history and future back pain risk.2,3 This is likely because endplate defects represent sites of structural weakness that can accentuate pro-inflammatory disc/vertebra crosstalk,4 which may trigger bone marrow neo-innervation and chemical sensitization.
A second important mediator of pain may be the paraspinal muscles (PSM) that serve to stabilize posture and limit excessive intervertebral movement.5,6 Poor PSM function can compromise lumbar spine alignment7 and impair spinal biomechanics8 that, in turn, increase disc stress and microtrauma potential.9,10 It is not surprising, therefore, that poor PSM quality (relative amounts of fat and lean muscle seen on MRI) and function is thought to be linked to low back pain and related disability.
Yet, the specific roles of MC and PSM in CLBP, either individually or in combination with other spinal pathologies, remains uncertain. For example, individual associations between MC and CLBP or between PSM quality and CLBP are inconsistent across numerous clinical studies.11,12 These inconsistencies could reflect differences in the study populations as well as the presence/absence of other spinal pathologies. For example, there is an association between reduced PSM quality and spinal pathologies linked to CLBP, including disc herniation,13 lumbar spinal stenosis,14 spondylolisthesis,15 and facet osteoarthritis.16
Another potential source of uncertainty regarding the roles of MC and PSM in CLBP is the unclear relationship between endplate defects (or MC) and adjacent PSM quality. Prior studies of CLBP patients have linked poor PSM composition with disc and vertebral pathologies such as MC17 and disc degeneration.18,19 Yet, because these imaging findings are also observed in asymptomatic cohorts,20–22 it is difficult to ascertain the clinical relevance of the relationship between these imaging findings.
Given the biomechanical interactions between PSM function and disc stress,8,23 as well as between disc stress and MC,24 we hypothesized that the strength of the association between endplate defects and CLBP depends on PSM quality. To investigate this, we conducted a study of PSM composition, endplate defects, MC, and their interaction in a cohort of patients with non-specific CLBP and age/sex-matched asymptomatic controls.
MATERIALS AND METHODS
Sample
With IRB approval, we enrolled and collected advanced MRI imaging on 52 adult subjects, including 38 CLBP patients and 14 age- and sex-matched asymptomatic control subjects. Inclusion criteria included patients with more than three continuous months of low back pain (VAS ≥ 4 or ODI≥30), between ages 18 and 70, and with a BMI under 40 kg/m2. Exclusion criteria for enrollment included pregnancy, diabetes, smoking, cancer, spondylolisthesis, scoliosis, prior lumbar surgery, disc herniation, compression fractures, taking osteoporosis medication. Volunteers were recruited locally and reported no prior history of back pain (VAS ≤ 1) or spinal pathology.
MR Imaging
Lumbar scans were performed on a 3T GE MR750 scanner and sequences included standard clinical T1- and T2-weighted MRI sequences and advanced sequences used for cartilage endplate detection (high-resolution 3D ultrashort echo time, UTE25 and fat fraction measurement (Iterative Decomposition of water and fat with Echo Asymmetry and Least-Squares Estimation, IDEAL26 (Figure 1). Specifications for the UTE sequence included TE=0.075 ms, TR=10 ms, voxel size of 0.22×0.22×0.80 mm3, and fat suppression. Specifications for the IDEAL sequence included TR=7ms, TE=2.1ms, flip angle=3°, rBW=±83.3kHz, FoV=22cm, in-plane resolution=1.3mm, and slice thickness=4mm).
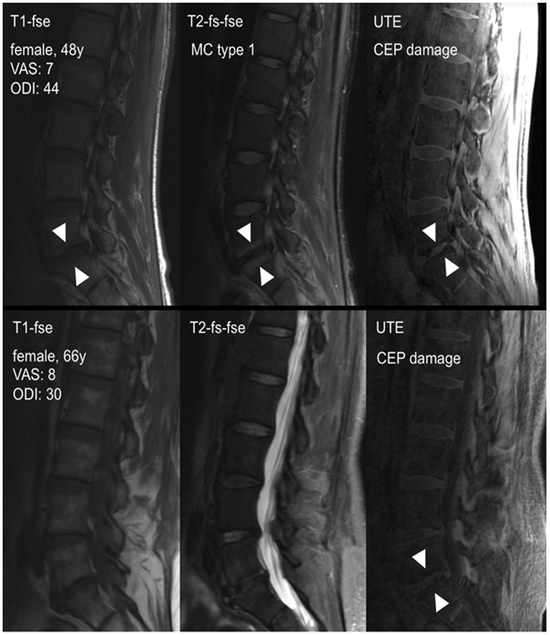
Figure 1. Identification of MC and CEP damage using conventional T1, fat-saturated T2-weighted, and UTE sequences.
The top panel shows CLBP patient with CEP damage and MC type 1 at L5/S1. The bottom panel shows a CLBP patient presenting CEP damage at L5S1 but no clear evidence of MC.
Muscle Segmentation
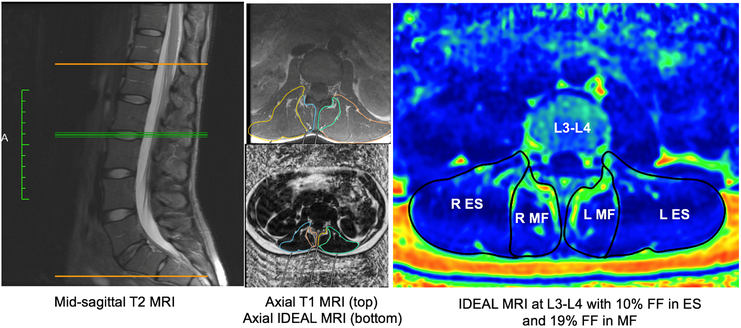
Multifidus (MF) and erector spinae (ES) were manually segmented from a combination of T1- and T2-axial images to conclude accurate facial boundaries, then segmentation regions of interest were transferred to IDEAL images for measuring mean fat fraction (FF; Figure 2). Intra- and inter-rater reliability was verified from muscle segmentation measurements from 5 subjects (Intra-rater ICC: 0.98, p<0.001; Inter-rater ICC: 0.99, p<0.001). Mean FF values were averaged both bilaterally and between two adjacent segments per lumbar disc level.
Figure 2: Quantifying PSM fat fraction from IDEAL MRI.
First with identification of disc level using mid-sagittal T2 MRI (left), then manually outlining muscle boundaries in corresponding axial T1 MRI (top middle), then transposing ROI to corresponding axial IDEAL MRI (bottom middle). Segmentation of separate MF and ES muscles on an IDEAL scan provides mean fat fraction values (right)
Outcomes
For paraspinal muscle quality, outcome measurements included level-specific and global lumbar (averaged from level-specific values between L1-L5) calculations of MF and ES fat fraction (MF FF, ES FF). For endplate pathology, outcome measurements included 1) presence or absence of cartilage endplate (CEP) damage within either superior or inferior endplates at a given lumbar disc level and 2) Modic change adjacent to either superior or inferior endplates at a given lumbar disc level. For mean disc degeneration, Pfirrmann grade was collected from a team of experienced physician spine-specialists (CO, ZM, DJ). For patient reported pain and disability, outcomes included the ODI (0–100) and VAS for back pain (0–10).
Statistical Analyses
Between group differences were assessed using bivariable and multivariable logistic regression. Differences in PSM FF between separate conditions (i.e. high/low ODI or presence/absence of adjacent CEP damage) were assessed using t-Tests. Significance was based on p<0.05. All analysis were done using Stata 15.0 (Stata Corp, College Station, TX).
RESULTS
Mean age and body mass index did not significantly differ between CLBP patients (47.9 ± 12.6 years, 25.6 ± 5.1 kg/m2) and healthy controls (45.7±12.3 years, 23.8 ± 4.8 kg/m2) (Table 1). Additionally, the percent representation of females to males was 43% for the controls and 50% for the CLBP patient group.
Table 1:
Demographics, PSM fat fraction, and CEP damage frequency.
| Controls (n=14) |
Patient subjects (n=38) |
Patient subjects, ODI<40 (n=23) |
Patient subjects, ODI>=40 (n=15) |
|
|---|---|---|---|---|
| Sex (%female) | 43% | 50% | 45% | 60% |
| Age (years) | 45.7 ± 12.3 | 47.9 ± 12.6 | 44.3 ± 13.1 | 53.5 ± 9.5 |
| BMI (kg/m2) | 23.8 ± 4.8 | 25.6 ± 5.1 | 24.3 ± 3.9 | 27.6 ± 6.2 |
| ODI (0-100) | 34.4 ± 14.5 | 24.4 ± 7.6 | 49.7 ± 7.0 | |
| VAS Back (0-10) | 6.6 ± 1.8 | 5.9 ± 1.4 | 7.7 ± 1.8 | |
| MF FF (L1-L5) | 20.9 ± 9.3 | 25.0 ± 9.9 | 23.4 ± 9.2 | 27.5 ± 10.7 |
| ES FF (L1-L5) | 16.8 ± 8.3 | 18.9 ± 8.5 | 17.6 ± 7.8 | 20.7 ± 9.3 |
| MF FF L1L2 | 24.9 ± 11.4 | 27.3 ± 10.1 | 26.6 ± 9.7 | 28.4 ± 10.8 |
| ES FF L1L2 | 11.6 ± 5.8 | 13.6 ± 7.2 | 12.7 ± 5.4 | 15.0 ± 9.5 |
| MF FF L2L3 | 20.4 ± 9.8 | 24.9 ± 10.4 | 23.0 ± 8.9 | 27.9 ± 12.2 |
| ES FF L2L3 | 12.4 ± 6.9 | 14.5 ± 8.0 | 13.4 ± 6.9 | 16.2 ± 9.4 |
| MF FF L3L4 | 18.8 ± 8.3 | 23.7 ± 10.9 | 21.5 ± 9.7 | 27.1 ± 12.0 |
| ES FF L3L4 | 16.8 ± 8.1 | 18.7 ± 8.1 | 17.3 ± 8.8 | 20.9 ± 9.3 |
| MF FF L4L5 | 19.5 ± 8.8 | 24.1 ± 9.9 | 22.6 ± 9.8 | 26.4 ± 9.8 |
| ES FF L4L5 | 26.2 ± 13.2 | 28.6 ± 11.5 | 27.2 ± 11.9 | 30.9 ± 10.9 |
| any CEP damage | 53.9% | 94.3% | 90.9% | 100.0% |
| any MC | 14.3% | 47.4% | 52.2% | 40.0% |
| Mean disc degen. | 1.7 ± 0.7 | 2.5 ± 0.7 | 2.5 ± 0.7 | 2.4 ± 0.7 |
| L1L2 CEP damage | 7.7% | 22.9% | 18.2% | 30.8% |
| L2L3 CEP damage | 15.4% | 28.6% | 27.3% | 30.8% |
| L3L4 CEP damage | 23.1% | 42.9% | 45.5% | 38.5% |
| L4L5 CEP damage | 23.1% | 60.0% | 54.5% | 69.2% |
| L5S1 CEP damage | 30.8% | 57.1% | 59.1% | 53.9% |
Independent predictors of CLBP
Independent predictors that a subject was in the CLBP group included: CEP damage (OR: 14.1, 95%CI: 2.3–85.2, p<0.01), any MC (OR: 5.4, 95%CI: 1.1–27.5, p=0.04), and mean disc degeneration (OR: 5.2, 95%CI: 1.4–18.9, p<0.01). Moreover, the likelihood of having CLBP significantly increased with each additional level with CEP damage (unit odds ratio 2.01, 1.12–3.61, p<0.01). After adjusting for any MC presence and mean disc degeneration, the predictive relationship between any CEP damage and CLBP strengthened (OR: 26.1, 95%CI: 1.1–639.1, p=0.04).
Paraspinal muscle and patient reported disability
The mean PSM FF values at each spinal level or for the whole lumbar spine were not significantly different between control and CLPB groups. However, when considering ODI scores, we observed that PSM FF was an independent predictor for patients with greater self-reported disability. Specifically, when stratifying CLBP patients based on low ODI (<40, n=23) or high ODI (>=40, n=15) scores, patients with high ODI scores had significantly greater MF FF values than the control group (Table 1). Specifically, global lumbar MF FF (Controls: 20.9%, High ODI subgroup: 27.5%, p=0.04), L2L3 (Controls: 20.4%; Higher ODI subgroup: 27.9%, p=0.04), L3L4 (Control: 18.8%, High ODI subgroup: 27.1%, p=0.02), and L4L5 (Control: 19.5%, High ODI subgroup: 26.4%, p=0.03). Muscle FF values did not differ between either the controls and low ODI subgroup or between separate the ODI patient subgroups.
Interactions between endplate defects and PSM quality within CLBP patients
To test our main hypothesis that PSM quality interacts with CEP damage to predict risk for CLBP, we examined level-specific relationships between paraspinal muscle quality and adjacent CEP damage.
For CEP damage, L4L5 had the highest incidence of damage (60% in CLBP group) compared to other lumbar disc levels (Table 1), and was the only level where CEP damage was significantly associated with CLBP (OR: 3.7, 95%CI: 1.2–21.5, p=0.03). Mean MF and ES FF at L4L5 was significantly higher in subjects with adjacent CEP damage (mean MF FF: 25.4%, mean ES FF: 30.7%) compared to subjects without adjacent CEP damage (mean MF FF: 19.1%, mean ES FF: 21.6%; Table 2). These mean values for paraspinal muscle FF at L4L5 without adjacent CEP damage are similar to the paraspinal muscle FF values at L4L5 in the control group (Table 1).
Table 2:
PSM FF adjacent to levels with or without CEP damage.
| No adjacent CEP damage |
Adjacent CEP damage present |
||||
|---|---|---|---|---|---|
| Mean | 95%CI | Mean | 95%CI | p-value | |
| MF FF L1L2 | 26.00% | 22.5-29.6% | 27.80% | 16.8-38.8% | 0.34 |
| ES FF L1L2 | 12.40% | 10.4-14.3% | 12.40% | 6.4-18.4% | 0.49 |
| MF FF L2L3 | 22.10% | 18.6-25.6% | 27.70% | 19.2-36.1% | 0.07 |
| ES FF L2L3 | 12.70% | 10.3-15.2% | 14.10% | 9.4-18.7% | 0.28 |
| MF FF L3L4 | 21.60% | 16.7-26.6% | 23.70% | 18.0-29.3% | 0.28 |
| ES FF L3L4 | 15.90% | 12.7-19.1% | 19.00% | 14.4-23.7% | 0.12 |
| MF FF L4L5 | 19.10% | 14.2-24.0% | 25.40% | 21.7-29.1% | 0.02 |
| ES FF L4L5 | 21.60% | 16.2-27.0% | 30.70% | 26.3-35.1% | p<0.01 |
Unpaired t-Test statistics comparing differences between PSM FF between CEP damage groups. At only L4L5 is PSM FF significantly different between CEP damage groups.
Among the CLBP patients, mean L4L5 MF FF was 24.1% and mean ES FF was 28.6%. After separating the patients into two equal-sized groups having the highest and lowest L4L5 MF FF and ES FF values, we found that the presence of CEP damage at L4L5 was predictive of CLBP in the patients with higher paraspinal FF values (MF, OR: 14.7, 95%CI: 1.2–185.2, p=0.04; ES, OR: 17.3, 95%CI: 1.4–216.6, p=0.03), but not the lower paraspinal FF values (MF, OR: 2.7, 95%CI: 0.4–16.8, p=0.4; ES, OR: 2.2, 95%CI: 0.4–13.8, p=0.3).
Discussion
The goals of our study were to explore the relationship between endplate damage and adjacent PSM quality, and to test the association of these anatomical changes with subjective report of disability in a cohort of CLBP patients and matched controls. We observed that at L4L5, PSM FF was greater when adjacent spinal endplate damage was present. We also found that CEP damage at L4L5 was predictive of CLBP when accompanied by relatively poorer PSM quality (MF, OR 14.7, p=0.04; ES, OR: 17.3, p=0.03), but not when PSM quality was relatively better and comparable to the asymptomatic values. L4L5 had the highest incidence of endplate damage and likely for that reason, revealed these relationships between endplate damage and adjacent PSM quality. These results demonstrate the clinically important reciprocity between passive and dynamic spinal stabilizers, and support the notion that therapies targeting the PSM may provide clinical benefit even in the presence of other spinal pathologies.
Our results also indicate a potential mechanistic interaction between the presence of vertebral endplate defects and poor PSM muscle quality in CLBP patients. In effort to elucidate imaging phenotypes that may be linked to CLBP symptoms, we investigated the presence of various structural features detectable lumbar spine MRI between non-specific CLBP patients and asymptomatic controls. First, we found that while the frequencies of MC, CEP damage, and worsening disc degeneration are higher in CLBP patients, CEP damage is the strongest predictor of non-specific CLBP after adjusting for MC and disc degeneration (Figure 1). Second, while PSM quality did not distinctly differ between the CLBP and control groups, PSM quality was worse for CLBP patients with more severe disability. Lastly, we found a level-specific association between CEP damage and PSM quality, and that the predictive association between CEP damage and CLBP was dependent on relatively higher levels of FF in adjacent PSMs (Figure 3).
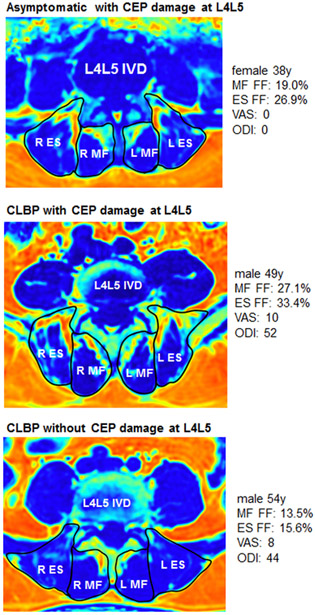
Figure 3: PSM FF and CEP damage.
Visual comparison using IDEAL MRI (fat fraction) of muscle quality differences at L4L5 between three subjects from our study: asymptomatic with L4L5 CEP damage (top), CLBP with L4L5 CEP damage (middle), CLBP without L4L5 CEP damage (bottom).
In our CLBP cohort, we found that endplate defects (specifically, CEP damage) were more predictive of symptoms than disc degeneration or MCs. Structural defects in the endplate are likely brought on by excessive biomechanical stresses from the adjacent disc, creating a channel for biological and biomechanical cross-talk between disc and vertebra that may trigger MCs in the neighboring vertebral bodies. The vertebral endplate is well innervated compared to the disc27 and added biomechanical stress upon endplate defects could provoke pain.28 Considering that endplate defects can be present alongside both disc degeneration and MCs in both symptomatic and asymptomatic individuals, added biomechanical stress associated with poor PSM function may be critical compounding factor. The spine is an integrated system, where the PSM (multifidus, quadratus lumborum, erector spinae, psoas) work in harmony with passive spinal structures (vertebra, discs, facets, ligaments) to support normal, pain-free biomechanical function. It is not surprising therefore, that altered loading through the spine related to poor PSM quality is a potential contributing factor to low back pain.
Our results reveal a level-specific association between the presence endplate defects and reduced PSM quality, with clinical cases including relatively poor PSM quality being predictive of CLBP. However, we did not find PSM quality to systematically differ between CLBP and asymptomatic groups. This is not surprising considering that muscle quality can diminish naturally with age regardless of pathology or symptoms.29 Beyond the effects of age, muscle quality can also be impacted by other subject-specific factors, such as biological sex19,30,31 and fitness/activity levels.32,33 These intrinsic and extrinsic factors create challenges for demonstrating an independent difference in PSM quality between CLBP and asymptomatic controls. A limitation in our current study is that our sample was not large enough to properly explore an effect of age and sex within separate CLBP and asymptomatic control groups. However, the separate groups are roughly matched for age and sexes. We confined most of our analyses to comparisons between groups to mitigate this effect.
The lack of distinct differences in PSM quality between groups suggests that PSM quality may not be an independent source of a pain, but rather a factor modulating the effect and amount of pain from other forms of spinal pathology, in this case endplate defects. Our results did show that CLBP patients that self-reported worse low back disability (ODI) had poorer PSM quality than those with lower disability scores. Although these values for PSM quality do not independently distinguish CLBP from asymptomatic controls, they do lend support to the proposed modulating effect of PSM quality on CLBP symptoms.
The level-specific effect between endplate defects and PSM quality in non-specific CLBP patients is distinct from the multi-level effect of PSM denervation that commonly arises from specific spinal conditions associated nerve root compression. With conditions related to nerve root compression (e.g. disc herniation), there is a multi-level effect running caudal from the affected spinal level. One animal study found that experimentally-induced nerve root injury led to an expected unilateral effect on PSM caudal to the injury. By contrast, experimentally-induced injury to the anterior aspect of the disc resulted lead to an unexpected effect on only the adjacent PSM at that disc level.34 The reason for the unexpected finding between disc injury (without nerve root compression) and deleterious changes to adjacent PSM at the same level is unclear, but may be due to a compensatory biomechanical response from the adjacent muscle in proprioceptive effort to stabilize the injured segment. There is also evidence that disc lesions in rodents can promote fat infiltration of adjacent multifidus muscles via a proinflammatory response35,36, however, the impact of this reduced muscle quality on segmental biomechanics and symptoms is unknown.
Compromised PSM quality can increase compressive load on the disc due to its reduced ability to actively stabilize the segments. Recent evidence from a longitudinal study on PSM quality changes in astronauts following 6-months in space demonstrated changes in muscle quality altered posture and spinal biomechanics due to unloading in space, which associated with CLBP and a heightened risk for disc herniation in subjects with pre-existing endplate pathology throughout their lumbar spine.8 This work supports that our results indicate a coupled biomechanical effect of poor PSM quality increasing intersegmental instability and compressive loading on endplate pathology. A limitation of our current study on non-specific CLBP lies in the cross-sectional study design, preventing us from observing how endplate pathology affects adjacent PSM quality and function, and whether or not changes in PSM quality affects symptoms. Future longitudinal studies seek to understand how changes in PSM quality relate to changes spinal alignment, intersegmental biomechanics, subject-reported pain and disability.
In conclusion, this study highlights a mechanistic association between CLBP phenotypes, beyond the mere presence or absence of structural abnormalities. Our data indicate that endplate defects were the primary predictor of non-specific CLBP after adjusting for MC and disc degeneration. Furthermore, we observed a level-specific association between endplate defects and PSM quality, and that the predictive association between endplate defects and CLBP was dependent on relatively higher levels of FF in adjacent PSMs. Importantly, our results support a potentially important relationship between reduced PSM quality and endplate defects that combine to provoke symptoms of CLBP. Non-specific CLBP is challenging to diagnose, in part, due to uncertainty regarding the source(s) of pain. Delineating mechanisms among phenotypes for CLBP may enhance clinical diagnosis using advanced imaging, as well as, support exercise rehabilitation and treatments targeting paraspinal muscle health.
ACKNOWLEDGMENTS:
We would like to thank the following individuals for their help with recruitment and data collection: Priya Nyayapati, Mariya Starostina, Alice Rochette, Kaitlyn Gary, Emel Ece Ozcan, Hemra Cil, and John Callander.
The manuscript submitted does not contain information about medical device(s)/drug(s). NIH (grant NIHR01AR63705) funds were received in support of this work. Relevant financial activities outside the submitted work: grants.
REFERENCES
- 1.Ringwalt C, Gugelmann H, Garrettson M, et al. Differential prescribing of opioid analgesics according to physician specialty for Medicaid patients with chronic noncancer pain diagnoses. Pain Res Manag 2014;19:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mok FPS, Samartzis D, Karppinen J, et al. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J 2016;16:32–41. [DOI] [PubMed] [Google Scholar]
- 3.Luoma K, Vehmas T, Kerttula L, et al. Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur Spine J 2016;25:2873–81. [DOI] [PubMed] [Google Scholar]
- 4.Dudli S, Sing DC, Hu SS, et al. Intervertebral disc/bone marrow cross-talk with Modic changes. Eur Spine J 2017;26:1362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macintosh JE & Bogduk N The Biomechanics of the Lumbar Multifidus. Clin Biomech (Bristol, Avon) 1986;1:205–13. [DOI] [PubMed] [Google Scholar]
- 6.Panjabi M, Abumi K, Duranceau J, et al. Spinal Stability and Intersegmental Muscle Forces: A Biomechanical Model. Spine 1989;14:194–200. [DOI] [PubMed] [Google Scholar]
- 7.Tamai K, Chen J, Stone M, et al. The evaluation of lumbar paraspinal muscle quantity and quality using the Goutallier classification and lumbar indentation value. Eur Spine J 2018;27:1005–12. [DOI] [PubMed] [Google Scholar]
- 8.Bailey JF, Miller SL, Khieu K, et al. From the international space station to the clinic: how prolonged unloading may disrupt lumbar spine stability. Spine J 2018;18:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilke HJ, Wolf S, Claes LE, et al. Stability Increase of the Lumbar Spine With Different Muscle Groups: A Biomechanical In Vitro Study. Spine 1995;20:192–8. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald DA, Moseley GL, & Hodges PW. The lumbar multifidus: Does the evidence support clinical beliefs? Man Ther 2006;11:254–63. [DOI] [PubMed] [Google Scholar]
- 11.Ranger TA, Cicuttini FM, Jensen TS, et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J 2017;17:1729–48. [DOI] [PubMed] [Google Scholar]
- 12.Jensen TS, Karppinen J, Sorensen JS, et al. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J 2008;17:1407–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshihara K, Shirai Y, Nakayama Y, et al. Histochemical changes in the multifidus muscle in patients with lumbar intervertebral disc herniation. Spine 2001;26:622–26. [DOI] [PubMed] [Google Scholar]
- 14.Fortin M, Lazáry À, Varga P, et al. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur Spine J 2017;26:2543–51. [DOI] [PubMed] [Google Scholar]
- 15.Kalichman L, Hodges PW, Li L, et al. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. Eur Spine J 2009;19:1136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooley JR, Walker BF, Ardakanim M, et al. Relationships between paraspinal muscle morphology and neurocompressive conditions of the lumbar spine: a systematic review with meta-analysis. BMC Musculoskelet Disord 2018;19:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teichtahl AJ, Urquhart DM, Wang Y, et al. Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community-based adults. Spine J 2015;15:1593–1601. [DOI] [PubMed] [Google Scholar]
- 18.Teichtahl AJ, Urquhart DM, Wang Y, et al. M. Lumbar disc degeneration is associated with modic change and high paraspinal fat content – a 3.0T magnetic resonance imaging study. BMC Musculoskelet Disord 2016;17:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urrutia J, Besa P, Lobos D, et al. Lumbar paraspinal muscle fat infiltration is independently associated with sex, age, and inter-vertebral disc degeneration in symptomatic patients. Skeletal Radiol 2018;47:955–61. [DOI] [PubMed] [Google Scholar]
- 20.Brinjikji W, Luetmer PH, Comstock B, et al. Systematic Literature Review of Imaging Features of Spinal Degeneration in Asymptomatic Populations. AJNR Am J Neuroradiol 2015;36:811–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. S. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994;331:69–73. [DOI] [PubMed] [Google Scholar]
- 22.Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990;72:403–8. [PubMed] [Google Scholar]
- 23.Shirazi-Adl A & Parnianpour M. Effect of changes in lordosis on mechanics of the lumbar spine-lumbar curvature in lifting. J Spinal Disord 1999;12:436–47. [PubMed] [Google Scholar]
- 24.Liu J, Hao L, Suyou L, et al. Biomechanical properties of lumbar endplates and their correlation with MRI findings of lumbar degeneration. J Biomech 2016;49:586–93. [DOI] [PubMed] [Google Scholar]
- 25.Fields AJ, Han M, Krug R & Lotz JC Cartilaginous End Plates: Quantitative MR Imaging with Very Short Echo Times-Orientation Dependence and Correlation with Biochemical Composition. Radiology 274, 482–489 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karampinos DC, Melkus G, Baum T, et al. Bone Marrow Fat Quantification in the Presence of Trabecular Bone: Initial Comparison Between Water-Fat Imaging and Single-Voxel MRS. Magn Reson Med 2014;71:1158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields AJ, Liebenberg EC, & Lotz JC. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J 2014;14:513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuslich SD, Ulstrom CL & Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am 1991;22:181–87. [PubMed] [Google Scholar]
- 29.Dahlqvist JR, Vissing CR, Hedermann G, et al. Fat Replacement of Paraspinal Muscles with Aging in Healthy Adults. Med Sci Sports Exerc 2017;49:595–601. [DOI] [PubMed] [Google Scholar]
- 30.Crawford RJ, Filli L, Elliott JM, et al. Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. AJNR Am J Neuroradiol 2016;37:742–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahidi B, Parra CL, Berry DB, et al. Contribution of Lumbar Spine Pathology and Age to Paraspinal Muscle Size and Fatty Infiltration. Spine 2017;42:616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasarn ML, Kostantinos V, Coyne E, et al. Does lumbar paraspinal muscle fatty degeneration correlate with aerobic index and Oswestry disability index? Surg Neurol Int 2015;6:S240–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teichtahl AJ, Urquhart DM, Wang Y, et al. M. Physical inactivity is associated with narrower lumbar intervertebral discs, high fat content of paraspinal muscles and low back pain and disability. Arthritis Research & Therapy 2015;17:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodges PW, Holm AK, Hansson T, et al. Rapid Atrophy of the Lumbar Multifidus Follows Experimental Disc or Nerve Root Injury. Spine 2006;31:2926–33. [DOI] [PubMed] [Google Scholar]
- 35.Hodges PW, James G, Blomster L, et al. Can proinflammatory cytokine gene expression explain multifidus muscle fiber changes after an intervertebral disc lesion? Spine 2014;39:1010–17. [DOI] [PubMed] [Google Scholar]
- 36.Hodges PW, James G, Blomster L, et al. Multifidus Muscle Changes After Back Injury Are Characterized by Structural Remodeling of Muscle, Adipose and Connective Tissue, but Not Muscle Atrophy. Spine 2015;40:1057–71. [DOI] [PubMed] [Google Scholar]