Abstract
Excessive alcohol use has adverse effects on the central nervous system (CNS) and can lead to alcohol use disorders (AUDs). Recent studies have implicated that myelin reductions may directly contribute to CNS dysfunctions associated with AUDs. Myelin consists of compact lipid membranes wrapping around axons to provide electrical insulation and trophic support. Regulation of myelin is considered as a new form of neural plasticity due to its profound impacts on the computation of neural networks. In this review, we first discuss experimental evidence showing how alcohol exposure causes demyelination in different brain regions, often accompanied by deficits in cognition and emotion. Next, we discuss postulated molecular and cellular mechanisms underlying alcohol’s impact on myelin. It is clear that more extensive investigations are needed in this important but underexplored research field in order to gain a better understanding of myelin-behavior relationship and to develop new treatment strategies for AUDs.
Keywords: Alcohol use disorder (AUD), central nervous system (CNS), alcohol addiction and dependence, myelin, oligodendrocyte (OL), oligodendrocyte progenitor cell (OPC), activity-dependent neural plasticity
Graphical abstract
Myelin consists of compact lipid membranes wrapping around axons to provide electrical insulation and trophic support, and can be regulated to profoundly alter brain functions. In this review, we discuss recent progress regarding how alcohol exposure causes demyelination in different brain regions, accompanied by deficits in cognition and emotion.
1. Introduction
Alcohol use disorders (AUDs) represent an enormous social and economic burden. Alcohol, also known by its chemical name ethanol (EtOH), is one of the oldest and most commonly consumed psychoactive substances worldwide. EtOH is typically classified as a depressant of the central nervous system (CNS) that affects multiple neurotransmitter systems, especially the GABAergic system, in various regions throughout the brain. Moreover, EtOH is a known teratogen, and particularly damaging during critical periods of rapid brain development. Consumption of high levels of EtOH is associated with its neurotoxic effects in the CNS, and alcohol addiction or AUDs in humans. AUD is a chronic relapsing disease of the brain characterized by compulsive alcohol use, loss of alcohol intake control, and a negative emotional state when not using [1]. EtOH-related problems can manifest themselves after both acute and chronic exposure as not only an impairment in neurophysiological functions, but also behaviorally, including craving, tolerance, physical dependence and withdrawal. Besides indirect effects on the brain through other organs/systems, such as the heart, liver, pancreas and the immune system, EtOH can directly impact brain cells. Chronic EtOH exposure can cause receptor up/down regulation and structural alterations of neurons and high doses of EtOH can lead to myelin cell death. For instance, EtOH has been shown to interfere with long-term potentiation and long-term depression, two potential mechanisms in the regulation of memory formation and storage [2,3]. These functional changes appear associated with changes in the formation of dendritic spines and the expression of neuronal genes [4,5]. Deficits in white matter and its related functions have also been reported. Recent studies show a strong link between EtOH consumption and the function of CNS myelin [6].
In the CNS, myelin is formed by processes that emanate from mature oligodendrocytes (OLs) that are differentiated from oligodendrocyte progenitor cells (OPCs) throughout the brain. As opposed to the Schwann cells that myelinate axons in the peripheral nervous system, OLs can myelinate multiple segments of multiple nearby axons. Myelin sheaths are made up of compact lipid membranes that wrap around the axon, providing electrical insulation and trophic support [7]. Myelin facilitates the propagation of action potentials along an axon in a saltatory fashion that is faster and more energy efficient compared to unmyelinated axons. Dense bundles of myelinated axons crossing the brain appear white to the naked eye and are thus known as white matter. While most research involving myelin has focused on these white matter tracts, many axons in gray matter are also myelinated. However, the function and mechanism of gray matter myelination remain poorly understood. Recent studies found that a significant proportion of gray matter myelination in the cortex is present on the axons of local inhibitory interneurons in both rodents and humans [8,9]. Besides potentially different intrinsic properties, it is anticipated that gray matter and white matter myelination may be regulated differently in the brain due to the distinct microenvironments.
Although myelin has traditionally been thought of as merely a passive insulator, recent reports highlight the role of OLs and myelination in the function and development of the CNS (for more details see recent review articles [10–12]). Disruption of myelin can lead to the dysregulation of various neural circuits and give rise to disease symptoms associated with not only sensory and motor functions, but also higher brain functions, including those manifested in multiple sclerosis (MS), bipolar disorder, and schizophrenia [13]. Uncovering the regulators of myelination has become increasingly important for diagnosis and treatment of these diseases, perhaps including AUDs as well in the near future.
2. Alcohol-induced myelin damage in human brains
Several recent studies have reported detrimental effects of alcohol on white matter integrity and function in humans. Using magnetic resonance imaging and diffusion tensor imaging techniques, Monnig et al (2015) found a significant association between severity of alcoholism ratings and white matter fractional anisotropy (FA). As AUD ratings increased, FA values, as a measure of white matter integrity, decreased. This relationship persisted even when controlling for the number of years drinking [14]. Similar results were reported in another study that utilized a longitudinal research design in subjects who sought treatment for AUDs and then either abstained from alcohol or relapsed after treatment. Interestingly, FA deficits reported in subjects with AUDs were reduced in those that abstained from alcohol compared to those that relapsed. Although FA values in the abstaining group were lower than controls, the trend in these individuals over the course of the study was positive and approaching control levels, while the opposite was true for those that relapsed. Furthermore, measures of FA and axial diffusivity in heavy-drinking relapse subjects demonstrate accelerated white matter aging [15]. Although these studies involved individuals from heavy-drinking human populations, even moderate EtOH consumption in adults has been shown to impact white matter microstructure. Structural MRI scans in a cohort of adults that were followed for 30 years found that moderate and heavy drinking subjects were significantly more susceptible to hippocampus atrophy and microstructural damage in the corpus callosum. These structural deficits were correlated with declining lexical fluency [16]. Of note, white matter reduction only suggests potential demyelination, since white matter also contains other cell types, including axons and astrocytes, in addition to mature OLs.
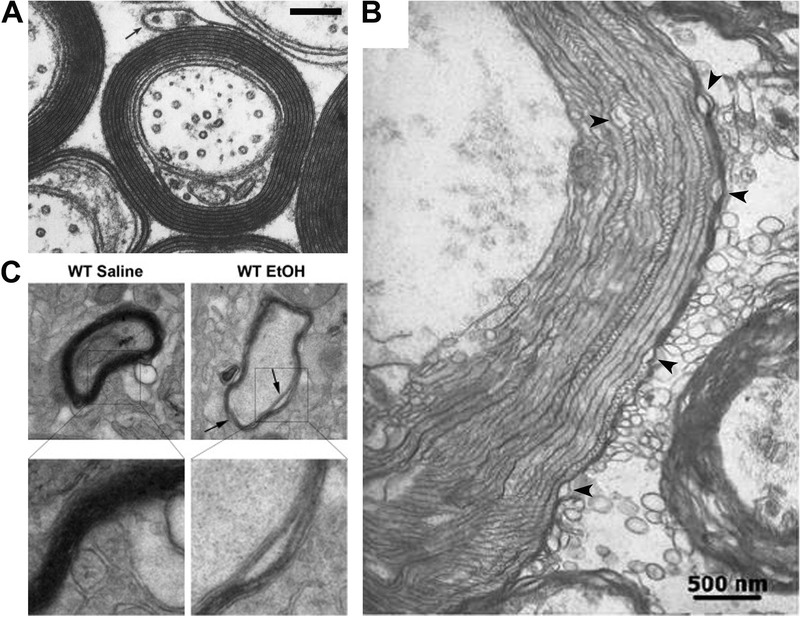
Postmortem studies have confirmed demyelination in humans with a history of AUDs. Multiple studies have reported reduced brain weight in individuals with AUDs, most of the decrease being in white matter (See more details in review articles [17,18]). A recent study showed that human AUDs are associated with substantial reduction of frontal lobe white matter lipid expression with regional variability, using MALDI imaging mass spectrometry to characterize lipid biochemistry for correlation with lifetime exposures and white matter degeneration, altered gene expression, and responses to abstinence or treatment [19]. Altered myelin ultrastructure in human AUDs was detected using transmission electron microscopy (TEM). In white matter from the brain of human alcoholics, there was an increase in the frequency of myelin membranes that were irregularly folded and split, indicating vacuoles between the myelin lamellae (Figure 1A, B), as well as swollen axons with enlarged mitochondria [20]. Other reports have noted changes in gray matter brain regions as well in subjects with AUDs [21]. Autoradiography studies used to detect specific neurotransmitter receptors in individuals with a history of alcohol use found decreased serotonin (5-HT) receptor binding in several brain regions, including the insular cortex, the cingulate cortex, the medial temporal lobe, and the raphe nucleus-the primary locus of 5-HT neurons in the CNS [22]. Significant increases in glutamate receptor (GluR) subtypes GluR2 and GluR3 were found in the hippocampus of alcoholics postmortem, suggesting alterations in glutamate signaling [23]. These brain areas are critical to limbic system function, and are implicated in various behaviors that are known to be affected by alcohol, including social behavior, learning, short- and long-term memory, and addiction. These regions also contain gray matter myelin, but the extent to which gray matter myelin is altered in AUDs remains unknown.
Figure 1. EtOH-induced alterations in myelin ultrastructure.
A, Transmission electron microscopy (TEM) image of myelinated axons in the normal CNS (modified from Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Siegel GJ, Agranoff BW, Albers RW, et al., editors. Philadelphia: Lippincott-Raven; 1999). B, TEM image of altered ultrastructure of myelin in substantia nigra of a human alcoholic brain (modified from [20]). Black arrowheads, vacuoles between the myelin lamellae. C, The representative TEM images from the PFC of adult (3 weeks after the last treatment administration) WT mice received intermittent ethanol or saline treatment in adolescence. Arrows indicate inter-laminar splitting of myelin sheaths (modified from [33]). Scale bars, 500 nm.
There are limitations associated with human studies, despite their necessity in understanding AUDs. First of all, most of these studies involve self-report of alcohol consumption. The timing of alcohol exposure and the type of alcohol consumed are highly variable and difficult to control for. In particular, developmental alcohol studies in humans have to rely on self-reports from subjects and families involved in prenatal or adolescent EtOH exposure that tend to be underestimated and unverifiable. Furthermore, it is difficult to carry out mechanistic studies with human subjects. Therefore, it is essential to use animal models to gain a better understanding of the role of myelination in AUDs.
3. EtOH-induced alterations of CNS myelin in animal models
The impact of EtOH on myelin in the brain has been established in various animal models. The extent of EtOH-induced demyelination varies in different brain regions and at different ages. Alcohol exposure during pregnancy can lead to fetal alcohol spectrum disorders (FASDs). Imaging studies revealed white matter abnormalities associated with cognitive impairment in children with FASDs [24,25]. Using a third trimester-equivalent mouse model of FASD, Newville and colleagues found a 58% decrease in the number of mature OLs and a 75% decrease in the number of proliferating OPCs within the corpus callosum of EtOH-exposed mice at postnatal day 16, but not the numbers of these cells derived from the postnatal subventricular zone [26]. Similar to the findings in the corpus callosum, a significant decrease in the number of prenatally derived mature OLs was observed in two other brain regions. These effects, albeit partially recoverable, can lead to long-lasting white matter injury, an observation that is consistent with FASD in humans [26]. In contrast, prenatal exposure of EtOH had no effect on myelination in the developing caudal pyramidal tract (i.e. corticospinal axons) in rats [27]. Progressive white matter atrophy in the corpus callosum with altered lipid profiles is partially reversed by short-term abstinence in a chronic binge model with adult male rats [28].
An important brain region that is affected by EtOH exposure is the medial prefrontal cortex (mPFC), which is critical for higher-order learning and social behavior. The mPFC has been shown to continue to develop well into adulthood, and numerous studies have been carried out to investigate the effects of EtOH exposure during adolescence on related neurobehavioral function [29]. Prolonged abstinence from EtOH leads to increases in myelin basic protein (MBP) expression in the mPFC [30], while acute exposure to EtOH during adolescence results in damaged myelin in the mPFC of rats [31]. This result is similar to the reductions in mPFC have been reported in mice that were socially isolated for up to 8 weeks [32]. It should be noted that re-socialization with their cage mates resulted in the stabilization of myelin-associated protein levels in these mice. Adolescent mice treated intermittently with EtOH for 2 weeks demonstrated ultrastructural alterations of myelin including inter-laminar splitting of myelin sheaths (Figure 1C) [33]. It would be interesting to investigate the extent to which EtOH and other stress (e.g. social isolation) affect myelination through a similar mechanism. Taken together, the studies using animal models showed EtOH-mediated effects on myelination, resembling some aspects of AUDs in humans. Experimental results from animal models lay a foundation for further mechanistic studies.
4. EtOH impacts the development of OLs
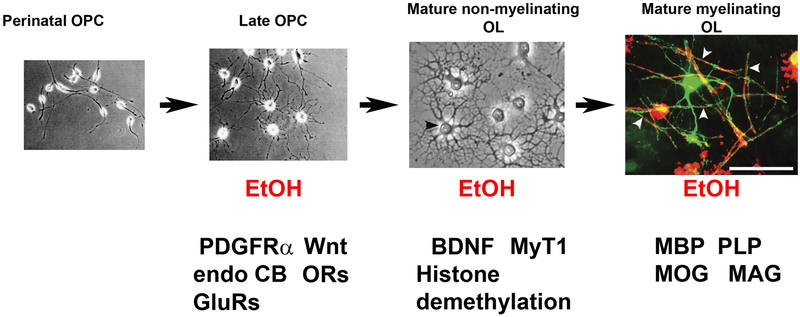
EtOH can affect myelination at multiple stages of OL development (Figure 2). Several lines of research have implicated multiple signaling pathways through which EtOH exposure may directly disrupt OPC differentiation and survival in the CNS. Developmental studies show that in vitro EtOH disrupted the expression of platelet-derived growth factor receptor alpha (PDGFRα) [34], which is crucial for the differentiation of OPCs into myelinating OLs. More recently, Vangipuram and Lyman [35] used human fetal brain tissue cell cultures to show that EtOH reduced Wnt3a and Wnt5a expression, while Wnt signaling can regulate OL cell fate specification, differentiation and proliferation, besides its role in regulating neurons [36][37,38]. Given that blocking Wnt signaling is associated with reduced expression of multiple myelin proteins and myelin sheath compaction [39], it appears that Wnt signaling might mediate the regulation of myelin by EtOH exposure.
Figure 2. EtOH can affect OLs at different developmental stages through different potential pathways.
Perinatal OPC and Late OPC were from purified OPC culture from rats (modified from [103]). Mature non-myelinating OL (shown by a black arrowhead) and Mature myelinating OL were from hippocampal myelin coculture (modified from [104]). In Mature myelinating OL, myelin coculture was infected with AAV9-GFP at 16 days in vitro (DIV), 2 d after OPCs were seeded. The coculture was fixed and stained after 28 DIV. Some mature OLs expressed GFP. One example of GFP-positive and mature OL (green) that developed multiple myelin basic protein (MBP)-positive (red) myelin segments [104]. White arrowheads, GFP- and MBP-positive myelin segments. Red arrows, potential impacts of EtOH on myelin have been implicated at different developmental stages. Scale bar, 100 μm. PDGFRα, platelet-derived growth factor receptor alpha. Wnt, Wingless/Integrated. Endo CB, endocannabinoid. GluRs, glutamate receptors. MyT1, the zinc finger protein Myelin Transcription Factor. ORs, opioid receptors. BDNF, brain-derived neurotrophic factor. PLP, proteolipid protein. MOG, myelin oligodendrocyte glycoprotein. MAG, myelin-associated glycoprotein.
Signaling pathways involved in early life brain development are also important in relation to the teratogenic effects of EtOH. The signaling pathway of brain derived neurotropic factor (BDNF) plays a key role in myelination [40–42], and can be altered by EtOH exposure. EtOH exposure and withdrawal were shown to bidirectionally alter BDNF levels in the amygdala via the expression of the immediate-early gene Arc. The blockade of Arc function on the BDNF pathway in the amygdala lead to increased EtOH consumption and anxiety-like behavior in rats [43]. The cannabinoid (CB) pathway is implicated in myelin function and impacted by EtOH as well. Postmortem studies found a link between alcoholism and CB receptor function. In the PFC, there was an increase in CB receptor 1 binding that coincided with reduced levels of monoacylglycerol lipase, extracellular signal-regulated kinase, and cyclic-AMP response element-binding protein, which are all critically involved in CB signaling [44]. WIN55212.2, a CB receptor agonist, increased OPC proliferation in vitro, as well as MBP mRNA and protein expression in mice [45], whereas blocking the metabolism of endogenous CBs decreased OL excitotoxicity and demyelination in vitro, and preserved myelin integrity in a demyelinating mouse model [46]. EtOH exposure alters CB ligands in several brain areas related to memory and addiction (e.g. PFC, Amygdala [47]). CB receptors on GABAergic neurons were implicated in modulating the effects of acute EtOH exposure [48] in the amygdala, and systemic blockade or deletion of CB receptors leads to reduced voluntary EtOH consumption and operant responding for EtOH in rodents [49]. Furthermore, using genomic profiling of transcripts in the PFC, a recent study showed that binge ethanol reduced myelin-related gene expression and altered chromatin modifying genes involved in histone demethylation at H3K9 and H3K36. Thus, EtOH may regulate histone methylation as a switch for regulation of myelin [50]. These are the potential pathways that EtOH exposure during critical periods of development can have long-lasting effects on neurobehavioral function and could, in part, explain the deficits noted in FASDs, as well as persistent cognitive and behavioral problems related to adolescent binge alcohol exposure. As an extension of this, it would also be interesting to investigate how changes in CB/myelin function might underlie subsequent EtOH consumption and addiction.
Recently, the endogenous opioid system was implicated in remyelination, or the active generation of new myelin sheaths around previously demyelinated axons. Opioid function has a long-established link to the rewarding effects of EtOH and other drugs of abuse [51–53], but recent evidence suggests that opioid function is related to remyelination. Specifically, antagonism of kappa opioid receptors led to increased differentiation of OPCs in both rodent and human cell culture, and also led to increased remyelination after focal demyelination in vivo [54]. Furthermore, the deletion of kappa opioid receptors in knockout mice lead to even greater myelin damage after induction of experimental autoimmune encephalitis, a mouse model of MS [55].
EtOH effects during development are particularly important, as OPCs that have yet to differentiate into OLs are more vulnerable to excitotoxic damage than mature OLs. Specifically, there are several factors that contribute to this increased sensitivity of OPCs to apoptosis, including higher AMPA/kainite GluR expression [56], elevated metabotropic GluR expression, and an increase in the expression of calcium-sensitive GluR subunits in OPCs. Consistent with this observation, a recent study in human brain tissue found elevated levels of NMDA GluR subunits in both gray matter and white matter regions of the brain. These NMDA subunits include one highly permeable to calcium influx, and the other insensitive to magnesium. These factors indicate that OPCs, as well as neurons, in the developing brain are particularly sensitive to Glu activation and thus more likely to suffer excitotoxicity and apoptosis [57]. In order to link this developmental sensitivity to excitotoxicity with EtOH exposure, Creeley et al [58] used non-human primates to examine the effects of prenatal EtOH exposure on OL apoptosis. Results showed that OL lineage cells were far more likely to be damaged/dying compared to other glial cells, and that this was directly related to EtOH exposure during the 3rd trimester period of development. These OLs appear to be specifically vulnerable to the teratogenic effects of EtOH when they are just beginning to express MBP prior to myelinating nearby axons. It is important to note that above-mentioned signaling pathways have also been shown to regulate neurons in addition to their impact on myelin cells. Further research is needed to identify myelin-specific regulatory pathways affected by EtOH.
5. EtOH impacts mature OLs
EtOH can affect mature myelinating OLs or myelin. Several studies have focused on the proteins that make up the actual myelin sheath, including MBP, proteolipid protein (PLP), myelin associated glycoprotein (MAG), and myelin oligodendrocyte glycoprotein (MOG). Brain tissue taken from the fetuses of pregnant BALB/C mice that were exposed to a liquid EtOH diet from gestational day 4–17 had reduced MBP expression compared to controls. There was no difference in the expression of neural cell adhesion molecule, suggesting that EtOH effects were specific to the myelin protein [59]. Similarly, Zoeller and coworkers [60] found that cerebellar MBP and MAG levels were not altered by prenatal alcohol exposure in rats at postnatal day 15. However, when rat pups were exposed to EtOH postnatally from days 4–10, a developmental period that is equivalent to the third-trimester of development in utero for humans, deficits in MBP and MAG expression were found that persisted into adulthood. This third-trimester equivalent exposure to EtOH occurs during a period of rapid development in the cerebellum [61], suggesting that myelin defects after EtOH exposure might be regionally and developmental-period dependent. Work utilizing primary glial cell cultures from Sprague-Dawley rats exposed to EtOH during the last two weeks of gestation found delayed MBP and transferrin expression that persisted for up to three weeks, without affecting other glial markers [62].
To evaluate the relationship between myelin and EtOH, one strategy is to investigate how overexpression of myelin-associated proteins impact EtOH consumption in rodents. A recent paper utilized this approach by virally overexpressing the zinc finger protein Myelin Transcription Factor (MyT1) in the dentate gyrus of rats. After recovery, MyT1 rats and sham controls were tested on a variety of behavioral tasks to assess anxiety-like behavior and EtOH consumption. MyT1 overexpression was associated with a decrease in anxious behavior on the elevated plus maze, and decreased voluntary EtOH consumption [63]. This result suggests that increasing myelination in the dentate gyrus may have an anxiolytic effect on behavior, while reducing EtOH consumption. This result is consistent with the human literature showing a negative correlation between white matter integrity and responsiveness to alcohol cues in individuals with AUDs [14,64].
How does EtOH carry out its toxic effect on OLs and myelin? Although EtOH itself has been shown to be neurotoxic, acetaldehyde, the primary metabolite of EtOH, might also contribute to OL excitotoxicity. In fact, a recent experiment in mouse primary OL cultures exposed to various concentrations of EtOH and acetaldehyde for 7 days found that OLs were far more sensitive to acetaldehyde than to EtOH. Significant cell death did not occur with EtOH until doses above 120 mM, while similar levels of OL toxicity were achieved at 50 μM in acetaldehyde [65]. Therefore, it is important to distinguish the effects between EtOH and acetaldehyde on myelination in future studies.
6. EtOH can indirectly impact myelin through its microenvironment
EtOH can affect microglia in the brain to indirectly alter myelination. Activation of glial toll-like receptor 4 (TLR4) can activate microglia to cause OL cell death and demyelination. In particular, chronic EtOH exposure was shown to activate TLR4 and the downregulation of PLP, MBP, MOG, and MAG protein expression, whereas knockout mice lacking TLR4 receptors did not [66]. To further investigate the relationship between EtOH and TLR4 function, Bajo et al [67] injected mice that were EtOH dependent with the TLR4 antagonist T5342126 for two weeks while measuring their voluntary consumption of EtOH in a 2-bottle choice paradigm. Results showed that blocking TLR4 reduced EtOH consumption while reducing the density of microglia in the central amygdala but not the dentate gyrus. It is important to note, however, that this reduction in EtOH consumption was accompanied by reduced locomotor activity, body temperature, and saccharine consumption. The issue of microglial activation in response to EtOH (and other brain insults) is complex, with both pro- and anti-inflammatory effects reported [68,69]. EtOH appears to promote microglial activation, such that a single binge exposure may be inconsequential, but subsequent EtOH exposure results in even greater activation of microglia [69]. Although a single exposure to EtOH may not have a dramatic effect on microglial activation, it may induce microglial priming, where the microglia are more susceptible to inflammatory insult [70]. Withdrawal from EtOH might be important for this partial activation of microglia, as Walter and Crews [71] report initial decreases in microglial gene expression, followed by increases during withdrawal. Similarly, EtOH exposure during key periods of development can have a long-lasting impact on the immune and inflammatory response on the brain [72,73]. With specific emphasis on myelin and OPCs/OLs, it was previously noted that EtOH activates TLR receptors, leading to an inflammatory response in microglia [66]. When TLR4 receptors are blocked by T5342126, this effect is blunted, and voluntary EtOH consumption is reduced as well [67]. Recent studies suggested that maternal binge-like alcohol consumption induces neuroinflammation (upregulation of TLR4 and NF-kB) and myelin damage in the brains of offspring and that such effects may underlie the persistent cognitive and behavioral impairments observed in FASDs [74,75]. It was further shown that nalmefene, an opioid antagonist, prevents neuroinflammation and brain damage by blocking the TLR4 response and support the role of central pro-inflammatory immune signaling in the modulation of alcohol addiction [76]. Although these studies highlight the complex nature of microglia activation (i.e. different subtypes of microglia mediating pro- or anti-inflammatory responses [77]), it is clear that EtOH can damage OL linage cells and myelin indirectly via microglia activation. TLR4 activity appears to be related to a wide range of functions in the brain, limiting the attractiveness of TLR4 as a therapeutic target for AUDs. Nonetheless, TLR4 signaling in reward circuits continues to be an important focus in relation to EtOH consumption and the progression of addiction in the CNS [78,79].
EtOH can impact the third major class of glial cells in the brain, astrocytes, while astrocytes can also regulate myelination and OL damage. The earliest systematic studies of the effect of EtOH on the development of the human CNS revealed impairments in the proliferation and migration of glial cells [80], and more recent studies suggest that, in addition to altering the brain inflammatory response, EtOH can also negatively impact astrocytes via oxidative stress (see more details in the review article [81]). The blood brain barrier (BBB) is an important brain structure that is dependent on astrocyte function that could also be impacted by EtOH exposure. Haorah et al [82] used human brain microvascular endothelial cells (that make up the BBB) in vitro to study the effects of EtOH and acetaldehyde on BBB integrity. EtOH and acetaldehyde exposure reduced barrier tightness, altered barrier permeability, and disrupted monocyte migration across the BBB by activation of protein tyrosine kinase. Such an effect could lead to increased susceptibility to neurotoxic insult or stroke in the brain, providing another indirect pathway by which EtOH could damage OLs and myelin function in the brain.
It is well established that ETOH alters neuronal function in a number of brain regions at both the system and molecular levels (See more details in a recent review article [83]). Given the recent interest in how neuronal function can influence myelination in both gray matter and white matter regions (See more details in recent review articles [11,12,84]), it is important to consider how EtOH might influence experience-dependent myelination via disrupted neuronal signaling. It is clear that functional activity can lead to preferential myelination of active axons [85,86], and the cellular processes involved have been the focus of recent research. Interestingly, glial cells, including OPCs/OLs, form unique non-synaptic connections with neurons, and these junctions are important for myelin formation related to neuronal function [85,87]. Operant responding for EtOH [88] and acute EtOH exposure in vitro [89] modulate gap junction function, providing an intriguing mechanism for EtOH to impact myelination. Acetylcholine signaling has long been studied with regard to myelin function [90] and is also implicated in the self-administration of EtOH [91,92]. It is clear from these studies that EtOH impacts on neuronal function can lead to alterations in OLs and myelin function in an indirect manner.
7. Conclusions and outlook
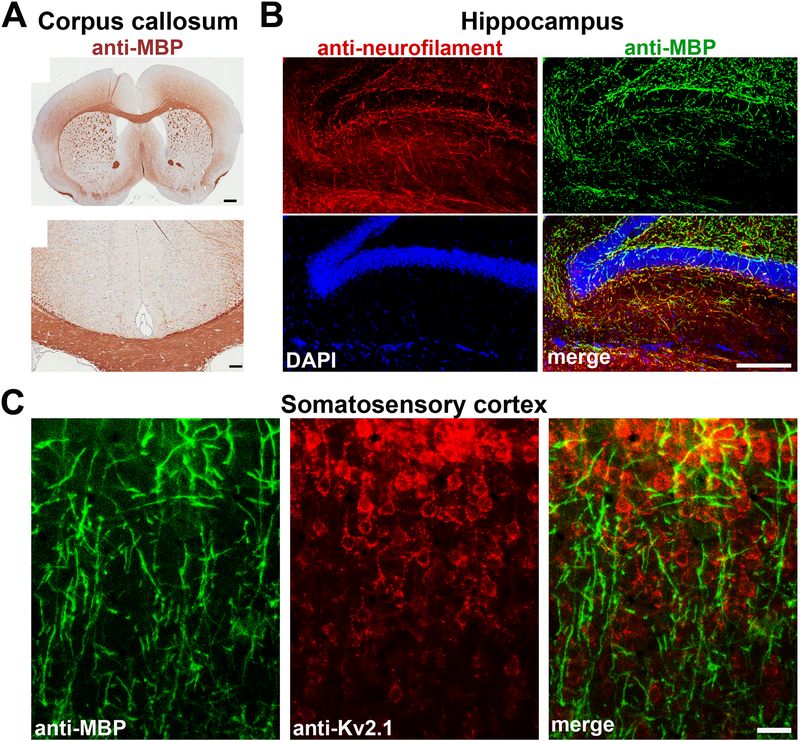
OLs and myelin have been implicated in the establishment of addictive behavior and alcoholism [88,93,94]. However, the causal relationship between EtOH-induced myelin alteration and behavioral change remains poorly understood. Regulation of myelin causes long-lasting changes in neural networks. Recent reports have associated myelin changes in the brain with behaviors like skill learning [95–98] and social isolation/defeat [32,99,100], shedding light on designing effective research strategies to address EtOH related questions. Several signaling pathways have been implicated in EtOH-induced alterations of myelin. However, the specificities of the cell type, brain region and mechanism involved often remain unclear. Of note, recent studies showed that besides highly bundled myelinated axons in white matter, there are extensive myelin networks in gray matter of the brain (Figure 3). These myelinated axons in gray matter are from both excitatory projection neurons and inhibitory GABAergic interneurons, especially the parvalbumin-positive fast-spiking GABAergic interneurons in both mice and humans [8,9]. Currently, little is known regarding the potential role of gray matter myelin in AUDs.
Figure 3. White matter and gray matter myelin in different regions of the mouse brain.
A, White matter bundles revealed by anti-MBP staining in the anterior cingulate cortex of the WT B6 mouse (top). Its corpus callosum is shown in an enlarged image at the bottom (modified from [105]). Scale bars, 500 μm (top) and 100 μm (bottom). B, Gray matter myelin segments in the mouse hippocampus that was stained for neurofilament (red), MBP (green) and DAPI nuclear dye (blue). C, Gray matter myelin segments in the mouse somatosensory cortex that was stained for MBP (green) and Kv2.1 (red). Kv2.1, a Shab voltage-gated potassium channel mainly expressed in the somatodendritic region of cortical pyramidal neurons (Image courtesy of Farida Eid). Scale bars, 200 μm in B and 100 μm in C.
The ultimate goal of understanding EtOH and myelin interactions is to improve the lives of individuals who suffer from AUDs. Promoting myelination may be developed into a new treatment strategy. For instance, choline treatment reversed the behavioral and neurobiological deficits of third-trimester equivalent prenatal EtOH exposure [61,101]. Given that choline is abundant in myelin, it is possible that this restorative effect may involve myelination. Antihistamines were shown to blunt deficits of prenatal EtOH on hippocampal function [102], and enhance myelin formation after social isolation [99], providing another attractive therapeutic approach. Taken together, this is an important but still underexplored research field. The findings from future research will not only contribute to a better understanding of myelin-behavior interactions but also the development of new treatment strategies for AUDs.
ACKNOWLEDGEMENTS
This work was supported in part by grants from NIH (R21AA024873. and R01NS093073) to CG. We apologize to authors whose work is not included in this review due to space constraints.
The List of Abbreviations
- 5-HT
serotonin
- AUD
alcohol use disorder
- BBB
blood-brain barrier
- BDNF
brain derived neurotrophic factor
- CB
cannabinoid
- CNS
central nervous system
- EtOH
ethanol
- FA
fractional anisotropy
- FASD
fetal alcohol spectrum disorder
- GluR
glutamate receptor
- MAG
myelin associated glycoprotein
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte glycoprotein
- mPFC
medial prefrontal cortex
- MS
multiple sclerosis
- MyT1
myelin transcription factor
- OL
oligodendrocyte
- OPC
oligodendrocyte progenitor cell
- PDGFRα
platelet-derived growth factor receptor alpha
- PFC
prefrontal cortex
- PLP
proteolipid protein
- TEM
transmission electron microscopy
- TLR4
glial toll-like receptor 4
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES:
- [1].Schuckit MA, Lancet 2009, 373, 492. [DOI] [PubMed] [Google Scholar]
- [2].Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, Valenzuela CF, Hansel C, J. Neurophysiol 2008, 100, 3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zorumski CF, Mennerick S, Izumi Y, Alcohol 2014, 48, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Romero AM, Renau-Piqueras J, Pilar Marin M, Timoneda J, Berciano MT, Lafarga M, Esteban-Pretel G, Neurotox Res 2013, 24, 532. [DOI] [PubMed] [Google Scholar]
- [5].Ryabinin AE, Wang YM, Freeman P, Risinger FO, Alcohol. Clin. Exp. Res 1999, 23, 1272. [DOI] [PubMed] [Google Scholar]
- [6].Perry CJ, J. Mol. Neurosci 2016, 60, 383. [DOI] [PubMed] [Google Scholar]
- [7].Baumann N, Pham-Dinh D, Physiol. Rev 2001, 81, 871. [DOI] [PubMed] [Google Scholar]
- [8].Micheva KD, Wolman D, Mensh BD, Pax E, Buchanan J, Smith SJ, Bock DD, Elife 2016, 5, DOI 10.7554/eLife.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stedehouder J, Couey JJ, Brizee D, Hosseini B, Slotman JA, Dirven CMF, Shpak G, Houtsmuller AB, Kushner SA, Cereb. Cortex 2017, 27, 5001. [DOI] [PubMed] [Google Scholar]
- [10].Fields RD, Science 2014, 344, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nickel M, Gu C, Neural Plast 2018, 2018, 6436453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mount CW, Monje M, Neuron 2017, 95, 743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Regenold WT, D’Agostino CA, Ramesh N, Hasnain M, Roys S, Gullapalli RP, Bipolar Disord 2006, 8, 188. [DOI] [PubMed] [Google Scholar]
- [14].Monnig MA, Yeo RA, Tonigan JS, McCrady BS, Thoma RJ, Sabbineni A, Hutchison KE, PLoS One 2015, 10, e0142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, Zahr NM, Sullivan EV, The Lancet. Psychiatry 2014, 1, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Topiwala A, Allan CL, Valkanova V, Zsoldos E, Filippini N, Sexton C, Mahmood A, Fooks P, Singh-Manoux A, Mackay CE, Kivimäki M, Ebmeier KP, BMJ 2017, 357, j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harper C, Alcohol Alcohol 2009, 44, 136. [DOI] [PubMed] [Google Scholar]
- [18].Harper C, J. Neuropathol. Exp. Neurol 1998, 57, 101. [DOI] [PubMed] [Google Scholar]
- [19].de la Monte SM, Kay J, Yalcin EB, Kril JJ, Sheedy D, Sutherland GT, Alcohol 2017, DOI 10.1016/j.alcohol.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Skuja S, Groma V, Ravina K, Tarasovs M, Cauce V, Teteris O, Ultrastruct Pathol 2013, 37, 346. [DOI] [PubMed] [Google Scholar]
- [21].Mann K, Agartz I, Harper C, Shoaf S, Rawlings RR, Momenan R, Hommer DW, Pfefferbaum A, Sullivan EV, Anton RF, Drobes DJ, George MS, Bares R, Machulla HJ, Mundle G, Reimold M, Heinz A, Alcohol. Clin. Exp. Res 2001, 25, 104S. [DOI] [PubMed] [Google Scholar]
- [22].Dillon KA, Gross-Isseroff R, Israeli M, Biegon A, Brain Res 1991, 554, 56. [DOI] [PubMed] [Google Scholar]
- [23].Breese CR, Freedman R, Leonard SS, Brain Res 1995, 674, 82. [DOI] [PubMed] [Google Scholar]
- [24].Lebel C, Roussotte F, Sowell ER, Neuropsychol Rev 2011, 21, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Norman AL, Crocker N, Mattson SN, Riley EP, Dev Disabil Res Rev 2009, 15, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Newville J, Valenzuela CF, Li L, Jantzie LL, Cunningham LA, Glia 2017, 65, 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miller MW, Brain Res 2017, 1672, 122. [DOI] [PubMed] [Google Scholar]
- [28].Yalcin EB, McLean T, Tong M, de la Monte SM, Alcohol 2017, 65, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Crews FT, Vetreno RP, Broadwater MA, Robinson DL, Pharmacol. Rev 2016, 68, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Navarro AI, Mandyam CD, Neuroscience 2015, 293, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vargas WM, Bengston L, Gilpin NW, Whitcomb BW, Richardson HN, J. Neurosci 2014, 34, 14777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P, Nat. Neurosci 2012, 15, 1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Montesinos J, Pascual M, Pla A, Maldonado C, Rodríguez-Arias M, Miñarro J, Guerri C, Brain Behav. Immun 2015, 45, 233. [DOI] [PubMed] [Google Scholar]
- [34].Luo J, Miller MW, Brain Res Brain Res Rev 1998, 27, 157. [DOI] [PubMed] [Google Scholar]
- [35].Vangipuram SD, Lyman WD, Alcohol. Clin. Exp. Res 2012, 36, 788. [DOI] [PubMed] [Google Scholar]
- [36].Ille F, Sommer L, Cell Mol. Life Sci 2005, 62, 1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu Y, Chen G, Ma C, Bower KA, Xu M, Fan Z, Shi X, Ke Z-J, Luo J, J. Neurosci. Res 2009, 87, 2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Luo J, Mol. Neurobiol 2009, 40, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tawk M, Makoukji J, Belle M, Fonte C, Trousson A, Hawkins T, Li H, Ghandour S, Schumacher M, Massaad C, J. Neurosci 2011, 31, 3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Huang EJ, Reichardt LF, Annu. Rev. Neurosci 2001, 24, 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fulmer CG, VonDran MW, Stillman AA, Huang Y, Hempstead BL, Dreyfus CF, J. Neurosci 2014, 34, 8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jukkola P, Gu Y, Lovett-Racke AE, Gu C, Front. Mol. Neurosci 2017, 10, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K, J. Neurosci 2008, 28, 2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Erdozain AM, Rubio M, Valdizan EM, Pazos A, Meana JJ, Fernández-Ruiz J, Alexander SPH, Callado LF, Addict Biol 2015, 20, 773. [DOI] [PubMed] [Google Scholar]
- [45].Tomas-Roig J, Wirths O, Salinas-Riester G, Havemann-Reinecke U, CNS Neurosci Ther 2016, 22, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bernal-Chico A, Canedo M, Manterola A, Victoria Sánchez-Gómez M, Pérez-Samartín A, Rodríguez-Puertas R, Matute C, Mato S, Glia 2015, 63, 163. [DOI] [PubMed] [Google Scholar]
- [47].Sanchez-Marin L, Pavon FJ, Decara J, Suarez J, Gavito A, Castilla-Ortega E, Rodriguez de Fonseca F, Serrano A, Front. Behav. Neurosci 2017, 11, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Talani G, Lovinger DM, Alcohol 2015, 49, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Henderson-Redmond AN, Guindon J, Morgan DJ, Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wolstenholme JT, Mahmood T, Harris GM, Abbas S, Miles MF, Front. Mol. Neurosci 2017, 10, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Peciña S, Physiol. Behav 2008, 94, 675. [DOI] [PubMed] [Google Scholar]
- [52].Crombag HS, Bossert JM, Koya E, Shaham Y, Philos. Trans. R. Soc. Lond. B, Biol. Sci 2008, 363, 3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wassum KM, Ostlund SB, Maidment NT, Balleine BW, Proc. Natl. Acad. Sci. USA 2009, 106, 12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mei F, Mayoral SR, Nobuta H, Wang F, Desponts C, Lorrain DS, Xiao L, Green AJ, Rowitch D, Whistler J, Chan JR, J. Neurosci 2016, 36, 7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Du C, Duan Y, Wei W, Cai Y, Chai H, Lv J, Du X, Zhu J, Xie X, Nat. Commun 2016, 7, 11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Butts BD, Houde C, Mehmet H, Cell Death Differ 2008, 15, 1178. [DOI] [PubMed] [Google Scholar]
- [57].Jantzie LL, Talos DM, Jackson MC, Park H-K, Graham DA, Lechpammer M, Folkerth RD, Volpe JJ, Jensen FE, Cereb. Cortex 2015, 25, 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Creeley CE, Dikranian KT, Johnson SA, Farber NB, Olney JW, Acta Neuropathol. Commun 2013, 1, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ozer E, Sarioglu S, Güre A, Clin Neuropathol 2000, 19, 21. [PubMed] [Google Scholar]
- [60].Zoeller RT, Butnariu OV, Fletcher DL, Riley EP, Alcohol. Clin. Exp. Res 1994, 18, 909. [DOI] [PubMed] [Google Scholar]
- [61].Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD, Behav. Neurosci 2007, 121, 120. [DOI] [PubMed] [Google Scholar]
- [62].Chiappelli F, Taylor AN, Espinosa A Monteros de los, Vellis J. de, Int. J. Dev. Neurosci 1991, 9, 67. [DOI] [PubMed] [Google Scholar]
- [63].Bahi A, Dreyer J-L, Psychopharmacology 2017, 234, 1829. [DOI] [PubMed] [Google Scholar]
- [64].Monnig MA, Thayer RE, Caprihan A, Claus ED, Yeo RA, Calhoun VD, Hutchison KE, Brain Behav 2014, 4, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Coutts DJC, Harrison NL, Alcohol. Clin. Exp. Res 2015, 39, 455. [DOI] [PubMed] [Google Scholar]
- [66].Alfonso-Loeches S, Pascual M, Gómez-Pinedo U, Pascual-Lucas M, Renau-Piqueras J, Guerri C, Glia 2012, 60, 948. [DOI] [PubMed] [Google Scholar]
- [67].Bajo M, Montgomery SE, Cates LN, Nadav T, Delucchi AM, Cheng K, Yin H, Crawford EF, Roberts AJ, Roberto M, Alcohol Alcohol 2016, 51, 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Montesinos J, Alfonso-Loeches S, Guerri C, Alcohol. Clin. Exp. Res 2016, 40, 2260. [DOI] [PubMed] [Google Scholar]
- [69].Marshall SA, Geil CR, Nixon K, Brain Sci 2016, 6, DOI 10.3390/brainsci6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Perry VH, Holmes C, Nat. Rev. Neurol 2014, 10, 217. [DOI] [PubMed] [Google Scholar]
- [71].Walter TJ, Crews FT, J. Neuroinflammation 2017, 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K, Brain Behav. Immun 2011, 25 Suppl 1, S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Boschen KE, Ruggiero MJ, Klintsova AY, Neuroscience 2016, 324, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pascual M, Montesinos J, Montagud-Romero S, Forteza J, Rodríguez-Arias M, Miñarro J, Guerri C, J. Neuroinflammation 2017, 14, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Cantacorps L, Alfonso-Loeches S, Moscoso-Castro M, Cuitavi J, Gracia-Rubio I, López-Arnau R, Escubedo E, Guerri C, Valverde O, Neuropharmacology 2017, 123, 368. [DOI] [PubMed] [Google Scholar]
- [76].Montesinos J, Gil A, Guerri C, Alcohol. Clin. Exp. Res 2017, 41, 1257. [DOI] [PubMed] [Google Scholar]
- [77].Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K, Neurobiol. Dis 2013, 54, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Crews FT, Walter TJ, Coleman LG, Vetreno RP, Psychopharmacology 2017, 234, 1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Crews FT, Lawrimore CJ, Walter TJ, Coleman LG, Neuropharmacology 2017, 122, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Clarren SK, Alvord EC, Sumi SM, Streissguth AP, Smith DW, J. Pediatr 1978, 92, 64. [DOI] [PubMed] [Google Scholar]
- [81].Guizzetti M, Zhang X, Goeke C, Gavin DP, Front. Pediatr 2014, 2, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Haorah J, Schall K, Ramirez SH, Persidsky Y, Glia 2008, 56, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Abrahao KP, Salinas AG, Lovinger DM, Neuron 2017, 96, 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fields RD, Nat. Rev. Neurosci 2015, 16, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD, Nat. Commun 2015, 6, 7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lee HU, Nag S, Blasiak A, Jin Y, Thakor N, Yang IH, ACS Chem. Neurosci 2016, 7, 1317. [DOI] [PubMed] [Google Scholar]
- [87].Nualart-Marti A, Solsona C, Fields RD, Biochim. Biophys. Acta 2013, 1828, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bull C, Freitas KCC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS, Neuropsychopharmacology 2014, 39, 2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Adermark L, Lovinger DM, Neuropharmacology 2006, 51, 1099. [DOI] [PubMed] [Google Scholar]
- [90].Fields RD, Dutta DJ, Belgrad J, Robnett M, Glia 2017, DOI 10.1002/glia.23101. [DOI] [PubMed] [Google Scholar]
- [91].Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE, Proc. Natl. Acad. Sci. USA 2007, 104, 12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kuzmin A, Jerlhag E, Liljequist S, Engel J, Psychopharmacology 2009, 203, 99. [DOI] [PubMed] [Google Scholar]
- [93].Feng Y, Neurochem. Res 2008, 33, 1940. [DOI] [PubMed] [Google Scholar]
- [94].Somkuwar SS, Staples MC, Galinato MH, Fannon MJ, Mandyam CD, Front. Pharmacol 2014, 5, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM, Nat. Neurosci 2009, 12, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lakhani B, Borich MR, Jackson JN, Wadden KP, Peters S, Villamayor A, MacKay AL, Vavasour IM, Rauscher A, Boyd LA, Neural Plast 2016, 2016, 7526135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD, Nat. Neurosci 2016, 19, 1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Keiner S, Niv F, Neumann S, Steinbach T, Schmeer C, Hornung K, Schlenker Y, Förster M, Witte OW, Redecker C, BMC Neurosci 2017, 18, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Liu J, Dupree JL, Gacias M, Frawley R, Sikder T, Naik P, Casaccia P, J. Neurosci 2016, 36, 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lehmann ML, Weigel TK, Elkahloun AG, Herkenham M, Sci. Rep 2017, 7, 46548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Thomas JD, Idrus NM, Monk BR, Dominguez HD, Birth Defects Res Part A Clin Mol Teratol 2010, 88, 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Varaschin RK, Rosenberg MJ, Hamilton DA, Savage DD, Alcohol. Clin. Exp. Res 2014, 38, 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Tang DG, Tokumoto YM, Raff MC, J. Cell Biol 2000, 148, 971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gardner A, Jukkola P, Gu C, Nat. Protoc 2012, 7, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Stephenson DT, O’Neill SM, Narayan S, Tiwari A, Arnold E, Samaroo HD, Du F, Ring RH, Campbell B, Pletcher M, Vaidya VA, Morton D, Mol. Autism 2011, 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]