Graphical Abstract
Sepsis is the leading cause of death in non-cardiac intensive care units (ICU) and accounts for 40% of ICU expenditures [1, 2]. Currently, no drugs are approved by the FDA for the treatment of sepsis due to the repeated failure of clinical trials [3, 4]. Patients that survive sepsis suffer long term physical and cognitive disabilities and the one year mortality rate for sepsis patients is unacceptably high [5, 6]. Secondary infections are a common cause of morbidity and mortality among sepsis patients, both in the hospital and post-discharge settings. It is generally accepted that sepsis-induced immunosuppression or immune paralysis is an important contributing factor to the increased susceptibility of septic patients to opportunistic and nosocomial infections. Specific alterations in monocyte and macrophage function, including decreased class II major histocompatibility complex expression, impaired phagocytic function and suppressed cytokine production (i.e. endotoxin tolerance) are associated with immune suppression and susceptibility to infection in human and animal models of sepsis. However, the underlying mechanisms contributing to monocyte dysfunction during sepsis are not well understood.
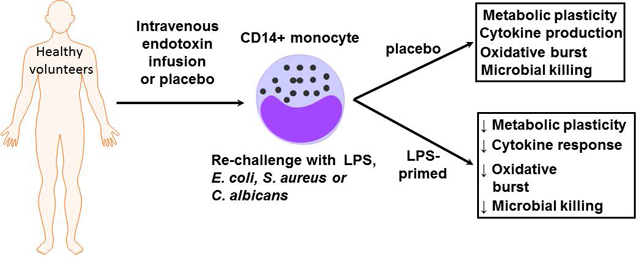
The paper by Grondman and colleagues in this issue of the Journal of Leukocyte Biology examines the effect of endotoxin treatment on human monocyte metabolic plasticity and antimicrobial functions. This is a prospective study in which human volunteers were treated with low dose endotoxin. Monocyte metabolism, cytokine production and anti-microbial functions were measured before and after endotoxin exposure. The contribution of specific metabolic pathways to monocyte cytokine production and reactive oxygen species generation were also measured following in vitro exposure of naïve monocytes to LPS. The authors report that naïve monocytes, but not monocytes from endotoxin-primed volunteers, exhibit metabolic plasticity in response to ex vivo challenge with LPS. Metabolic plasticity was characterized by induction of glycolysis, the pentose phosphate pathway, TCA cycle and metabolism of pyruvate, glutamate, glutathione and amino acids in conjunction with decreased lipid metabolism. Monocytes from endotoxin-primed subjects also showed altered antimicrobial functions and cytokine production when compared to naïve monocytes. The authors conclude that in vivo exposure to LPS induces an immunotolerant phenotype characterized by loss of metabolic plasticity and antimicrobial efficacy (Figure 1). The authors speculate that a similar loss of metabolic plasticity in the immune cells of septic patients may be a fundamental mechanism underlying sepsis-induced immunoparalysis. The authors postulate that the identified metabolic pathways have the potential to reveal novel therapeutic targets to ameliorate or reverse the immunotolerant state and perhaps sepsis-induced immune dysfunction.
Figure 1.
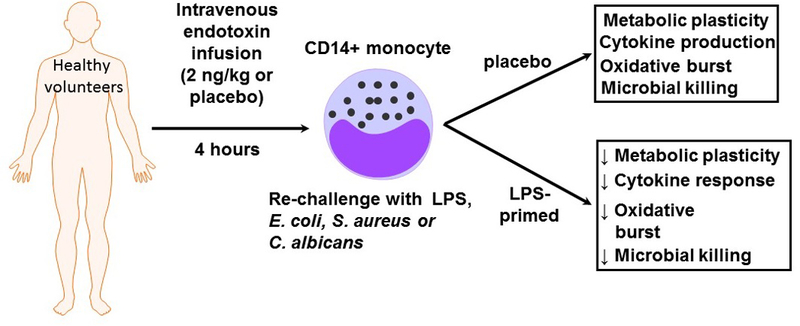
Endotoxin induced immunotolerant monocytes show a loss of metabolic plasticity and antimicrobial function. In this study, Grondman and colleagues administered endotoxin (2 ng/kg) or placebo to normal, healthy male volunteers in an ICU setting. CD14+ monocytes were harvested at 4 hrs and 7 days after endotoxin infusion and treated with microbial stimuli. At four hours after endotoxin infusion, immunotolerant CD14+ monocytes showed a loss of metabolic plasticity which correlated with decreased antimicrobial activity. The authors postulate that these findings may have important implications for our understanding of sepsis induced immune paralysis.
The authors also investigated the contributions of metabolic pathways to microbe-induced pro-inflammatory cytokine production in naïve monocytes. They observed that inhibition of glutaminolysis significantly suppressed both S. aureus and C. albicans induced TNFα production, while inhibition of glycolysis reduced C. albicans stimulated TNFα and IL-1β production. However, none of the metabolic inhibitors significantly attenuated E. coli induced proinflammatory cytokine production in naïve monocytes. Interestingly, stimulation of the TCA cycle with dichloroacetate (DCA) enhanced cytokine production in naïve monocytes stimulated with either E. coli, S. aureus, or C. albicans. The data suggests that aerobic glycolysis regulates monocyte responses to different microbial stimuli and that those responses are altered at 4 hours after LPS exposure.
The authors are to be commended for their critical investigation into the impact of endotoxin exposure on the metabolic response of human monocytes to secondary LPS challenge and its association with cytokine production and antimicrobial functions. Although numerous alterations in immune function have been reported in septic patients, i.e. T effector cell apoptosis, generation of myeloid-derived suppressor cells (MDSCs) and regulatory T cells, there is also significant evidence documenting monocyte dysfunction [7, 8]. The study by Grondman et al advances our understanding of the metabolic response of monocytes to endotoxin challenge and how prior endotoxin exposure changes that response. Execution of this study in human volunteers is attractive since questions have been raised regarding the validity of extrapolating results from sepsis studies performed in experimental animals into humans [4]. This study circumvents that concern. However, the use of human systems limits the scope and depth of mechanistic studies that can be performed. Thus, animal models may be useful for gaining a deeper understanding of the cellular and molecular mechanisms underlying the reported findings.
An interesting aspect of this research is that metabolic plasticity is specific to the individual. Individual variability between healthy subjects is well known to investigators involved in translational or clinical research. When studying septic patients individual variability can be far greater depending on a number of factors. While the number of subjects in this study is too small to draw any meaningful conclusions, it is interesting to speculate that healthy subjects and septic patients might group into responders and non-responders.
An important factor to consider when interpreting these data is the timing of sample harvesting. Samples were taken at 4 hours and 7 days after endotoxin exposure. At the 4 hour time point, it is likely that monocytes are still in an acute state of activation, which is likely to make them refractory to further stimulation. By 7 days, the measured endpoints had returned to baseline status. Addition of 24 and/or 48 hour samples would be very informative since this would allow monocytes to have progressed beyond the acute activation window, but they would still retain memory of the endotoxin exposure. Sepsis is a progressive and evolving condition in which the immune phenotype changes over time. By focusing on the 4 hour and 7 day time points the authors have provided the reader with important insights into the early and late phases of immunotolerance.
Although this study provides new information about the monocyte response to endotoxin exposure and challenge, several important points must be considered before applying these findings to patients with sepsis-induced immune paralysis. First of all, this is a small study which should be viewed as foundational in nature. Of equal importance, low dose endotoxin administration does not fully mimic the pathophysiology of sepsis. The endotoxin infusion protocol employed in this study is a useful and controlled model but induces a much less severe insult than that suffered by septic patients which, by definition, results in organ injury and prolonged hospitalization. The endotoxin-treated volunteers in this study recover fully without residual functional deficits and most of the measured endpoints return to baseline at 7 days after endotoxin exposure indicating that the alterations induced by this model are transient. In contrast, a large proportion of septic patients develop severe and long lasting functional disabilities. Most septic patients develop secondary infections weeks to months after diagnosis with many dying in the first year after hospital discharge. Therefore, it is difficult to extrapolate the global and immunological impact of this model to what happens in a septic patient. In addition, the present study was performed in healthy male volunteers 18–35 years of age. According to the Sepsis Alliance, approximately 65% of sepsis cases occur in patients over the age of 65. Therefore, the cohort employed in this study is not representative of patient populations that most commonly develop sepsis.
When considered as a whole, the study of Grondman and colleagues provides important insights into metabolic and antimicrobial changes that occur in human monocytes early after low dose endotoxin exposure. However, it is difficult to say whether these findings have significant implications for our understanding of the immune paralysis that plagues many sepsis patients. Future studies evaluating metabolic plasticity and antimicrobial function in monocytes and other immunocytes derived from sepsis patients would be valuable for validating and extending the findings reported in the present study.
Acknowledgements
This work was supported, in part, by NIH GM083016 (Li and Williams), HL071837 (Li), GM119197 (Sherwood and Williams) and C06RR0306551 (ETSU).
Footnotes
Disclosure
The authors have no conflict of interest.
References
- 1.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K, International Forum of Acute Care T: Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 2016, 193(3):259–272. [DOI] [PubMed] [Google Scholar]
- 2.Mayr FB, Yende S, Angus DC: Epidemiology of severe sepsis. Virulence 2014, 5(1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall JC: Why have clinical trials in sepsis failed? Trends Mol Med 2014, 20(4):195–203. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC: The search for effective therapy for sepsis: back to the drawing board? Jama 2011, 306(23):2614–2615. [DOI] [PubMed] [Google Scholar]
- 5.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ: Late mortality after sepsis: propensity matched cohort study. Bmj 2016, 353:i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angus DC, Opal S: Immunosuppression and Secondary Infection in Sepsis: Part, Not All, of the Story. Jama 2016, 315(14):1457–1459. [DOI] [PubMed] [Google Scholar]
- 7.Boomer JS, Green JM, Hotchkiss RS: The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence 2014, 5(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bomans K, Schenz J, Sztwiertnia I, Schaack D, Weigand MA, Uhle F: Sepsis Induces a Long-Lasting State of Trained Immunity in Bone Marrow Monocytes. Front Immunol 2018, 9:2685. [DOI] [PMC free article] [PubMed] [Google Scholar]


