Abstract
Hepatic encephalopathy (HE) is associated with poor quality of life, sharply increased mortality, repeated hospitalizations, falls, and motor vehicle accidents. HE manifests with a dynamic spectrum of severity. Overt HE is clinically obvious disorientation, even coma. Although multiple strategies are available to characterize early stage HE, data are limited validating these methods in predicting overt HE, many are impractical in clinical practice, and test cutoffs relevant to the average patient clinicians manage are lacking. In order to accurately and efficiently classify the risk of overt HE in the population with cirrhosis, novel strategies may be needed. Herein, we review the potential competing strategies for the prediction of overt HE. We propose refining diagnostic cutoffs for tests designed to define early HE using overt HE as a gold standard and expanding prediction tools by using measures of components from the risk pathway for HE.
Keywords: Portal Hypertension, Psychometric testing, Falls, Motor vehicle accidents
Introduction
The prevalence of cirrhosis is rising.(1) Owing to epidemic obesity and nonalcoholic fatty liver disease, this trend is expected to accelerate, substantially increasing the global burden of persons with cirrhosis and its complications.(2) Among the complications of cirrhosis, none are more complex than hepatic encephalopathy (HE). It is associated with poor quality of life (for both patients and caregivers), sharply increased mortality, repeated hospitalizations, falls, and motor vehicle accidents.(3–6) A volatile condition, HE is characterized by unpredictable changes in cognitive function and progressive disability.(6, 7) HE manifests with a dynamic spectrum of severity.(8) Overt HE is clinically obvious; disorientation to person or place or time, asterixis, lethargy (grade 2); complete disorientation or somnolence (grade 3);and coma (grade 4).(9) Early or covert HE is subtler, including deficits in executive function and attention (minimal HE) and decreased awareness (grade 1). Compared to patients with cirrhosis without HE, even those with early HE are at higher risk of adverse outcomes.(10, 11) Classification of a patient’s risk for overt HE may allow for closer monitoring, lifestyle modification, earlier treatment, and the opportunity to prevent associated complications such as falls and motor vehicle accidents.(12) Although multiple strategies are available to characterize early stage HE, data are limited validating these methods in predicting overt HE, many are impractical in clinical practice, and test cutoffs relevant to the average patient clinicians manage on a daily basis are lacking. In order to accurately and efficiently classify the risk of overt HE in the population with cirrhosis, novel strategies may be needed. Herein, we review the potential competing strategies for the prediction of overt HE. We propose refining diagnostic cutoffs for tests designed to define early HE using overt HE as a gold standard and expanding prediction tools by using measures of components from the risk pathway for HE.
The HE risk pathway
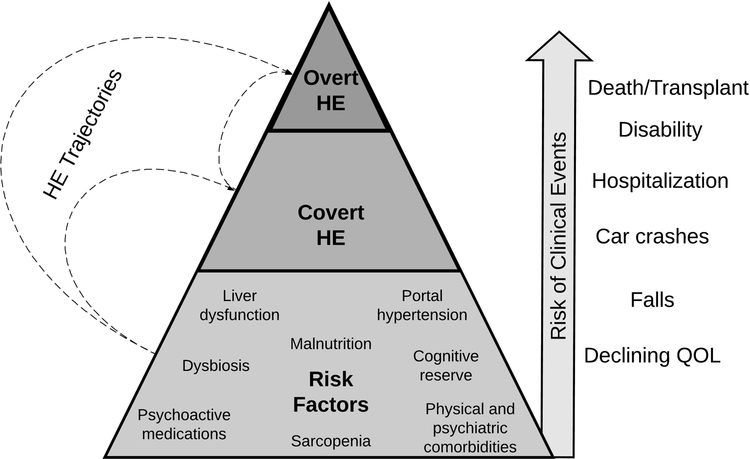
Although they are distinct in their clinical presentation, covert and overt HE share a common biology. The spectrum of cognitive dysfunction in cirrhosis is predated substantially by the development of the risk pathway for HE. Clinically apparent HE is caused by a combination of adverse trends in a patient’s peripheral ammonia concentration, burden of inflammation, and inter-organ glutamine trafficking.(13) These mechanisms, however, are secondary to other, earlier processes, most of which are readily measurable.(Figure 1)
Figure: The Spectrum of Hepatic Encephalopathy-Related Risks.
HE = hepatic encephalopathy, QOL = quality of life
Above all, as liver dysfunction progresses, the risk of HE rises. This risk can be quantified using simple labs and examination findings – the model for endstage liver disease (MELD), Child classification, and a score including bilirubin and albumin have each been shown to predict the development of HE.(14–16)
Beyond measures of liver function, portal hypertension (as captured by thrombocytopenia, varices, portal manometry) is independently associated with the risk of HE,(17) reflecting increased systemic distribution of neurotoxic substances from the splanchnic circulation via portosystemic shunting.
Owing to skeletal muscle’s role in ammonia metabolism, sarcopenia is associated with hyperammonemia and can be observed clinically,(18) measured directly using conventional imaging tools.
The peripheral (shunted) burden of gut bacteria is pro-inflammatory and strongly linked to the development of cognitive dysfunction in patients with cirrhosis.(19, 20) The gastrointestinal microbiome is accessible at least in the context of research studies and its specific constituents are associated with (or causally linked to) the risk of HE.(21, 22) Inflammatory cytokines are not routinely measured in clinical practice, however should they become commercially available they may discriminate risk for HE.(20)
Medication lists are easily abstracted from the medical record. Some medication classes may modify the gut’s production of ammonia by altering microbial characteristics (i.e. proton pump inhibitors), modulating enteric glutaminase activity (i.e. metformin), or by altering gut motility (i.e. opioids).(23–25) Other medication classes, namely gabapentinoids and benzodiazepines, may exacerbate the neurocognitive effects of cerebral ammonia exposure.
Predicting the risk of overt HE by identifying covert HE
Covert HE is a risk factor for the development of overt HE. For this reason, the American Association for the Study of Liver Disease recommends that patients with cirrhosis should be evaluated for the presence of early grade HE by experienced examiners.(26) Many tools are available (Table 1). These include paper-pencil tests (e.g. Portosystemic HE Score; PHES), computer programs (EncephalApp), electroencephalography (EEG), and critical flicker fusion (CFF). Several factors, however, complicate this recommendation’s clinical implementation.
Table:
Strengths and Limits of Previously Validated Strategies for the Prediction of Hepatic Encephalopathy
| Domains | Factors | Competing Strategies for the Evaluation of the Risk of Hepatic Encephalopathy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EEG | CFF | PHES | Encephal-app | ICT | SIP | ANT | BABS Score | Child Class / MELD score | Physical exam for grade 1 HE | ||
| Ease of use | Can be performed at point-of-care | - | ● | - | ● | ● | ● | ● | ● | ● | ● |
| Takes < 5 minutes | - | - | - | - | - | ● | ● | ● | ● | ● | |
| Takes < 10 minutes | - | ● | - | ● | - | ● | ● | ● | ● | ● | |
| Requires trained staff | ● | ● | ● | - | - | - | - | - | - | ● | |
| Special equipment | ● | ● | ● | - | - | - | - | - | - | - | |
| Quality of data | Validated using established psychometric tests | ● | ● | ● | ● | ● | ● | ● | - | - | ● |
| Cutoffs validated to predict outcomes prediction | - | - | - | - | - | - | - | ● | ● | ● | |
| Used to predict outcomes | ● | ● | ● | ● | ● | - | ● | ● | ● | ● | |
| Important subgroups excluded from prior study | ● | ● | ● | ● | ● | ● | ● | - | - | - | |
| Test characteristics | Applicable to large populations | - | - | - | - | - | - | - | ● | ● | ● |
| High positive predictive value for outcomes | ● | - | - | - | ● | - | - | - | - | - | |
| High negative predictive value | ● | - | - | ● | ● | - | - | ● | ● | ● | |
ANT= animal naming test (number of unique animals named in 60 seconds(43)); BABS = Bilirubin, Albumin, Beta-Blocker(16), Statin; CFF= critical flicker fusion; EEG = electroencephalography; ICT = inhibitory control test; MELD = Model for Endstage Liver Disease, PHES = psychometric hepatic encephalopathy score, SIP = sickness impact profile (age, sex, and questions about irritability, appetite, interest in activities, and balance(44)).
First, HE is not always a linear progression from normal to overt HE through covert stages.(Figure 1) Many patients without covert HE are at risk for overt HE.(10, 11, 27) In a study of 170 patients without a history of overt HE who underwent neuropsychological testing for covert HE, Patidar et al found that the 1-year risk of overt HE was 34% in patients with covert HE compared to 18% in those without.(10) Although refinements in the evaluation of covert HE could improve risk capture, the classification of early grade HE is fundamentally complicated by the lack of a true gold-standard.
Second, population-based strategies for the evaluation of covert HE are lacking. Having been excluded from studies of covert HE, many at-risk patients are not suitable candidates for the tools validated to identify cognitive dysfunction in the setting of cirrhosis. This includes patients with alcohol use, psychoactive medications, and cardiopulmonary and renal comorbidities,(28) clinical factors that may be present in the majority of contemporary patients with cirrhosis.(16) The result is a clinically meaningful chasm between efficacy (what can be shown in experimental conditions free of confounders) and effectiveness (how a test performs for the patients encountered in practice).
Third, cutoffs for neuropsychological or neurophysiological assessments to predict overt HE among real-world patients have not been established. As shown by Bajaj et al(29), tests of cognitive function may retain their predictive power in less controlled cohorts, but not with the same diagnostic cutoffs or test characteristics. Even in highly selected cohorts, cutoffs suggestive of minimal or covert HE vary widely.(30) Each test is internally valid and capable of distinguishing covert HE from normal controls in the experimental context but poorly generalizable across studies. Insufficiently harmonized test characteristics therefore sharply limits external validity. The consequence is unacceptably imprecise outcome prediction. Flud and Duarte-Rojo found in a review that the proportion who developed overt HE after a diagnosis of minimal HE varied from 10% to 40%.(31)
Fourth, the grading of neurocognitive status is highly variable between studies. Standard psychometric tests (such as the PHES) are graded relative to performance by age and sex matched controls.(32) However, normal controls from one center could be interpreted as cognitively impaired relative to control performance from another.(27, 30) In our analysis of a nationally representative cohort who underwent psychometric testing, we found that factors which are unmatched in studies of minimal HE such as education, comorbidities (e.g. diabetes, obesity), smoking, and remote alcohol history significantly impact psychometric test performance.(30, 33) These differences in control selection between studies are compounded by inter-rater variation of test interpretation within studies.(34)
Fifth, most clinicians do not use neurocognitive tests for a variety of reasons including the time required and that the recommended “experienced examiners” are scarce resources.(35)
Finally, as it relates to its prognostic implications, the very construct of covert HE which lumps minimal with grade 1 HE, is controversial. In two recent prospective studies, a diagnosis of grade 1 HE by physical examination has significantly greater long-term prognostic significance than a diagnosis of minimal HE determined using psychometric testing.(11, 36) In these studies, patients with minimal HE experienced risks of decompensation and death no different from those without cognitive impairment.(11, 36) It is, however, challenging for the average clinician to discern normal from abnormal cognitive function based on routine clinical assessment. In a study examining the classification of standardized patients with various grades of HE presented by video,(37) Reuter et al found that half of the hepatologists enrolled (from experienced transplant centers) could not distinguish between standardized patients with cirrhosis and no HE and those with grade 1 HE. Given these data, ‘covert HE’ may misclassify risk through over-diagnosis while particularly diagnoses of grade 1 HE may have imperfect inter-rater reliability limiting generalizeability. To resolve this conflict, prospective, multicenter comparisons of the relative ability for covert and grade 1 HE to accurately classify the risk of overt HE are needed.
Predicting the risk of overt HE along HE risk pathways
An alternative to using the presence of minimal or grade 1 HE as the principle predictor of overt HE is risk-pathway based assessments. There are multiple examples.
The oral glutamine challenge is a physiologic test which captures glutaminase activity and excessive peripheral ammonia (reflecting microbial ‘function’) after a glutamine load. Elevated ammonia levels after the challenge can predict overt HE.(38) The remaining risk-pathway based assessments require prospective validation.
Clinical scores based on routinely available measures of severity of liver disease are effective predictors of overt HE. Either Child class or MELD alone can predict the development of overt HE and other important clinical outcomes.(14, 15) We recently developed a risk score – the BABS score (Table 1) – based on bilirubin, albumin, nonselective beta-blocker use (reflecting varices), and statin use.(16) Patients with low scores (≤0) had an 89% negative predictive value for the development of overt HE over the following year.
Sarcopenia (e.g. low skeletal muscle index at the level of the 3rd lumbar vertebra) has been linked with the development of overt HE in a cohort of portosystemic shunt recipients.(18) Though promising, data are limited regarding the role of bedside measures of muscle bulk and function in this context. Given mounting interest in sarcopenia as a general risk biomarker in cirrhosis, such studies are likely highly feasible by collecting data on new HE (and other decompensations) in addition to conventional outcomes such as transplant-free survival.
Medication burden is also associated with the development of HE. Prior studies have implicated proton pump inhibitors, benzodiazepines, nonselective betablockers.(16, 39, 40) Whether these findings causally related or correlated is debatable. Regardless, they are effective biomarkers of risk that can be efficiently abstracted at the population level for risk-assessment.
Implementing Outcome Prediction
Calibration of cutoffs in existing modalities
Outcomes should be used to calibrate psychometric test cutoffs. However, each modality may need multiple test cutoffs for two reasons. First, there are multiple HE-related outcomes of value for at-risk patients including overt HE, falls, poor health-related quality of life, and mortality.(Figure 1) Second, even the same outcome may need cutoffs tailored to the clinical context. Scores that are predictive in decompensated cirrhosis may not provide risk-discrimination in patients with compensated disease. Furthermore, cutoffs should be lower to maximize sensitivity and reduce the risk of false negatives among, for example, transplant-waitlisted patients with Child C cirrhosis. Conversely, cutoffs should be higher to maximize specificity and minimize the risk of a false positive among highly functional patients with Child A cirrhosis. Mirroring recommendations for the diagnosis of covert HE,(41) some patients may benefit from ‘screening’ using simple tests with cutoffs conditioned to provide high sensitivity/negative predictive value followed by tests with cutoffs that aim for specificity/positive predictive value.
New directions
Prediction of HE can utilize established psychometric and neurophysiologic tools but could be expanded. First, many elements of the risk pathway for HE can be ascertained at the bedside and incorporated as predictors. These include measures of liver function (or medications consistent with advanced liver disease), sarcopenia (clinical muscle depletion or radiographic evidence),(18) frailty (weakness or disability),(42) portal hypertension (the presence of varices or portal pressure), and burden of psychoactive medications.(29) Studies to validate such biomarkers must be prospective cohort studies that employ rigorous definitions of HE outcomes and should, preferably, compare multiple biomarkers/modalities simultaneously. Second, these factors may also serve as targets for therapeutic interventions including improved nutrition (to improve or maintain muscle mass), physical therapy or exercise (to improve strength and balance to prevent falls), and strategic de-prescribing of psychoactive medications. Accordingly, to validate alternative, risk-pathway based predictors of HE in the context of an intervention study would involve demonstrating decreased incident HE in patients without (but at-risk for) HE (primary prophylaxis) or reduced hospital-days or readmissions in patients with prior overt HE (secondary prophylaxis).
Pitfalls for outcome prediction
Using outcome prediction as a gold-standard poses 3 main pitfalls. First, existing data for overt HE prediction are limited. New prospective studies will be needed but can be supplemented with patient-level meta-analyses of published studies. For example, multiple small cohorts have been followed after baseline assessment (e.g. inhibitory control test)(30); these cohorts can be combined and the pooled risk of overt HE can be used to refine test cutoffs (to one that is not defined by cognitive performance but outcome prediction). Second, for each strategy there are tradeoffs in accuracy and inclusion related to the test’s simplicity, cost, and resource availability. It is unclear how this will impact comparisons across tests. Tests which have not been validated in patients taking psychoactive medications, for example, exclude from their denominator an important component of the at-risk population. Conversely, tests which employ administrative data (such as our score based on billing codes, standard laboratory tests, and pharmacy records(16)), apply to more patients but lack potentially important measures of baseline cognitive function. Third, generalizable test-cutoffs are dependent on standardized definitions of outcomes which challenging even in the clinical trial setting.(9) “Overt HE” defined using administrative data may differ in important ways from “overt HE” discovered in prospective research, affecting predictive model characteristics. Similar pitfalls in outcome definition will be present for alternative end-points such as motor-vehicle accidents (i.e. self-reported versus driver registry-based(4)) or quality of life (which can be dynamic).
Conclusions
The goal of predicting overt HE is to inform patients and implement interventions that mitigate the risk of progression. In order to predict overt HE in the population of patients with cirrhosis whom we encounter in our clinics, we need new or recalibrated methods that are broadly applicable, and validated to predict meaningful outcomes. New data are needed to distinguish competing strategies on the basis of their ability to discern risk for adverse events that range from the development of overt HE to poor quality of life, falls, admissions, and death. An enhanced ability to risk stratify HE will improve the design of intervention studies to mitigate these risks.
Acknowledgements:
The author would like to thank several people for their thoughtful, constructive criticisms: Drs. Anna Lok, Andres Duarte-Rojo, Vilas Patwardhan, and Neil Sengupta
Funding: Elliot Tapper receives funding from the National Institutes of Health through the Michigan Institute for Clinical and Health Research (KL2TR002241).
Footnotes
Disclosure:
Elliot Tapper is the guarantor of this article
Conflicts of interest: Dr. Tapper served on an advisory council for Salix in 2018.
References
- 1.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015;149:1471–1482. e1475. [DOI] [PubMed] [Google Scholar]
- 2.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of hepatology 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj JS, Saeian K, Schubert CM, Hafeezullah M, Franco J, Varma RR, Gibson DP, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009;50:1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezaz G, Murphy SL, Mellinger J, Tapper EB. Increased Morbidity and Mortality Associated with Falls among Patients with Cirrhosis. The American journal of medicine 2018. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, Stravitz RT, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol 2011;106:1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapper EB, Halbert B, Mellinger J. Rates of and Reasons for Hospital Readmissions in Patients with Cirrhosis: A Multistate Population-based Cohort Study. Clinical Gastroenterology and Hepatology 2016. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology 2009;50:2014–2021. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj J, Frederick R, Bass N, Ghabril M, Coyne K, Margolis M, Santoro M, et al. Overt hepatic encephalopathy: development of a novel clinician reported outcome tool and electronic caregiver diary. Metabolic brain disease 2016;31:1081–1093. [DOI] [PubMed] [Google Scholar]
- 10.Patidar KR, Thacker LR, Wade JB, Sterling RK, Sanyal AJ, Siddiqui MS, Matherly SC, et al. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. The American journal of gastroenterology 2014;109:1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomsen KL, Macnaughtan J, Tritto G, Mookerjee RP, Jalan R. Clinical and pathophysiological characteristics of cirrhotic patients with grade 1 and minimal hepatic encephalopathy. PloS one 2016;11:e0146076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajaj JS, Pinkerton SD, Sanyal AJ, Heuman DM. Diagnosis and treatment of minimal hepatic encephalopathy to prevent motor vehicle accidents: A cost‐effectiveness analysis. Hepatology 2012;55:1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: a physiology-driven approach to the treatment of hepatic encephalopathy. In: Mayo Clinic Proceedings; 2015: Elsevier; 2015. p. 646–658. [DOI] [PubMed] [Google Scholar]
- 14.Gomez EV, Rodriguez YS, Bertot LC, Gonzalez AT, Perez YM, Soler EA, Garcia AY, et al. The natural history of compensated HCV-related cirrhosis: a prospective long-term study. Journal of hepatology 2013;58:434–444. [DOI] [PubMed] [Google Scholar]
- 15.Greinert R, Ripoll C, Hollenbach M, Zipprich A. Stepwise diagnosis in covert hepatic encephalopathy: critical flicker frequency and MELD‐score as a first‐step approach. Alimentary pharmacology & therapeutics 2016;44:514–521. [DOI] [PubMed] [Google Scholar]
- 16.Tapper EB, Parikh N, Sengupta N, Mellinger J, Ratz D, Lok AS, Su GL. A Risk Score to Predict the Development of Hepatic Encephalopathy in a Population‐Based Cohort of Patients with Cirrhosis. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 17.Lens S, Rincón D, García-Retortillo M, Albillos A, Calleja JL, Bañares R, Abraldes JG, et al. Association between severe portal hypertension and risk of liver decompensation in patients with hepatitis C, regardless of response to antiviral therapy. Clinical Gastroenterology and Hepatology 2015;13:1846–1853. e1841. [DOI] [PubMed] [Google Scholar]
- 18.Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, Merli M, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clinical Gastroenterology and Hepatology 2017;15:934–936. [DOI] [PubMed] [Google Scholar]
- 19.Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2010;51:1062–1069. [DOI] [PubMed] [Google Scholar]
- 20.Montoliu C, Piedrafita B, Serra MA, del Olmo JA, Urios A, Rodrigo JM, Felipo V. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. Journal of clinical gastroenterology 2009;43:272–279. [DOI] [PubMed] [Google Scholar]
- 21.Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 2017;66:1727–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj JS, Matin P, White MB, Fagan A, Deeb JG, Acharya C, Dalmet SS, et al. Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. American Journal of Physiology-Gastrointestinal and Liver Physiology 2018;315:G824–G837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acharya C, Betrapally N, Gillevet P, Sterling R, Akbarali H, White M, Ganapathy D, et al. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Alimentary pharmacology & therapeutics 2017;45:319–331. [DOI] [PubMed] [Google Scholar]
- 24.Bajaj JS, Acharya C, Fagan A, White MB, Gavis E, Heuman DM, Hylemon PB, et al. Proton Pump Inhibitor Initiation and Withdrawal affects Gut Microbiota and Readmission Risk in Cirrhosis. The American journal of gastroenterology 2018:1. [DOI] [PubMed] [Google Scholar]
- 25.Romero-Gómez M, Jover M, Del Campo JA, Royo JL, Hoyas E, Galán JJ, Montoliu C, et al. Variations in the promoter region of the glutaminase gene and the development of hepatic encephalopathy in patients with cirrhosis: a cohort study. Annals of internal medicine 2010;153:281–288. [DOI] [PubMed] [Google Scholar]
- 26.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 27.Allampati S, Duarte-Rojo A, Thacker LR, Patidar KR, White MB, Klair JS, John B, et al. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. The American journal of gastroenterology 2016;111:78–86. [DOI] [PubMed] [Google Scholar]
- 28.Tapper EB PN, Waljee AK, Volk M, Carlozzi NE, Lok AS. Diagnosis of Minimal Hepatic Encephalopathy: A Systematic Review of Point-of-Care Diagnostic Tests. Am J Gastroenterol 2018. [DOI] [PubMed] [Google Scholar]
- 29.Bajaj JS, Thacker LR, Heuman DM, Sterling RK, Stravitz RT, Sanyal AJ, Luketic V, et al. Cognitive performance as a predictor of hepatic encephalopathy in pretransplant patients with cirrhosis receiving psychoactive medications: a prospective study. Liver Transplantation 2012;18:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tapper EB, Parikh ND, Waljee AK, Volk M, Carlozzi NE, Lok AS. Diagnosis of Minimal Hepatic Encephalopathy: A Systematic Review of Point-of-Care Diagnostic Tests. The American Journal of Gastroenterology 2018. [DOI] [PubMed] [Google Scholar]
- 31.Flud CR, Duarte-Rojo A. Prognostic implications of minimal/covert hepatic encephalopathy: large-scale validation cohort studies. Journal of Clinical and Experimental Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissenborn K, Ennen JC, Schomerus H, Rückert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. Journal of hepatology 2001;34:768–773. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Matos MC, Jiang ZG, Tapper EB. Factors That Affect Results of Psychometric Tests to Identify Patients With Minimal Hepatic Encephalopathy. Clinical Gastroenterology and Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amodio P, Pellegrini A, Ubiali E, Mathy I, Del Piccolo F, Orsato R, Gatta A, et al. The EEG assessment of low-grade hepatic encephalopathy: comparison of an artificial neural network-expert system (ANNES) based evaluation with visual EEG readings and EEG spectral analysis. Clinical Neurophysiology 2006;117:2243–2251. [DOI] [PubMed] [Google Scholar]
- 35.Bajaj JS, Etemadian A, Hafeezullah M, Saeian K. Testing for minimal hepatic encephalopathy in the United States: an AASLD survey. Hepatology 2007;45:833–834. [DOI] [PubMed] [Google Scholar]
- 36.Montagnese S, Balistreri E, Schiff S, De Rui M, Angeli P, Zanus G, Cillo U, et al. Covert hepatic encephalopathy: agreement and predictive validity of different indices. World Journal of Gastroenterology: WJG 2014;20:15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuter B, Walter K, Bissonnette J, Leise MD, Lai J, Tandon P, Kamath PS, et al. Assessment of the spectrum of hepatic encephalopathy: A multicenter study. Liver Transplantation 2018;24:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero-Gómez M, Grande L, Camacho I, Benitez S, Irles JA, Castro M. Altered response to oral glutamine challenge as prognostic factor for overt episodes in patients with minimal hepatic encephalopathy. Journal of hepatology 2002;37:781–787. [DOI] [PubMed] [Google Scholar]
- 39.Nardelli S, Gioia S, Ridola L, Farcomeni A, Merli M, Riggio O. Proton pump inhibitors are associated to minimal and overt hepatic encephalopathy and increase mortality in cirrhotics. Hepatology. [DOI] [PubMed] [Google Scholar]
- 40.Grønbæk L, Watson H, Vilstrup H, Jepsen P. Benzodiazepines and risk for hepatic encephalopathy in patients with cirrhosis and ascites. United European gastroenterology journal 2018;6:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patidar KR, Bajaj JS. Covert and overt hepatic encephalopathy: diagnosis and management. Clinical Gastroenterology and Hepatology 2015;13:2048–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tapper EB, Konerman M, Murphy S, Sonnenday CJ. Hepatic encephalopathy impacts the predictive value of the Fried Frailty Index. American Journal of Transplantation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campagna F, Montagnese S, Ridola L, Senzolo M, Schiff S, De Rui M, Pasquale C, et al. The animal naming test: an easy tool for the assessment of hepatic encephalopathy. Hepatology 2017;66:198–208. [DOI] [PubMed] [Google Scholar]
- 44.Nabi E, Thacker LR, Wade JB, Sterling RK, Stravitz RT, Fuchs M, Heuman DM, et al. Diagnosis of covert hepatic encephalopathy without specialized tests. Clinical Gastroenterology and Hepatology 2014;12:1384–1389. e1382. [DOI] [PMC free article] [PubMed] [Google Scholar]